Abstract
Species of Sarcocystis are Apicomplexan parasites requiring intermediate and definitive hosts to complete their life cycle. Humans are one of many natural host species and may serve as both intermediate and definitive hosts. However, the extent and public health significance of human Sarcocystis infection are incompletely known. In this minireview, we provide an update on the epidemiology and diagnosis of human sarcocystosis and propose some tools that could contribute to a better understanding of the clinical significance and epidemiology of Sarcocystis infections.
INTRODUCTION
Humans are natural hosts of multiple Apicomplexan genera and usually serve as either the intermediate (e.g., Toxoplasma, Plasmodium) or definitive (e.g., Cryptosporidium [monoxenous life cycle]) host. Meanwhile, Sarcocystis comprises Apicomplexan species with a two-host life cycle, including an intermediate host (prey) and a definitive host (predator), and humans may be both intermediate and definitive hosts for some of these species (1–3) (Fig. 1). Sarcocystis undergoes sexual reproduction in the intestinal epithelium, and oocysts are subsequently shed in host stool in definitive hosts, while the parasite forms the so-called “sarcocysts” in muscle tissue of distinct types, including striated, cardiac, and smooth muscle, in intermediate hosts. At least two species, namely, Sarcocystis hominis and Sarcocystis suihominis, exist with humans as definitive hosts and hence as potential causes of human intestinal sarcocystosis, with cattle and pigs as intermediate hosts, respectively. Humans can also be accidental intermediate hosts and develop muscular sarcocystosis.
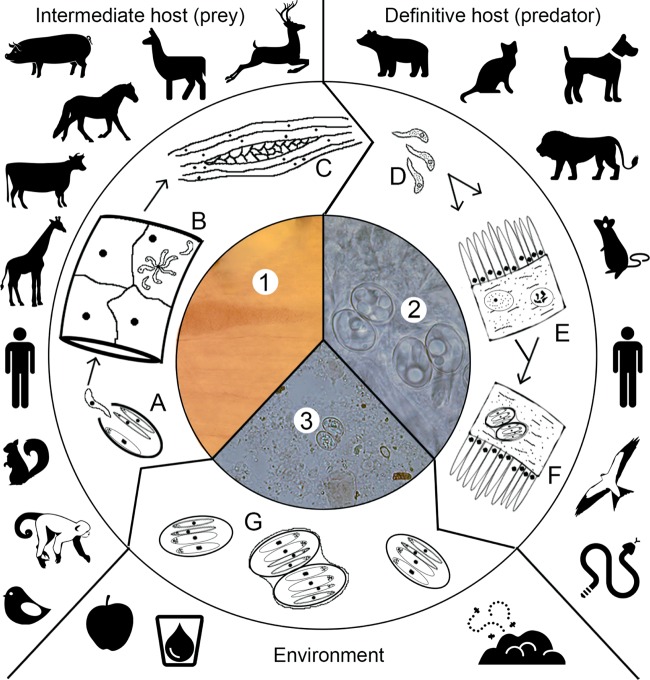
FIG 1.
Life cycle of Sarcocystis. Intermediate hosts ingest oocysts/sporocysts which release sporozoites (A) that enter endothelial cells (B) with various numbers of merogony generations, eventually releasing merozoites. In muscle cells (myocytes), and inside a parasitophorous vacuole, merozoites develop into sarcocysts containing infective bradyzoites (C and 1). Definitive hosts ingest meat containing infectious sarcocysts (C and 1) which in turn release the bradyzoites (D) that invade gut epithelial cells and perform gametogony in the lamina propria or goblet cells, producing micro- and macrogametes (E). The gametes fuse and develop into oocysts (F and 2). Oocysts/sporocysts are excreted in feces and may be distributed in water and food potentially immediately infectious for the intermediate host (G and 3).
Muscular sarcocystosis in the farming industry is a well-documented problem. A high prevalence of Sarcocystis infection is seen in cattle, pigs, and sheep in both developing and industrialized countries. Infection with Sarcocystis in the intermediate host is known to cause mortality, morbidity, abortions, lower meat yield, and meat condemnation due to visible sarcocysts (1, 2). Meanwhile, the extent and importance of human sarcocystosis remain less well described despite the potential for muscular or intestinal sarcocystosis to afflict a large number of people (3–6).
In this minireview, a short introduction to Sarcocystis taxonomy has been included. A systematic literature search has been performed on the prevalence of both intestinal and muscular sarcocystosis in humans. Lastly, diagnostic methods and their limitations are summarized with suggestions for development and application of molecular diagnostic tools to facilitate detection and a better understanding of the epidemiology and clinical significance of Sarcocystis infections of humans.
TAXONOMY
The taxonomy of Sarcocystis was updated in 1998 by Odening (7), who included 189 species. Since then, the number of species has increased, owing in part to an expanding interest in Sarcocystis in wildlife. The taxonomy of Sarcocystis is currently under revision; a common source of confusion has been the classification of identical Sarcocystis species from intermediate and definitive hosts as separate species. Traditionally, a new species of Sarcocystis is accepted when described in a new intermediate or definitive host (8). The sarcocyst wall structure is utilized for species identification due to the variability between Sarcocystis species (2); however, the wall structure is subject to change depending on the age of the sarcocyst (3) and so may have limited applicability as a morphological criterion. Molecular characterization combined with phenotypic description is critical to the ongoing revision of species in the genus Sarcocystis and is useful to study Sarcocystis transmission patterns in intermediate and definitive hosts, circumventing the need for expensive and laborious experimental infections (9, 10). This approach has been employed to some extent and recently led to the division of Sarcocystis in cervids into multiple species (10).
With the utilization of molecular methods for species identification, there is a need for guidelines as to what genes are useful for this purpose. Currently, the small-subunit (SSU) rRNA, cytochrome oxidase 1 (cox1), and internal transcribed spacer (ITS) genes are used for species identification (9, 10). There appear to be no guidelines, however, with respect to the amount of genetic diversity necessary to separate species and how to best analyze genetic diversity in terms of genetic markers, amount of DNA sequence data needed (e.g., sequence read length), and phylogenetic models. Currently, however, maximum-parsimony analysis of cox1 data appears to be the best possible method for delineation of closely related species, which may in part be due to the fact that cox1 DNA sequences are less difficult to align than SSU ribosomal DNA (rDNA) sequences (10).
With molecular confirmation pending, it is unknown whether Sarcocystis species other than S. hominis and S. suihominis can cause intestinal sarcocystosis (3). Until recently, the Sarcocystis species causing muscular sarcocystosis in humans were unknown (3, 5). Recently, 18S ribosomal DNA sequencing performed on a human muscle biopsy specimen containing Sarcocystis identified the species as Sarcocystis nesbitti (11). S. nesbitti has been described in muscle tissue of nonhuman primates (NHP), implying that NHP may indeed serve as reservoir hosts for Sarcocystis species causing muscular sarcocystosis in humans.
EPIDEMIOLOGY AND CLINICAL SIGNIFICANCE
Natural enteric infections with S. hominis and S. suihominis are described mostly as asymptomatic (4, 12, 13), although Yu (14) reported that 10% of infected individuals had symptoms associated with Sarcocystis infection. Meanwhile, experimental infections have been associated with gastroenteritis and eosinophilia (2, 3, 15, 16).
The prevalence of intestinal Sarcocystis infection in humans has been estimated to be between 1.1% and 10.4% in Europe, between 0.4% and 23.2% in Asia, 0.5% in Australia, and 0% in Argentina (Table 1). In general, caution must be taken when comparing prevalence figures reported in these studies due to the differences in diagnostic approaches. In addition, population bias may have been a factor, since the individuals in the study groups were not selected randomly from the population. A relatively high prevalence was observed in most of the studies, indicating that a large population was infected, but there is a need for further studies to confirm this. There is tendency of recent studies to report prevalence figures lower than those reported in older studies (Table 1).
TABLE 1.
Prevalence data of intestinal and muscular sarcocystosis in humans
| Reference and sarcocystosis category | Country location and study group | % prevalence value(s) (species; no. of samples examined) | Method(s) |
|---|---|---|---|
| Intestinal | |||
| 17 | Poland | 10.4 (125) | Kato-Miur |
| 13 | France | 2.0 (S. hominis; 3,500); 0.7 (S. suihominis; 700) | Ether treatment followed by flotation |
| 18 | Germany, routine fecal samples from hospitals and clinics from patients with intestinal complaints | 2 (403) | Modified hematoxylin dye, Kato and sodium-nitrate flotation |
| 14 | Tibet in 3 counties | 20.5, 22.5, and 22.9 (S. hominis; 926); 0, 0.6, and 7.0 (S. suihominis; 926) | Zinc sulfate flotation |
| 19 | Laos | 7.9 (767) | Kato-Katz & merthiolate-iodine-formaldehyde concn |
| 12 | Central Slovakia, workers from Vietnam | 1.1 (1,228) | Fecal smear |
| 20 | Western Australia, Aboriginal communities | 0.5 (385) | Direct microscopy |
| 4 | Thailand, Thai laborers | 23.2 (362) | Formalin-ether concn |
| 21 | Argentina, children | 0 (69) | Modified Telemann and Fülleborn |
| 22 | Thailand, rural communities | 7.0 (1,603) | Kato thick smear & direct smear |
| 23 | Northeast Thailand, rural communities | 0.4 (253) | Formalin-ethyl acetate |
| Muscular | |||
| 24 | United States, California, autopsy samples | 0 (297) | Digestion of (sieved and centrifuged) pepsin followed by microscopic examination |
| 25 | Malaysia, blood samples from blood donors | 19.7 (243) | IFAT; 1:64 serum dilution considered positive |
| 26 | Yugoslavia, blood samples obtained from cases suspected to be infected with Toxoplasma gondii | 44.4 (341) | IFAT; 1:40 serum dilution |
| 27 | Denmark, autopsy samples | 3.6 (112) | Trichinoscopical examination of crus diaphragmatica |
| 28 | Australia, blood samples | 34 (50) | ELISA |
| 29 | Malaysia, autopsy samples | 21 (100) | Examination of formalin-fixed, paraffin-embedded sections of tongue tissues |
| 30 | Australia, Sydney, autopsy samples | 0 (50) | Two methods: (i) examination of formalin-fixed, paraffin-embedded sections from the psoas and diaphragm and (ii) digestion of (sieved and centrifuged) pepsin followed by microscopic examination |
| 31 | Northern Ireland, autopsy samples | 0 (50) | Examination of formalin-fixed, paraffin-embedded sections of tongue and diaphragm tissues |
Potential geographical variation in the prevalence of intestinal sarcocystosis is difficult to assess due to many of the prevalence studies not being available in English, and so the methodology cannot be evaluated systematically.
Recent prevalence studies based on morphology and molecular data on S. hominis in cattle revealed that the occurrence of S. hominis infections is rather common, with prevalences of 6.2% to 97.4% (32–34), 53.5% (35), and 94% (16) in Europe, Asia, and South America, respectively. However, no cases of S. hominis infection were observed in a study performed in North America (36). Data on the prevalence of S. suihominis infection is scarce compared to those on the prevalence of S. hominis infection, and so recent prevalence studies estimating the prevalence of S. suihominis infection in pigs appear not to be available. Again, it should be emphasized that studies vary with regard to the type of sample (for instance, processed or unprocessed meat), type of muscle tissue examined, animal age, and number of samples analyzed. The fact that S. hominis or S. suihominis is regularly detected in intermediate hosts means that humans must be infected with these parasites.
Possible risk factors associated with acquiring intestinal Sarcocystis infection include eating raw or undercooked beef and pork and unsanitary disposal of human feces that can infect the intermediate host and maintain the life cycle of Sarcocystis species causing intestinal sarcocystosis in humans (4).
Symptoms of muscular sarcocystosis include acute fever, myalgias, myositis, vasculitis, bronchospasm, and pruritic rashes (3, 5, 6, 37, 38). Muscular sarcocystosis has also been associated with nonspecific rheumatic diseases associated with myositis (39). No experimental-infection experiments have been performed with humans as intermediate hosts to our knowledge.
Treatment with albendazole alone or in combination with prednisone may lead to improvement in some cases (6, 37). However, as with intestinal sarcocystosis, there are currently no recommendations regarding treatment of muscular sarcocystosis, despite the severity in some cases, mainly due to lack of experience (3, 5).
The prevalence of muscular sarcocystosis in humans has been estimated to range between 0% and 3.6% in western countries and 21% in Malaysia from postmortem examination (Table 1). The high prevalence in Malaysia compared with western countries is likely due to NHPs being common in Malaysia, and humans can serve as intermediate hosts for the Sarcocystis species found in NHPs (40). Serological methods such as enzyme-linked immunosorbent assays (ELISA) and indirect fluorescent antibody tests (IFAT) have also been used to estimate the seroprevalence in Australia, the former Social Federal Republic of Yugoslavia, and Malaysia, producing seroprevalence figures of 34%, 22.5% to 44.4%, and 19.7%, respectively (Table 1) (25, 26, 28). The discrepancy between the higher prevalences observed in serological studies and those observed by direct detection in postmortem studies might be explained by low diagnostic sensitivity of direct detection and/or by low diagnostic specificity of serology. Another explanation could be persistence of a serological response after clearance of infection. There is a need for further studies to clarify this.
In 2004, Fayer (3) calculated the total number of muscular sarcocystosis cases described to be 92, but that many cases were probably unreported. Recently, the largest outbreak of human muscular sarcocystosis reported to date, with 100 people infected, was investigated on Tioman Island, Malaysia (5). The outbreak has received attention due to the severity of disease and because the infected individuals were almost exclusively European tourists (5, 6). Also supported by the prevalence studies in Malaysia (25, 29), this indicates that the indigenous population is at substantial risk of contracting disease. In addition, an outbreak of muscular sarcocystosis was observed in 89 Malayan college students who visited Pangkor Island (38).
Little is known about the risk factors associated with acquiring muscular sarcocystosis. Humans have most likely been infected by consuming water or food contaminated with oocysts/sporocysts from definitive hosts (5, 6).
DIAGNOSIS OF INTESTINAL SARCOCYSTOSIS
A suspicion of intestinal sarcocystosis may be based on a history of gastroenteritis combined with habits of eating raw or undercooked meat that might contain infective Sarcocystis. The “diagnostic window” is limited to the time of oocyst/sporocyst excretion. The prepatent periods for S. hominis and S. suihominis are 14 to 18 days and 11 to 13 days, respectively, and oocyst/sporocyst excretion can last for at least 1 month (1–3). Importantly, this may imply that Sarcocystis may not be shed in feces in the acute stage of intestinal infection.
Methods for coprological examination include different flotation techniques (2, 3), modified Kato thick smear, the formalin-ether technique, and direct smear (22). The sensitivity of these methods is to a large extent unknown due the lack of studies addressing this specifically, but it should be possible to determine sensitivity by spiking samples with known amounts of oocysts/sporocysts. In animals, intestinal Sarcocystis infection is often detected postmortem by examination of mucosal scrapings rather than stool due to light fecal shedding (8).
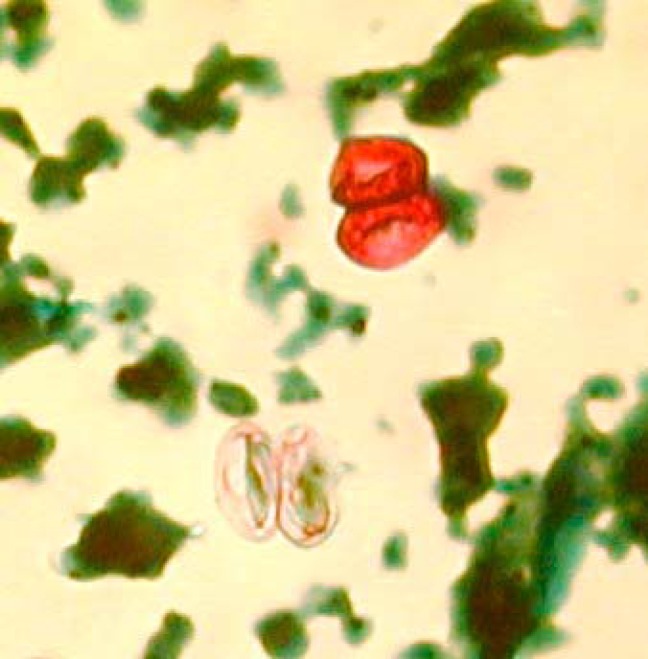
Oocysts of S. suihominis measure 12.3 to 14.6 μm by 18.5 to 20.0 μm; fewer detailed data are available for oocysts of S. hominis, which may measure 20 to 23 μm in diameter. Sporocysts of S. hominis average 9.3 by 14.7 μm, and those of S. suihominis average 10.5 by 13.5 μm (3, 41). Both oocysts and sporocysts may be seen in fecal samples from definitive hosts; the oocyst wall may be difficult to discern (Fig. 2), but the use of stains such as periodic acid-Schiff stain may enable its detection. Given the shape and size of the oocysts and sporocysts, there should be little risk of confusing them with oocysts of Cryptosporidium, Cyclospora, and Cystoisospora. Still, a number of predicaments may impede our ability to detect Sarcocystis in human fecal samples on the basis of traditional parasitological methods and conventional reasoning. The relatively long prepatent period has already been highlighted, and it is reasonable to believe that fecal samples are sometimes being analyzed from patients with gastroenteritis before oocyst shedding commences. Moreover, oocysts/sporocysts do not consistently take up acid-fast stain (Fig. 2), and so they may be missed by the Ziehl-Neelsen staining commonly used for traditional parasitological detection of Apicomplexan parasites in stool. Moreover, in a recent study, we showed that none of 16 of 889 fecal samples positive for Cryptosporidium by real-time PCR were positive by Ziehl-Neelsen staining, which was probably due to the presence of only light infections, as evidenced by high threshold cycle (CT) values (42). Since fecal shedding of Sarcocystis is presumably usually light, the parasite may therefore easily be overlooked in Ziehl-Neelsen preparations, and given the variability in uptake of acid-fast stain, the Ziehl-Neelsen method should not be recommended for detecting Sarcocystis.
FIG 2.
Sarcocystis oocysts in a Ziehl-Neelsen preparation; two sporocysts are seen in each oocyst. Note that only one of the two oocysts has taken up stain.
To detect Sarcocystis in stool, a DNA detection-based approach therefore appears relevant. Real-time PCR assays have been published for several intestinal protozoa, but so far, none has been published for Sarcocystis. This may be due to a variety of reasons. Generally, if laboratory methods fail to enable detection of a given parasite, the parasite will probably be thought of as rare, and so the incentive to develop DNA-based methods for its detection may remain low. More specifically, regions of the small-subunit (SSU) rRNA gene conserved across the Sarcocystis genus may be so genetically similar to those found in competing templates in fecal genomic DNA that the applicability of this marker in genus-specific PCR analyses performed on DNA extracted directly from feces is reduced due to decreased PCR specificity and sensitivity. Nevertheless, the first molecular diagnostic assay to diagnose DNA of oocysts/sporocysts of Sarcocystis in fecal samples was developed only recently. The described method included seminested amplification of the SSU rRNA gene with subsequent restriction fragment length polymorphism (RFLP) analysis, making species differentiation possible in cattle (43). The primers target conserved regions; however, the forward primer “18s 2L” given in a paper by Xiang et al. (43) and used in both first and second PCR reads 5′-GGATAAACCGTGGTAATTCTATG-3′, while the SSU rDNA template reads 5′-GGATAACCGTGGTAATTCTATG-3′. The two primer combinations used in the nested PCR appear relatively nonspecific, and in silico analysis suggests that they may amplify a variety of Apicomplexan parasites. A different gene target, cytochrome oxidase 1 (cox1), exhibits extensive genetic diversity and can be used to discriminate between even closely related species of Sarcocystis (10). Unfortunately, cox1 DNA sequences are unavailable for the two species commonly thought to be involved in human sarcocystosis, namely, S. hominis and S. suihominis. Once those sequences are available, and given the amount of sequence data in GenBank available for multiple species of Sarcocystis, it should be possible to develop diagnostic species- and group-specific PCRs based on the cox1 gene in a real-time or nested PCR format to increase sensitivity. Relevant pretreatment of fecal samples could include purification and concentration of oocysts using gradient (zinc sulfate, sodium chloride, sucrose, etc.) methods, inherently increasing the chances of detecting other single-celled parasites of similar densities as well, with subsequent microscopy and DNA extraction of the meniscus. At present, nearly full-length SSU rDNA sequences (∼1.9 kbp) and partial cox1 sequences (∼1.0 kbp) of about 40 and at least 20 Sarcocystis species, respectively, are available (10), potentially facilitating the design of relevant primers and probes for conventional or quantitative PCR-based detection of oocysts/sporocysts in feces.
DIAGNOSIS OF MUSCULAR SARCOCYSTOSIS
Muscular sarcocystosis is suspected when compatible symptoms are present and especially if traveling has occurred to places where native wildlife includes NHPs. The identification of sarcocysts in muscle is considered the sole definitive diagnostic criterion (5). It is, however, rarely possible to obtain a biopsy specimen containing sarcocysts due both to issues related to obtaining consent from the infected individual and to the low chance of retrieving a specimen that contains sarcocysts.
ELISA and IFAT methods are used in serodiagnosis of muscular sarcocystosis (39, 44). There has been no report of cross-reactivity with, e.g., Toxoplasma, indicating that methods in use have high specificity (44). One IFAT method showed complete agreement with morphological examination of freshly slaughtered cattle (45). Similar prevalence figures in humans were also obtained in Malaysia, where Wong and Pathmanathan (29) used microscopy to examine tongue tissue while Thomas and Dissanaike (25) used IFAT, reporting prevalences of 21% and 19.7%, respectively. However, the two studies were performed on two different groups of people. Ideally, ELISA and IFAT methods should not be able to detect intestinal sarcocystosis and classify it as muscular sarcocystosis (44); however, the high prevalence figures observed in the former Socialist Federal Republic of Yugoslavia and Australia where no species of NHP is autochthonous indicate that this may not be the case (26, 46). A Western blot analysis for the confirmation of muscular sarcocystosis is currently under development (5).
An inherent problem with the utilization of antibodies as a principle of diagnosis is the lack of differentiation between early-stage infection and late-stage infection (47). This is of particular importance in relation to the development and clearance of sarcocysts in humans. Among animals, an ELISA enabled detection of IgM in acute-phase infections in mice and sheep; however, the IgG response was detected earlier than the IgM response in infections in pigs (48). No data on IgM development in humans are available. A different approach to detecting acute muscular sarcocystosis using nested PCR on blood samples containing circulating merozoites has been developed for testing in sheep (47). Molecular studies are warranted to identify the species that can cause muscular sarcocystosis to enable making epidemiological inferences that can be used to assess the extent and importance of human muscular sarcocystosis plus the modes of transmission.
CONCLUDING REMARKS
Diagnostic limitations have impeded our ability to increase our understanding of the epidemiology and public health significance of Sarcocystis infections in humans. Due to light shedding in feces, PCR-based methods for detection of DNA from oocysts/sporocysts in feces appear relevant; multiple species of Sarcocystis have now been molecularly characterized, and available sequence data should greatly enhance the possibilities for development of relevant diagnostic PCR assays and assays serving to map host specificity and transmission modes. Meanwhile, direct detection of the parasite in cases of human muscular sarcocystosis is hampered by the apparent absence of a predilection site, and so indirect detection by serological methods is preferred to testing of tissue biopsy specimens.
Biography

Christen Rune Stensvold is a Senior Scientist and Public Health Microbiologist with a specialty in parasitology. He has a Bachelor's degree in medical sciences, an M.Sc. in parasitology, and a Ph.D. in health sciences. He has been based at Statens Serum Institut, Copenhagen, Denmark, since 2004 and spent two years of postdoctoral studies at the London School of Hygiene and Tropical Medicine, United Kingdom, from 2009 to 2011. Since 2006, he has authored or coauthored more than 60 articles that have been published in internationally distributed, peer-reviewed scientific journals. In 2013, he was rewarded the Fritz Kauffmann prize for his contribution to clinical microbiology in Denmark. He specializes in the development and implementation of diagnostic methods for parasite detection in clinical and environmental samples, molecular epidemiology of parasitic infections, and detection and description of novel ribosomal lineages of parasites in humans and animals. For many years, he has been exploring the role of common intestinal microeukaryotes in human health and disease.
Footnotes
Published ahead of print 23 April 2014
REFERENCES
- 1.Markus MB. 1978. Sarcocystis and sarcocystosis in domestic animals and man. Adv. Vet. Sci. Comp. Med. 22:159–193 [PubMed] [Google Scholar]
- 2.Dubey JP, Speer CA, Fayer R. 1989. Sarcocystosis of animals and man. CRC Press, Inc., Boca Raton, FL [Google Scholar]
- 3.Fayer R. 2004. Sarcocystis spp. in human infections. Clin. Microbiol. Rev. 17:894–902. 10.1128/CMR.17.4.894-902.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilairatana P, Radomyos P, Radomyos B, Phraevanich R, Plooksawasdi W, Chanthavanich P, Viravan C, Looareesuwan S. 1996. Intestinal sarcocystosis in Thai laborers. Southeast Asian J. Trop. Med. Public Health 27:43–46 [PubMed] [Google Scholar]
- 5.Esposito DH, Freedman DO, Neumayr A, Parola P. 2012. Ongoing outbreak of an acute muscular Sarcocystis-like illness among travellers returning from Tioman Island, Malaysia, 2011–2012. Euro Surveill. 17:20310 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=20310 [PMC free article] [PubMed] [Google Scholar]
- 6.Tappe D, Ernestus K, Rauthe S, Schoen C, Frosch M, Müller A, Stich A. 2013. Initial patient cluster and first positive biopsy findings in an outbreak of acute muscular Sarcocystis-like infection in travelers returning from Tioman island, Peninsular Malaysia, in 2011. J. Clin. Microbiol. 51:725–726. 10.1128/JCM.03063-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Odening K. 1998. The present state of species-systematics in Sarcocystis Lankester, 1882 (Protista, Sporozoa, Coccidia). Syst. Parasitol. 41:209–233. 10.1023/A:1006090232343 [DOI] [Google Scholar]
- 8.Heckeroth A, Tenter AM. 2007. Sarcocystiosis, p 172–232 In Ortega-Mora LM, Gottstein B, Conraths FJ, Buxton D. (ed), Protozoal abortion in farm ruminants. CABI, Gateshead, United Kingdom [Google Scholar]
- 9.Stojecki K, Karamon J, Sroka J, Cencek T. 2012. Molecular diagnostics of Sarcocystis spp. infections. Pol. J. Vet. Sci. 15:589–596 [DOI] [PubMed] [Google Scholar]
- 10.Gjerde B. 2013. Phylogenetic relationships among Sarcocystis species in cervids, cattle and sheep inferred from the mitochondrial cytochrome c oxidase subunit I gene. Int. J. Parasitol. 43:579–591. 10.1016/j.ijpara.2013.02.004 [DOI] [PubMed] [Google Scholar]
- 11.Lau YL, Chang PY, Tan CT, Fong MY, Mahmud R, Wong KT. 2014. Sarcocystis nesbitti infection in human skeletal muscle: possible transmission from snakes. Am. J. Trop. Med. Hyg. 90:361–364. 10.4269/ajtmh.12-0678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Straka S, Skraciková J, Konvit I, Szilágyiová M, Michal L. 1991. Sarcocystis species in Vietnamese workers. Cesk. Epidemiol. Mikrobiol. Imunol. 40:204–208 (In Slovak.) [PubMed] [Google Scholar]
- 13.Deluol AM, Mechali D, Cenac J, Savel J, Coulaud JP. 1980. Incidence and clinical aspects of intestinal coccidiosis in a tropical medicine practice. Bull. Soc. Pathol. Exot. Filiales 73:259–265 (In French.) [PubMed] [Google Scholar]
- 14.Yu S. 1991. Field survey of Sarcocystis infection in the Tibet autonomous region. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 13:29–32 (In Chinese.) [PubMed] [Google Scholar]
- 15.Chen X, Zuo Y, Zuo W. 1999. Observation on the clinical symptoms and sporocyst excretion in human volunteers experimentally infected with Sarcocystis hominis. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi 17:25–27 (In Chinese.) [PubMed] [Google Scholar]
- 16.Pena HF, Ogassawara S, Sinhorini IL. 2001. Occurrence of cattle Sarcocystis species in raw kibbe from Arabian food establishments in the city of São Paulo, Brazil, and experimental transmission to humans. J. Parasitol. 87:1459–1465. 10.1645/0022-3395(2001)087[1459:OOCSSI]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- 17.Plotkowiak J. 1976. The results of continued investigations on the occurrence and epidemiology of Isospora hominis (Railliet and Lucet, 1891) invasion. Ann. Parasitol. 22:137–147 [PubMed] [Google Scholar]
- 18.Gauert B, Jungmann R, Hiepe T. 1983. Relationship between intestinal disturbances and infections with intestinal parasites in man, with particular reference to Sarcocystis infections. Deutsch. Gesundheitsw. 38:62–66 [Google Scholar]
- 19.Giboda M, Ditrich O, Scholz T, Viengsay T, Bouaphanh S. 1991. Current status of food-borne parasitic zoonoses in Laos. Southeast Asian J. Trop. Med. Public Health 22(Suppl):56–61 [PubMed] [Google Scholar]
- 20.Meloni BP, Thompson RC, Hopkins RM, Reynoldson JA, Gracey M. 1993. The prevalence of Giardia and other intestinal parasites in children, dogs and cats from aboriginal communities in the Kimberley. Med. J. Aust. 158:157–159 [DOI] [PubMed] [Google Scholar]
- 21.Soriano SV, Barbieri LM, Pierángeli NB, Giayetto AL, Manacorda AM, Castronovo E, Pezzani BC, Minvielle M, Basualdo JA. 2001. Intestinal parasites and the environment: frequency of intestinal parasites in children of Neuquén, Patagonia, Argentina. Rev. Latinoam. Microbiol. 43:96–101 [PubMed] [Google Scholar]
- 22.Tungtrongchitr A, Chiworaporn C, Praewanich R, Radomyos P, Boitano JJ. 2007. The potential usefulness of the modified Kato thick smear technique in the detection of intestinal sarcocystosis during field surveys. Southeast Asian J. Trop. Med. Public Health 38:232–238 [PubMed] [Google Scholar]
- 23.Boonjaraspinyo S, Boonmars T, Kaewsamut B, Ekobol N, Laummaunwai P, Aukkanimart R, Wonkchalee N, Juasook A, Sriraj P. 2013. A cross-sectional study on intestinal parasitic infections in rural communities, northeast Thailand. Korean J. Parasitol. 51:727–734. 10.3347/kjp.2013.51.6.727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Theis JH, Ikeda RM, Ruddell CR, Tay S. 1978. Apparent absence of Sarcocystis and low prevalence of Trichinella in artificially digested diaphragm muscle removed during post-mortem examination at a Sacramento (California) medical center. Am. J. Trop. Med. Hyg. 27:837–839 [DOI] [PubMed] [Google Scholar]
- 25.Thomas V, Dissanaike AS. 1978. Antibodies to Sarcocystis in Malaysians. Trans. R. Soc. Trop. Med. Hyg. 72:303–306. 10.1016/0035-9203(78)90211-0 [DOI] [PubMed] [Google Scholar]
- 26.Sibalic D, Bordoski A, Conic V, Durkovic-Dakovic O. 1983. A study of Toxoplasma gondii and Sarcocystis sp. infections in humans suspected of acquired toxoplasmosis. Acta Vet. Yugoslav. 33:39–48 [Google Scholar]
- 27.Greve E. 1985. Sarcosporidiosis–an overlooked zoonosis. Man as intermediate and final host. Dan. Med. Bull. 32:228–230 [PubMed] [Google Scholar]
- 28.Murrell TG, O'Donoghue PJ, Ellis T. 1986. A review of the sheep-multiple sclerosis connection. Med. Hypotheses 19:27–39. 10.1016/0306-9877(86)90134-9 [DOI] [PubMed] [Google Scholar]
- 29.Wong KT, Pathmanathan R. 1992. High prevalence of human skeletal muscle sarcocystosis in south-east Asia. Trans. R. Soc. Trop. Med. Hyg. 86:631–632. 10.1016/0035-9203(92)90161-5 [DOI] [PubMed] [Google Scholar]
- 30.Troedsen C, Pamphlett R, Collins H. 1992. Is sarcocystosis common in Sydney? Med. J. Aust. 156:136. [PubMed] [Google Scholar]
- 31.Wong KT, Leggett PF, Heatley M. 1993. Apparent absence of Sarcocystis infection in human tongue and diaphragm in Northern Ireland. Trans. R. Soc. Trop. Med. Hyg. 87:496. [DOI] [PubMed] [Google Scholar]
- 32.Moré G, Pantchev A, Skuballa J, Langenmayer MC, Maksimov P, Conraths FJ, Venturini MC, Schares G. 4 April 2014. Sarcocystis sinensis is the most prevalent thick-walled Sarcocystis species in beef on sale for consumers in Germany. Parasitol. Res. 10.1007/s00436-014-3877-x [DOI] [PubMed] [Google Scholar]
- 33.Domenis L, Peletto S, Sacchi L, Clementi E, Genchi M, Felisari L, Felisari C, Mo P, Modesto P, Zuccon F, Campanella C, Maurella C, Guidetti C, Acutis PL. 2011. Detection of a morphogenetically novel Sarcocystis hominis-like in the context of a prevalence study in semi-intensively bred cattle in Italy. Parasitol. Res. 109:1677–1687. 10.1007/s00436-011-2441-1 [DOI] [PubMed] [Google Scholar]
- 34.Vangeel L, Houf K, Chiers K, Vercruysse J, D'Herde K, Ducatelle R. 2007. Molecular-based identification of Sarcocystis hominis in Belgian minced beef. J. Food Prot. 70:1523–1526 [DOI] [PubMed] [Google Scholar]
- 35.Jehle C, Dinkel A, Sander A, Morent M, Romig T, Luc PV, De TV, Thai VV, Mackenstedt U. 2009. Diagnosis of Sarcocystis spp. in cattle (Bos taurus) and water buffalo (Bubalus bubalis) in Northern Vietnam. Vet. Parasitol. 166:314–320. 10.1016/j.vetpar.2009.08.024 [DOI] [PubMed] [Google Scholar]
- 36.Pritt B, Trainer T, Simmons-Arnold L, Evans M, Dunams D, Rosenthal BM. 2008. Detection of sarcocystis parasites in retail beef: a regional survey combining histological and genetic detection methods. J. Food Prot. 71:2144–2147 [DOI] [PubMed] [Google Scholar]
- 37.Arness MK, Brown JD, Dubey JP, Neafie RC, Granstrom DE. 1999. An outbreak of acute eosinophilic myositis attributed to human Sarcocystis parasitism. Am. J. Trop. Med. Hyg. 61:548–553 [DOI] [PubMed] [Google Scholar]
- 38.Abubakar S, Teoh BT, Sam SS, Chang LY, Johari J, Hooi PS, Lakhbeer-Singh HK, Italiano CM, Omar SF, Wong KT, Ramli N, Tan CT. 2013. Outbreak of human infection with Sarcocystis nesbitti, Malaysia, 2012. Emerg. Infect. Dis. 19:1989–1991. 10.3201/eid1912.120530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Abdul-Rahman SM, Rashad SM, Doma MA. 2002. Human muscle sarcocystosis in relation to non-specific rheumatic diseases and rheumatoid arthritis. Egypt. Rheumatol. Rehab. 29:743–753 http://applications.emro.who.int/imemrf/egypt_rheum_regabil_2002_29_5_743.pdf [Google Scholar]
- 40.Dissanaike AS. 1994. Human Sarcocystis infection. Trans. R. Soc. Trop. Med. Hyg. 88:364. [DOI] [PubMed] [Google Scholar]
- 41.Bunyaratvej S, Bunyawongwiroj P, Nitiyanant P. 1982. Human intestinal sarcosporidiosis: report of six cases. Am. J. Trop. Med. Hyg. 31:36–41 [DOI] [PubMed] [Google Scholar]
- 42.Stensvold CR, Nielsen HV. 2012. Comparison of microscopy and PCR for detection of intestinal parasites in Danish patients supports an incentive for molecular screening platforms. J. Clin. Microbiol. 50:540–541. 10.1128/JCM.06012-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xiang Z, Chen X, Yang L, He Y, Jiang R, Rosenthal BM, Luan P, Attwood SW, Zuo Y, Zhang YP, Yang Z. 2009. Non-invasive methods for identifying oocysts of Sarcocystis spp. from definitive hosts. Parasitol. Int. 58:293–296. 10.1016/j.parint.2009.03.004 [DOI] [PubMed] [Google Scholar]
- 44.Habeeb YSM, Selim MA, Ali MS, Mahmoud LA, Hadi AMA, Shafei A. 1996. Serological diagnosis of extraintestinal sarcocystosis. J. Egypt. Soc. Parasitol. 26:393–400 [PubMed] [Google Scholar]
- 45.Moré G, Abrahamovich P, Jurado S, Bacigalupe D, Marin JC, Rambeaud M, Venturini L, Venturini MC. 2011. Prevalence of Sarcocystis spp. in Argentinean cattle. Vet. Parasitol. 177:162–165. 10.1016/j.vetpar.2010.11.036 [DOI] [PubMed] [Google Scholar]
- 46.Pamphlett R, O'Donoghue P. 1992. Antibodies against Sarcocystis and Toxoplasma in humans with the chronic fatigue syndrome. Aust. N. Z. J. Med. 22:307–308 [DOI] [PubMed] [Google Scholar]
- 47.Heckeroth AR, Tenter AM. 1999. Development and validation of species-specific nested PCRs for diagnosis of acute sarcocystiosis in sheep. Int. J. Parasitol. 29:1331–1349. 10.1016/S0020-7519(99)00111-3 [DOI] [PubMed] [Google Scholar]
- 48.O'Donoghue PJ, Weyreter H. 1984. Examinations on the serodiagnosis of Sarcocystis infections. II. Class-specific immunoglobulin responses in mice, pigs, and sheep. Zentralbl. Bakteriol. Mikrobiol. Hyg. A 257:168–184 [PubMed] [Google Scholar]