Abstract
High-fat diets lead to obesity, inflammation, and dysglycemia. 12-Lipoxygenase (12-LO) is activated by high-fat diets and catalyzes the oxygenation of cellular arachidonic acid to form proinflammatory intermediates. We hypothesized that 12-LO in the pancreatic islet is sufficient to cause dysglycemia in the setting of high-fat feeding. To test this, we generated pancreas-specific 12-LO knockout mice and studied their metabolic and molecular adaptations to high-fat diets. Whereas knockout mice and control littermates displayed identical weight gain, body fat distribution, and macrophage infiltration into fat, knockout mice exhibited greater adaptive islet hyperplasia, improved insulin secretion, and complete protection from dysglycemia. At the molecular level, 12-LO deletion resulted in increases in islet antioxidant enzymes Sod1 and Gpx1 in response to high-fat feeding. The absence or inhibition of 12-LO led to increases in nuclear Nrf2, a transcription factor responsible for activation of genes encoding antioxidant enzymes. Our data reveal a novel pathway in which islet 12-LO suppresses antioxidant enzymes and prevents the adaptive islet responses in the setting of high-fat diets.
INTRODUCTION
Obesity, typically a result of diets high in saturated fat content, is directly correlated to insulin resistance and prediabetes. Macrovascular disease consequences, including stroke, myocardial infarction, and mortality increase even as blood sugars rise in the prediabetic phase (1), thus emphasizing the perils of glycemic dysregulation in the absence of frank diabetes. High-fat diets (HFDs) and their consequent insulin resistance lead to adaptive islet hyperplasia, in which basal secretion of insulin is increased in an attempt to compensate for insulin resistance (2). However, this compensatory mechanism can eventually fail in the face of prolonged insulin resistance. Inflammation and oxidative stress are major factors thought to contribute to dysfunction of β cells in the setting of insulin resistance (3, 4). In this regard, β cells have low expression and activity levels of the antioxidant enzymes compared to other metabolic tissues (5), and the pathway(s) that endogenously suppresses production of these antioxidant enzymes in the β cell remains unclear.
The lipoxygenases represent a group of enzymes that catalyzes the oxygenation of polyunsaturated fatty acids to form inflammatory lipid intermediates, which have been shown to contribute to oxidative stress (6). In the mouse, 12-lipoxygenase (12-LO) is expressed in several cell types, including macrophages, white adipocytes, and pancreatic islets (7). 12-LO oxygenates membrane-derived arachidonic acid primarily at the 12-position carbon atom to form 12-hydroperoxyeicosatetraenoic acid (12-HPETE), which is then converted to the more stable form 12-hydroxyeicosatetraenoic acid (12-HETE) (7). The role of 12-LO in the setting of HFDs and obesity has been studied primarily in the context of whole-body 12-LO knockout mice. Recent studies showed that 12-LO knockout mice fed a HFD exhibit reduced macrophage infiltration into adipose tissue, reduced insulin resistance, enhanced β cell function, and improved glucose tolerance compared to controls (8, 9). Tissue-specific deletion of 12-LO driven by the aP2-Cre transgene in mice led to similar protection from HFD-induced glucose intolerance (10), suggesting that the effects seen in the whole-body knockout might be attributable to effects of 12-LO deletion in adipocytes or macrophages. Although 12-LO expression in pancreatic islets is low to undetectable at baseline, its expression in islet β cells is increased in the setting of metabolic stresses, such as hyperglycemia, cytokine-mediated damage, and partial pancreatectomy (11–13). In none of the knockout animal models to date is it clear whether 12-LO in the islet could contribute to insulin secretory defects.
To address more directly the actions of 12-LO in the islet, we generated mice with specific deletion of 12-LO in islets and examined their response to cytokines and obesity-induced glucose intolerance. We show that deletion of 12-LO in the islet protected mice from glucose intolerance seen in both multiple low doses of streptozotocin (STZ) and from HFD feeding. Deletion of 12-LO increased antioxidant enzyme levels in islets and decreased islet oxidative stress, in part, through increased nuclear translocation of Nrf2. Our results indicate for the first time that 12-LO plays an important role in metabolic stress-induced dysfunction in islet β cells, and 12-LO activity in the islet itself is sufficient to induce whole-body glucose intolerance under conditions of HFD consumption.
MATERIALS AND METHODS
Animals and genotyping.
Experiments involving mice were performed under protocols approved by the Indiana University Institutional Animal Care and Use Committee. CD1 mice (for procurement of mouse islets) were purchased from Charles River Laboratories. Alox15Loxp/+ mice, in which Cre recombinase recognition sequences (Loxp) flank exons 2 to 5, were generated by Ozgene on a C57BL/6 genetic background. Alox15Loxp/+ mice were bred to Pdx1-Cre transgenic mice (14) to generate mice with a pancreas-specific knockout of 12-LO (Pdx1-Cre; Alox15Loxp/Loxp, referred to as pLO-KO mice).
Allele-specific genotyping was performed by PCR using primers flanking the 5′ LoxP site: 5′-CATGGGTTCTCTGCCTTAGTGG-3′, and 5′-TCACCTCAACCAAGAGTGAGAGGG-3′. Using these primers, the wild-type Alox15 allele generates an amplicon of 175 bp, and the mutant allele generates an amplicon of 251 bp. For analysis of genomic recombination in islets, hypothalamus, muscle, fat, and liver, DNA was extracted from these tissues using a commercially available kit (Sigma) and subjected to PCR using primers designed to amplify the recombined allele: 5′-TTTCCCATCGTCCATCGTCCATCC-3′ and 5′-TCCCTACTTGACCCAGACTACCAG-3′. The PCR product (173 bp) was analyzed using a 2% agarose gel, and its identify was confirmed by automated sequencing.
HFD feeding and animal procedures.
For experiments involving defined diet feeding, 8-week-old male wild-type and pLO-KO mice were placed on a normal chow diet (NCD) containing 18% kcal from fat, 58% kcal from carbohydrate, and 24% kcal from protein (2018S, Harlan Laboratories), or an HFD consisting of 42% kcal from fat, 43% kcal from carbohydrate (mostly sucrose), and 15% kcal from protein (TD.88137, Harlan Laboratories).
Blood glucose was measured weekly for 4 weeks using an AlphaTRAK glucometer (Abbott Laboratories). Body weights were measured weekly. Body composition was measured by using dual energy X-ray absorptiometry analysis (Lunar PIXImusII densitometer; GE Medical Systems). Glucose-stimulated insulin secretion in vivo, glucose tolerance tests (GTTs), and insulin tolerance tests were performed as described previously (15) with the following modifications: for the GTTs mice were injected intraperitoneally with 2 g/kg glucose after an overnight fast, and for the insulin tolerance tests mice were injected intraperitoneally with 0.75 U/kg insulin. For studies involving insulin signaling, mice were injected intraperitoneally with 10 U/kg regular human insulin or saline for 5 min prior to euthanasia, as described previously (16).
Islets and assays.
Mouse islets were isolated from collagenase-digested pancreas (17) and were allowed to recover overnight prior to experimentation. Islets were treated with a mixture of cytokines (10 ng/ml tumor necrosis factor alpha [TNF-α], 100 ng/ml gamma interferon [IFN-γ], and 5 ng/ml interleukin-1β [IL-1β]) or 100 nM 12-HETE for either 4 or 24 h.
Static glucose-stimulated insulin secretion assays using islets were performed as previously described (15), and insulin released into the medium was measured using a mouse insulin enzyme-linked immunosorbent assay (ELISA) kit (Alpco Diagnostics). 12-HETE released into the medium was measured by using a 12-HETE ELISA kit (Enzo Life Sciences). Insulin in mouse serum was measured by using an ultrasensitive mouse insulin ELISA kit (Crystal Chem), and serum proinsulin was measured using a mouse proinsulin ELISA kit (ALPCO Diagnostics), both according to the manufacturer's protocols. The preproinsulin unmethylation index from mouse serum was performed as described previously (18). Reactive oxygen species (ROS) were stained in isolated islets using CellROX Deep Red reagent (Life Technologies) and counterstained with Hoechst dye.
Immunostaining and morphometric assessment of β cell area.
Pancreata and adipose tissue from at least three different mice per group were fixed in 4% paraformaldehyde, paraffin embedded, and sectioned onto glass slides. The β cell area as a percentage of total pancreas area (β cell area%) was calculated as previously detailed (19). Chop and Ki67 staining was quantified by counting the number of Chop+ and Ki67+ nuclei in insulin+ cells per islet. 12-LO, ROS, 4-HNE, GPx1, SOD1, and catalase were quantified by measuring pixel density per insulin+ cell. Nrf2 was quantified by measuring nuclear and cytoplasmic pixel density per insulin+ cell. Images were acquired by using an Axio-Observer Z1 (Zeiss) inverted fluorescence microscope or an LSM 700 (Zeiss) confocal equipped with an Orca ER charge-coupled device camera (Hamamatsu).
Antibodies and immunoblots.
Immunohistochemistry of pancreas sections were performed using anti-insulin antibodies (sc-9168 [Santa Cruz Biotechnology]; 1:250) and horseradish peroxidase-conjugated secondary antibodies (Vector Laboratories). Adipose tissue sections were immunostained using anti-F4/80 antibodies (14-4801 [eBioscience]; 1:100) and horseradish peroxidase-conjugated secondary antibodies (Vector Laboratories). For immunofluorescence of pancreas sections, sections were stained with anti-12-LO (sc-27357 [Santa Cruz Biotechnology]; 1:100), anti-Ki67 (ab66155 [Abcam]; 1:200), anti-Chop antibody (sc-575 [Santa Cruz Biotechnology]; 1:200), anti-4-HNE antibody (Ab464545 [Abcam]; 1:100), anti-Gpx1 antibody (sc-22145 [Santa Cruz Biotechnology]; 1:200), anti-Sod1 antibody (sc-11407 [Santa Cruz Biotechnology]; 1:200), anticatalase antibody (sc-34285 [Santa Cruz Biotechnology]; 1:200), anti-Nrf2 antibody (sc-722 [Santa Cruz Biotechnology]; 1:200), and/or anti-insulin antibodies (Life Technologies; 1:250). Alexa Fluor 568–donkey anti-rabbit antibody and Alexa Fluor 488–donkey anti-guinea pig antibody were used as secondary antibodies (Invitrogen).
Whole-cell extracts from islets were resolved by electrophoresis on a 4 to 20% sodium dodecyl sulfate-polyacrylamide gel, followed by immunoblotting with anti-phospho-Akt (9271 [Cell Signaling]),anti-Akt (2920 [Cell Signaling]), antiactin (691001 [ImmunO]), anti-histone H3 (07-030 [Upstate]), anti-Nrf2 (sc-722 [Santa Cruz Biotechnology]), anti-Gpx1 (sc-22145 [Santa Cruz Biotechnology]), and anti-Chop (sc-575 [Santa Cruz Biotechnology]) primary antibodies and fluorophore-labeled secondary antibodies. Immunoblots were visualized by using the LiCor Odyssey system (LiCor Biosciences).
Quantitative real-time reverse transcription-PCR (RT-PCR).
Real-time PCR from reverse-transcribed RNA was performed using SYBR green technology and primers previously described as follows: Alox15 (20); Gpx1 and Sod1 (21); Catalase (22); Chop, Bip, Xbp1, Xbp1s, and Nos2 (23); and Nfe212 (24).
Statistical analysis.
All data are presented as means ± the standard errors of the mean. A Student t test was performed for comparisons involving two conditions and a one-way analysis of variance (with a Dunnett's post test) was performed for comparisons involving more than two conditions. Area-under-the-curve analyses were performed using the trapezoid method. GraphPad Prism (version 5.0) software was used for all statistical analyses. P values of <0.05 were considered significant.
RESULTS
Generation of pancreatic islet 12-LO knockout (pLO-KO) mice.
To obtain an islet-specific deletion of the mouse gene encoding 12-LO (Alox15), we first generated mice in which exons 2 to 5 of an Alox15 allele were flanked by Cre recombinase recognition sequences (Loxp), as shown in Fig. S1A in the supplemental material. The resultant mice were bred to homozygosity and then crossed to mice harboring the Pdx1-Cre transgene to remove 12-LO at the inception of pancreas development (henceforth referred to as pLO-KO mice). Because 12-LO is absent in the exocrine pancreas (25) (see Fig. S2A in the supplemental material), pLO-KO mice are essentially islet-specific 12-LO knockouts. PCR genotyping was used to confirm mice of differing genotypes (Alox15+/+, Alox15Loxp/+, and Alox15Loxp/Loxp) (see Fig. S1B in the supplemental material). Quantitative RT-PCR analysis showed significant reduction of the mRNA encoding 12-LO in islets but not in fat or liver; the hypothalamus does not exhibit detectable levels of the mRNA in control or pLO-KO animals (see Fig. S1C in the supplemental material).
Islets from pLO-KO mice exhibit resistance to cytokine toxicity in vitro.
Intraperitoneal GTTs were indistinguishable between 8-week-old pancreas-specific male and female pLO-KO and heterozygous knockout (pLO-HET) mice compared to control littermates (Alox15Loxp/Loxp mice not carrying the Pdx-Cre transgene) (see Fig. S1D and E in the supplemental material). Islets isolated from pLO-KO and control male mice exhibited identical glucose-stimulated insulin secretion in vitro (see Fig. S1F in the supplemental material). These data, consistent with those of the global 12-LO knockouts (26), suggest that the absence of 12-LO in pancreas has little or no impact on the development/allocation of islet cell types and islet function or on glucose homeostasis.
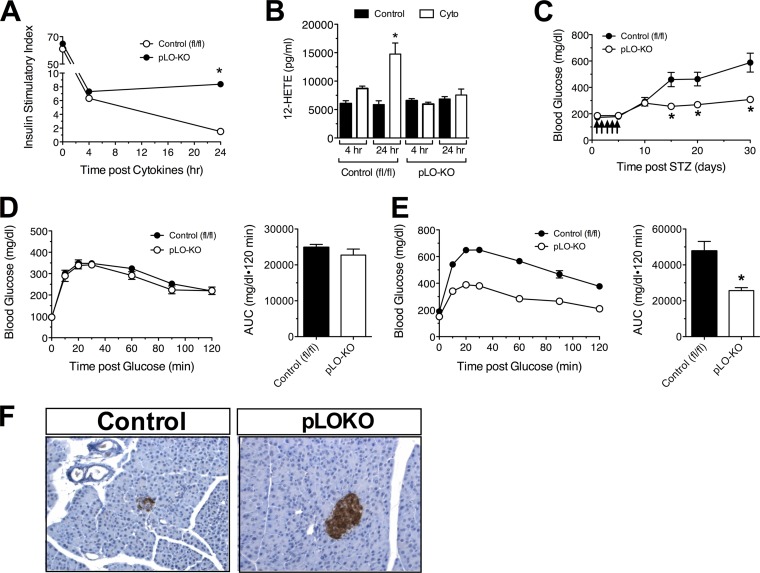
Most forms of diabetes involve proinflammatory cytokine-mediated dysfunction of islet β cells (27). In particular, it has been shown that in mouse models of obesity and type 2 diabetes, elevations in levels of proinflammatory cytokines IL-1-β, TNF-α, and IFN-γ occur (likely released from macrophages and adipose tissue) (28–30). To determine whether deletion of 12-LO confers resistance to proinflammatory cytokines, we next incubated islets from pLO-KO mice or control littermates with a cocktail of proinflammatory cytokines (IL-1β, TNF-α, and IFN-γ) in vitro at concentrations previously used in the literature (19, 31). As shown in Fig. S1F in the supplemental material, after 4 h of cytokine exposure, islets from both control and pLO-KO animals exhibited increased basal (low glucose) insulin secretion and diminished stimulated (high glucose) insulin secretion compared to islets not exposed to cytokines. However, after 24 h of cytokine treatment, islets from pLO-KO mice exhibited relatively lower insulin secretion at low glucose and greater insulin secretion at high glucose compared to islets from control littermates (see Fig. S1F in the supplemental material). The resulting insulin stimulatory index at 24 h was unchanged in pLO-KO islets compared to 4 h, whereas the index in control islets at 24 h continued to decline (Fig. 1A). These data suggest that deletion of 12-LO from islets results in delayed (>4 h) protection from cytokine toxicity. To clarify the mechanism underlying the role of 12-LO at 4 h versus 24 h of cytokine treatment, we examined 12-HETE (the product of 12-LO activity) secretion from islets. As shown in Fig. 1B, no 12-HETE production was detected in control or pLO-KO islets after 4 h of cytokine treatment. However, 12-HETE production was significantly increased in control islets after 24 h of cytokine treatment but not in pLO-KO islets (Fig. 1B). These data suggest that accumulation of the downstream proinflammatory lipid product of 12-LO, 12-HETE, is correlated with the severe dysfunction of islets seen after 24 h of cytokine exposure.
FIG 1.
Susceptibility of pLO-KO islets and mice to cytokine-induced dysfunction. (A) Insulin stimulatory index (ratio of insulin secretion at 2.5G:25G) from islets isolated from male control Alox15Loxp/Loxp (fl/fl) and pancreatic 12-LO knockout (pLO-KO) mice after 0, 4, and 24 h of cytokine treatment (n = 3 or 4 per group); (B) 12-HETE secretion from isolated control and pLO-KO islets after 0, 4, and 24 h of cytokine treatment (n = 3 or 4 per group); (C) male control and pLO-KO mice were subjected to multiple low-dose injections of STZ (55 mg/kg daily for 5 days), and random glucoses were monitored for 30 days (n = 5 or 6 animals per group); (D) glucose tolerance test at 10 days after the start of STZ injections (left panel) and the corresponding area under the curve (AUC) (right panel) (n = 5 or 6 animals per group); (E) glucose tolerance test at 30 days after the start of STZ injections (left panel), and the corresponding AUC (right panel) (n = 5 or 6 animals per group); (F) representative images of pancreata immunostained for insulin (brown) and counterstained with hematoxylin (blue). Magnification, ×100. *, P < 0.05 for the value compared to the corresponding control.
pLO-KO mice are protected from glycemic deterioration following low-dose streptozotocin injections.
To test whether resistance of pLO-KO islets to cytokine stress translates to resistance to dysglycemia in vivo, we subjected male control and pLO-KO mice to five daily low doses of STZ (55 mg/kg given intraperitoneally) and monitored the blood glucose levels over the next 25 days. In this model, the recruitment of dendritic cells/macrophages into the vicinity of the islet causes the local release of proinflammatory cytokines, which leads to islet dysfunction and eventual death (19, 32). Prior studies have shown that global 12-LO knockout mice are protected from low-dose STZ treatment (26), but it remains unclear whether this effect is secondary to loss of 12-LO in β cells or immune cells. As shown in Fig. 1C, control mice developed hyperglycemia (≥300 mg/dl) by day 10, which gradually worsened over the next 20 days. In contrast, pLO-KO mice showed the same initial hyperglycemia by day 10, but thereafter blood glucoses remained stable. Intraperitoneal GTTs performed at day 10 demonstrated indistinguishable glucose tolerance between the two groups of mice (Fig. 1D). However, consistent with the blood glucose values at 25 days after the administration of STZ, glucose tolerance of pLO-KO mice was significantly improved compared to the control animals (Fig. 1E). Immunohistochemical analysis of pancreata of STZ-treated mice at the end of the study revealed significantly greater insulin staining intensity in pLO-KO mice compared to control animals (Fig. 1F). These data suggest that the absence of 12-LO specifically in the pancreas is sufficient to protect against the long-term deterioration of glycemic control with multiple low-dose STZ.
pLO-KO mice are protected from glycemic deterioration in a model of obesity-induced β cell dysfunction.
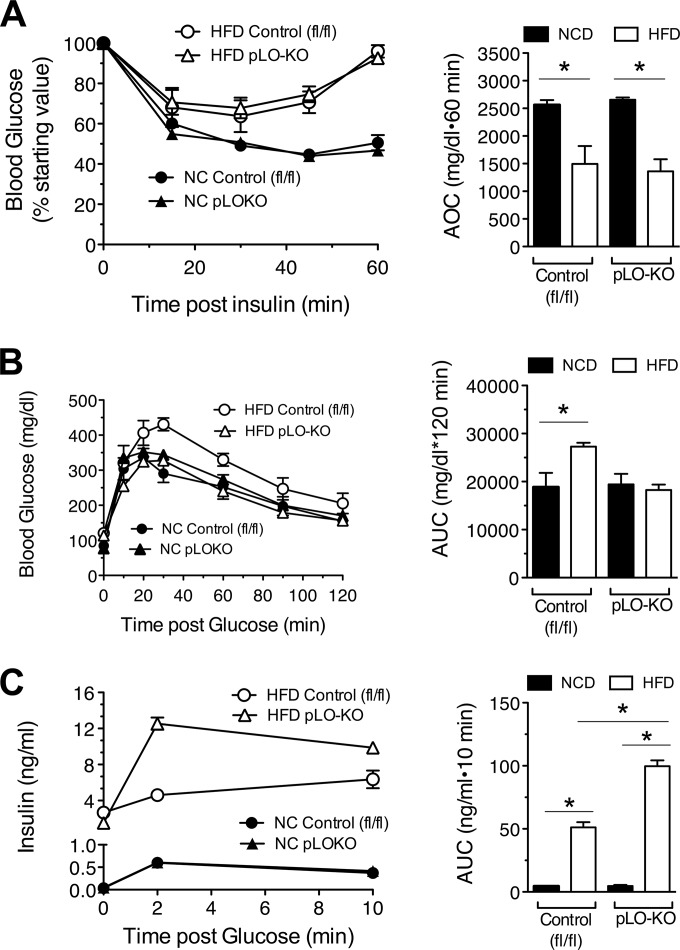
HFDs lead to polarization of adipose tissue macrophages to a more proinflammatory phenotype, resulting in the release of inflammatory cytokines that impair β cell function (33, 34). To test resistance of pLO-KO islets to cytokine stress in vivo, we subjected pLO-KO mice and control littermates (Alox15Loxp/Loxp animals) to HFD feeding. We placed 8-week-old mice on either the Western HFD (42% kcal from fat) or normal chow diet (NCD, 17% kcal from fat) for 20 weeks. At the end of the feeding, examination of pancreatic islets revealed an increase in 12-LO staining in control animals placed on a HFD compared to animals on a NCD, with no staining in extra-islet tissues (see Fig. S2A in the supplemental material). As expected, 12-LO immunoreactivity was significantly reduced in the pLO-KO animals (see Fig. S2A and B in the supplemental material). During the feeding study, pLO-KO mice gained equivalent weight and percent fat mass compared to control mice on a HFD, both parameters of which were greater than the mice placed on a NCD (see Fig. S2C and D in the supplemental material). HFD feeding, however, did not significantly alter lean body mass in any of the animals (see Fig. S2E in the supplemental material). Insulin tolerance tests revealed that both control and pLO-KO mice exhibited impairments in insulin sensitivity compared to animals on a NCD (Fig. 2A). Similarly, insulin signaling measured by phosphorylated Akt in muscle was not different in HFD-fed pLO-KO mice compared to controls (see Fig. S3A in the supplemental material). Prior studies of HFD feeding of global 12-LO knockout mice revealed reductions in adipocyte size and infiltration of adipocytes by macrophages compared to control mice (8, 9). However, in HFD-fed pLO-KO mice, we observed no differences in adipocyte size or F4/80+ macrophage infiltration into adipocytes compared to control animals fed a HFD (see Fig. S3B and C in the supplemental material). Collectively, these data demonstrate that pancreas-specific knockout of 12-LO did not affect cellular or whole-body insulin resistance, as acquired during HFD feeding.
FIG 2.
Effect of high-fat diet feeding on insulin sensitivity, glycemic control, and insulin secretion. At 8 weeks of age, control (fl/fl) and pLO-KO male mice were placed on a normal chow diet (NCD) or a high-fat diet (HFD) for 20 weeks. (A) Insulin tolerance tests and corresponding area over the curve (AOC) (n = 4 to 8 animals per group); (B) glucose tolerance tests and the corresponding AUC (n = 4 to 8 animals per group); (C) insulin levels measured at the indicated times after intraperitoneal glucose injection and the corresponding AUC (n = 3 or 4 animals per group). *, P < 0.05 for the comparisons indicated.
To assess glucose homeostasis, we next performed intraperitoneal GTTs. As shown in Fig. 2B, whereas HFD-fed control mice exhibited impaired glucose tolerance compared to those fed NCD, pLO-KO mice fed a HFD displayed glucose tolerance that was indistinguishable from those fed a NCD. Although HFD feeding (and consequent insulin resistance) led to higher insulin levels within the first 10 min after glucose injections in both control and pLO-KO animals, the insulin levels in pLO-KO animals were significantly greater than in control mice during this time frame (Fig. 2C). The greater insulin levels in HFD-fed pLO-KO animals suggest that these animals have enhanced β cell insulin secretory function, a finding consistent with our observations in vitro that pLO-KO islets secrete more insulin in the face of proinflammatory cytokines.
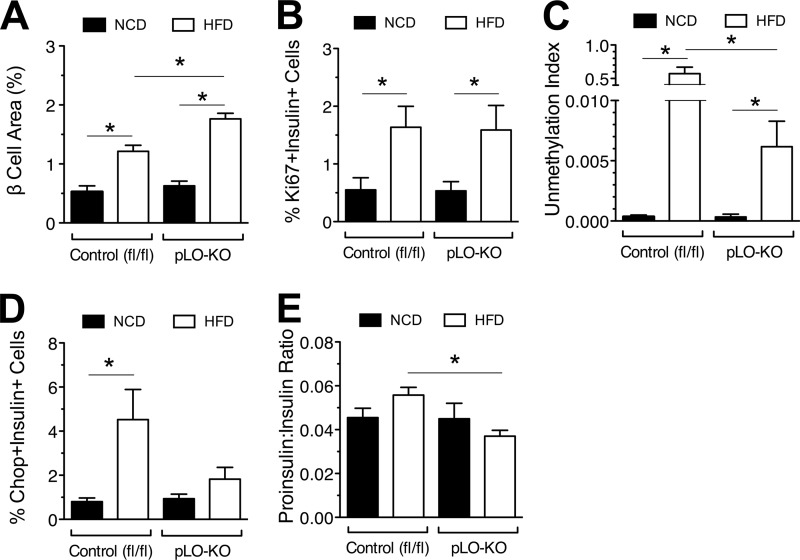
HFD-fed pLO-KO mice exhibit enhanced β cell mass and reduced β cell death.
Improvements in insulin secretion in HFD-fed pLO-KO mice led us to examine the morphology of islets. As shown in Fig. 3A, the β cell area% (relative to the total pancreas area) was greater in HFD-fed control and pLO-KO mice than in their NCD-fed counterparts. However, the β cell area% of HFD-fed pLO-KO animals was significantly higher than that of HFD-fed control animals (Fig. 3A), a finding concordant with the higher insulin levels observed in these animals. To determine whether the increased β cell area% of HFD-fed pLO-KO animals resulted from changes to β cell proliferation, pancreatic sections were stained for Ki67. Both control and pLO-KO HFD-fed mice had increased, Ki67+/insulin+ compared to NCD-fed mice but were undistinguishable from each other (Fig. 3B). Instead, β cell death was reduced in pLO-KO mice, since unmethylated preproinsulin DNA in the serum (the “unmethylation index,” a specific marker of β cell death) (18) was reduced in these mice compared to controls (Fig. 3C). Interestingly, this reduction in β cell death was associated with reductions in insulin+/Chop+ cells in pLO-KO mice (Fig. 3D), suggestive of reductions in endoplasmic reticulum (ER) stress-induced apoptosis. Consistent with this possibility, pLO-KO mice exhibited reductions in the proinsulin/insulin ratio (Fig. 3E), which may be reflective of reduced ER stress (23).
FIG 3.
Effect of high-fat diet feeding on β cell replication, survival, and function. At 8 weeks of age, control (fl/fl) and pLO-KO male mice were placed on a normal chow diet (NCD) or a high-fat diet (HFD) for 20 weeks. (A) Relative β cell area as a percentage of total pancreatic area after 20 weeks of feeding (n = 3 animals per group); (B) Ki67+/insulin+ cells as a percentage of total insulin+ cells in islets after 20 weeks of feeding (n = 3 animals per group); (C) serum unmethylation index after 20 weeks of feeding (n = 3 animals per group); (D) Chop+/insulin+ cells as a percentage of total insulin+ cells in islets after 20 weeks of feeding (n = 3 animals per group); (E) serum proinsulin to insulin ratio after 20 weeks of feeding (n = 4 animals per group). *, P < 0.05 for the comparisons indicated.
12-LO deletion protects against chronic cytokine-induced oxidative and ER stress in vitro.
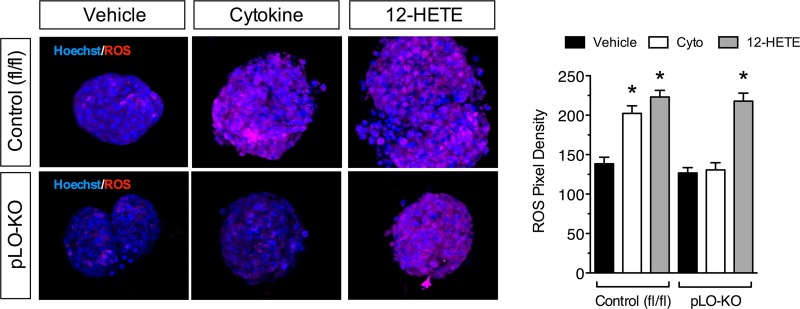
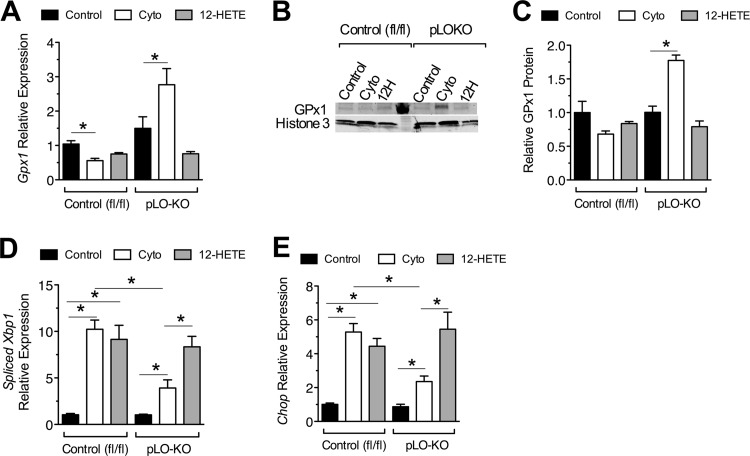
Because improved glucose tolerance in HFD-fed pLO-KO mice was attributable to improved β cell function and survival, we next investigated signaling pathways that contribute to β cell survival in these animals. The 12-HETE and other lipid peroxides produced by 12-LO leads to oxidative stress (35). Immunofluorescence analysis using a fluorescent dye-based free radical sensor (CellROX) demonstrated an increase in ROS upon incubation of control islets with cytokines for 24 h (Fig. 4). In contrast, ROS production was almost completely blocked in pLO-KO islets upon incubation with cytokines for 24 h. Notably, this effect of cytokines was reproduced upon incubation with 100 nM the 12-LO product 12-HETE for 24 h regardless of genotype, suggesting that cytokine-induced ROS production at 24 h may result from downstream products of 12-LO activity. Because of low antioxidant enzyme production, islet β cells have limited ability to counter ROS production. As shown in Fig. 5A, incubation of control mouse islets with cytokines for 24 h resulted in a reduction in the mRNA encoding glutathione peroxidase (Gpx1). In contrast, cytokine incubation of pLO-KO islets for 24 h led to an increase in Gpx1 levels. The reduction in Gpx1 mRNA by cytokines was mimicked by the addition of 12-HETE, regardless of genotype (Fig. 5A). Figure 5B and C show that protein levels of GPx1 by immunoblot paralleled the changes in mRNA levels.
FIG 4.
ROS production after cytokine exposure. Islets from 8-week-old control (fl/fl) and pLO-KO mouse islets were treated with or without cytokines (Cyto) or with or without 12-hydroxyeicosotetranoic acid (12-HETE) for 24 h. Isolated islets were stained for ROS (red) and Hoechst (blue). Shown are representative images (left panel) and pixel density of ROS staining (right panel). Magnification, ×200. *, P < 0.05 compared to the control in each group.
FIG 5.
Expression of genes encoding antioxidant enzymes and ER stress markers in response to cytokine treatment. Islets from 8-week-old control (fl/fl) and pLO-KO mice were isolated and treated with or without cytokines and with or without 12-HETE for 24 h and processed for RNA or protein (n = 3 per group). (A) Relative levels of Gpx1 mRNA in control (fl/fl) and pLO-KO islets (normalized to Actb mRNA); (B) representative immunoblot for GPx1 protein and histone 3; (C) quantitation of immunoblots (B) for GPx1 protein normalized to histone 3 (n = 3); (D and E) relative levels of spliced Xbp1 and Chop mRNA, respectively (normalized to Actb mRNA). *, P < 0.05 for the comparisons shown.
Oxidative stress is closely linked to activation of the unfolded protein response (UPR) and ER stress, since increased oxidation of proteins can lead to the misfolding of proteins in the ER lumen (36). Figure 5D and E show that incubation of control islets with cytokines for 24 h results in an increase in Xbp1s and Chop mRNA levels, respectively, and that these increases were blunted in pLO-KO islets. Incubation with 100 nM 12-HETE produced increases in Xbp1s and Chop similar to cytokines in both control and pLO-KO islets, suggesting that the development of ER stress by cytokines at 24 h may be largely mediated by 12-LO products (Fig. 5D and E). Collectively, these data indicate that the increased oxidative stress of cytokines appears to be closely linked to ER stress and that the absence of 12-LO in islets is partially protective against these changes.
HFD-fed pLO-KO mice showed decreased islet oxidative stress and increased antioxidant enzyme levels.
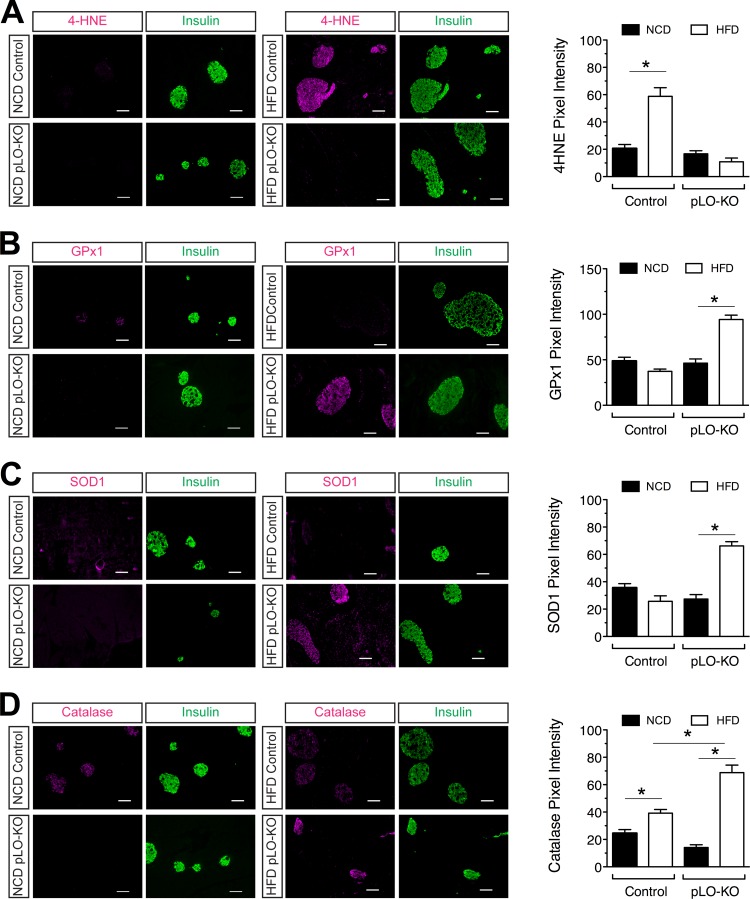
The activation of oxidative stress in cytokine-treated islets led us to explore whether oxidative stress is correlated with islet dysfunction in HFD-fed mice and whether deletion of 12-LO is protective. We costained pancreas sections of NCD- and HFD-fed mice for insulin and 4-hydroxynonenal (4-HNE), a product of endogenous lipid peroxidation and a marker of oxidative stress-induced cellular damage. Mice fed NCD had little to no 4-HNE staining in β cells, whereas HFD-fed control mice had intense 4-HNE staining (Fig. 6A). In contrast, HFD-fed pLO-KO mice had little to no 4-HNE staining, similar to NCD-fed mice (Fig. 6A). These data parallel findings from isolated islet studies (Fig. 4), suggesting that the absence of 12-LO protects against the development of oxidative stress.
FIG 6.
Oxidative stress and antioxidant enzyme staining in islets after HFD feeding. Fixed pancreatic sections from control (fl/fl) and pLO-KO mice fed a normal chow diet (NCD) or a high-fat diet (HFD) for 20 weeks were subjected to immunofluorescence staining. Shown are representative images (left panel) and the pixel density (right panel) from analysis of at least three animals from each group stained for 4-hydroxynoneal (4-HNE, red) and insulin (green) (A), glutathione peroxidase (GPx1, red) and insulin (green) (B), superoxide dismutase (SOD1, red) and insulin (green) (C), and catalase (Cat, red) and insulin (green) (D). Magnification, ×200. *, P < 0.05 compared to the control in each group.
To determine whether the reduction in apparent islet oxidative stress in pLO-KO mice resulted from increases in antioxidant enzyme levels, we next costained pancreatic sections for insulin and antioxidant enzymes. The β cells of NCD-fed animals showed minimally detectable levels of Gpx1 (Fig. 6B), superoxide dismutase (SOD1) (Fig. 6C), and catalase (Fig. 6D). Whereas Gpx1 staining intensity decreases slightly in β cells upon HFD feeding in control mice, levels increase substantially after HFD feeding in pLO-KO mice (Fig. 6B). Similar results were observed with SOD1 and catalase (Fig. 6C and D), wherein staining intensities of these enzymes increased in pLO-KO mice upon HFD feeding. Taken together, with the data obtained from cytokine incubations in vitro, it appears that 12-LO promotes oxidative stress by preventing adaptive increases in antioxidant enzymes in islet β cells.
12-LO inhibits nuclear accumulation of the Nrf2 antioxidant gene activator.
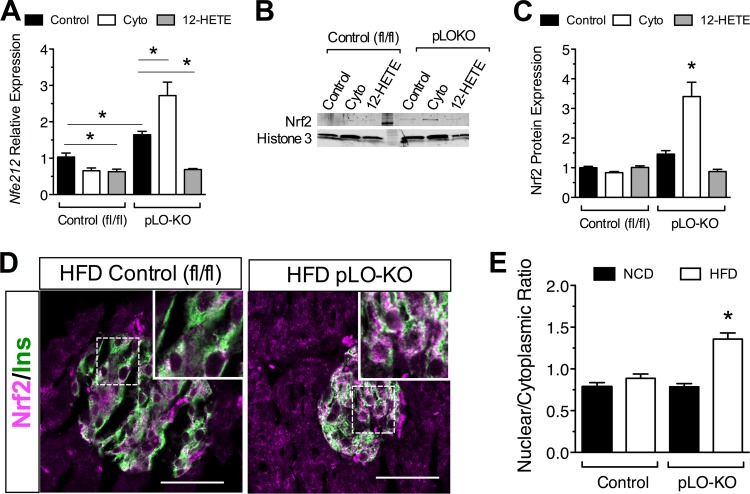
The Nrf2 transcription factor is an important activator of antioxidant genes, and the promoters of Gpx1 and Sod1 contain Nrf2 recognition sequences (37). To understand better the mechanism for the increased antioxidant gene activity in pLO-KO mice, we examined levels of Nrf2 protein and its corresponding mRNA (Nfe212). As shown in Fig. 7A, treatment of mouse islets with cytokines for 24 h resulted in a reduction in Nfe212 mRNA levels, which were not only restored but increased in islets isolated from pLO-KO mice. Incubation with 100 nM 12-HETE reduced Nfe212 mRNA in both control and pLO-KO islets, suggesting that the negative effect of cytokines on Nfe212 transcription is mediated by 12-HETE. Immunoblots of islet cell extract showed that Nrf2 protein levels paralleled the observed transcript levels (Fig. 7B and C).
FIG 7.
Effect of 12-LO on Nrf2 levels in vitro and in vivo. Islets from 8-week-old control (fl/fl) and pLO-KO mice were isolated and treated with or without cytokines and with or without 12-HETE for 24 h and processed for mRNA or protein (n = 3 per group). Pancreas sections from control and pLO-KO mice fed a NCD or HFD for 20 weeks were immunostained for Nrf2. (A) Relative expression level of Nfe212 mRNA in control and pLO-KO mouse islets (normalized to Actb); (B) representative immunoblot from control and pLO-KO mouse islet extract for Nrf2 and histone 3; (C) quantitation of immunoblots (B) for Nrf2 protein normalized to histone 3 (n = 3); (D) representative immunofluorescence images for Nrf2 (red) and insulin (green) (magnification, ×200); (E) quantitation of the nuclear/cytoplasmic Nrf2 pixel density from islets of HFD-fed control and pLO-KO animals (n = 300 total insulin+ cells from three animals per group). *, P < 0.05 compared to control or comparisons indicated.
To determine whether the effects 12-LO deletion on Nrf2 are observed in vivo, we costained pancreas sections from HFD-fed mice. As shown in Fig. 7D and E, pLO-KO mice had increased Nrf2 in the nuclear and perinuclear region compared to control animals. Taken together, these data suggest that 12-LO and its products, notably 12-HETE, inhibit activation and nuclear localization of Nrf2 in the setting of cytokine-mediated stress.
DISCUSSION
Prediabetes is characterized by inflammation involving macrophages, adipose tissue, and the pancreatic islet (27). The enzyme 12-LO is expressed in key tissues involved in metabolic homeostasis—leukocytes, adipose tissue, and pancreatic islets—and is thought to contribute to the inflammation and insulin resistance seen in prediabetic states. Rodent and human pancreatic islets have been shown to express 12-LO and its products in the context of inflammation (35, 38, 39), but whether 12-LO plays an intrinsic role in islet dysfunction during HFD feeding in vivo has never been addressed until the present study. To interrogate both a role and a mechanism for islet-intrinsic 12-LO in the setting of insulin resistance, we crossed Pdx1 promoter-driven Cre recombinase transgenic mice to Alox15 “floxed” mice. Pdx1-Cre is expressed in pancreatic progenitors beginning at embryonic day ∼9.0, and the resultant mice from this cross are technically pancreas-specific 12-LO knockouts (pLO-KO). However, because 12-LO is absent in pancreatic exocrine tissue (the present study and reference 25), we considered these mice as islet-specific knockouts. We show that pLO-KO mice gain weight normally on a chow diet and exhibit no overt metabolic phenotype. Our data support whole-body knockout studies that suggest that 12-LO is dispensable for pancreas development and islet mass accrual and function postdevelopmentally (26).
HFDs have been shown to provoke inflammation in both adipose tissue and islets, in part through the recruitment of macrophages and their local release of proinflammatory cytokines (33). We show here that pLO-KO mice exhibit improved glucose tolerance, with greater insulin secretion and β cell mass compared to HFD-fed control littermates. Given the specificity of our knockout for the islet, we accordingly observed no differences in weight gain and body fat distribution, adipocyte size, or macrophage infiltration into adipose tissue between control and pLO-KO animals. Importantly, no differences in insulin sensitivity (either by ITT or phospho-Akt signaling in muscle) were observed between control and pLO-KO mice, a finding that is also consistent with the islet specificity of the knockout. We therefore conclude that the improvement in glycemic control in pLO-KO animals is attributable to an islet-intrinsic effect of 12-LO deletion. This conclusion is also consistent with the known specificity of the Pdx1-Cre transgene for pancreas compared to adipose tissue, macrophage, liver, and brain. Notably, a recent study demonstrated expression of the Pdx1-Cre transgene in brain (40); however, based on genomic DNA analysis of Alox15 gene recombination, we did not observe evidence for recombination in brain tissue from our animals.
To verify the resistance of pLO-KO-derived islets to inflammation, we performed cytokine incubation studies in vitro. Interestingly, following an initial reduction in glucose-stimulated insulin secretion (equivalent to that seen in control islets), pLO-KO islets subsequently maintained their level of glucose responsiveness. These findings were also reflected in the multiple low-dose STZ studies of pLO-KO mice in vivo, wherein an initial worsening of blood glucose levels was followed by subsequent stabilization of glucoses at this slightly higher, but nondiabetic, level. In prior studies, we and others have shown that an initial effect of cytokine signaling (or STZ administration in vivo) is the upregulation of iNOS, which leads to short-term defects in stimulus-secretion coupling (19, 31, 41). Indeed, there is no evidence from our studies that this initial effect of cytokines is mitigated by loss of 12-LO. These findings are unlikely to result from β cell loss in the 4- to 24-h period, since death of β cells does not occur until >24 h incubation with cytokines (42). Nevertheless, the longer-term signaling effects of cytokines was clearly blunted in our studies. At least two observations might account for this finding: first, it has been reported that nitric oxide (the product of iNOS activity) can increase substrate (nonesterified arachidonic acid) availability to 12-LO in β cells, resulting in increased 12-HETE production and reduced stimulus-secretion coupling. Thus, in the absence of 12-LO, this effect of nitric oxide would be blunted. Second, it has been shown that the longer term effects of cytokines include activation of the UPR in β cells (42, 43). It is likely that these two observations are causally linked by 12-HETE. In this respect, our observations that 12-HETE activates the UPR in β cell lines despite 12-LO deletion suggests a role for 12-LO-derived lipid peroxides in the development of islet ER stress in vitro.
The development of ER stress is closely linked to processes that promote production of ROS (36). We show that whereas cytokines can lead to increased β cell ROS in islets, at least part of this effect can be attributed to 12-LO activity because its elimination diminishes ROS production. 12-LO activity, particularly 12-HETE itself, can increase NADPH oxidase activity in β cell lines, with concomitant production of ROS, superoxide, and hydrogen peroxide (35). The protection from HFD-induced β cell dysfunction in pLO-KO mice likely reflects this reduction in ROS production, since we observed dramatically reduced 4-HNE staining in islets from these mice. Therefore, we feel our studies provide important new insights, since we demonstrate that 12-LO deletion enhances the expression of antioxidant proteins Gpx1 and SOD1 and therefore may improve the β cell capacity to remediate oxidative stress. In this context, the overexpression of Gpx1 in islets of db/db diabetic mice protect against long-term hyperglycemia, partly through the restoration of β cell transcription factor expression (44, 45).
Because of the increases in Gpx1 and SOD1 in pLO-KO mice, we considered the possibility that 12-LO and its product 12-HETE affect the levels and/or nuclear localization of the transcription factor Nrf2, which regulates the primary gene expression program in response to oxidative stress (46). The β cell-specific Nrf2 knockout mouse exhibits enhanced sensitivity to oxidative stress (47). Nrf2 is normally sequestered in the cytoplasm by two proteins, Cul3 and Keap1, that lead to its ubiquitin-mediated degradation. In the setting of oxidative stress, key cysteine residues in Keap1 are oxidized, causing disruption of ubiquitination and accumulation of Nrf2 in both the cytoplasm and the nucleus (48–50). In islets, we show that total Nrf2 levels are reduced by cytokine incubation and restored upon the deletion of 12-LO. The effect of cytokines is recapitulated with 12-HETE alone. We therefore believe that 12-HETE is the key factor linking 12-LO to Nrf2. Similarly, we observed in pancreas sections of HFD-fed pLO-KO mice that not only do the Nrf2 levels in β cells appear to increase but also that the perinuclear staining of Nrf2 is enhanced. Whether this effect on Nrf2 is a direct consequence of 12-HETE effects on Nrf2 itself or upon its regulatory proteins is an ongoing area of investigation in our group.
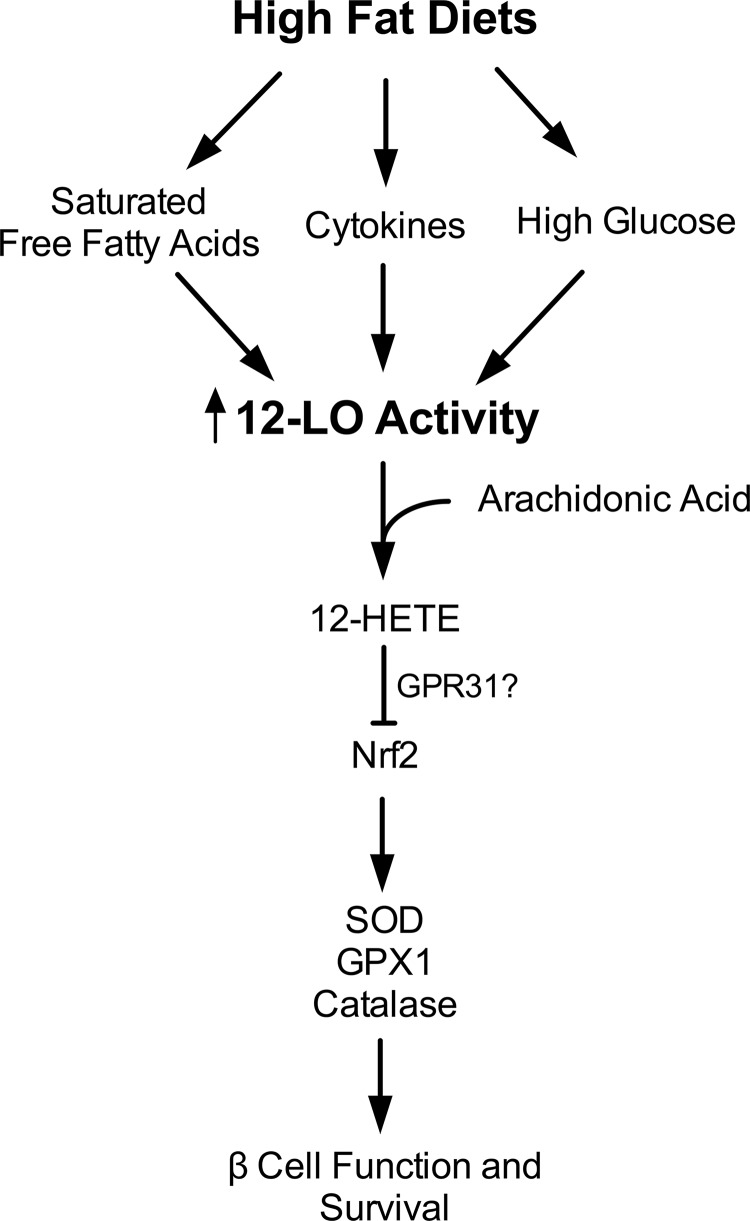
Taken together, our studies provide the first evidence that an intrinsic action of 12-LO in islets is central to insulin secretory defects and the development of glucose intolerance in the setting of obesity and insulin resistance. Our studies present a conceptually innovative approach to understanding how different metabolically active tissues might be linked to one another by a single protein in the setting of obesity and insulin resistance. Based on the data presented here, we propose the model depicted in Fig. 8. In the setting of HFDs, cytokine signaling, and likely hyperglycemia from impaired peripheral glucose uptake and elevated systemic free fatty acids as well, promotes nitric oxide production and stimulates 12-LO activity. As the former provides added substrate to the latter, the intracellular production of lipid peroxide intermediates (e.g., 12-HETE) is increased. 12-HETE promotes oxidative stress and inhibits Nrf2 function, the result of which is diminished stimulus-secretion coupling and eventual β cell apoptosis. Undoubtedly, aspects of this model will require further refinement, since the mechanisms by which 12-HETE impacts Nrf2 remain unclear. The recent identification of a potential 12-HETE receptor (GPR31 [51]) raises the possibility that these and other effects of 12-HETE are mediated through a precise signaling pathway involving G protein-coupled receptors. Nevertheless, our studies suggest that the inhibition of 12-LO, possibly through clinical application of recently identified selective inhibitors (52), may have applicability in the preservation of islet β cell function in prediabetes or established type 2 diabetes.
FIG 8.
Proposed model of 12-LO action in the islet β cell. The figure depicts evidence from both published literature and the present study. High-fat diets (via cytokines, free fatty acids, and elevated blood glucose) activate 12-LO activity in β cells, which then leads to increased 12-HETE production. In the present study, we propose that 12-HETE and other lipid peroxide products of 12-LO suppresses the otherwise adaptive increase Nrf2 transcription and protein levels. As a result, antioxidant genes and their encoded proteins are suppressed, leading to failure of β cell adaptation and to glucose intolerance.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by an institutional National Institutes of Health (NIH) Clinical and Translational Sciences Award KL2 award (to S.A.T.), an American Diabetes Association Junior Faculty Award (to S.A.T.), NIH grant R01 HL112605 (to J.L.N.), and NIH grants R01 DK060581 and R01 DK080583 (both to R.G.M.).
We thank B. Tersey, N. Stull, and K. Benninger for technical assistance and the Indiana Diabetes Research Center Islet and Rodent Cores for the provision of primary tissues from mice.
Footnotes
Published ahead of print 28 July 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/MCB.00157-14.
REFERENCES
- 1.Lawes CMM, Parag V, Bennett DA, Suh I, Lam TH, Whitlock G, Barzi F, Woodward M, Asia Pacific Cohort Studies Collaboration 2004. Blood glucose and risk of cardiovascular disease in the Asia Pacific region. Diabetes Care 27:2836–2842. 10.2337/diacare.27.12.2836 [DOI] [PubMed] [Google Scholar]
- 2.Prentki M, Nolan CJ. 2006. Islet β cell failure in type 2 diabetes. J. Clin. Invest. 116:1802–1812. 10.1172/JCI29103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lerner AG, Upton J-P, Praveen PVK, Ghosh R, Nakagawa Y, Igbaria A, Shen S, Nguyen V, Backes BJ, Heiman M, Heintz N, Greengard P, Hui S, Tang Q, Trusina A, Oakes SA, Papa FR. 2012. IRE1α induces thioredoxin-interacting protein to activate the NLRP3 inflammasome and promote programmed cell death under irremediable ER stress. Cell Metab. 16:250–264. 10.1016/j.cmet.2012.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oslowski CM, Hara T, O'Sullivan-Murphy B, Kanekura K, Lu S, Hara M, Ishigaki S, Zhu LJ, Hayashi E, Hui ST, Greiner D, Kaufman RJ, Bortell R, Urano F. 2012. Thioredoxin-interacting protein mediates ER stress-induced β cell death through initiation of the inflammasome. Cell Metab. 16:265–273. 10.1016/j.cmet.2012.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lenzen S, Drinkgern J, Tiedge M. 1996. Low antioxidant enzyme gene expression in pancreatic islets compared with various other mouse tissues. Free Radic. Biol. Med. 20:463–466. 10.1016/0891-5849(96)02051-5 [DOI] [PubMed] [Google Scholar]
- 6.Brash AR. 1999. Lipoxygenases: occurrence, functions, catalysis, and acquisition of substrate. J. Biol. Chem. 274:23679–23682. 10.1074/jbc.274.34.23679 [DOI] [PubMed] [Google Scholar]
- 7.Dobrian AD, Lieb DC, Cole BK, Taylor-Fishwick Da Chakrabarti ASK, Nadler JL. 2011. Functional and pathological roles of the 12- and 15-lipoxygenases. Prog. Lipid Res. 50:115–131. 10.1016/j.plipres.2010.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nunemaker CS, Chen M, Pei H, Kimble SD, Keller SR, Carter JD, Yang Z, Smith KM, Wu R, Bevard MH, Garmey JC, Nadler JL. 2008. 12-Lipoxygenase-knockout mice are resistant to inflammatory effects of obesity induced by Western diet. Am. J. Physiol. Endocrinol. Metab. 295:E1065–E1075. 10.1152/ajpendo.90371.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sears DD, Miles PD, Chapman J, Ofrecio JM, Almazan F, Thapar D, Miller YI. 2009. 12/15-lipoxygenase is required for the early onset of high fat diet-induced adipose tissue inflammation and insulin resistance in mice. PLoS One 4:e7250. 10.1371/journal.pone.0007250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cole BK, Morris MA, Grzesik WJ, Leone KA, Nadler JL. 2012. Adipose tissue-specific deletion of 12/15-lipoxygenase protects mice from the consequences of a high-fat diet. Mediat. Inflamm. 2012:851798. 10.1155/2012/851798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laybutt DR, Sharma A, Sgroi DC, Gaudet J, Bonner-Weir S, Weir GC. 2002. Genetic regulation of metabolic pathways in β-cells disrupted by hyperglycemia. J. Biol. Chem. 277:10912–10921. 10.1074/jbc.M111751200 [DOI] [PubMed] [Google Scholar]
- 12.Ma Z, Ramanadham S, Corbett JA, Bohrer A, Gross RW, McDaniel ML, Turk J. 1996. Interleukin-1 enhances pancreatic islet arachidonic acid 12-lipoxygenase product generation by increasing substrate availability through a nitric oxide-dependent mechanism. J. Biol. Chem. 271:1029–1042. 10.1074/jbc.271.2.1029 [DOI] [PubMed] [Google Scholar]
- 13.Natarajan R, Gu JL, Rossi J, Gonzales N, Lanting L, Xu L, Nadler J. 1993. Elevated glucose and angiotensin II increase 12-lipoxygenase activity and expression in porcine aortic smooth muscle cells. Proc. Natl. Acad. Sci. U. S. A. 90:4947–4951. 10.1073/pnas.90.11.4947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gu G, Dubauskaite J, Melton DA. 2002. Direct evidence for the pancreatic lineage: NGN3+ cells are islet progenitors and are distinct from duct progenitors. Development 129:2447–2457 [DOI] [PubMed] [Google Scholar]
- 15.Evans-Molina C, Robbins RD, Kono T, Tersey SA, Vestermark GL, Nunemaker CS, Garmey JC, Deering TG, Keller SR, Maier B, Mirmira RG. 2009. Peroxisome proliferator-activated receptor γ activation restores islet function in diabetic mice through reduction of endoplasmic reticulum stress and maintenance of euchromatin structure. Mol. Cell. Biol. 29:2053–2067. 10.1128/MCB.01179-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ramalingam L, Oh E, Yoder SM, Brozinick JT, Kalwat MA, Groffen AJ, Verhage M, Thurmond DC. 2012. Doc2b is a key effector of insulin secretion and skeletal muscle insulin sensitivity. Diabetes 61:2424–2432. 10.2337/db11-1525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stull ND, Breite A, McCarthy RC, Tersey SA, Mirmira RG. 2012. Mouse islet of Langerhans isolation using a combination of purified collagenase and neutral protease. J. Vis Exp. 67:e4137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fisher MM, Perez Chumbiauca CN, Mather KJ, Mirmira RG, Tersey SA. 2013. Detection of islet β cell death in vivo by multiplex PCR analysis of differentially methylated DNA. Endocrinology 154:3476–3481. 10.1210/en.2013-1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maier B, Ogihara T, Trace AP, Tersey SA, Robbins RD, Chakrabarti SK, Nunemaker CS, Stull ND, Taylor CA, Thompson JE, Dondero RS, Lewis EC, Dinarello CA, Nadler JL, Mirmira RG. 2010. The unique hypusine modification of eIF5A promotes islet β cell inflammation and dysfunction in mice. J. Clin. Invest. 120:2156–2170. 10.1172/JCI38924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McDuffie M, Maybee NA, Keller SR, Stevens BK, Garmey JC, Morris MA, Kropf E, Rival C, Ma K, Carter JD, Tersey SA, Nunemaker CS, Nadler JL. 2008. Nonobese diabetic (NOD) mice congenic for a targeted deletion of 12/15-lipoxygenase are protected from autoimmune diabetes. Diabetes 57:199–208. 10.2337/db07-0830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Song B, Scheuner D, Ron D, Pennathur S, Kaufman RJ. 2008. Chop deletion reduces oxidative stress, improves β cell function, and promotes cell survival in multiple mouse models of diabetes. J. Clin. Invest. 118:3378–3389. 10.1172/JCI34587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mao P, Manczak M, Calkins MJ, Truong Q, Reddy TP, Reddy AP, Shirendeb U, Lo H-H, Rabinovitch PS, Reddy PH. 2012. Mitochondria-targeted catalase reduces abnormal APP processing, amyloid β production and BACE1 in a mouse model of Alzheimer's disease: implications for neuroprotection and lifespan extension. Hum. Mol. Genet. 21:2973–2990. 10.1093/hmg/dds128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tersey SA, Nishiki Y, Templin AT, Cabrera SM, Stull ND, Colvin SC, Evans-Molina C, Rickus JL, Maier B, Mirmira RG. 2012. Islet β-cell endoplasmic reticulum stress precedes the onset of type 1 diabetes in the nonobese diabetic mouse model. Diabetes 61:818–827. 10.2337/db11-1293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee S, Hur E, Ryoo I, Jung K-A, Kwak J, Kwak M-K. 2012. Involvement of the Nrf2-proteasome pathway in the endoplasmic reticulum stress response in pancreatic β-cells. Toxicol. Appl. Pharmacol. 264:431–438. 10.1016/j.taap.2012.08.021 [DOI] [PubMed] [Google Scholar]
- 25.Shannon VR, Ramanadham S, Turk J, Holtzman MJ. 1992. Selective expression of an arachidonate 12-lipoxygenase by pancreatic islet β-cells. Am. J. Physiol. 263:E828–E836 [DOI] [PubMed] [Google Scholar]
- 26.Bleich D, Chen S, Zipser B, Sun D, Funk CD, Nadler JL. 1999. Resistance to type 1 diabetes induction in 12-lipoxygenase knockout mice. J. Clin. Invest. 103:1431–1436. 10.1172/JCI5241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Velloso LA, Eizirik DL, Cnop M. 2013. Type 2 diabetes mellitus-an autoimmune disease? Nat. Rev. Endocrinol. 9:750–755. 10.1038/nrendo.2013.131 [DOI] [PubMed] [Google Scholar]
- 28.Wang B, Sun J, Li X, Zhou Q, Bai J, Shi Y, Le G. 2013. Resveratrol prevents suppression of regulatory T-cell production, oxidative stress, and inflammation of mice prone or resistant to high-fat diet-induced obesity. Nutr. Res. N. Y. N 33:971–981. 10.1016/j.nutres.2013.07.016 [DOI] [PubMed] [Google Scholar]
- 29.Heitmeier MR, Kelly CB, Ensor NJ, Gibson KA, Mullis KG, Corbett JA, Maziasz TJ. 2004. Role of cyclooxygenase-2 in cytokine-induced β-cell dysfunction and damage by isolated rat and human islets. J. Biol. Chem. 279:53145–53151. 10.1074/jbc.M410978200 [DOI] [PubMed] [Google Scholar]
- 30.Liu X, Xue Y, Liu C, Lou Q, Wang J, Yanagita T, Xue C, Wang Y. 2013. Eicosapentaenoic acid-enriched phospholipid ameliorates insulin resistance and lipid metabolism in diet-induced-obese mice. Lipids Health Dis. 12:109. 10.1186/1476-511X-12-109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nishiki Y, Adewola A, Hatanaka M, Templin AT, Maier B, Mirmira RG. 2013. Translational control of inducible nitric oxide synthase by p38 MAPK in islet β-cells. Mol. Endocrinol. 27:336–349. 10.1210/me.2012-1230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Calderon B, Suri A, Miller MJ, Unanue ER. 2008. Dendritic cells in islets of Langerhans constitutively present β cell-derived peptides bound to their class II MHC molecules. Proc. Natl. Acad. Sci. U. S. A. 105:6121–6126. 10.1073/pnas.0801973105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eguchi K, Manabe I, Oishi-Tanaka Y, Ohsugi M, Kono N, Ogata F, Yagi N, Ohto U, Kimoto M, Miyake K, Tobe K, Arai H, Kadowaki T, Nagai R. 2012. Saturated fatty acid and TLR signaling link β cell dysfunction and islet inflammation. Cell Metab. 15:518–533. 10.1016/j.cmet.2012.01.023 [DOI] [PubMed] [Google Scholar]
- 34.Lumeng CN, Bodzin JL, Saltiel AR. 2007. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J. Clin. Invest. 117:175–184. 10.1172/JCI29881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weaver JR, Holman TR, Imai Y, Jadhav A, Kenyon V, Maloney DJ, Nadler JL, Rai G, Simeonov A, Taylor-Fishwick DA. 2012. Integration of proinflammatory cytokines, 12-lipoxygenase and NOX-1 in pancreatic islet β cell dysfunction. Mol. Cell. Endocrinol. 358:88–95. 10.1016/j.mce.2012.03.004 [DOI] [PubMed] [Google Scholar]
- 36.Malhotra JD, Kaufman RJ. 2007. Endoplasmic reticulum stress and oxidative stress: a vicious cycle or a double-edged sword? Antioxid. Redox Signal. 9:2277–2293. 10.1089/ars.2007.1782 [DOI] [PubMed] [Google Scholar]
- 37.Banning A, Deubel S, Kluth D, Zhou Z, Brigelius-Flohé R. 2005. The GI-GPx gene is a target for Nrf2. Mol. Cell. Biol. 25:4914–4923. 10.1128/MCB.25.12.4914-4923.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen M, Yang ZD, Smith KM, Carter JD, Nadler JL. 2005. Activation of 12-lipoxygenase in proinflammatory cytokine-mediated β cell toxicity. Diabetologia 48:486–495. 10.1007/s00125-005-1673-y [DOI] [PubMed] [Google Scholar]
- 39.Ma K, Nunemaker CS, Wu R, Chakrabarti SK, Taylor-Fishwick DA, Nadler JL. 2010. 12-Lipoxygenase products reduce insulin secretion and β-cell viability in human islets. J. Clin. Endocrinol. Metab. 95:887–893. 10.1210/jc.2009-1102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wicksteed B, Brissova M, Yan W, Opland DM, Plank JL, Reinert RB, Dickson LM, Tamarina NA, Philipson LH, Shostak A, Bernal-Mizrachi E, Elghazi L, Roe MW, Labosky PA, Myers MM, Gannon M, Powers AC, Dempsey PJ. 2010. Conditional gene targeting in mouse pancreatic β-cells: analysis of ectopic Cre transgene expression in the brain. Diabetes 59:3090–3098. 10.2337/db10-0624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hughes KJ, Chambers KT, Meares GP, Corbett JA. 2009. Nitric oxides mediates a shift from early necrosis to late apoptosis in cytokine-treated β-cells that is associated with irreversible DNA damage. Am. J. Physiol. Endocrinol. Metab. 297:E1187–E1196. 10.1152/ajpendo.00214.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chambers KT, Unverferth JA, Weber SM, Wek RC, Urano F, Corbett JA. 2008. The role of nitric oxide and the unfolded protein response in cytokine-induced β-cell death. Diabetes 57:124–132. 10.2337/db07-0944 [DOI] [PubMed] [Google Scholar]
- 43.Cardozo AK, Ortis F, Storling J, Feng Y-M, Rasschaert J, Tonnesen M, Van Eylen F, Mandrup-Poulsen T, Herchuelz A, Eizirik DL. 2005. Cytokines downregulate the sarcoendoplasmic reticulum pump Ca2+ ATPase 2b and deplete endoplasmic reticulum Ca2+, leading to induction of endoplasmic reticulum stress in pancreatic β-cells. Diabetes 54:452–461. 10.2337/diabetes.54.2.452 [DOI] [PubMed] [Google Scholar]
- 44.Guo S, Dai C, Guo M, Taylor B, Harmon JS, Sander M, Robertson RP, Powers AC, Stein R. 2013. Inactivation of specific β cell transcription factors in type 2 diabetes. J. Clin. Invest. 123:3305–3316. 10.1172/JCI65390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Harmon JS, Bogdani M, Parazzoli SD, Mak SSM, Oseid EA, Berghmans M, Leboeuf RC, Robertson RP. 2009. β-Cell-specific overexpression of glutathione peroxidase preserves intranuclear MafA and reverses diabetes in db/db mice. Endocrinology 150:4855–4862. 10.1210/en.2009-0708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pi J, Zhang Q, Fu J, Woods CG, Hou Y, Corkey BE, Collins S, Andersen ME. 2010. ROS signaling, oxidative stress and Nrf2 in pancreatic β-cell function. Toxicol. Appl. Pharmacol. 244:77–83. 10.1016/j.taap.2009.05.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yagishita Y, Fukutomi T, Sugawara A, Kawamura H, Takahashi T, Pi J, Uruno A, Yamamoto M. 2014. Nrf2 protects pancreatic β-cells from oxidative and nitrosative stress in diabetic model mice. Diabetes 63:605–618. 10.2337/db13-0909 [DOI] [PubMed] [Google Scholar]
- 48.Kobayashi A, Kang M-I, Okawa H, Ohtsuji M, Zenke Y, Chiba T, Igarashi K, Yamamoto M. 2004. Oxidative stress sensor Keap1 functions as an adaptor for Cul3-based E3 ligase to regulate proteasomal degradation of Nrf2. Mol. Cell. Biol. 24:7130–7139. 10.1128/MCB.24.16.7130-7139.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sekhar KR, Rachakonda G, Freeman ML. 2010. Cysteine-based regulation of the CUL3 adaptor protein Keap1. Toxicol. Appl. Pharmacol. 244:21–26. 10.1016/j.taap.2009.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yamamoto T, Suzuki T, Kobayashi A, Wakabayashi J, Maher J, Motohashi H, Yamamoto M. 2008. Physiological significance of reactive cysteine residues of Keap1 in determining Nrf2 activity. Mol. Cell. Biol. 28:2758–2770. 10.1128/MCB.01704-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Guo Y, Zhang W, Giroux C, Cai Y, Ekambaram P, Dilly A-K, Hsu A, Zhou S, Maddipati KR, Liu J, Joshi S, Tucker SC, Lee M-J, Honn KV. 2011. Identification of the orphan G protein-coupled receptor GPR31 as a receptor for 12-(S)-hydroxyeicosatetraenoic acid. J. Biol. Chem. 286:33832–33840. 10.1074/jbc.M110.216564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kenyon V, Rai G, Jadhav A, Schultz L, Armstrong M, Jameson JB, Perry S, Joshi N, Bougie JM, Leister W, Taylor-Fishwick Da Nadler AJL, Holinstat M, Simeonov A, Maloney DJ, Holman TR. 2011. Discovery of potent and selective inhibitors of human platelet-type 12-lipoxygenase. J. Med. Chem. 54:5485–5497. 10.1021/jm2005089 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.