LETTER
The main cause of fosfomycin resistance in extended-spectrum β-lactamase (ESBL)-producing Escherichia coli isolates is glutathione S-transferase responsible for fosfomycin resistance (FR-GST), which includes FosA3, FosA4, and FosC2 (1, 2). Fosfomycin is expected to become an option for treating urinary tract infections caused by ESBL-producing E. coli (3), but FR-GST genes have already spread among E. coli isolates in a variety of clinical and veterinary settings, which limits the potential use of fosfomycin (1, 4–7). Detection of FR-GST-producing bacteria by clinical microbiology laboratories is, therefore, necessary to prevent their further spread in clinical settings. To easily identify the production of FR-GST, we have recently developed a simple, cost-effective disk potentiation test, using phosphonoformate (PPF), a specific inhibitor of FR-GST (2, 8). The test uses Kirby-Bauer (KB) disks containing 50 μg fosfomycin and 5 μg glucose-6-phosphate (G6P), in combination with Mueller-Hinton (MH) agar plates containing 25 μg/ml G6P (MH-G6P plates). The addition of G6P to the agar plates increased the inhibitory effect by PPF, compared to MH plates without G6P supplementation, enabling unambiguous identification of FR-GST production (2).
Clinical and Laboratory Standards Institute (CLSI) guidelines recommend the use of KB disks containing 200 μg fosfomycin and 50 μg G6P when performing disk diffusion susceptibility testing (9). These amounts are 4- and 10-fold higher, respectively, than those we have used (2). In this study, we have incorporated the high-dosage fosfomycin-G6P KB disks into the disk potentiation test to evaluate their effectiveness in screening for FR-GST production.
Fifteen FR-GST-positive E. coli isolates (12 isolates with fosA3, 2 isolates with fosA4, and 1 isolate with fosC2) and 22 FR-GST-negative E. coli isolates were used for the evaluation (2). The strains tested were prepared according to the CLSI guidelines (9) and spread on MH plates and MH-G6P plates. Two blank disks were placed on the agar plates; the first disk was loaded with 200 μg fosfomycin and 50 μg G6P (20 μl was loaded with the solution containing 10-mg/ml fosfomycin and 2.5-mg/ml G6P dissolved in water), and the second disk was additionally loaded with 1 mg PPF (Fig. 1). After 18 h of incubation at 37°C, the growth inhibitory zone around each disk was measured, and the results were summarized in Fig. 2. Fifteen FR-GST-positive isolates exhibited 11- to 19-mm (average 14-mm) expansion and 10- to 14-mm (average 12-mm) expansion in the growth inhibition zone by the addition of PPF on MH-G6P and MH plates, respectively (P < 0.05 by the Wilcoxon signed-rank test). When the FR-GST-positive strains were grown on MH-G6P plates, the growth inhibition zone was larger than that on MH plates. Thus, the addition of G6P to MH plates resulted in a larger inhibitory zone, confirming the results of our previous study (2). However, MH plates without the addition of G6P resulted in enough growth inhibition zone expansion to enable the detection of FR-GST production (Fig. 2). The FR-GST-negative strains showed no growth inhibition zone expansion by PPF, although a slight reduction in the size of the zone was observed, as shown by our previous study (2). Therefore, the use of high-dosage fosfomycin-G6P disks containing 200 μg fosfomycin and 50 μg G6P could eliminate the need for additional G6P supplementation in the potentiation test for identifying FR-GST producers.
FIG 1.
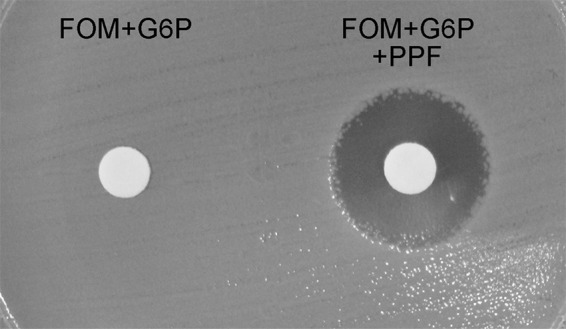
Potentiation test for a FosA3-producing E. coli isolate on an MH plate. The left disk contains 200 μg fosfomycin (FOM) and 50 μg glucose-6-phosphate (G6P), and the right disk contains 200 μg fosfomycin, 50 μg G6P, and 1 mg phosphonoformate (PPF).
FIG 2.
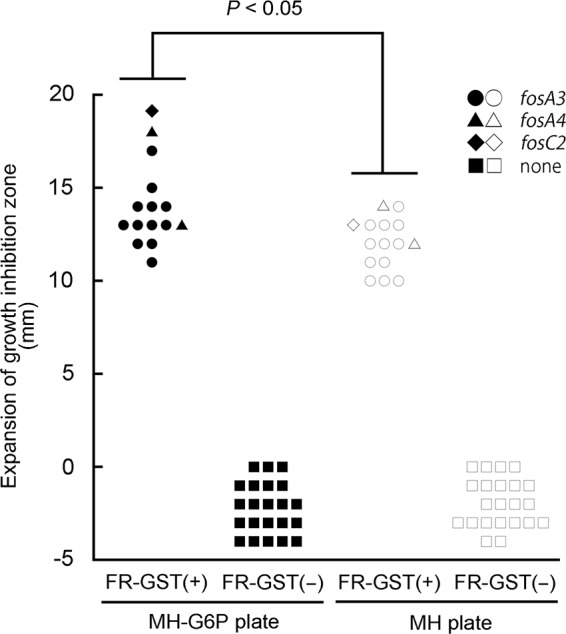
Changes to growth inhibition zone diameter by PPF on MH-G6P and MH plates. The y axis shows enlargement of the growth inhibition zone (in millimeters) by PPF. FR-GST-positive [FR-GST(+)] and FR-GST-negative [FR-GST(−)] isolates are shown.
Together with our previous results (2), we confirmed that both the Western standard disks (high-dosage fosfomycin-G6P) with MH plates and Japanese standard disks (low-dosage fosfomycin-G6P) with MH plates supplemented with 25 μg/ml G6P can detect FR-GST-producing E. coli isolates.
ACKNOWLEDGMENTS
This study was supported by grants from the Japanese Ministry of Health, Labour and Welfare (H24-Shinkou-Ippan-010).
Footnotes
Published ahead of print 13 August 2014
REFERENCES
- 1.Wachino J, Yamane K, Suzuki S, Kimura K, Arakawa Y. 2010. Prevalence of fosfomycin resistance among CTX-M-producing Escherichia coli clinical isolates in Japan and identification of novel plasmid-mediated fosfomycin-modifying enzymes. Antimicrob. Agents Chemother. 54:3061–3064. 10.1128/AAC.01834-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nakamura G, Wachino J-I, Sato N, Kimura K, Yamada Y, Jin W, Shibayama K, Yagi T, Kawamura K, Arakawa Y. 20 June 2014. Practical agar-based disk potentiation test to detect fosfomycin-non-susceptible Escherichia coli clinical isolates producing glutathione S-transferases. J. Clin. Microbiol. 10.1128/JCM.01094-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Neuner EA, Sekeres J, Hall GS, van Duin D. 2012. Experience with fosfomycin for treatment of urinary tract infections due to multidrug-resistant organisms. Antimicrob. Agents Chemother. 56:5744–5748. 10.1128/AAC.00402-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sato N, Kawamura K, Nakane K, Wachino JI, Arakawa Y. 2013. First detection of fosfomycin resistance gene fosA3 in CTX-M-producing Escherichia coli isolates from healthy individuals in Japan. Microb. Drug Resist. 19:477–482. 10.1089/mdr.2013.0061 [DOI] [PubMed] [Google Scholar]
- 5.Hou J, Yang X, Zeng Z, Lv L, Yang T, Lin D, Liu JH. 2013. Detection of the plasmid-encoded fosfomycin resistance gene fosA3 in Escherichia coli of food-animal origin. J. Antimicrob. Chemother. 68:766–770. 10.1093/jac/dks465 [DOI] [PubMed] [Google Scholar]
- 6.Hou J, Huang X, Deng Y, He L, Yang T, Zeng Z, Chen Z, Liu JH. 2012. Dissemination of the fosfomycin resistance gene fosA3 with CTX-M β-lactamase genes and rmtB carried on IncFII plasmids among Escherichia coli isolates from pets in China. Antimicrob. Agents Chemother. 56:2135–2138. 10.1128/AAC.05104-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ho PL, Chan J, Lo WU, Law PY, Li Z, Lai EL, Chow KH. 2013. Dissemination of plasmid-mediated fosfomycin resistance fosA3 among multidrug-resistant Escherichia coli from livestock and other animals. J. Appl. Microbiol. 114:695–702. 10.1111/jam.12099 [DOI] [PubMed] [Google Scholar]
- 8.Rigsby RE, Rife CL, Fillgrove KL, Newcomer ME, Armstrong RN. 2004. Phosphonoformate: a minimal transition state analogue inhibitor of the fosfomycin resistance protein, FosA. Biochemistry 43:13666–13673. 10.1021/bi048767h [DOI] [PubMed] [Google Scholar]
- 9.Clinical and Laboratory Standards Institute. 2013. Performance standards for antimicrobial susceptibility testing: twenty-third informational supplement. M100-S23 Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]


