Abstract
Although pertussis disease is vaccine preventable, Washington State experienced a substantial rise in pertussis incidence beginning in 2011. By June 2012, the reported cases reached 2,520 (37.5 cases per 100,000 residents), a 1,300% increase compared with the same period in 2011. We assessed the molecular epidemiology of this statewide epidemic using 240 isolates collected from case patients reported from 19 of 39 Washington counties during 2012 to 2013. The typing methods included pulsed-field gel electrophoresis (PFGE), multilocus variable number tandem repeat analysis (MLVA), multilocus sequence typing (MLST), and pertactin gene (prn) mutational analysis. Using the scheme PFGE-MLVA-MLST-prn mutations-Prn deficiency, the 240 isolates comprised 65 distinct typing profiles. Thirty-one PFGE types were found, with the most common types, CDC013 (n = 51), CDC237 (n = 44), and CDC002 (n = 42), accounting for 57% of them. Eleven MLVA types were observed, mainly comprising type 27 (n = 183, 76%). Seven MLST types were identified, with the majority of the isolates typing as prn2-ptxP3-ptxA1-fim3-1 (n = 157, 65%). Four different prn mutations accounted for the 76% of isolates exhibiting pertactin deficiency. PFGE provided the highest discriminatory power (D = 0.87) and was found to be a more powerful typing method than MLVA and MLST combined (D = 0.67). This study provides evidence for the continued predominance of MLVA 27 and prn2-ptxP3-ptxA1 alleles, along with the reemergence of the fim3-1 allele. Our results indicate that the Bordetella pertussis population causing this epidemic was diverse, with a few molecular types predominating. The PFGE, MLVA, and MLST profiles were consistent with the predominate types circulating in the United States and other countries. For prn, several mutations were present in multiple molecular types.
INTRODUCTION
Once a major cause of illness and death among infants and children, with over 160,000 cases annually during the 1920s and 1930s, pertussis, or whooping cough, has become the most frequently reported vaccine-preventable bacterial disease in the United States (1). With the introduction of whole-cell vaccines in the 1940s, the number of reported cases decreased by >99% to a record low in the late 1970s (1–3). Acellular vaccines that contained inactivated pertussis toxin (Ptx), one or more additional bacterial components (i.e., filamentous hemagglutinin [Fha], pertactin [Prn], and fimbriae [Fim] types 2 and 3), and diphtheria and tetanus toxoids were subsequently licensed. These acellular vaccines were recommended for the entire childhood booster series of DTaP (diphtheria, tetanus, and acellular pertussis vaccine) by the end of the 1990s, and in 2005, an additional single dose of diphtheria, tetanus, and acellular pertussis adolescent and adult booster (Tdap) was recommended (4–6). Recently, the Advisory Committee on Immunization Practices (ACIP) expanded booster recommendations to include vaccination of pregnant women to protect mothers and infants (7). Despite these recommendations, pertussis notifications have been steadily increasing since the 1980s, especially among adolescents and adults (8, 9).
During late 2011 and early 2012, several counties in western Washington State observed marked and sustained increases in pertussis over several weeks, prompting the Washington State Department of Health (WA DOH) to declare a statewide epidemic in April 2012. By 16 June, the reported number of cases in 2012 reached 2,520 (37.5 cases per 100,000 residents), a 1,300% increase compared to the same time period in 2011. By the end of the year, over 4,900 cases were reported, the highest number in 70 years. The incidence was greatest in infants of <1 year and in 10-year-olds and was elevated in 13- and 14-year-olds, the first birth cohort vaccinated solely with acellular vaccines for the childhood series and adolescent booster (10). Because WA DOH school entry data for 2011 to 2012 showed that only 3.5% of students were exempted from pertussis-containing vaccine, suggesting high immunization coverage, and the majority of pediatric cases were reported as vaccinated in this epidemic, it was important to assess how current populations of Bordetella pertussis were changing at a molecular level in comparison to the current vaccine (10).
To evaluate the molecular epidemiology of circulating strains of B. pertussis in this epidemic, we implemented 3 molecular typing methods that are currently used in the United States and other countries (11–15): pulsed-field gel electrophoresis (PFGE), multilocus variable number tandem repeat analysis (MLVA), and multilocus sequence typing (MLST). These methods are used to differentiate B. pertussis isolates based on whole-genome analysis and sequencing at variable loci throughout the genome (11, 12, 14, 15). Considering that prn mutational analysis for this statewide epidemic has been previously described (16), here we combined that prn analysis with other molecular typing methods to assess whether those prn mutations corresponded to specific molecular types. Our aims were to characterize the molecular epidemiology of the circulating strains of B. pertussis during the 2012 Washington State epidemic and for our findings to contribute to the ongoing discussion concerning possible reasons for the reemergence and increased incidence of pertussis in the United States over the past 30 years.
MATERIALS AND METHODS
Bacterial isolate and case reporting.
According to the Washington Administrative Code (WAC), health care providers, laboratories, and health care facilities in Washington State are required to report cases of suspected and confirmed pertussis to the local health jurisdiction (LHJ) where the patient resides. In addition, clinical laboratories are also required to submit pertussis isolates to the Washington State Public Health Laboratories (WAPHL). The LHJ performs case investigations and transmits data on case demographics, clinical and laboratory characteristics, and vaccination status to WA DOH using the state's electronic disease reporting surveillance system. Epidemiologists in the WA DOH Office of Communicable Disease Epidemiology (CDE) review these electronic case reports, monitor the reported vaccination status of cases, assign case classifications, transmit data to CDC, and monitor statewide pertussis activity and trends.
Data from surveillance reports and the percentage of cases with isolates submitted to WA PHL suggest that most WA health care providers order pertussis PCR only, reflecting clinical testing trends at the national level. During the study period, the majority of pertussis isolates in WA were obtained at a pediatric academic hospital laboratory, two laboratories serving large hospital systems, and WAPHL.
Two hundred thirty B. pertussis isolates from 2012 were forwarded from the WAPHL to the Centers for Disease Control and Prevention (CDC) for molecular characterization, along with 13 isolates submitted in late 2011 and early 2013. Of the 243 isolates sent, 3 were obtained from out-of-state residents and were not included in the analysis. Isolates were cultured on Regan-Lowe agar without cephalexin for 72 h at 35°C.
PFGE.
PFGE was conducted using previously described methods (16–18). Colonies were suspended in agarose and formed into plugs for PFGE. Plugs were treated with 20 mg/ml proteinase K for 2 h and then washed several times to remove excess proteinase K. Slices of each plug were digested with XbaI restriction endonuclease for 1.5 h at 37°C. Electrophoresis was performed with a CHEF-Mapper (Bio-Rad Laboratories, Hercules, CA) using the following conditions: gradient, 6 V/cm; included angle, 120°; initial switch time, 2.16 s; final switch time, 35.07 s; ramping factor, linear; temperature, 14°C; run time, 18 h. After electrophoresis, gels were stained with ethidium bromide and DNA bands were visualized with UV light. Tagged image file format (TIFF) images of gels were analyzed, and PFGE profiles were assigned to isolates based on a database of U.S. isolates maintained at CDC, using BioNumerics software version 5.01 (Applied Maths, Austin, TX).
MLVA.
MLVA was performed using a duplex reaction targeting variable number tandem repeats (VNTRs) 3 and 4 and a multiplex reaction targeting VNTRs 1, 5, and 6 as previously described (11–13). A HotStarTaq polymerase kit (Qiagen, Valencia, CA) was used to amplify the VNTRs from 60 ng total DNA in each reaction mixture, yielding a final volume of 24 μl (see Table S1 in the supplemental material for primer sequences and concentrations). The amplified products were diluted 1:50 and 1:100 and mixed with 0.5 μl of MapMarker X-rhodamine-labeled 400-bp ladder (BioVentures, Murfreesboro, TN). Sizes were determined by using the Prism 3130xl genetic analyzer (Applied Biosystems, Foster City, CA); VNTR sizes were determined using GeneMapper version 4.0 software (Applied Biosystems). Amplified product sizes for all strains were compared with those found for B. pertussis reference strain Tohama I to determine the repeat count for each locus. The assignment of MLVA type was based on the combination of repeat counts for VNTRs 1, 3a, 3b, 4, 5, and 6 and was consistent with international nomenclature (http://www.mlva.net/).
MLST and prn sequence analysis.
Our MLST algorithm consisted of 4 DNA targets: fragments of the pertactin gene (prn), the first gene in the pertussis toxin operon and its promoter (ptxA and ptxP), and the fimbrial protein-encoding gene (fim3). The primers used for PCR and sequencing are described in Table S1 in the supplemental material. For prn typing, PCR was performed using the Expand high-fidelity PCR system (Roche Applied Sciences, Indianapolis, IN). After a 15-min incubation at 95°C, prn was amplified, using the primers PRN-AF and PRN-BR, to produce a 1.4-kb PCR product (see Fig. S1 and Table S1) in 30 cycles, each cycle consisting of 30 s at 95°C, 30 s at 55°C, and 2.5 min at 72°C, with a final extension at 72°C for 5 min. For fim3, ptxP, and ptxA typing, PCR amplification was performed using HotStarTaq master mix with the primers indicated in the supplemental material (see Table S1). After a 15-min incubation at 95°C, ptxP, ptxA, and fim3 were amplified in 25 cycles consisting of 45 s at 94°C, 45 s at 57°C (ptxP and fim3) or 58°C (ptxA), and a 1-min extension at 72°C. The ptxP, ptxA, fim3, and prn sequencing was performed with the primers indicated in Table S1, using the BigDye terminator version 3.1 sequencing kit (Applied Biosystems) as previously described (19). Products were separated on an AB Prism 3130xl genetic analyzer (Applied Biosystems), and sequences were compared to known alleles for each locus. The compositions of these loci were expressed in an allelic profile designated prn-ptxP-ptxA-fim3.
Sequencing of prn for identification of mutations leading to the lack of pertactin expression was conducted as described previously (16). To identify the G insert at nucleotide 1185 and the stop codon at nucleotide 1273, additional prn sequencing was performed with the primers PRN-1627R and PRN-A2F individually for complete coverage of nt 982 to nt 1627 from the PRN-AF and PRN-BR 1.4-kb PCR product (reference to Tohama I) (see Fig. S1 and Table S1 in the supplemental material) (20).
The final molecular typing profile is represented as PFGE-MLVA-MLST-prn mutations-Prn deficiency.
Population analysis and index of diversity.
The geographic distribution of 2012 cases with and without isolates, by ZIP Code, was analyzed using ArcMap, version 10.1 (ESRI, Redlands, CA). Typing data were compiled using Bionumerics version 5.01. Minimum spanning trees were generated using default settings and either the categorical or binary coefficient, as indicated in the figure legends. The Simpson index of diversity (DI) was calculated as described by Hunter and Gaston (21). The DI is the assessment of the relative frequencies of types defined by a specific molecular typing method (21). In this study, this value indicates the probability of two isolates having different types for each molecular typing method and any combination of typing methods. DI was calculated for MLVA, MLST, PFGE, prn mutations, Prn deficiency, and the combinations of all molecular typing methods. All typing data were combined into overarching molecular typing profiles for this study. For example, for the combination PFGE plus MLVA plus MLST, CDC002-MLVA 27-prn2-ptxP3-ptxA1-fim3-1 was considered a unique type different from CDC002-MLVA 27-prn2-ptxP3-ptxA1-fim3-2.
RESULTS
Descriptive characteristics of the epidemic.
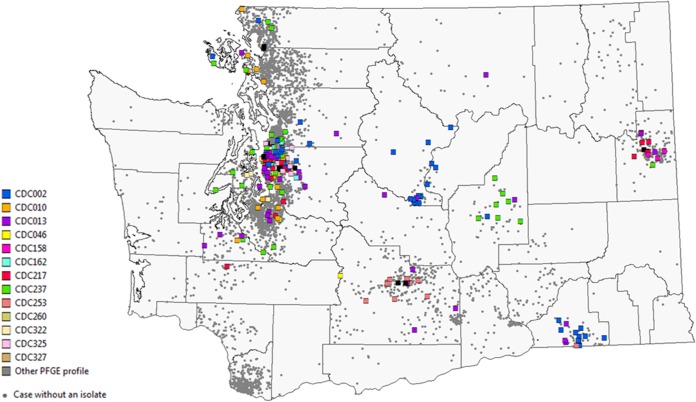
Of the 4,935 pertussis cases included in this analysis, 4,427 cases were classified as confirmed (84.8% had laboratory confirmation, and 15.2% were epidemiologically linked). Of the 3,602 laboratory-confirmed cases, 90.7% were confirmed by PCR alone, 2.3% by culture alone, and 7.0% by both PCR and culture. Among 2,020 cases aged 3 months to 10 years and 367 cases aged 11 to 12 years, 71.2% and 74.6%, respectively, were up to date for age on their pertussis vaccinations. Among 1,038 adolescents aged 13 to 19 years, 77.5% reported receipt of a Tdap dose. The case patients with isolates (240/4,935) are representative of the overall epidemic case patient population with respect to gender, age, and geographic distribution (Fig. 1 and Table 1). A majority of the isolates were collected during the peak months of the epidemic, April through June of 2012 (Table 1). Cases that were hospitalized were more likely to have had an isolate obtained than cases that were tested in an outpatient setting (37.5% and 18.4%, respectively) (Table 1), which may be consistent with provider practices to conduct PCR testing, as well as more specific testing via culture for infants with severe illness. Differences by race/ethnicity reflect the distribution of the catchment area served by Seattle Children's Hospital Laboratory, which submitted the majority of isolates (59%). Geographic region is defined as counties of case residence east (Eastern Washington) and west (Western Washington) of the Cascade Mountains. Cases residing in Eastern Washington were less likely to have had an isolate obtained than cases in Western Washington, which reflects differences in clinic provider testing practices (e.g., reliance on clinical diagnosis or confirmation by PCR only) between the two regions and the primary catchment area served by Seattle Children's Hospital Laboratory, which is located in Western Washington. However, among the 223 isolates with the case patient's ZIP Code known, the isolates tested were representative of the geographic distribution of the epidemic. Spatial clustering of PFGE types is observed among isolates from Central and Eastern Washington (Fig. 1).
FIG 1.
Map of the geographic distribution of cases with and without isolates collected in 2012. Cases were mapped by ZIP Code of residence. Squares represent the locations of case patients with isolates for whom the ZIP Codes and PFGE profiles are known (n = 233), with the color corresponding to the PFGE profile of the isolate. Gray circles represent the locations of cases without isolates but with the ZIP Code known (n = 4,681). The symbols are placed only within the ZIP Code and do not correspond to the actual location of each case patient's residence.
TABLE 1.
Demographic and clinical characteristics of 2012 Washington State pertussis cases with and without an isolate available for molecular characterization testing
| Characteristic | No. (%) without isolate (n = 4,695)a | No. (%) with isolate tested (n = 240) | P valueb |
|---|---|---|---|
| Gender | |||
| Female | 2,529 (53.9) | 135 (56.3) | 0.470 |
| Male | 2,166 (46.1) | 105 (43.8) | |
| Age group (yr) | |||
| <1 | 359 (7.7) | 24 (10.0) | 0.523 |
| 1–6 | 909 (19.4) | 34 (14.2) | |
| 7–10 | 972 (20.7) | 46 (19.2) | |
| 11–12 | 378 (8.1) | 21 (8.8) | |
| 13–14 | 559 (11.9) | 32 (13.3) | |
| 15–19 | 543 (11.6) | 31 (12.9) | |
| ≥20 | 975 (20.8) | 52 (21.7) | |
| Race/Ethnicity | |||
| White | 2,441 (52.0) | 141 (59.0) | 0.0002 |
| Hispanic | 872 (18.6) | 33 (13.8) | |
| Other | 226 (4.8) | 23 (9.6) | |
| Unknown | 1,156 (24.6) | 43 (17.9) | |
| Geographic region | |||
| Western WA | 3,641 (77.6) | 163 (67.9) | 0.0005 |
| Eastern WA | 1,054 (22.5) | 77 (32.1) | |
| Quarter of onset (n = 4,918) | n = 4,695 | n = 228 | |
| January-March | 1,110 (23.6) | 16 (7.0) | <0.0001 |
| April-June | 2,050 (43.7) | 127 (55.7) | |
| July-September | 1,077 (22.9) | 64 (28.1) | |
| October-December | 458 (9.8) | 21 (9.2) | |
| Hospitalization among infants (n = 383) | n = 359 | n = 24 | |
| Yes | 66 (18.4) | 9 (37.5) | 0.022 |
| No | 293 (81.6) | 15 (62.5) |
One hundred nine cases had a positive culture documented in the case report, but the respective isolates were not submitted to the WAPHL.
χ2 test for independence was used to determine differences between cases with and without isolates. P values of <0.05 were considered statistically significant.
Molecular typing with PFGE, MLST, and MLVA.
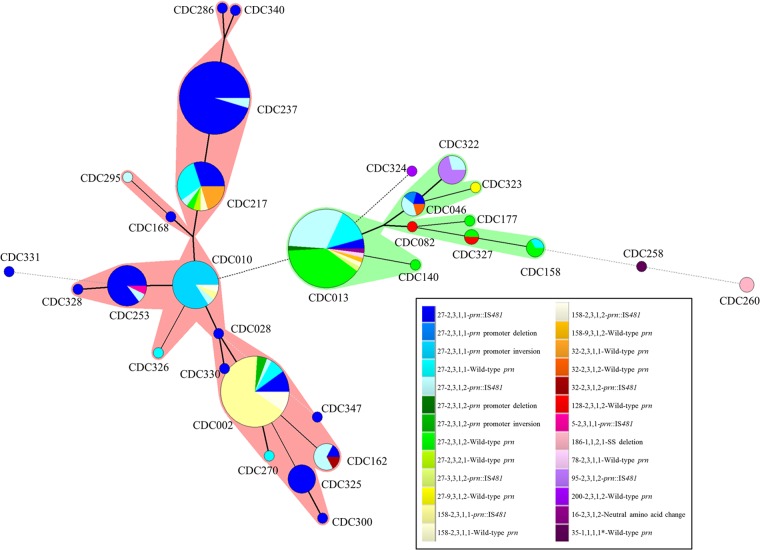
Sixty-five molecular typing profiles were identified. WA1 and WA2 were the most common profiles, representing 18% and 12% of the total isolates, respectively (Table 2). Forty-two profiles (65%) were represented by a single isolate (Table 2). Thirty-one PFGE types were identified, with the most common types, CDC013 (n = 51; 10 profiles), CDC237 (n = 44; 2 profiles), and CDC002 (n = 42; 6 profiles), accounting for 57% of total isolates (Table 2; Fig. 2). For a majority of PFGE profiles, only a small number of isolates were identified (Table 2; Fig. 2).
TABLE 2.
Molecular typing profiles of B. pertussis (n = 240) isolates obtained during the 2012 Washington State epidemic
| Profile | PFGEa | MLVAb | Allele of: |
prn mutation | Expresses Prn | Total no. of isolates | |||
|---|---|---|---|---|---|---|---|---|---|
| prn | ptxP | ptxA | fim3 | ||||||
| WA1 | 237 | 27 | 2 | 3 | 1 | 1 | IS481 insertionc | − | 42 |
| WA2 | 2 | 158 | 2 | 3 | 1 | 1 | IS481 insertion | − | 28 |
| WA3 | 13 | 27 | 2 | 3 | 1 | 2 | IS481 insertion | − | 19 |
| WA4 | 13 | 27 | 2 | 3 | 1 | 2 | Wild-type prn | + | 17 |
| WA5 | 10 | 27 | 2 | 3 | 1 | 1 | Promoter inversion (−74 nt) | − | 16 |
| WA6 | 253 | 27 | 2 | 3 | 1 | 1 | IS481 insertion | − | 12 |
| WA7 | 13 | 27 | 2 | 3 | 1 | 1 | Wild-type prn | + | 7 |
| WA8 | 13 | 27 | 2 | 3 | 1 | 1 | IS481 insertion | − | 2 |
| WA9 | 325 | 27 | 2 | 3 | 1 | 1 | IS481 insertion | − | 7 |
| WA10 | 217 | 27 | 2 | 3 | 1 | 1 | Wild-type prn | + | 6 |
| WA11 | 217 | 27 | 2 | 3 | 1 | 1 | IS481 insertion | − | 6 |
| WA12 | 2 | 27 | 2 | 3 | 1 | 1 | IS481 insertion | − | 5 |
| WA13 | 322 | 95 | 2 | 3 | 1 | 2 | IS481 insertion | − | 5 |
| WA14 | 2 | 158 | 2 | 3 | 1 | 2 | IS481 insertion | − | 4 |
| WA15 | 162 | 27 | 2 | 3 | 1 | 2 | IS481 insertion | − | 4 |
| WA16 | 217 | 32 | 2 | 3 | 1 | 1 | Wild-type prn | + | 4 |
| WA17 | 2 | 27 | 2 | 3 | 1 | 2 | Promoter inversion (−74 nt) | − | 2 |
| WA18 | 2 | 27 | 2 | 3 | 1 | 1 | Wild-type prn | + | 2 |
| WA19 | 46 | 27 | 2 | 3 | 1 | 2 | IS481 insertion | − | 2 |
| WA20 | 158 | 27 | 2 | 3 | 1 | 2 | Wild-type prn | + | 2 |
| WA21 | 237 | 27 | 2 | 3 | 1 | 2 | IS481 insertion | − | 2 |
| WA22 | 260 | 186 | 1 | 1 | 2 | 1 | Signal sequence deletion (nt 26 to 109) | − | 2 |
| WA23 | 322 | 27 | 2 | 3 | 1 | 2 | IS481 insertion | − | 2 |
| WA24 | 2 | 27 | 2 | 3 | 1 | 2 | IS481 insertion | − | 1 |
| WA25 | 10 | 27 | 2 | 3 | 1 | 2 | IS481 insertion | − | 1 |
| WA26 | 10 | 158 | 2 | 3 | 1 | 1 | IS481 insertion | − | 1 |
| WA27 | 10 | 158 | 2 | 3 | 1 | 2 | IS481 insertion | − | 1 |
| WA28 | 13 | 27 | 2 | 3 | 1 | 2 | Deletion (nt −2090 to −478) | − | 1 |
| WA29 | 13 | 27 | 3 | 3 | 1 | 2 | IS481 insertion | − | 1 |
| WA30 | 13 | 158 | 2 | 3 | 1 | 1 | Wild-type prn | + | 1 |
| WA31 | 13 | 158 | 9 | 3 | 1 | 2 | Wild-type prn | + | 1 |
| WA32 | 13 | 16 | 2 | 3 | 1 | 2 | T at nt 638; neutral amino acid change | + | 1 |
| WA33 | 13 | 78 | 2 | 3 | 1 | 1 | Wild-type prn | + | 1 |
| WA34 | 28 | 27 | 2 | 3 | 1 | 1 | IS481 insertion | − | 1 |
| WA35 | 46 | 27 | 2 | 3 | 1 | 1 | IS481 insertion | − | 1 |
| WA36 | 46 | 27 | 2 | 3 | 1 | 1 | Deletion (nt −2090 to 478) | − | 1 |
| WA37 | 46 | 32 | 2 | 3 | 1 | 2 | Wild-type prn | + | 1 |
| WA38 | 82 | 128 | 2 | 3 | 1 | 2 | Wild-type prn | + | 1 |
| WA39 | 140 | 27 | 2 | 3 | 1 | 2 | Wild-type prn | + | 1 |
| WA40 | 158 | 27 | 2 | 3 | 1 | 1 | Wild-type prn | + | 1 |
| WA41 | 162 | 27 | 2 | 3 | 1 | 1 | IS481 insertion | − | 1 |
| WA42 | 162 | 32 | 2 | 3 | 1 | 2 | IS481 insertion | − | 1 |
| WA43 | 168 | 27 | 2 | 3 | 1 | 1 | IS481 insertion | − | 1 |
| WA44 | 177 | 27 | 2 | 3 | 1 | 2 | Wild-type prn | + | 1 |
| WA45 | 217 | 27 | 2 | 3 | 1 | 2 | Wild-type prn | + | 1 |
| WA46 | 217 | 27 | 2 | 3 | 1 | 2 | IS481 insertion | − | 1 |
| WA47 | 217 | 27 | 2 | 3 | 2 | 1 | Wild-type prn | + | 1 |
| WA48 | 217 | 158 | 2 | 3 | 1 | 1 | Wild-type prn | + | 1 |
| WA49 | 253 | 27 | 2 | 3 | 1 | 2 | IS481 insertion | − | 1 |
| WA50 | 253 | 5 | 2 | 3 | 1 | 1 | IS481 insertion | − | 1 |
| WA51 | 258 | 35 | 1 | 1 | 1 | 1*d | Wild-type prn | + | 1 |
| WA52 | 270 | 27 | 2 | 3 | 1 | 1 | Wild-type prn | + | 1 |
| WA53 | 286 | 27 | 2 | 3 | 1 | 1 | IS481 insertion | − | 1 |
| WA54 | 295 | 27 | 2 | 3 | 1 | 2 | IS481 insertion | − | 1 |
| WA55 | 300 | 27 | 2 | 3 | 1 | 1 | IS481 insertion | − | 1 |
| WA56 | 323 | 27 | 9 | 3 | 1 | 2 | Wild-type prn | + | 1 |
| WA57 | 324 | 200 | 2 | 3 | 1 | 2 | Wild-type prn | + | 1 |
| WA58 | 326 | 27 | 2 | 3 | 1 | 1 | Wild-type prn | + | 1 |
| WA59 | 327 | 27 | 2 | 3 | 1 | 2 | Wild-type prn | + | 1 |
| WA60 | 327 | 128 | 2 | 3 | 1 | 2 | Wild-type prn | + | 1 |
| WA61 | 328 | 27 | 2 | 3 | 1 | 1 | IS481 insertion | − | 1 |
| WA62 | 330 | 27 | 2 | 3 | 1 | 1 | IS481 insertion | − | 1 |
| WA63 | 331 | 27 | 2 | 3 | 1 | 1 | IS481 insertion | − | 1 |
| WA64 | 340 | 27 | 2 | 3 | 1 | 1 | IS481 insertion | − | 1 |
| WA65 | 347 | 27 | 2 | 3 | 1 | 1 | IS481 insertion | − | 1 |
PFGE, pulsed-field gel electrophoresis.
MLVA, multilocus variable tandem repeat analysis.
prn::IS481.
fim3-1* has a T at nucleotide 87 instead of a C (4).
FIG 2.
Minimum spanning tree based on PFGE. A binary coefficient was used for clustering. Each circle in the tree represents a different PFGE type, and the type number is indicated within or near the circle. The size of the circle indicates the number of isolates with the particular PFGE type. The lines connecting the circles represent the number of band differences between connecting PFGE types. Heavy solid lines connecting two PFGE types denote types differing by a single band, thin solid lines connect types that differ by two bands, and dotted lines indicate types differing by three or more bands. Each color represents a different MLVA-MLST (prn-ptxP-ptxA-fim3)-prn genotype profile, as indicated in the key. Shadows around circles indicate PFGE types that are closely related based on banding patterns.
Seven MLST types were identified, with the majority of the isolates typing as prn2-ptxP3-ptxA1-fim3-1 (n = 157, 65%; 32 profiles) or prn2-ptxP3-ptxA1-fim3-2 (n = 76, 32%; 27 profiles) (Table 2; Fig. 2 and 3). Isolates with the PFGE type CDC013 were predominately prn2-ptxP3-ptxA1-fim3-2 (n = 38, 75%), while isolates with the PFGE types CDC002 and CDC0237 were predominately prn2-ptxP3-ptxA1-fim3-1 (n = 35, 83%, and n = 42, 95%, respectively) (Table 2; Fig. 2).
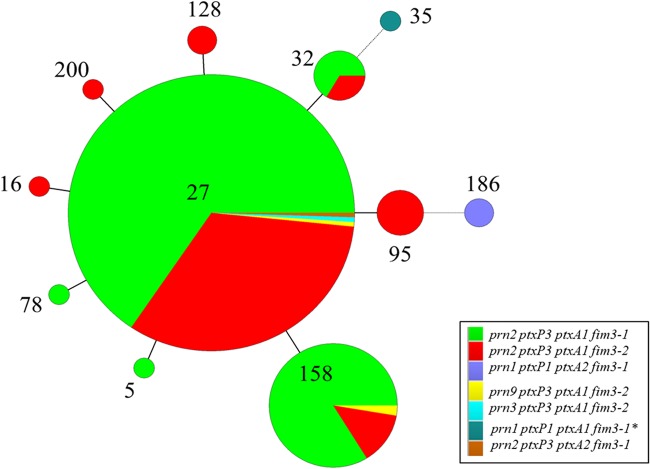
FIG 3.
Minimum spanning tree based on MLVA. A categorical coefficient was used for clustering. Each circle in the tree represents a different MLVA type, and the type number is indicated within the circle. The size of the circle indicates the number of isolates with the particular MLVA type. Solid lines connecting circles represent 1 VNTR difference between connecting MLVA types, while dotted lines represent 2 VNTR differences. Each color represents a different MLST type, as indicated in the key.
Eleven MLVA types were observed, mainly comprising MLVA 27 (n = 183, 76%; 46 profiles) and MLVA 158 (n = 37, 15%; 7 profiles) (Table 2; Fig. 2 and 3). Of the isolates with PFGE types CDC013 and CDC237, 92% and 100%, respectively, were MLVA 27. Seventy-six percent of isolates with the PFGE type CDC002 were MLVA 158 (n = 32). Twenty-two of 31 PFGE types were of a single MLVA type.
In comparing MLVA and MLST, we found that a majority of isolates with MLVA 27 and MLVA 158 had the MLST type prn2-ptxP3-ptxA1-fim3-1 (66% and 84%, respectively) (Table 2; Fig. 3). The 3 isolates typing as WA22 and WA51 were the only isolates observed with the ptxP1 allele (Table 2; Fig. 2 and 3).
Among pertussis isolates from this statewide epidemic, PFGE alone (D = 0.87) accounted for a higher discriminatory power than the combination of MLVA and MLST (D = 0.67) (Table 3). The fim3 locus contributed the most to the discriminatory power of MLST (D = 0.45).The combination of PFGE, MLVA, and MLST had an index of diversity of 0.92 (Table 3).
TABLE 3.
Simpson's diversity indices for typing strategies
| Typing methoda | Type count | Index of diversity |
|---|---|---|
| PFGE | 31 | 0.87 |
| MLVA | 11 | 0.40 |
| MLST | 7 | 0.47 |
| PFGE + MLST | 43 | 0.90 |
| PFGE + MLVA | 43 | 0.91 |
| MLVA + MLST | 18 | 0.67 |
| PFGE + MLVA + MLST | 57 | 0.92 |
| prn mutations | 6 | 0.51 |
| PFGE + prn mutations | 40 | 0.91 |
| MLVA + prn mutations | 16 | 0.70 |
| MLST + prn mutations | 14 | 0.71 |
| Prn deficiency | 2 | 0.36 |
| PFGE + MLVA + MLST + prn mutations + Prn deficiency | 65 | 0.94 |
PFGE, pulsed-field gel electrophoresis; MLVA, multilocus variable number tandem repeat analysis; MLST, multilocus sequence typing; prn, pertactin gene.
prn mutations.
Seventy-six percent (n = 183) of the isolates collected during this statewide epidemic were pertactin deficient, as previously described (16). Of the 65 molecular typing profiles identified, only 25 (38%) produced Prn (Table 2). Of the 3 major PFGE profiles, all PFGE type CDC237 isolates (n = 44) and 88% of the isolates with PFGE profile CDC002 (n = 37) had an IS481 insertion in the prn gene (Table 2). In contrast, 61% of the isolates with PFGE type CDC013 were wild type and produced Prn (n = 31). The IS481 insertion, which can be found in 3 different locations within prn, was seen in 35 molecular profiles and associated with five different MLVA types (27, 158, 95, 32, and 5), along with three MLST types (Table 2; see also Fig. S1 in the supplemental material). The prn 22-kb promoter inversion, at nucleotide −74, was present in profiles WA5 and WA17, which corresponded to 84% of isolates with PFGE type CDC010 and 5% of isolates with PFGE type CDC002 (Table 2; see also Fig. S1). Furthermore, the promoter inversion was only seen in the 18 isolates with MLVA 27 and two MLST types that differ by the fim3 allele (Table 2). The signal sequence (SS) deletion of nucleotides 22 to 109 in prn was only seen in two isolates with profile WA22 (Table 2; see also Fig. S1). The prn promoter and 5′-end deletion was only seen in the two isolates with profiles WA28 and WA36 (Table 2). These isolates were MLVA 27 and harbored two MLST profiles that differ by the fim3 allele (Table 2). A point mutation in prn resulting in a neutral amino acid change was seen in the isolate with the profile WA32 and did not lead to Prn deficiency (Table 2; see also Fig. S1). Figure 2 shows the clustering of predominately Prn-deficient PFGE types (indicated by red shadows) and predominately Prn-expressing PFGE types (indicated by green shadows).
The combination of PFGE and the prn mutations (D = 0.91) demonstrated a higher discriminatory power than the combinations of MLVA and the prn mutations (D = 0.70) and MLST and the prn mutations (D = 0.71) (Table 3). The combination of all molecular typing data into the scheme PFGE-MLVA-MLST-prn mutations-Prn expression accounted for the highest discriminatory power of all combinations of typing methods (D = 0.94) (Table 3).
DISCUSSION
We investigated the molecular epidemiology of the largest pertussis epidemic in Washington State in 70 years. Given that the majority of cases were fully vaccinated, we assessed the current population of B. pertussis at a molecular level and compared the results to the current vaccine. By PFGE, we found 65 distinct typing profiles that demonstrated high strain diversity with no predominate clone and a large proportion of isolates with pertactin deficiency, and we found a higher discriminatory power of PFGE alone than of MLVA plus MLST. Although it is likely that multiple, possibly interrelated reasons for the recent resurgence of pertussis will ultimately be identified, this study sheds light on the importance of considering bacterial adaption to current vaccines as one possible factor (22–25). This is evident with the surge in strains deficient in pertactin, a known pertussis acellular vaccine immunogen.
The predominate MLVA-MLST type circulating in Washington State was MLVA 27 prn2-ptxP3-ptxA1-fim3-1, which aside from the fim3 allele, coincides with the predominate strain found during the early 1990s to 2009 in a similar study conducted in the United States (11). This strain is currently predominate in Japan, Australia, and parts of Europe (11, 13, 26–28). The molecular profile for 2 strains used to produce the current U.S. vaccine are MLVA 38 prn1-ptxP1-ptxA2-fim3-1 (Tohama I) and MLVA 167 prn1-ptxP1-ptxA4-fim3-1 (strain 10536). Few isolates were observed with the ptxP1 allele, which coincides with the decreased frequency of this allele in circulating strains in the United States, Netherlands, Canada, Sweden, Australia, and Denmark (27–31). MLVA 186 was first seen in the United States in 2007, yet the two isolates with this MLVA type in this epidemic are associated with the MLST profile prn1-ptxP1-ptxA2-fim3-1 that was observed in the Tohama I strain (11).
Three PFGE profiles predominated within the Washington State epidemic: CDC013, CDC237, and CDC002 (Fig. 1; see also Fig. S2 in the supplemental material). CDC013 was the most common PFGE profile in the United States between 2000 and 2009 (40%) but decreased to 11% in 2010 to 2012 (32). The PFGE profile CDC237 was first seen in the United States in 2009 and has rapidly increased to 25% of the 2012 isolates tested (32). Interestingly, 95% of isolates with the profile CDC237 were MLVA 27 and prn2-ptxP3-ptxA1-fim3-1, which are also the predominate MLVA and MLST profiles overall. Unlike the other predominate PFGE profiles, CDC002 represented <10% of U.S. isolates from 2000 to 2009 before rapidly increasing to 24% in 2010 to 2012 (32). Sixty-seven percent of isolates with the profile CDC002 were MLVA 158 and prn2-ptxP3-ptxA1-fim3-1.
CDC013 continues to have a majority of isolates with the fim3-2 allele, while the majority of the CDC237 and CDC002 isolates possess the fim3-1 allele (Fig. 2). Until 2009, increased pertussis case reporting was correlated with the emergence of fim3-2 (designated fim3B in reference 11) in the United States. That is not the case for this epidemic, where the reemergence of the fim3-1 allele (designated fim3A in reference 11) was seen. The fim3-1 allele has not predominated in U.S. isolates since the early 2000s (11).
By comparing the index of diversity values of the molecular typing methods implemented in this study, it was evident that different methods demonstrated very different snapshots of diversity. Similar to what is seen in the United States as a whole, as well as in other countries, this epidemic does not demonstrate the presence of a large number of alleles for the prn, ptxP, and ptxA genes, indicating a low level of diversity based on these MLST loci (11, 26, 29). The fim3 locus contributed most to the diversity of the MLST profiles. Our finding of a higher discriminatory power for PFGE than for the combination of MLVA and MLST was also seen in a molecular typing study conducted on Swedish isolates (15). Seeing that PFGE is a depiction of diversity on a genomic scale provides evidence that these isolates are changing more rapidly on a genomic level than within the individual genes and regions targeted in MLVA and MLST. With the indication that diversity is occurring at a genomic level, as seen by PFGE and prn mutations (specifically the IS481 insertion), it is important to address the need for a whole-genome analysis to make more definitive conclusions about correlation. With the lack of a unified typing scheme and the diversity seen in this one epidemic, it has become increasingly necessary to develop a universally applicable format for reporting the molecular epidemiology of B. pertussis populations that is consistent with the guidelines for bacterial genetic nomenclature. This will allow for more efficient comparisons across multiple epidemics in multiple countries.
Though it was first identified in an isolate from 1994, it was not until 2010 that pertactin deficiency began steadily increasing in B. pertussis isolates in the United States (16, 33). To date, the United States has seen a significantly higher number of pertactin-deficient B. pertussis strains than other countries, such as France in 2007 (7%), Japan from 1990 to 2009 (27%), or Finland from 2006 to 2011 (2%) (34–36). The proportion of pertactin-deficient isolates has begun to steadily increase in other countries, including France, increasing from 2% in 2005 to 14% in 2012, which is similar to the progressive increases seen in the United States and Australia (16, 37–39). A large proportion of isolates (76%) in this study were pertactin deficient due to one of four different mutations previously identified in the prn gene (16, 20, 34–37). The presence of the SS deletion in the 2 isolates with PFGE profile CDC260, MLVA 186, and MLST profile prn1-ptxP1-ptxA2-fim3-1 does not correlate with what was seen in Japan from 1990 to 2009, where the prn1 SS deletion predominated among pertactin-deficient isolates (73%) and was seen in 3 different MLVA types (MLVA 186, MLVA 194, and MLVA 226) (35). The prn1 SS deletion was first seen in Japan in 2000 and has shown more MLVA diversity than was seen in the Washington State epidemic (35). One isolate (collected during 2007) out of 600 in the United States had MLVA type 186 and had the same PFGE and MLST profiles as seen with the 2 isolates from the Washington State epidemic (11). The only two pertactin-deficient isolates collected in Finland from 2006 to 2011 also harbored the prn1 SS deletion (36). It should be noted that while IS481 insertions are seen throughout multiple MLVA types and MLST profiles, the additional three mutations loosely associate with two MLVA types and two MLST profiles, which may be due in part to the low number of isolates collected in this study (Table 2).
Since the majority of cases in this statewide epidemic occurred among school-aged children that were up to date for their age for pertussis-containing vaccine as recommended by the ACIP, it is important to consider what factors may have led to this surge in reported cases in Washington State, as well as in the United States as a whole. One current hypothesis centers on the idea that B. pertussis strains harboring prn mutations (i.e., escape mutants) possibly have a selective advantage in individuals vaccinated with acellular vaccines (40, 41). As previously mentioned, the majority of strains circulating in the Washington State epidemic are divergent from the two current U.S. vaccine strains, which both express prn1. A study conducted in Italy indicated that the prn vaccine allele (prn1, which encodes the Prn1 antigenic variant) was identified at a lower frequency in vaccinated individuals and was seen in an increased number of isolates collected from areas of low vaccination (40). prn1 and prn2 differ by the presence of 5 amino acid repeats in variant region 1 of prn but are recognized equally efficiently by the T-cell response in mice and humans (42). Here, we see prn2 predominating in a highly vaccinated population, which would provide evidence in support of this hypothesis. Epidemiological analysis suggests that vaccinated persons have increased susceptibility to pertactin-deficient strains compared to their susceptibility to strains expressing pertactin (40, 41). There has been no direct evidence that pertactin-deficient strains are more virulent in humans than strains expressing pertactin (38, 40). Through our analysis, it is clear that the currently circulating strains are diverse on a genomic level. The rapidly increasing availability of whole-genome sequencing and analyses, specifically through analysis of additional virulence-related genes, will allow direct investigation of this hypothesis by determining whether pertactin is a major virulence-related gene affected by mutations and will likewise provide answers with regard to what genes may provide these strains associated with pertussis disease with a selective advantage in vaccinated populations.
In an era of increasing provider reliance on pertussis PCR for rapid diagnosis and treatment of suspected cases, this study demonstrates the importance of maintaining culture testing practices. With the highest number of pertussis cases reported in 2012 in the United States since the 1950s, understanding the molecular epidemiology of currently circulating strains of B. pertussis is critical. Providers should be made aware that isolation of B. pertussis is necessary so that genetic characterization and analysis can be conducted to monitor trends in strain circulation. This evaluation of isolates from a large pertussis epidemic in Washington State has contributed further to our understanding of strain evolution in the United States and suggests that as current molecular typing techniques advance, our understanding of the B. pertussis diversity seen on a genomic level will continue to evolve.
Supplementary Material
Footnotes
Published ahead of print 16 July 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JCM.01189-14.
REFERENCES
- 1.Tanaka M, Vitek CR, Pascual FB, Bisgard KM, Tate JE, Murphy TV. 2003. Trends in pertussis among infants in the United States, 1980–1999. JAMA 290:2968–2975. 10.1001/jama.290.22.2968 [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. 2002. Pertussis—United States, 1997–2000. Morb. Mortal. Weekly Rep. 51:73–76 [PubMed] [Google Scholar]
- 3.Davis SF, Strebel PM, Cochi SL, Zell ER, Hadler SC. 1992. Pertussis surveillance—United States, 1989–1991. Morb. Mortal. Wkly. Rep. 41:11–19 [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. 1997. Pertussis vaccination: use of acellular pertussis vaccines among infants and young children. Recommendations of the Advisory Committee on Immunization Practices (ACIP). Morb. Mortal. Weekly Rep. 416:1–25 [PubMed] [Google Scholar]
- 5.Broder KR, Cortese MM, Iskander JK, Kretsinger K, Slade BA, Brown KH, Mijalski CM, Tiwari T, Weston EJ, Cohn AC, Srivastava PU, Moran JS, Schwartz B, Murphy TV, Advisory Committee on Immunization Practices (ACIP) 2006. Preventing tetanus, diphtheria, and pertussis among adolescents: use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccines recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm. Rep. 55(RR-3):1–34 [PubMed] [Google Scholar]
- 6.Kretsinger K, Broder KR, Cortese MM, Joyce MP, Ortega-Sanchez I, Lee GM, Tiwari T, Cohn AC, Slade BA, Iskander JK, Mijalski CM, Brown KH, Murphy TV, Centers for Disease Control and Prevention; Advisory Committee on Immunization Practices; Healthcare Infection Control Practices Advisory Committee 2006. Preventing tetanus, diphtheria, and pertussis among adults: use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccine recommendations of the Advisory Committee on Immunization Practices (ACIP) and recommendation of ACIP, supported by the Healthcare Infection Control Practices Advisory Committee (HICPAC), for use of Tdap among health-care personnel. MMWR Recomm. Rep. 55(RR-17):1–37 [PubMed] [Google Scholar]
- 7.Sawyer M, Liang JL, Messonnier N, Clark TA. 2013. Updated recommendations for use of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine (Tdap) in pregnant women—Advisory Committee on Immunization Practices (ACIP), 2012. MMWR Morb. Mortal. Wkly. Rep. 62:131–135 [PMC free article] [PubMed] [Google Scholar]
- 8.Farizo KM, Cochi SL, Zell ER, Brink EW, Wassilak SG, Patriarca PA. 1992. Epidemiological features of pertussis in the United States, 1980–1989. Clin. Infect. Dis. 14:708–719. 10.1093/clinids/14.3.708 [DOI] [PubMed] [Google Scholar]
- 9.Guris D, Strebel PM, Bardenheier B, Brennan M, Tachdjian R, Finch E, Wharton M, Livengood JR. 1999. Changing epidemiology of pertussis in the United States: increasing reported incidence among adolescents and adults, 1990–1996. Clin. Infect. Dis. 28:1230–1237. 10.1086/514776 [DOI] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention. 2012. Pertussis epidemic—Washington, 2012. Morb. Mortal. Weekly Rep. 61:517–522 [PubMed] [Google Scholar]
- 11.Schmidtke AJ, Boney KO, Martin SW, Skoff TH, Tondella ML, Tatti KM. 2012. Population diversity among Bordetella pertussis isolates, United States, 1935–2009. Emerg. Infect. Dis. 18:1248–1255. 10.3201/eid1808.120082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schouls LM, van der Heide HGJ, Vauterin L, Vauterin P, Mooi FR. 2004. Multiple-locus variable-number tandem repeat analysis of Dutch Bordetella pertussis strains reveals rapid genetic changes with clonal expansion during the late 1990s. J. Bacteriol. 186:5496–5505. 10.1128/JB.186.16.5496-5505.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Litt DJ, Neal SE, Fry NK. 2009. Changes in genetic diversity of the Bordetella pertussis population in the United Kingdom between 1920 and 2006 reflect vaccination coverage and emergence of a single dominant clonal type. J. Clin. Microbiol. 47:680–688. 10.1128/JCM.01838-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kurniawan J, Maharjan RP, Chan W-F, Reeves PR, Sintchenko V, Gilbert GL, Mooi FR, Lan R. 2010. Bordetella pertussis clones identified by multilocus variable-number tandem-repeat analysis. Emerg. Infect. Dis. 16:297–300. 10.3201/eid1602.081707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Advani A, Van der Heide HGJ, Hallander HO, Mooi FR. 2009. Analysis of Swedish Bordetella pertussis isolates with three typing methods: characterization of an epidemic lineage. J. Microbiol. Methods 78:297–301. 10.1016/j.mimet.2009.06.019 [DOI] [PubMed] [Google Scholar]
- 16.Pawloski LC, Queenan AM, Cassiday PK, Lynch AS, Harrison M, Shang W, Williams MM, Bowden KE, Burgos-Rivera B, Qin X, Messonnier N, Tondella ML. 2014. Prevalence and molecular characterization of pertactin-deficient Bordetella pertussis in the United States. Clin. Vaccine Immunol. 21:119–125. 10.1128/CVI.00717-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bisgard KM, Christie CDC, Reising SF, Sanden GN, Cassiday PK, Gomersall C, Wattigney WA, Roberts NE, Strebel PM. 2001. Molecular epidemiology of Bordetella pertussis by pulsed-field gel electrophoresis profile: Cincinnati, 1989–1996. J. Infect. Dis. 183:1360–1367. 10.1086/319858 [DOI] [PubMed] [Google Scholar]
- 18.Hardwick TH, Cassiday P, Weyant RS, Bisgard KM, Sanden GN. 2002. Changes in predominance and diversity of genomic subtypes of Bordetella pertussis isolated in the United States, 1935–1999. Emerg. Infect. Dis. 8:44–49. 10.3201/eid0801.010021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mooi FR, van Oirschot H, Heuvelman K, van der Heide HGJ, Gaastra W, Willems RJL. 1998. Polymorphism in the Bordetella pertussis virulence factors P.69/pertactin and pertussis toxin in the Netherlands: temporal trends and evidence for vaccine-driven evolution. Infect. Immun. 66:670–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Queenan AM, Cassiday PK, Evangelista A. 2013. Pertactin-negative variants of Bordetella pertussis in the United States. N. Engl. J. Med. 368:583–584. 10.1056/NEJMc1209369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hunter PR, Gaston MA. 1988. Numerical index of the discriminatory ability of typing systems—an application of Simpsons index of diversity. J. Clin. Microbiol. 26:2465–2466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Korppi M. 2013. Whooping cough—still a challenge. J. Pediatr. (Rio J.) 89:520–522. 10.1016/j.jped.2013.09.001 [DOI] [PubMed] [Google Scholar]
- 23.Guiso N. 2014. Bordetella pertussis: Why is it still circulating? J. Infect. 68:S119–S124. 10.1016/j.jinf.2013.09.022 [DOI] [PubMed] [Google Scholar]
- 24.Mooi FR, Van Der Maas NA, De Melker HE. 2014. Pertussis resurgence: waning immunity and pathogen adaptation—two sides of the same coin. Epidemiol. Infect. 142:685–694. 10.1017/S0950268813000071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Allen A. 2013. Public health. The pertussis paradox. Science 341:454–455. 10.1126/science.341.6145.454 [DOI] [PubMed] [Google Scholar]
- 26.Miyaji Y, Otsuka N, Toyoizumi-Ajisaka H, Shibayama K, Kamachi K. 2013. Genetic analysis of Bordetella pertussis isolates from the 2008–2010 pertussis epidemic in Japan. PLoS One 8:e77165. 10.1371/journal.pone.0077165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Octavia S, Sintchenko V, Gilbert GL, Lawrence A, Keil AD, Hogg G, Lan R. 2012. Newly emerging clones of Bordetella pertussis carrying prn2 and ptxP3 alleles implicated in Australian pertussis epidemic in 2008–2010. J. Infect. Dis. 205:1220–1224. 10.1093/infdis/jis178 [DOI] [PubMed] [Google Scholar]
- 28.Petersen RF, Dalby T, Dragsted DM, Mooi F, Lambertsen L. 2012. Temporal trends in Bordetella pertussis populations, Denmark, 1949–2010. Emerg. Infect. Dis. 18:767–774. 10.3201/eid1805.110812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Gent M, Bart MJ, van der Heide HGJ, Heuvelman KJ, Mooi FR. 2012. Small mutations in Bordetella pertussis are associated with selective sweeps. PLoS One 7:e46407. 10.1371/journal.pone.0046407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Advani A, Gustafsson L, Ahren C, Mooi FR, Hallander HO. 2011. Appearance of fim3 and ptxP3-Bordetella pertussis strains, in two regions of Sweden with different vaccination programs. Vaccine 29:3438–3442. 10.1016/j.vaccine.2011.02.070 [DOI] [PubMed] [Google Scholar]
- 31.Shuel M, Jamieson FB, Tang P, Brown S, Farrell D, Martin I, Stoltz J, Tsang RSW. 2013. Genetic analysis of Bordetella pertussis in Ontario, Canada reveals one predominant clone. Int. J. Infect. Dis. 17:E413–E417. 10.1016/j.ijid.2012.12.015 [DOI] [PubMed] [Google Scholar]
- 32.Cassiday P, Skoff T, Faulkner A, Connolly L, Jawahir S, Tondella M. 2013. Temporal changes in the predominance of pulsed-field gel electrophoresis profiles of Bordetella pertussis isolates from United States, 2000–2012, abstr P12 Abstracts of the 10th International Symposium on Bordetella, Dublin, Ireland, 8 to 11 September 2013 [Google Scholar]
- 33.Quinlan T, Musser KA, Currenti SA, Zansky SM, Halse TA. 2014. Pertactin-negative variants of Bordetella pertussis in New York State: a retrospective analysis, 2004–2013. Mol. Cell. Probes 28:138–140. 10.1016/j.mcp.2013.12.003 [DOI] [PubMed] [Google Scholar]
- 34.Bouchez V, Brun D, Cantinelli T, Dore G, Njamkepo E, Guiso N. 2009. First report and detailed characterization of B. pertussis isolates not expressing pertussis toxin or pertactin. Vaccine 27:6034–6041. 10.1016/j.vaccine.2009.07.074 [DOI] [PubMed] [Google Scholar]
- 35.Otsuka N, Han HJ, Toyoizumi-Ajisaka H, Nakamura Y, Arakawa Y, Shibayama K, Kamachi K. 2012. Prevalence and genetic characterization of pertactin-deficient Bordetella pertussis in Japan. PLoS One 7:e31985. 10.1371/journal.pone.0031985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barkoff A-M, Mertsola J, Guillot S, Guiso N, Berbers G, He Q. 2012. Appearance of Bordetella pertussis strains not expressing the vaccine antigen pertactin in Finland. Clin. Vaccine Immunol. 19:1703–1704. 10.1128/CVI.00367-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hegerle N, Paris AS, Brun D, Dore G, Njamkepo E, Guillot S, Guiso N. 2012. Evolution of French Bordetella pertussis and Bordetella parapertussis isolates: increase of bordetellae not expressing pertactin. Clin. Microbiol. Infect. 18:E340–E346. 10.1111/j.1469-0691.2012.03925.x [DOI] [PubMed] [Google Scholar]
- 38.Bodilis H, Guiso N. 2013. Virulence of pertactin-negative Bordetella pertussis isolates from infants, France. Emerg. Infect. Dis. 19:471–474. 10.3201/1903.121475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lam C, Octavia S, Ricafort L, Sintchenko V, Gilbert GL, Wood N, McIntyre P, Marshall H, Guiso N, Keil AD, Lawrence A, Robson J, Hogg G, Lan R. 2014. Rapid increase in pertactin-deficient Bordetella pertussis isolates, Australia. Emerg. Infect. Dis. 20:626–633. 10.3201/eid2004.131478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mastrantonio P, Spigaglia P, van Oirschot H, van der Heide HGJ, Heuvelman K, Stefanelli P, Mooi FR. 1999. Antigenic variants in Bordetella pertussis strains isolated from vaccinated and unvaccinated children. Microbiology 145(Pt 8):2069–2075. 10.1099/13500872-145-8-2069 [DOI] [PubMed] [Google Scholar]
- 41.Martin S, Pawloski L, Williams M, Weening K, DeBolt C, Qin X, Reynolds L, Kenyon C, Giambrone G, Kudish K, Miller L, Selvage D, Lee A, Skoff T, Kamiya H, Cassiday P, Clark T, Tondella M. 2013. Pertactin-negative B. pertussis strains: evidence for a selective advantage?, abstr P69 Abstracts of the 10th International Symposium on Bordetella, Dublin, Ireland, 8 to 11 September 2013 [Google Scholar]
- 42.Stenger RM, Poelen MCM, Moret EE, Kuipers B, Bruijns SCM, Hoogerhout P, Hijnen M, King AJ, Mooi FR, Boog CJP, van Els CACM. 2009. Immunodominance in mouse and human CD4+ T-cell responses specific for the Bordetella pertussis virulence factor P.69 pertactin. Infect. Immun. 77:896–903. 10.1128/IAI.00769-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.