Abstract
Using crude whole-genome assemblies, we analyzed 25 isolates of Neisseria gonorrhoeae by using a high-resolution single nucleotide polymorphism (SNP) approach for nine housekeeping genes, characterizing penA alleles, and antimicrobial susceptibility phenotypes coupled with population structure analysis. Two clonal complexes, characterized by their spatial and geographical persistence, were identified. In addition, the clonal spread of penicillin-resistant/intermediate phenotypes and a novel introduction of the azithromycin resistance phenotype in Saskatchewan, Canada, were ascertained using this method.
TEXT
Neisseria gonorrhoeae causes 106 million new gonorrhea infections globally each year (1). In Canada, the reported number of cases of gonorrhea more than doubled from 14.9 per 100,000 in 1997 to 33.1 per 100,000 in 2011 (2). A significant concern is the emergence and dissemination of antimicrobial resistance in the gonococcal population worldwide, which has consequently resulted in a significant limitation of treatment options (3). The MICs of gonococcal isolates to third-generation extended-spectrum cephalosporins (ESCs) (e.g., cefixime and ceftriaxone), the last of the single-dose treatment options for gonorrhea, have been increasing since the early 2000s (4). N. gonorrhoeae isolates with probable resistance (MICs, ≥25 μg/ml) to third-generation cephalosporins, linked with treatment failures, have been reported in Japan, Europe, the United States, Canada, and South Africa (5–10). This global trend in gonococcal resistance to antibiotics may result in the emergence of untreatable strains. Presently, there is no vaccine against N. gonorrhoeae. Therefore, preventive measures, based on accurate information on gonococcal transmission and the spread and evolution of antimicrobial resistance among the pathogen population, play a crucial role in interventions designed to limit the spread of gonorrhea.
In this study, we exploited whole-genome data to ascertain the single nucleotide polymorphisms (SNPs) in 9 housekeeping genes and penA alleles in 25 N. gonorrhoeae isolates. These genomic analyses were combined with the antimicrobial susceptibilities of N. gonorrhoeae isolates and molecular evolutionary analysis. Our objective was to apply a high-resolution typing method, without the influence of homologous recombinations, in order to resolve the ancestral relationship of predominant N. gonorrhoeae strains from Saskatchewan, a Canadian province with a high rate of gonorrhea infections (2), and potentially to identify evolutionary events that led to the spread of antibiotic-resistant phenotypes. We sequenced the genomes of 23 previously characterized N. gonorrhoeae isolates collected from Saskatchewan between 2005 and 2008 (11). The gonococcal isolates were selected based on their high-prevalence, so-called circulating strains and their single-locus variants (SLVs). Three random isolates (SK 17973, SK 16942, and SK 29471) of circulating strain ST-1 were selected together with its four SLVs, ST-17 (SK 28355), ST-28 (SK 14515), ST-8 (SK 16259), and ST-23 (SK 12684). Also, three other random isolates (SK 29344, SK 6987, and SK 25532) of circulating strain ST-3 were selected with its one SLV, ST-22 (SK 7842). The remaining gonococcal isolates were selected based on their unique antimicrobial phenotypes. Two other genomes of spatially and temporally unrelated gonococcal strains, CH 811 (Chile, 1982) and GC1-182 (Canada, 1985), were sequenced (12, 13). Gonococcal DNA was extracted using the Qiagen DNeasy tissue kit (Qiagen, Inc., Valencia, CA) according to the manufacturer's instructions. Genomes of the 25 isolates of N. gonorrhoeae were sequenced using an Illumina HiSeq platform according to established procedure, and contigs were assembled using the CLC Genomics Workbench. In all, 10 loci (penA, abcZ, adk, gdh, glnA, gnd, fumC, pilA, pyrD, and serC) were extracted from each of the 25 genome assemblies using additional scripts. The regions of homologous recombinations within the housekeeping genes were identified using a nonparametric recombination detection method, SiScan (14), as described earlier (15). The DNA sequences were incorporated into Molecular Evolutionary Genetics Analysis (MEGA) version 4 (16) for the identification of SNPs. Phylogeny, based on SNPs, was inferred using the minimum evolution method (17). The neighbor-joining algorithm (18) was used to generate the initial phylogenetic tree. The antimicrobial susceptibilities of N. gonorrhoeae isolates to penicillin, tetracycline, spectinomycin, ciprofloxacin, cefixime, azithromycin, and ceftriaxone were assessed, in duplicate, by the agar dilution method as described previously (19, 20). Structure (21) evolutionary analysis was performed using a combination of SNPs, antimicrobial susceptibilities, and penA alleles. The programs CLUMPP (22) and DISTRUCT (23) were used to generate the initial graphs depicting the population structure of the gonococcal strains.
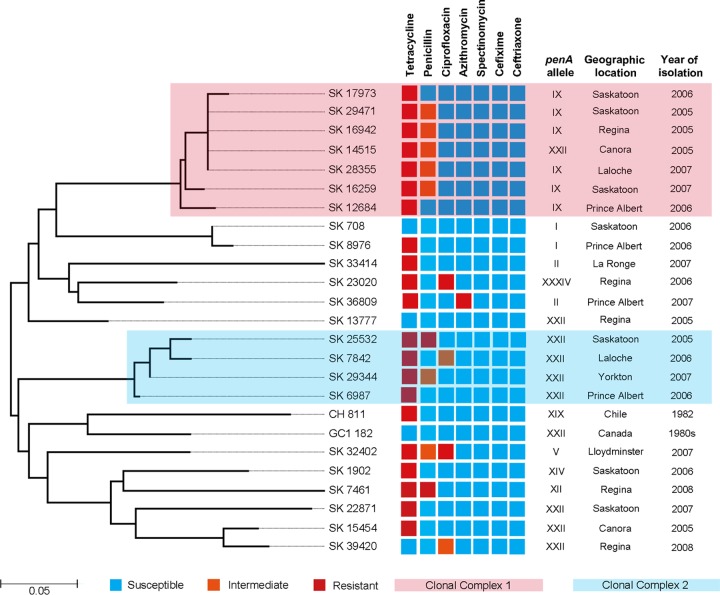
To minimize the negative effect of homoplasy on gonococcal phylogeny, all regions affected by homologous recombinations were removed from further analysis of the 9 housekeeping genes (11,494 bp for each isolate was used for the comparative analysis). A total of 70 SNPs were identified among 25 gonococcal isolates for the nine loci, ranging from the lowest SNP density of 361 (1 SNP per 361 bp) in gdh to the highest SNP density of 71 (1 SNP per 71 bp) in pilA. The minimum evolution method revealed the existence of two major clades (Fig. 1). The first clade comprised a cluster of homogenous strains (SK 17973, SK 29471, SK 16942, SK 14515, SK 28355, SK 16259, and SK 12684) separated by 5 SNPs, hereafter referred to as clonal complex 1 (CC1), and a group of diverse gonococcal strains separated by 41 SNPs (Fig. 1). The second clade included another group of genetically homogenous strains, CC2 (SK 25532, SK 7842, SK 29344, and SK 6987), separated by 4 SNPs, and a group of genetically distant strains, including reference strains CH 811 and GC1-182, which are separated by 56 SNPs (Fig. 1). The overall average pairwise distance, an estimate of evolutionary divergence between sequences, for all the gonococcal isolates was 0.252, indicating that any two gonococcal strains would diverge, on average, ∼25% from each other based on their SNPs. While diverse isolates might differ by 42.9%, when these extreme values are averaged, the evolutionary divergence is 25%. The isolates within CC1 and CC2 showed profound genetic homogeneity, diverging from each other by only 2.3% and 3.1% of the counted SNPs, respectively.
FIG 1.
Minimum evolution phylogenetic tree based on 70 SNPs of gonococcal isolates annotated with their antimicrobial susceptibility phenotypes and epidemiological data. The antimicrobial susceptibilities of each isolate for seven different antibiotics are indicated by the color of the antimicrobial phenotype. The scale bar represents substitutions per SNP.
All the isolates were susceptible to spectinomycin and ESCs. A majority of the isolates (80%, 20/25) were resistant to tetracycline; far fewer were resistant to penicillin (12%, 3/25), ciprofloxacin (8%, 2/25), and azithromycin (4%, 1/25). The majority of penicillin-resistant/intermediate phenotypes (77.7%, 7/9) were distributed among the two CCs. In contrast, ciprofloxacin-resistant/intermediate phenotypes (75%, 3/4) were mainly found among isolates that did not belong to the CCs (Fig. 1). Azithromycin resistance was identified in the genetically distant isolate SK 36809 (Fig. 1). The sequenced gonococcal isolates possessed 9 penA alleles, in keeping with the classification scheme developed by others (24, 25), including allele XXII (40%, 10/25), allele IX (24%, 6/25), allele II (8%, 2/25), allele I (8%, 2/25), mosaic penA allele XXXIV (4%, 1/25), allele XIX (4%, 1/25), allele XIV (4%, 1/25), allele XII (4%, 1/25), and allele V (4%, 1/25) (Fig. 1).
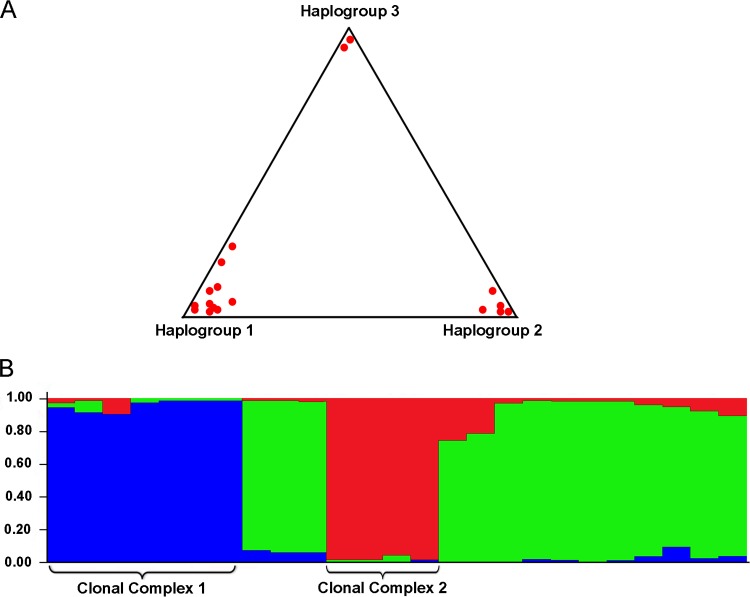
To better define the population structure of these gonococcal isolates, we analyzed their SNPs, penA alleles, and antibiotic phenotypes using structure evolutionary analysis. The results of multiple analyses strongly suggest that the collection of gonococcal isolates consisted of two large haplogroups, 1 and 2, and one small haplogroup, 3 (Fig. 2A). The mean of the pairwise distances indicated that haplogroup 1 is the most heterogeneous group (0.329), whereas haplogroups 2 (0.059) and 3 (0.035) are more homogeneous. Point estimate analyses suggested that haplogroup 3 diverged 0.214 and 0.120 from haplogroups 2 and 1, respectively, whereas haplogroups 1 and 2 diverged from each other 0.094, indicating that haplogroup 3 is the most distant group (Fig. 2A).
FIG 2.
Population structure of N. gonorrhoeae strains. (A) Triangle plot of a Bayesian cluster analysis reveals the existence of three distinct haplogroups (K = 3) within the examined collection of N. gonorrhoeae strains. Haplogroup 1 is the most diverse group of gonococcal strains, whereas haplogroup 3 is most distant. Clonal complex 1 (CC1) was associated with haplogroup 2, while clonal complex 2 (CC2) associated itself with haplogroup 1. (B) Population structure analysis carried out using structure revealed the existence of two genetically unique groups (CC1 and CC2) of gonococcal isolates based on a combination of SNPs, penA alleles, and antimicrobial phenotypes. Each strain is represented by a single vertical bar, partitioned into three colored segments which represent its overall contribution to each of the three haplogroups.
Previously, we reported that the majority of gonorrhea infections from Saskatchewan, one of the most affected regions of Canada (26), were caused by the three circulating strains (11). Using SNP analysis, a high-resolution typing method, with a significantly reduced influence of homoplasy (i.e., all regions of homologous recombination were removed prior to SNP analysis), we showed that two circulating strains have formed clonal complexes spanning time and a vast area. The population structure analysis clearly indicated that each of these two clonal complexes exhibited distinct combinations of SNPs, penA alleles, and antimicrobial phenotypes among the gonococcal population (Fig. 2B). The unique genetic backgrounds of these two clonal complexes are likely associated with their temporal and spatial expansions. Molecular surveillance of clinical N. gonorrhoeae isolates in Russia during a 1-year period revealed a high clonality of gonococcal isolates in which four clonal complexes were identified with high prevalence rates (27). Our results suggest that the gonococcal clonal complexes found in Saskatchewan are characterized not only by a high prevalence rate but also by their profound temporal persistence, indicating that these highly successful clonal genotypes have likely acquired a biological fitness advantage over other gonococcal genotypes. Another possible explanation for the clonal expansion of these two clonal complexes may lie in the fact that the clonal genotypes could enter into the major sexual networks of the high-risk population, thus affecting a large number of people via usually very complex and extensive sexual networks (28, 29) and at the same time creating the reservoir of asymptomatically infected individuals (30). Interestingly, the majority of penicillin-resistant/intermediate phenotypes were found among the clonally associated genotypes, further suggesting that after acquisition of this antimicrobial phenotype, it was disseminated by clonal spread. In a sharp contrast to this antimicrobial phenotype, the ciprofloxacin resistance phenotype was identified in several genetically distant gonococcal genotypes, spanning over both clades, clearly indicating nonclonal spread. Resistance to azithromycin, suggested by some as a possible alternative monotherapeutic antibiotic (31), as well as being an antibiotic used presently in dual therapy (32), was present in a single strain, SK 36809, which was clustered within a group of genetically distant strains, suggesting that the azithromycin resistance was most likely introduced into the province. This finding is further supported by the point estimate analyses, which revealed that strain SK 36809, based on SNPs, the antimicrobial profile, and the penA allele, is one of the most divergent gonococcal strains in the studied collection, suggesting the introduction of this antimicrobial resistance phenotype into Saskatchewan from somewhere else.
In summary, using crude whole-genome assemblies as a starting point and a combination of SNPs, penA alleles, antimicrobial susceptibility profiles, and molecular population structure analysis, we have shown that the tested gonococcal population consisted of two clonal complexes. These clonal complexes were characterized by temporal and geographic persistence in Saskatchewan, which has very high gonorrhea infection rates, further highlighting the public health importance of these clonal complexes. In addition, we reported the clonal spread of penicillin-resistant/intermediate phenotypes and introduction of azithromycin resistance among the gonococcal population from western Canada.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the Saskatchewan Health Research Foundation (grant 9127, Research Alliance for the Prevention of Infectious Disease [RAPID]) and by funding from the University of Saskatchewan (to J.R.D.). Genome sequencing was funded by the U.S. National Institutes of Health (grant A1096768).
Mathew Ezewudo, Sandeep Joseph, William Shafer, Deborah Dean, and Richard Seldon contributed to the genome sequencing effort. We thank the Saskatchewan Disease Control Laboratory (Regina, SK, Canada) for providing characterized gonococcal isolates. We are grateful to Daniela Vidovic for help with preparation of the figures.
J.R.D. and S.V. designed the experiment and performed strain selection, T.D.R. performed genome sequencing and assembly, A.K., C.C., and A.T. mapped the genomes and extracted the gene data, S.V. performed primary data analysis and prepared the manuscript, S.D.T. performed antimicrobial susceptibility tests, and J.R.D. provided team organization, approval, and final editing of manuscript. All the authors contributed to editing the manuscript.
Published ahead of print 23 July 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JCM.01502-14.
REFERENCES
- 1.World Health Organization. 2012. Global action plan to control the spread and impact of antimicrobial resistance in Neisseria gonorrhoeae. World Health Organization, Geneva, Switzerland [Google Scholar]
- 2.Public Health Agency of Canada. 2014. National surveillance of antimicrobial susceptibilities of Neisseria gonorrhoeae annual summary 2012. Public Health Agency of Canada, Winnipeg, Canada [Google Scholar]
- 3.Tapsall J. 2006. Antibiotic resistance in Neisseria gonorrhoeae is diminishing available treatment options for gonorrhea: some possible remedies. Expert Rev. Anti Infect. Ther. 4:619–628. 10.1586/14787210.4.4.619 [DOI] [PubMed] [Google Scholar]
- 4.Martin I, Jayaraman G, Wong T, Liu G, Gilmour M, Canadian Public Health Laboratory Network 2011. Trends in antimicrobial resistance in Neisseria gonorrhoeae isolated in Canada: 2000–2009. Sex. Transm. Dis. 38:892–898. 10.1097/OLQ.0b013e31822c664f [DOI] [PubMed] [Google Scholar]
- 5.Bolan GA, Sparling PF, Wasserheit JD. 2012. The emerging threat of untreatable gonococcal infection. N. Engl. J. Med. 366:485–487. 10.1056/NEJMp1112456 [DOI] [PubMed] [Google Scholar]
- 6.Martin I, Sawatzky P, Allen V, Hoang L, Lefebvre B, Mina N, Wong T, Gilmour M. 2012. Emergence and characterization of Neisseria gonorrhoeae isolates with decreased susceptibilities to ceftriaxone and cefixime in Canada: 2001–2010. Sex. Transm. Dis. 39:316–323. 10.1097/OLQ.0b013e3182401b69 [DOI] [PubMed] [Google Scholar]
- 7.Ohnishi M, Saika T, Hoshina S, Iwasaku K, Nakayama S, Watanabe H, Kitawaki J. 2011. Ceftriaxone-resistant Neisseria gonorrhoeae, Japan. Emerg. Infect. Dis. 17:148–149. 10.3201/eid1701.100397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Unemo M, Golparian D, Stary A, Eigentler A. 2011. First Neisseria gonorrhoeae strain with resistance to cefixime causing gonorrhoea treatment failure in Austria, 2011. Euro Surveill. 16:pii=19998 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19998 [PubMed] [Google Scholar]
- 9.Unemo M, Golparian D, Nicholas R, Ohnishi M, Gallay A, Sednaoui P. 2012. High-level cefixime- and ceftriaxone-resistant N. gonorrhoeae in Europe (France): novel penA mosaic allele in a successful international clone causes treatment failure. Antimicrob. Agents Chemother. 56:1273–1280. 10.1128/AAC.05760-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lewis DA, Sriruttan C, Muller EE, Golparian D, Gumede L, Fick D, de Wet J, Maseko V, Coetzee J, Unemo M. 2013. Phenotypic and genetic characterization of the first two cases of extended-spectrum-cephalosporin-resistant Neisseria gonorrhoeae infection in South Africa and association with cefixime treatment failure. J. Antimicrob. Chemother. 68:1267–1270. 10.1093/jac/dkt034 [DOI] [PubMed] [Google Scholar]
- 11.Vidovic S, Thakur SD, Horsman GB, Levett PN, Anvari V, Dillon JR. 2012. Longitudinal analysis of the evolution and dissemination of Neisseria gonorrhoeae strains (Saskatchewan, Canada 2005–2008) reveals three major circulating strains and convergent evolution of ciprofloxacin and azithromycin resistance. J. Clin. Microbiol. 50:3823–3830. 10.1128/JCM.01402-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yeung KH, Dillon JR, Pauze M, Wallace E. 1986. A novel 4.9-kilobase plasmid associated with an outbreak of penicillinase-producing Neisseria gonorrhoeae. J. Infect. Dis. 153:1162–1165. 10.1093/infdis/153.6.1162 [DOI] [PubMed] [Google Scholar]
- 13.Ruddock PS, Charland M, Ramirez S, Lopez A, Towers NGH, Arnason JT, Liao M, Dillon JR. 2011. Antimicrobial activity of flavonoids from Piper lanceaefolium and other Colombian medicinal plants against antibiotic susceptible and resistant strains of Neisseria gonorrhoeae. Sex. Transm. Dis. 38:82–88. 10.1097/OLQ.0b013e3181f0bdbd [DOI] [PubMed] [Google Scholar]
- 14.Gibbs MJ, Armstrong JS, Gibbs AJ. 2000. Sister-scanning: a Monte Carlo procedure for assessing signals in recombinant sequences. Bioinformatics 16:573–582. 10.1093/bioinformatics/16.7.573 [DOI] [PubMed] [Google Scholar]
- 15.Vidovic S, Horsman GB, Liao M, Dillon JR. 2011. Influence of conserved and hypervariable genetic markers on genotyping circulating strains of Neisseria gonorrhoeae. PLoS One 6:e28259. 10.1371/journal.pone.0028259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tamura K, Dudley J, Nei M, Kumar S. 2007. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol. Biol. Evol. 24:1596–1599. 10.1093/molbev/msm092 [DOI] [PubMed] [Google Scholar]
- 17.Rzhetsky A, Nei M. 1992. A simple method for estimating and testing minimum evolution trees. Mol. Biol. Evol. 9:945–967 [Google Scholar]
- 18.Saitou N, Nei M. 1987. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4:406–425 [DOI] [PubMed] [Google Scholar]
- 19.Clinical and Laboratory Standards Institute. 2006. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 7th ed, approved document M7-A7 Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 20.Thakur SD, Starnino S, Horsman GB, Levett PN, Dillon JR. 2014. Unique combined penA/mtrR/porB mutations and NG-MAST strain types associated with ceftriaxone and cefixime MIC increases in a “susceptible” Neisseria gonorrhoeae population. J. Antimicrob. Chemother. 69:1510–1516. 10.1093/jac/dkt543 [DOI] [PubMed] [Google Scholar]
- 21.Pritchard JK, Stephens M, Donnelly P. 2000. Inference of population structure using multilocus genotype data. Genetics 155:945–959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jakobsson M, Rosenberg NA. 2007. CLUMPP: a cluster matching and permutation program for dealing with label switching and multimodality in analysis of population structure. Bioinformatics 23:1801–1806. 10.1093/bioinformatics/btm233 [DOI] [PubMed] [Google Scholar]
- 23.Rosenberg NA. 2004. DISTRUCT: a program for the graphical display of population structure. Mol. Ecol. Notes 4:137–138. 10.1046/j.1471-8286.2003.00566.x [DOI] [Google Scholar]
- 24.Whiley DM, Limnios EM, Ray S, Sloots TP, Tapsall JW. 2007. Diversity of penA alterations and subtypes in Neisseria gonorrhoeae strains from Sydney, Australia, that are less susceptible to ceftriaxone. Antimicrob. Agents Chemother. 51:3111–3116. 10.1128/AAC.00306-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ohnishi M, Golparian D, Shimuta K, Saika T, Hoshima S, Iwasaki K, Nakayama S, Kitawaki J, Unemo M. 2011. Is Neisseria gonorrhoeae initiating a future era of untreatable gonorrhea? Detailed characterization of the first strain with high-level resistance to ceftriaxone. Antimicrob. Agents Chemother. 55:3538–3545. 10.1128/AAC.00325-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Public Health Agency of Canada. 2011. Report on sexually transmitted infections in Canada: 2009. Centre for Communicable Diseases and Infection Control, Infectious Disease Prevention and Control Branch, Public Health Agency of Canada, Ottawa, Canada: http://www.catie.ca/sites/default/files/2009%20Report%20on%20STI%20in%20Canada_EN.pdf [Google Scholar]
- 27.Ilina EN, Oparina NY, Shitikov EA, Borovskaya AD, Govorun VM. 2010. Molecular surveillance of clinical Neisseria gonorrhoeae isolates in Russia. J. Clin. Microbiol. 48:3681–3689. 10.1128/JCM.00565-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wylie LJ, Jolly A. 2001. Patterns of chlamydia and gonorrhea infection in sexual networks in Manitoba, Canada. Sex. Transm. Dis. 28:14–24. 10.1097/00007435-200101000-00005 [DOI] [PubMed] [Google Scholar]
- 29.Wylie LJ, Cabral T, Jolly AM. 2005. Identification of networks of sexually transmitted infection: a molecular, geographic, and social network analysis. J. Infect. Dis. 191:899–906. 10.1086/427661 [DOI] [PubMed] [Google Scholar]
- 30.Detels R, Green AM, Klausner JD, Katzenstein D, Gaydos C, Handsfield HH, Pequegnat W, Mayer K, Hartwell TD, Quinn TC. 2011. The incidence and correlates of symptomatic and asymptomatic Chlamydia trachomatis and Neisseria gonorrhoeae infections in selected populations in five countries. Sex. Transm. Dis. 38:503–509 [PMC free article] [PubMed] [Google Scholar]
- 31.Bignell C, Garley J. 2010. Azithromycin in the treatment of infection with Neisseria gonorrhoeae. Sex. Transm. Infect. 86:422–426. 10.1136/sti.2010.044586 [DOI] [PubMed] [Google Scholar]
- 32.Bignell C, Fitzgerald M, Guideline Development Group, British Association for Sexual Health and HIV UK 2011. UK national guideline for the management of gonorrhoea in adults, 2011. Int. J. STD AIDS. 22:541–547. 10.1258/ijsa.2011.011267 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.