Abstract
Multidrug-resistant Acinetobacter baumannii is among the most prevalent bacterial pathogens associated with trauma-related wound and bloodstream infections. Although septic shock and disseminated intravascular coagulation have been reported following fulminant A. baumannii sepsis, little is known about the protective host immune response to this pathogen. In this study, we examined the role of PTX3, a soluble pattern recognition receptor with reported antimicrobial properties and stored within neutrophil granules. PTX3 production by murine J774a.1 macrophages was assessed following challenge with A. baumannii strains ATCC 19606 and clinical isolates (CI) 77, 78, 79, 80, and 86. Interestingly, only CI strains 79, 80, and 86 induced PTX3 synthesis in murine J774a.1 macrophages, with greatest production observed following CI 79 and 86 challenge. Subsequently, C57BL/6 mice were challenged intraperitoneally with CI 77 and 79 to assess the role of PTX3 in vivo. A. baumannii strain CI 79 exhibited significantly (P < 0.0005) increased mortality, with an approximate 50% lethal dose (LD50) of 105 CFU, while an equivalent dose of CI 77 exhibited no mortality. Plasma leukocyte chemokines (KC, MCP-1, and RANTES) and myeloperoxidase activity were also significantly elevated following challenge with CI 79, indicating neutrophil recruitment/activation associated with significant elevation in serum PTX3 levels. Furthermore, 10-fold-greater PTX3 levels were observed in mouse serum 12 h postchallenge, comparing CI 79 to CI 77 (1,561 ng/ml versus 145 ng/ml), with concomitant severe pathology (liver and spleen) and coagulopathy. Together, these results suggest that elevation of PTX3 is associated with fulminant disease during A. baumannii sepsis.
INTRODUCTION
Since the initial widespread use of antibiotics in hospitals during the 1930s and subsequently on multiple battlefields, Gram-negative bacteria resistant to many first-generation cell wall-targeting antibiotics are now the predominant cause of traumatic wound and burn infections (1–3). Complications encountered during treatment arise in part due to the emergence of multidrug-resistant (MDR) A. baumannii isolates (4–7) whose resistance allows them to disseminate, giving rise to septic shock and disseminated intravascular coagulation (DIC). Consequently, mortality rates range from 30 to 75% depending on the route of infection (8). Although specific virulence factors (lipopolysaccharide [LPS] and membrane glycosylation) and a robust cellular innate immune response (neutrophil infiltration) contribute to disease severity and clearance during A. baumannii sepsis, respectively, very little is known about differences in virulence or mortality between strains and the overall protective host immune response necessary for protection against A. baumannii infection (9–15).
Neutrophils contain a variety of antimicrobial molecules stored within cytoplasmic azurophilic granules (16–18). One of these molecules is the soluble pattern recognition receptor designated pentraxin 3 (PTX3), which recognizes and interacts with a variety of pathogen/damage-associated molecular patterns (PAMP/DAMP) eliciting protection against select pathogens, e.g., Pseudomonas aeruginosa and Aspergillus fumigatus, but not Escherichia coli (2, 19–22). Additionally, PTX3 has been shown to opsonize pathogens, thus enhancing complement activation and phagocytosis during bacterial and fungal infections, aiding in pathogen clearance through recruitment of C1q and stimulation of the Fcγ receptor, respectively (16, 18–20, 23–28). Despite immunoprotective properties, prolonged elevation of patient PTX3 levels has been reported to correlate with increased morbidity and mortality in severe sepsis (29–35) thought to arise from increased tissue factor (TF) expression on the surface of monocytic phagocytes and vascular endothelial cells observed in vitro following LPS stimulation (36, 37). Although neutrophils store PTX3 in a preformed active state within cytoplasmic granules, giving rise to short-lived spikes in serum PTX3 levels following degranulation, prolonged elevation can arise from induced expression by monocytic cells, e.g., monocytes, macrophages, dendritic cells, and endothelial cells (17, 19, 25, 28, 38, 39). Data presented here suggest that prolonged elevation of PTX3 during A. baumannii sepsis is associated with more severe disease.
MATERIALS AND METHODS
Ethics statement.
All animal experiments were performed in compliance with the Animal Welfare Act, the U.S. Public Health Service Policy on Humane Care and Use of Laboratory Animals, and the Guide for the care and use of laboratory animals published by the National Research Council. All animal work was carried out under approved protocol MU070-10/14A0 in accordance with the guidelines set forth by the University of Texas at San Antonio Institutional Animal Care and Use Committee (IACUC) and Institutional Biosafety Committee (IBC).
Cell lines.
Murine J774a.1 peritoneal macrophages were grown in Dulbecco's modified Eagle medium (DMEM) (Life Technologies, Carlsbad, CA) supplemented with 10% fetal bovine serum (FBS) (Thermo Scientific, Rockford, IL) as well as amphotericin B and gentamicin (final concentrations, 0.25 μg/ml and 0.01 μg/ml, respectively). Growth medium was replaced with medium lacking antibiotics 24 h prior to bacterial challenge experiments.
Bacterial strains.
Acinetobacter baumannii clinical isolates (CI) lacking personal identifiers (designated CI 77, 78, 79, 80, and 86) were retained by the San Antonio Military Medical Center (SAMMC) (Fort Sam Houston, San Antonio, TX) from injured military personnel and graciously provided by James Jorgensen (University of Texas Health Science Center at San Antonio, San Antonio, TX). A. baumannii (ATCC 19606) was obtained from the American Type Culture Collection (Manassas, VA). All strains were grown in Luria-Bertani (LB) broth.
Mice.
All animal experiments were performed utilizing 6- to 8-week-old pathogen-free C57BL/6 mice purchased from Charles River Laboratories (Frederick, MD).
LD50 comparison of A. baumannii strains.
Mice were challenged by intraperitoneal injection with 100 μl bacterial phosphate-buffered saline (PBS) suspension. For 50% lethal dose (LD50) comparison experiments, each group was challenged with increasing amounts of the A. baumannii clinical isolates CI 77 and 79, ranging from 104 to 108 CFU, and monitored for 1 month.
Tissue histology.
Tissues (liver, spleen, and kidney) were collected from mice sacrificed 24 h postchallenge with either CI 77 or 79. Tissues were subsequently prepared and analyzed for histopathology at the U.S. Army Institute of Surgical Research (JBSA-Fort Sam Houston, San Antonio, TX). Briefly, tissues were paraffin embedded, and 5-μm-thick sections were cut using a rotary microtome. Respective sections were placed on slides, transferred to an oven, heated for 30 min, and stained (standard hematoxylin-and-eosin (H&E) staining, Shandon automatic stainer; Thermo Scientific, Rockford, IL). Finally, pathology was assessed and scored in liver and kidney tissue sections as described by Dalle Lucca et al. (40).
Whole-blood collections.
Whole blood collected from the submandibular vein of anesthetized challenged mice was used for cytokine enzyme-linked immunosorbent assay (ELISA) and blood culture. For blood cultures, 10 μl whole blood was diluted and plated for enumeration of CFU burden on LB agar supplemented with 50 μg/ml chloramphenicol. The remaining blood was allowed to clot at room temperature and centrifuged at 1,200 × g to obtain serum for ELISA.
Whole blood collected by cardiac puncture for complete blood count (CBC) and coagulation studies was transferred to either 500-μl Capiject capillary blood collection tubes containing EDTA (Terumo Medical Corp., Elkton, MD) for CBC analysis or 500-μl microcentrifuge tubes containing 109 mM sodium citrate (8:1 blood-to-anticoagulant ratio; Becton, Dickinson, Franklin Lakes, NJ) for rotational thromboelastometry (ROTEM). Whole blood used for flow cytometry, prothrombin time (PT), D dimer, fibrinogen, and cytokine assays was collected from the descending aorta in 200 mM sodium citrate (9:1 blood-to-anticoagulant ratio) to accommodate increased blood volumes necessary for the assays while preventing spontaneous coagulation. In all cases, 100 μg/ml corn trypsin inhibitor was used to prevent clotting during collection.
Cytokine and myeloperoxidase activity assays.
Human and murine PTX3 (PTX3 Duoset ELISA; R&D Systems, Minneapolis, MN) levels in mouse serum and cell culture supernatants, as well as murine plasma D2D (D dimer) and fibrinogen (BioTang Inc., Waltham, MA) levels, were determined by ELISA. Additionally, myeloperoxidase activity in serum from challenged mice was assessed as previously described (41). Briefly, 2 μl serum was added to a 200-μl freshly prepared solution of 5 mM o-dianisidine dihydrochloride and 0.0005% hydrogen peroxide (Sigma-Aldrich, St. Louis, MO). Color development was determined after 1 to 3 min at 450 nm. A custom Bio-Plex Pro cytokine panel (Bio-Rad, Hercules, CA) was used to quantitate plasma levels of the leukocyte chemokines KC, MCP-1, and RANTES.
Complete blood count and coagulation studies.
EDTA-treated whole blood was subject to CBC analysis using an Advia 120 CBC analyzer (Siemens, Sacramento, CA) calibrated to mouse parameters. EXTEM and FIBTEM analyses were performed by ROTEM (TEM Systems Inc., Durham, NC) to monitor the extrinsic coagulation pathway and fibrin polymerization, respectively, using 105 μl whole blood collected in 109 mM sodium citrate (8:1 ratio). Prothrombin times were assessed on a Stago ST4 coagulation analyzer using 25 μl whole blood collected in 200 mM sodium citrate (9:1 ratio).
Flow cytometry.
Whole blood collected in 200 mM sodium citrate (9:1 ratio) was subjected to flow cytometry using the BD FACSCanto flow cytometer (Becton, Dickinson and Company, San Jose, CA) equipped with 2 lasers (488 nm and 633 nm). Briefly, 5 μl whole blood was treated with mouse Fc block (Becton, Dickinson and Company, San Jose, CA) for 5 min at room temperature, followed by either a 10-min incubation in the dark with allophycocyanin (APC)-conjugated anti-mouse CD41 (eBioscience, San Diego, CA) and fluorescein isothiocyanate (FITC)-conjugated lactadherin (Hematologic Technologies, Inc., Essex Junction, VT) to monitor platelet activation or a 15-min incubation in the dark with FITC-conjugated anti-mouse CD45 and phycoerythrin (PE)-Cy7-conjugated anti-mouse CD11b (eBioscience, San Diego, CA) to monitor myelocytic leukocyte activation. Appropriate isotype controls were used to account for nonspecific fluorescence. Data acquisition and analysis were performed using BD FACS Diva v6.13 software.
Statistics.
Generally, statistical differences were assessed by one-way analysis of variance (ANOVA) with Holm-Sidak correction for multiple comparisons or the Welch t test. Error bars represent standard deviation. Due to the skewed nature of PT times, D-dimer, and fibrinogen levels, statistical significance between samples was assessed by the Kruskal-Wallis test with Dunn correction for multiple comparisons. All statistics were performed using GraphPad Prism statistical software. All data presented are representative of at least two independent experiments.
RESULTS
Assessment of PTX3 production in monocytic cell lines.
Release of PTX3 following neutrophil degranulation is nonspecific and short-lived (17, 42). As a result, prolonged elevation of PTX3 levels in circulation requires induced de novo production in monocytic cells (38). Thus, we sought to determine if A. baumannii induced PTX3 production in the murine J774a.1 macrophage cell line using a variety of Acinetobacter calcoaceticus-baumannii complex strains isolated from military service personnel injured while serving in Iraq and Afghanistan (Table 1). Interestingly, of the six tested strains, only CI 79 (P < 0.0005), 80 (P < 0.05), and 86 (P < 0.0005) induced detectable PTX3 production 24 h postchallenge, with greatest levels of PTX3 production observed following challenge with strains CI 79 and 86 (Fig. 1). Given the clear phenotypic differences observed between A. baumannii strains with regard to the ability to induce PTX3 production, we selected strains CI 77 and 79 for in vivo survival comparison in a murine intraperitoneal sepsis model.
TABLE 1.
Acinetobacter species strains used in this study
| Species | Strain | Origina | Source | MDRb |
|---|---|---|---|---|
| A. baumannii | ATCC 19606 | ATCC | Urine | N |
| A. baumannii | CI 77 | OIF | Superficial wound | Y |
| A. calcoaceticus | CI 78 | OEF | Respiratory | Y |
| A. baumannii | CI 79 | OIF | Respiratory | Y |
| A. baumannii | CI 80 | OIF | Superficial wound | Y |
| A. baumannii | CI 86 | OIF | Superficial wound | Y |
ATCC, American Type Culture Collection; OIF, Operation Iraqi Freedom (Iraq); OEF, Operation Enduring Freedom (Afghanistan).
N, no; Y, yes.
FIG 1.
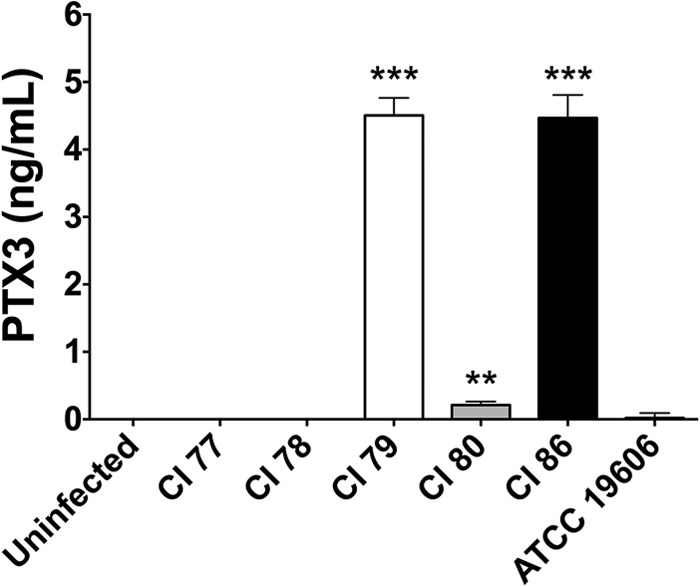
A. baumannii induction of PTX3 production in monocytic cells. Levels of PTX3 in J774a.1 macrophage cell supernatants were assessed, as previously described in Materials and Methods, 24 h post-challenge with several A. baumannii strains. Error bars represent ±SD for triplicate wells in all graphs. Statistical differences were determined using one-way ANOVA with Holm-Sidak correction; *, P < 0.05; **, P < 0.005; ***, P < 0.0005.
In vivo pathogenesis comparison between A. baumannii strains.
Traditionally, bacterial sepsis models used for study of A. baumannii utilize porcine mucin to enhance virulence of the bacterium through inhibition of phagocytosis (13, 43, 44). Consequently, LD50 values as low as 103 to 104 CFU have been reported, with time to death (TTD) near 1 week postchallenge (13, 14). However, given that published data (18, 24, 26, 27) indicate that PTX3 opsonizes the bacteria to promote bacterial phagocytosis, contributing to clearance, we chose not to use porcine mucin in the bacterial preparation for our sepsis model. Although the observed LD50 was elevated (data not shown), the TTD in these mice was greatly reduced. All mice succumbed to challenge with 107 CFU within 24 h regardless of strain (Fig. 2, CI 77 and CI 79 High). Although 105 CFU corresponded to the LD50 for strain CI 79, which induced the highest levels of PTX3 in vitro (Fig. 2, CI 79 Low), surprisingly, a significant (P < 0.0005) difference in survival was observed following challenge with an equivalent number of CFU of the PTX3-noninducing strain CI 77, giving rise to zero mortality (Fig. 2, CI 77 Low). Interestingly, while 100% of CI 79-challenged mice exhibited positive blood cultures, with an average burden of 2.4 × 106 CFU/ml whole blood, less than 10% of mice challenged with CI 77 had any detectable bacteria in circulation 24 h postchallenge. Additionally, all mice challenged with 107 CFU UV- or heat-killed (HK) bacteria of either strain survived the challenge, which was 100% lethal with live organisms (Fig. 2, UV and HK), indicating that live bacteria are necessary for virulence.
FIG 2.
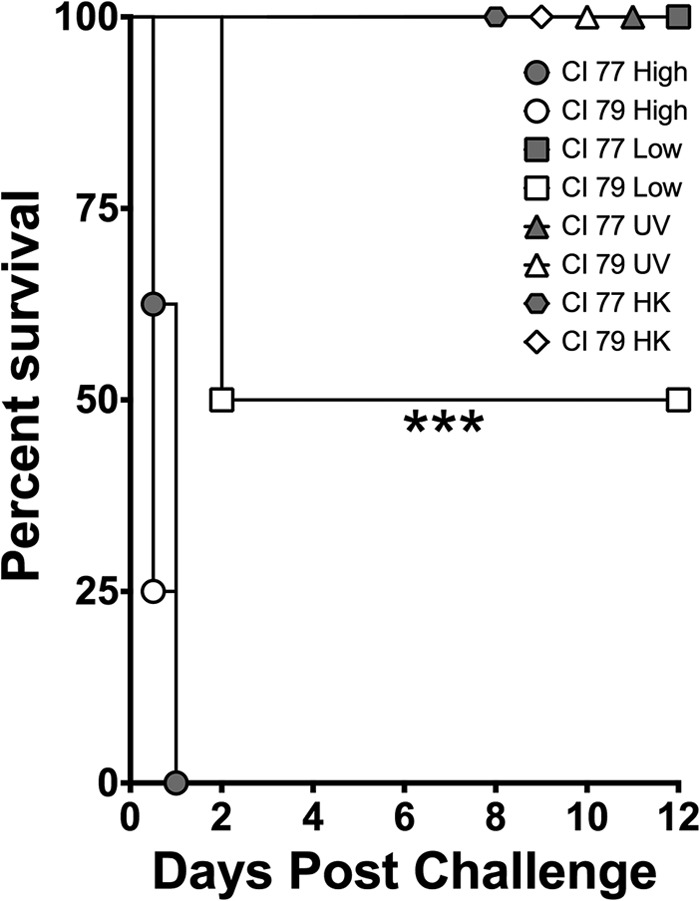
Comparison of survival following challenge with A. baumannii CI 77 and 79 challenge. Mice were assessed for survival following challenge with either the PTX3-noninducing strain CI 77 or the PTX3-inducing strain CI 79, using an in vivo mouse intraperitoneal sepsis model as previously described in Materials and Methods. Mice were challenged with a high dose (designated CI 77/79 High; 107 CFU; n = 8), a 100-fold-lower dose (designated CI 77/79 Low; 105 CFU; n = 8), UV-inactivated bacteria (designated CI 77/79 UV; equivalent to 107 CFU; n = 8) or heat-killed (HK) bacteria (designated CI 77/79 HK; equivalent to 107 CFU; n = 4). Statistical differences were determined using the Mantel-Cox log rank test; ***, P < 0.0005.
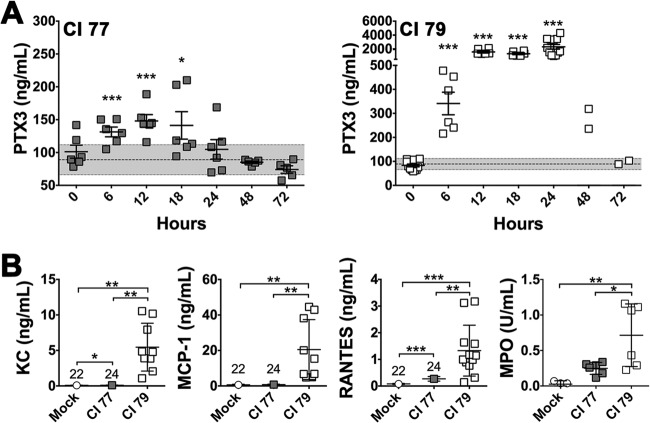
Sodium citrate-treated mouse plasma collected 24 h postchallenge with 105 CFU CI 77 or 79 was subjected to cytokine analysis. Although PTX3 levels in both CI 77- and 79-challenged mice peaked 12 h postchallenge (Fig. 3A), 10-fold-higher levels were observed in mice challenged with CI 79 (145 ng/ml versus 1,561 ng/ml, respectively). Additionally, while PTX3 levels in mice challenged with CI 77 return to basal levels observed in mock-challenged mice 24 h postchallenge (Fig. 3A), peak PTX3 levels observed 12 h postchallenge with mice challenged with CI 79 persisted through 24 h, at which time mice either succumbed to infection or survived (Fig. 3A). Furthermore, PTX3 levels in surviving mice following CI 79 challenge did not return to basal levels observed in the mock-challenge control until 72 h postchallenge.
FIG 3.
Chemokine and PTX3 levels in A. baumannii-challenged mice. In vivo production of PTX3 and chemokines was determined as described in Materials and Methods. (A) Serum PTX3 levels were monitored over a 24-h period (n = 6) in mice challenged with A. baumannii strain CI 77 or 79. The gray bar indicates basal PTX3 production observed in the mock control group over 24 h (mean ± 1 SD). (B) Chemokines KC, MCP-1, and RANTES (mock, n = 22; CI 77, n = 24; CI 79, n = 12) were assessed 24 h postchallenge, as were serum MPO levels (n = 6). Error bars represent ±SD in all graphs; statistical differences were determined by the Welch t test; *, P < 0.05; **, P < 0.005; ***, P < 0.0005.
Since the greatest difference in PTX3 levels was observed 24 h postchallenge between CI 77- and 79-challenged mice, the leukocyte chemokines KC, MCP-1, and RANTES, known to be involved in neutrophil and T cell recruitment, were also assessed 24 h postchallenge. As expected, all were significantly (P < 0.005) elevated in CI 79-challenged mice (Fig. 3B). Although KC and RANTES were also significantly (P < 0.05) increased in mice challenged with CI 77, this elevation was minimal compared to levels observed with strain CI 79. Sera from mice collected 24 h postchallenge also exhibited significantly (P < 0.05) elevated myeloperoxidase (MPO) activity following CI 79 challenge compared to that of both CI 77- and mock-treated mice, corresponding to increased neutrophil activation (Fig. 3B). This increase in MPO activity also corresponded to the elevated PTX3 levels observed in mice challenged with strain CI 79 compared to those for CI 77-challenged animals (Fig. 3A). Thus, significant elevation in PTX3 production appeared to be associated with increased disease severity, consistent with published case studies (29–35).
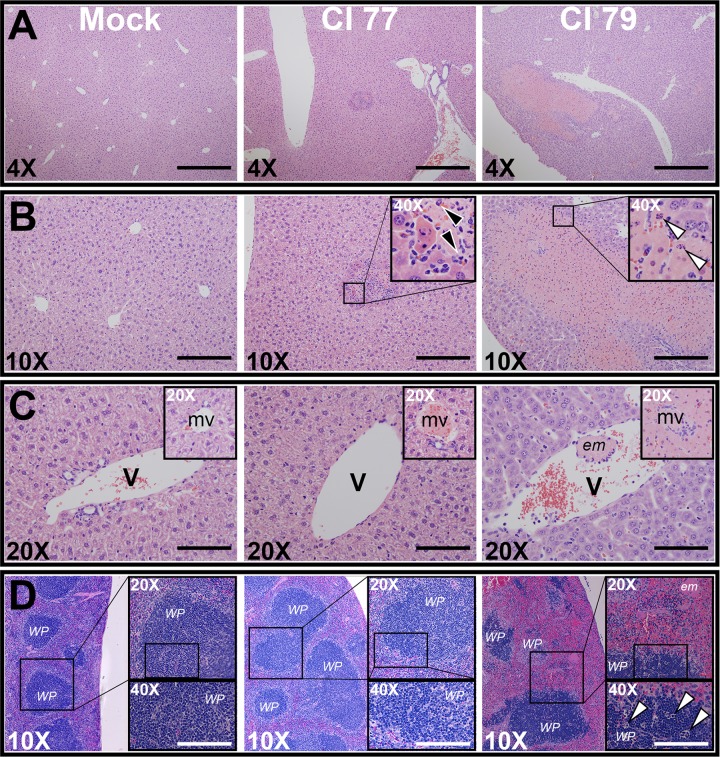
In addition to plasma cytokine analysis, tissue sections (liver, spleen, and kidney) obtained 24 h postchallenge were assessed for organ pathology associated with A. baumannii sepsis. Although tissue damage was observed in the kidneys of both CI 77- and 79-challenged mice relative to results for mock-challenged control mice (data not shown), there was no significant difference in pathology between the two challenge groups. However, significant (P < 0.005) differences in liver pathology between the two tested strains were observed. As expected, no pathology was observed in mock-challenged controls (Fig. 4A and B, left). Mice challenged with CI 77 (average liver pathology score = 3.33) exhibited small inflammatory foci in the liver (Fig. 4B, center) with polymorphonuclear cell infiltration (Fig. 4B, center; insert, black arrows), while those challenged with CI 79 (average liver pathology score = 8.67) exhibited distinct apoptotic nuclei (Fig. 4B, right; insert, white arrows) within large zones of tissue necrosis measuring more than a millimeter in diameter (Fig. 4A, right). Although mice challenged with both CI 77 and 79 exhibited microvessel occlusion, indicating some level of hypercoagulopathy compared to results for the mock control group (Fig. 4C), only CI 79 exhibited zones of necrosis surrounding occluded microvessels, as well as large venous emboli, indicating more severe disease (Fig. 4C, right). Additionally, major pathology was observed in splenic sections obtained from CI 79-challenged mice, including white pulp depletion, leukocyte apoptosis, and embolus formation, which were not observed in CI 77- or mock-challenged mice (Fig. 4D).
FIG 4.
Pathology associated with A. baumannii challenge. Tissues were collected 24 h after A. baumannii low-dose (105) challenge and assessed and scored for pathology by H&E staining as previously described in Materials and Methods. (A and B) 4× (A) or 10× (B) objective magnification of representative liver tissue pathology from mock-challenged (left), CI 77-challenged (center), and CI 79-challenged (right) mice. Polymorphonuclear cell infiltration and apoptotic nuclei are designated by black and white arrows, respectively. (C) Respective hepatic veins and microvessels from mock-challenged (left), CI 77-challenged (center), and CI 79-challenged (right) mice. (D) Representative spleen tissue pathology from mock-challenged (left), CI 77-challenged (center), and CI 79-challenged (right) mice. Images are representative of 3 biological samples per treatment. Abbreviations: WP, splenic white pulp; em, emboli; mv, hepatic microvessel; V, hepatic vein. Scale bars: 4×, 1 mm; 10×, 400 μm; 20×, 200 μm; 40×, 100 μm.
Studies have attributed septic shock associated with A. baumannii infections to bacterial LPS and outer membrane shedding in secreted outer membrane vesicles by the bacterium (14, 44, 45). However, data presented here suggest that this may not be the case, but rather, elevated PTX3 production induced by select A. baumannii strains appears to correspond with increased susceptibility of mice to infection. Furthermore, in agreement with previously published patient case study data, prolonged elevation of PTX3 levels was associated with development of hypercoagulopathy and DIC in challenged animals (29–35).
A. baumannii sepsis-induced hypercoagulopathy.
Since histological studies indicated that mice challenged with A. baumannii developed hypercoagulopathy, blood from mice challenged with PBS (mock), CI 77, or CI 79 was collected, and coagulation parameters were assessed. Complete blood count (CBC) analyses of EDTA-treated whole blood revealed a significant (P < 0.0005) decrease in both platelet and white blood cell counts following CI 79 challenge compared to results for both mock- and CI 77-challenged mice (Table 2). In agreement with the observed increase in serum MPO activity, the relative neutrophil count was significantly elevated in mice challenged with CI 79; however, in contrast, the lymphocyte population was significantly reduced. Furthermore, flow cytometry revealed a significant (P < 0.0005) increase in CD11b expression on the surface of circulating leukocytes following CI 79 challenge (CD11b mean fluorescence intensity [MFI] ± standard deviation [SD]: mock challenge, 11,200 ± 5,450 [n = 10]; CI 77 challenge, 9,300 ± 1,900 [n = 11]; CI 79 challenge, 24,000 ± 7,750 [n = 3]), consistent with neutrophil and monocyte activation (46). Loss of platelets in CI 79-challenged mice was not due to spontaneous sample clotting, as evidenced by no significant differences being observed in complete blood cell counts or indices, i.e., hematocrit (HCT), hemoglobin (HGB), mean cell volume (MCV), etc., (cf. Table 2) compared to mock- and CI 77-challenged mice. Additionally, a significant (P < 0.005) increase in lactadherin binding of phosphatidylserine, i.e., a marker of procoagulant activation (47), on the surface of platelets obtained from CI 79-challenged mice was observed by flow cytometry (lactadherin MFI ± SD: mock challenge, 1,550 ± 200 [n = 10]; CI 77 challenge, 1,600 ± 450 [n = 11]; CI 79 challenge, 2,600 ± 1,000 [n = 4]).
TABLE 2.
CBC profiles of A. baumannii-challenged mice
| CBC parametera | Value for challenge group (nb)c |
||
|---|---|---|---|
| Mock (3) | CI 77 (5) | CI 79 (6) | |
| RBC (×106 cells/μl) | 9.88 | 9.82 | 10.20 |
| HGB (g/dl) | 14.9 | 14.7 | 15.4 |
| HCT (%) | 52.6 | 52.7 | 53.5 |
| MCV (fl) | 53.3 | 53.7 | 52.5 B |
| RDW (%) | 13.7 | 13.5 | 14.3 |
| WBC (×103 cells/μl) | 9.28 | 8.36 | 3.89 A,B |
| % lymph | 85.5 | 82.5 | 57.5 A,B |
| % neut | 8.4 | 9.4 | 23.2 A,B |
| % eos | 2.5 | 3.2 A | 1.5 A,B |
| % baso | 1.2 | 1.5 | 2.8 A,B |
| % mono | 0.7 | 1.0 | 2.8 |
| PLT (×103 cells/μl) | 1,140 | 1,130 | 374 A,B |
| MPV (fl) | 7.1 | 7.5 | 7.9 A,B |
CBC, complete blood count; RBC, red blood cells; WBC, white blood cells; PLT, platelets; HGB, hemoglobin; HCT, hematocrit; MCV, mean corpuscular volume; RDW, red blood cell distribution width; lymph, lymphocytes; neut, neutrophils; eos, eosinophils; baso, basophils; mono, monocytes; MPV, mean platelet volume.
n, no. of mice.
A, significantly different from mock-challenged animals (P < 0.005); B, significantly different from CI 77-challenged animals (P < 0.005). Statistical differences were determined by the Welch t test.
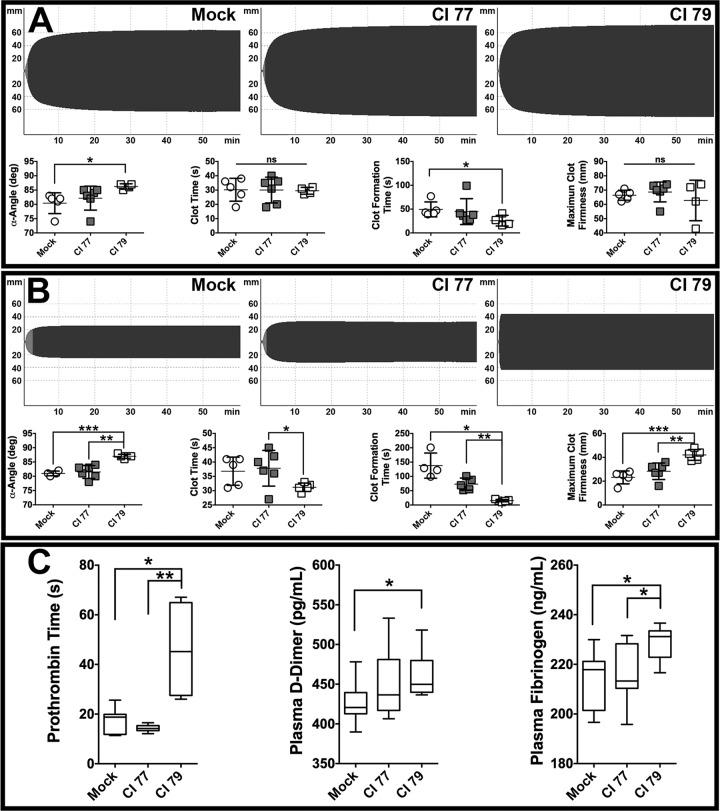
To further assess differences in coagulation parameters, sodium citrate-treated whole blood was subjected to rotational thromboelastometry (ROTEM) analysis using EXTEM and FIBTEM assays. The EXTEM assay measures hemostatic potential of whole blood activated with TF and phospholipid, i.e., the extrinsic coagulation pathway. The FIBTEM assay is a modified EXTEM assay utilizing cytochalasin D (an inhibitor of actin polymerization) to minimize platelet contribution, thus allowing measurement of fibrin polymerization during clot formation. Although visually indistinguishable, EXTEM tracings (Fig. 5A, top) revealed significant (P < 0.05) differences in α-angle and clot formation time (CFT) between mock-and CI 79-challenged mice (Fig. 5A, bottom). In contrast, FIBTEM tracings (Fig. 5B, top) exhibited pronounced visual differences that translated to a significant (P < 0.0005) increase in α-angle and maximum clot firmness (MCF) as well as significantly (P < 0.05) reduced CFT in mice challenged with CI 79 compared to CI 77 and mock challenges (Fig. 5B, bottom). Furthermore, significantly (P < 0.05) elevated D-dimer levels and prolonged PT were observed in blood samples collected from CI 79-challenged mice, as were fibrinogen levels (Fig. 5C), consistent with hypercoagulability or nonovert DIC in severe sepsis observed in humans (48). These data, in concert with thrombotic changes observed in liver tissue sections (Fig. 4), indicate evolving DIC in CI 79-challenged mice, closely resembling clinical symptoms observed in patients with Gram-negative sepsis (5, 29, 48).
FIG 5.
Assessing effects of A. baumannii infection on coagulation. Coagulation parameters ROTEM (EXTEM and FIBTEM), prothrombin time (PT), and ELISA for plasma D-dimer and fibrinogen determinations were performed as previously described in Materials and Methods. (A) EXTEM tracings (top) and corresponding α-angle, CF, CFT, and MCF parameters (bottom) assessing hemostatic potential of blood activated by tissue factor and phospholipid obtained from mock-challenged (n = 5), CI 77-challenged (n = 6), and CI 79-challenged (n = 4) mice. (B) FIBTEM tracings (top) and corresponding α-angle, CF, CFT, and MCF parameters (bottom) assessing the contribution of fibrin polymerization to clot formation with platelet function inhibited in mock-challenged (n = 5), CI 77-challenged (n = 6), and CI 79-challenged (n = 5) mice. (C) Clinical coagulation profiles, including PT, D-dimer, and fibrinogen assays from mock-challenged (n = 8), CI 77-challenged (n = 9), and CI 79-challenged (n = 4) mice represented in box whisker plots. Error bars represent ±SD in panels A and B. Statistical differences were determined using either the Welch t test (A and B) or the Kruskal-Wallis test with Dunn correction (C); *, P < 0.05; **, P < 0.005.
DISCUSSION
The emergence of MDR A. baumannii as a primary cause of traumatic wound and bloodstream infections (2, 49–51), with increasing prevalence in hospitals and clinics worldwide (5, 8, 52–62), underscores the need for a better understanding of the host immune response toward this pathogen. As part of the humoral arm of the innate immune system, pentraxins (e.g., C-reactive protein [CRP], serum amyloid P component [SAP], PTX3, etc.) are thought to serve as ancestral precursors to antibodies (63), and have been reported to have a nonredundant role in protection against both fungal and bacterial pathogens (2, 19–22, 28, 63, 64). Despite numerous reported protective functions of PTX3 in infection, female fertility, myocardial infarction, and trauma (18–20, 23, 28, 39, 42, 63–68), sustained elevation in critically ill patients has been associated with increased morbidity and mortality (29–35). Similarly, data presented here suggest that prolonged elevation of PTX3 levels, possibly arising from de novo production in peritoneal macrophage cells (Fig. 1), is associated with significant differences in survival and disease severity between the two strains tested (Fig. 2). A similar association between elevated PTX3 production and disease severity has been observed following both Gram-positive and Gram-negative sepsis in human patients (29–33, 35); however, at this time we cannot discern if the effects observed here are due in part to other entities, e.g., inflammatory molecules. Interestingly, although studies examining A. baumannii bloodstream infections involving hypercoagulability and DIC have attributed these symptoms to bacterial LPS and outer membrane shedding (14, 44, 45), data presented here indicate that disease severity was dependent on interaction of live bacteria with the immune system, as evidenced by the complete lack of mortality in mice challenged with UV-killed or HK bacteria administered at high inocula (Fig. 2). The significant increase in mortality observed in mice challenged with CI 79 corresponded with very high (i.e., 10-fold-elevated) serum PTX3 levels sustained over 24 h (Fig. 3A), as well as neutrophil recruitment and activation (Fig. 3B). Additionally, when challenged with another PTX3-inducing strain (i.e., CI 86), mice exhibited comparable (P = 0.313) levels of PTX3 production 24 h postchallenge with respect to CI 79-challenged mice, and all succumbed to infection within 48 h (data not shown). Although positive blood cultures were observed in 100% of mice challenged with CI 79 at 24 h postchallenge, with an average systemic burden of 2.4 × 106 CFU/ml blood, surprisingly the elevated PTX3 levels observed in these mice 24 h postchallenge did not appear to be directly related to the bacterial burden, since surviving mice (n = 2) exhibited an average burden of only 450 CFU/ml blood yet still produced microgram quantities of PTX3.
Mice challenged with CI 79 also exhibited more severe pathology in the liver and spleen inclusive of features consistent with hypercoagulability, i.e., large emboli causing vessel blockage and tissue damage not observed in mock- or CI 77-challenged mice (Fig. 4). Mice challenged with A. baumannii strain CI 79 exhibited CBC and coagulation profiles consistent with severe hypercoagulopathy and DIC (Table 2 and Fig. 5). These observations appear to be in agreement with previous observations associating hyperocagulopathy and disease severity arising from increased TF expression (29–37, 69); however, the causal link between PTX3 production and pathology/disease severity in A. baumannii sepsis requires further elucidation.
To date, this is the first study examining the possible role of PTX3 in A. baumannii sepsis. Although we do observe an association between PTX3 production and disease severity in A. baumannii sepsis in a wild-type C57BL/6 murine sepsis model, a larger cohort of A. baumannii clinical isolates, human patient samples, and studies involving PTX3 KO mice are required to firmly establish its utility as a biomarker or if a cause/effect relationship exists between PTX3 levels and disease severity in A. baumannii sepsis.
ACKNOWLEDGMENTS
We thank the Coagulation and Blood Research Program of the U.S. Army Institute of Surgical Research (JBSA-Fort Sam Houston) for providing access to and technical assistance for the Avida 120 CBC, ROTEM, and Stago ST4 analyzers.
This research was partially funded by the U.S. Army Medical Research and Material Command, the Army Research Office of the Department of Defense (contract W911NF-11-1-0136), and the Alvarez Graduate Research Education Excellence Fund.
The opinions or assertions contained herein are the private views of the authors and are not to be construed as official or as reflecting the views of the Department of the Army or the Department of Defense.
Footnotes
Published ahead of print 7 July 2014
REFERENCES
- 1.Lindberg RBLC, Wetzler TF, Marshall JDC, Newton AM, Strawitz JGFL, Howard JMC. 1955. The bacterial flora of battle wounds at the time of primary debridement. A study of the Korean battle casualty. Ann. Surg. 141:369–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Petersen K, Riddle MS, Danko JR, Blazes DL, Hayden R, Tasker SA, Dunne JR. 2007. Trauma-related infections in battlefield casualties from Iraq. Ann. Surg. 245:803–811. 10.1097/01.sla.0000251707.32332.c1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lewis K. 2013. Platforms for antibiotic discovery. Nat. Rev. Drug Discov. 12:371–387. 10.1038/nrd3975 [DOI] [PubMed] [Google Scholar]
- 4.Scott P, Petersen K, Fishbain J, Craft D, Ewell A, Moran K, Hack D, Deye G, Riddell S, Christopher G, Mancuso J, Petruccelli B, Endy T, Lindler L, Davis K, Milstrey E, Brosch L, Pool J, Blankenship C, Witt C, Malone J, Tornberg D, Srinivasan A. 2004. Acinetobacter baumannii infections among patients at military medical facilities treating injured U.S. service members, 2002–2004. MMWR Morb. Mortal. Wkly. Rep. 53:1063–1066 [PubMed] [Google Scholar]
- 5.Cisneros JM, Reyes MJ, Pachón J, Becerril B, Caballero FJ, García Garmendia JL, Ortiz C, Cobacho AR. 1996. Bacteremia due to Acinetobacter baumannii: epidemiology, clinical findings, and prognostic features. Clin. Infect. Dis. 22:1026–1032. 10.1093/clinids/22.6.1026 [DOI] [PubMed] [Google Scholar]
- 6.Rustigian R, Cipriani A. 1947. Bacteriology of open wounds. JAMA 133:7. [DOI] [PubMed] [Google Scholar]
- 7.Tong MJ. 1972. Septic complications of war wounds. JAMA 219:1044–1047. 10.1001/jama.1972.03190340050011 [DOI] [PubMed] [Google Scholar]
- 8.Bergogne-Bérézin E, Towner KJ. 1996. Acinetobacter spp. as nosocomial pathogens: microbiological, clinical, and epidemiological features. Clin. Microbiol. Rev. 9:148–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diebel LN, Liberati DM, Amin PB, Diglio CA. 2009. Cleavage of SIgA by Gram negative respiratory pathogens enhance neutrophil inflammatory potential. J. Trauma Acute Care Surg. 66:1336–1342. 10.1097/TA.0b013e31819dc577 [DOI] [PubMed] [Google Scholar]
- 10.Qiu H, KuoLee R, Harris G, Chen W. 2009. High susceptibility to respiratory Acinetobacter baumannii infection in A/J. mice is associated with a delay in early pulmonary recruitment of neutrophils. Microbes Infect. 11:946–955. 10.1016/j.micinf.2009.06.003 [DOI] [PubMed] [Google Scholar]
- 11.van Faassen H, KuoLee R, Harris G, Zhao X, Conlan JW, Chen W. 2007. Neutrophils play an important role in host resistance to respiratory infection with Acinetobacter baumannii in mice. Infect. Immun. 75:5597–5608. 10.1128/IAI.00762-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Breslow JM, Meissler JJ, Jr, Hartzell RR, Spence PB, Truant A, Gaughan J, Eisenstein TK. 2011. Innate immune responses to systemic Acinetobacter baumannii infection in mice: neutrophils, but not IL-17, mediate host resistance. Infect. Immun. 79:3317–3327. 10.1128/IAI.00069-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iwashkiw JA, Seper A, Weber BS, Scott NE, Vinogradov E, Stratilo C, Reiz B, Cordwell SJ, Whittal R, Schild S, Feldman MF. 2012. Identification of a general O-linked protein glycosylation system in Acinetobacter baumannii and its role in virulence and biofilm formation. PLoS Pathog. 8:e1002758. 10.1371/journal.ppat.1002758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin L, Tan B, Pantapalangkoor P, Ho T, Baquir B, Tomaras A, Montgomery JI, Reilly U, Barbacci EG, Hujer K, Bonomo RA, Fernandez L, Hancock REW, Adams MD, French SW, Buslon VS, Spellberg B. 2012. Inhibition of LpxC protects mice from resistant Acinetobacter baumannii by modulating inflammation and enhancing phagocytosis. mBio 3(5):e00312–12. 10.1128/mBio.00312-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eveillard M, Soltner C, Kempf M, Saint-André J-P, Lemarié C, Randrianarivelo C, Seifert H, Wolff M, Joly-Guillou M-L. 2010. The virulence variability of different Acinetobacter baumannii strains in experimental pneumonia. J. Infect. 60:154–161. 10.1016/j.jinf.2009.09.004 [DOI] [PubMed] [Google Scholar]
- 16.Soares AC, Souza DG, Pinho V, Vieira AT, Nicoli JR, Cunha FQ, Mantovani A, Reis LFL, Dias AAM, Teixeira MM. 2006. Dual function of the long pentraxin PTX3 in resistance against pulmonary infection with Klebsiella pneumoniae in transgenic mice. Microbes Infect. 8:1321–1329. 10.1016/j.micinf.2005.12.017 [DOI] [PubMed] [Google Scholar]
- 17.Jaillon S, Peri G, Delneste Y, Frémaux I, Doni A, Moalli F, Garlanda C, Romani L, Gascan H, Bellocchio S, Bozza S, Cassatella MA, Jeannin P, Mantovani A. 2007. The humoral pattern recognition receptor PTX3 is stored in neutrophil granules and localizes in extracellular traps. J. Exp. Med. 204:793–804. 10.1084/jem.20061301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moalli F, Doni A, Deban L, Zelante T, Zagarella S, Bottazzi B, Romani L, Mantovani A, Garlanda C. 2010. Role of complement and Fcγ receptors in the protective activity of the long pentraxin PTX3 against Aspergillus fumigatus. Blood 116:5170–5180. 10.1182/blood-2009-12-258376 [DOI] [PubMed] [Google Scholar]
- 19.Deban L, Jaillon S, Garlanda C, Bottazzi B, Mantovani A. 2011. Pentraxins in innate immunity: lessons from PTX3. Cell Tissue Res. 343:237–249. 10.1007/s00441-010-1018-0 [DOI] [PubMed] [Google Scholar]
- 20.Garlanda C, Hirsch E, Bozza S, Salustri A, De Acetis M, Nota R, Maccagno A, Riva F, Bottazzi B, Peri G, Doni A, Vago L, Botto M, De Santis R, Carminati P, Siracusa G, Altruda F, Vecchi A, Romani L, Mantovani A. 2002. Non-redundant role of the long pentraxin PTX3 in anti-fungal innate immune response. Nature 420:182–186. 10.1038/nature01195 [DOI] [PubMed] [Google Scholar]
- 21.Moalli F, Paroni M, Véliz Rodriguez T, Riva F, Polentarutti N, Bottazzi B, Valentino S, Mantero S, Nebuloni M, Mantovani A, Bragonzi A, Garlanda C. 2011. The therapeutic potential of the humoral pattern recognition molecule PTX3 in chronic lung infection caused by Pseudomonas aeruginosa. J. Immunol. 186:5425–5434. 10.4049/jimmunol.1002035 [DOI] [PubMed] [Google Scholar]
- 22.Paroni M, Moalli F, Nebuloni M, Pasqualini F, Bonfield T, Nonis A, Mantovani A, Garlanda C, Bragonzi A. 2013. Response of CFTR-deficient mice to long-term chronic Pseudomonas aeruginosa infection and PTX3 therapy. J. Infect. Dis. 208:130–138. 10.1093/infdis/jis636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gaziano R, Bozza S, Bellocchio S, Perruccio K, Montagnoli C, Pitzurra L, Salvatori G, De Santis R, Carminati P, Mantovani A, Romani L. 2004. Anti-Aspergillus fumigatus efficacy of pentraxin 3 alone and in combination with antifungals. Antimicrob. Agents Chemother. 48:4414–4421. 10.1128/AAC.48.11.4414-4421.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nauta AJ, Bottazzi B, Mantovani A, Salvatori G, Kishore U, Schwaeble WJ, Gingras AR, Tzima S, Vivanco F, Egido J, Tijsma O, Hack EC, Daha MR, Roos A. 2003. Biochemical and functional characterization of the interaction between pentraxin 3 and C1q. Eur. J. Immunol. 33:465–473. 10.1002/immu.200310022 [DOI] [PubMed] [Google Scholar]
- 25.Deban L, Russo RC, Sironi M, Moalli F, Scanziani M, Zambelli V, Cuccovillo I, Bastone A, Gobbi M, Valentino S, Doni A, Garlanda C, Danese S, Salvatori G, Sassano M, Evangelista V, Rossi B, Zenaro E, Constantin G, Laudanna C, Bottazzi B, Mantovani A. 2010. Regulation of leukocyte recruitment by the long pentraxin PTX3. Nat. Immunol. 11:328–334. 10.1038/ni.1854 [DOI] [PubMed] [Google Scholar]
- 26.Inforzato A, Peri G, Doni A, Garlanda C, Mantovani A, Bastone A, Carpentieri A, Amoresano A, Pucci P, Roos A, Daha MR, Vincenti S, Gallo G, Carminati P, De Santis R, Salvatori G. 2006. Structure and function of the long pentraxin PTX3 glycosidic moiety: fine-tuning of the interaction with C1q and complement activation. Biochemistry 45:11540–11551. 10.1021/bi0607453 [DOI] [PubMed] [Google Scholar]
- 27.Diniz SN, Nomizo R, Cisalpino PS, Teixeira MM, Brown GD, Mantovani A, Gordon S, Reis LFL, Dias AAM. 2004. PTX3 function as an opsonin for the dectin-1-dependent internalization of zymosan by macrophages. J. Leukoc. Biol. 75:649–656. 10.1189/jlb.0803371 [DOI] [PubMed] [Google Scholar]
- 28.Mantovani A, Garlanda C, Bottazzi B. 2003. Pentraxin 3, a non-redundant soluble pattern recognition receptor involved in innate immunity. Vaccine 21(Suppl 2):S43–S47. 10.1016/S0264-410X(03)00199-3 [DOI] [PubMed] [Google Scholar]
- 29.Mauri T, Bellani G, Patroniti N, Coppadoro A, Peri G, Cuccovillo I, Cugno M, Iapichino G, Gattinoni L, Pesenti A, Mantovani A. 2010. Persisting high levels of plasma pentraxin 3 over the first days after severe sepsis and septic shock onset are associated with mortality. Intensive Care Med. 36:621–629. 10.1007/s00134-010-1752-5 [DOI] [PubMed] [Google Scholar]
- 30.Sprong T, Peri G, Neeleman C, Mantovani A, Signorini S, van der Meer JWM, van Deuren M. 2009. Pentraxin 3 and C-reactive protein in severe Meningococcal disease. Shock 31:28–32. 10.1097/SHK.0b013e31817fd543 [DOI] [PubMed] [Google Scholar]
- 31.Uusitalo-Seppälä R, Huttunen R, Aittoniemi J, Koskinen P, Leino A, Vahlberg T, Rintala EM. 2013. Pentraxin 3 (PTX3) is associated with severe sepsis and fatal disease in emergency room patients with suspected infection: a prospective cohort study. PLoS One 8:e53661. 10.1371/journal.pone.0053661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wagenaar JFP, Goris MGA, Gasem MH, Isbandrio B, Moalli F, Mantovani A, Boer KR, Hartskeerl RA, Garlanda C, van Gorp ECM. 2009. Long pentraxin PTX3 is associated with mortality and disease severity in severe leptospirosis. J. Infect. 58:425–432. 10.1016/j.jinf.2009.04.004 [DOI] [PubMed] [Google Scholar]
- 33.Huttunen R, Hurme M, Aittoniemi J, Huhtala H, Vuento R, Laine J, Jylhävä J, Syrjänen J. 2011. High plasma level of long pentraxin 3 (PTX3) is associated with fatal disease in bacteremic patients: a prospective cohort study. PLoS One 6:e17653. 10.1371/journal.pone.0017653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vänskä M, Koivula I, Hämäläinen S, Pulkki K, Nousiainen T, Jantunen E, Juutilainen A. 2011. High pentraxin 3 level predicts septic shock and bacteremia at the onset of febrile neutropenia after intensive chemotherapy of hematologic patients. Haematologica 96:1385–1389. 10.3324/haematol.2011.044925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Muller B, Peri G, Doni A, Torri V, Landmann R, Bottazzi B, Mantovani A. 2001. Circulating levels of the long pentraxin PTX3 correlate with severity of infection in critically ill patients. Crit. Care Med. 29:1404–1407. 10.1097/00003246-200107000-00017 [DOI] [PubMed] [Google Scholar]
- 36.Napoleone E, Di Santo A, Peri G, Mantovani A, de Gaetano G, Donati MB, Lorenzet R. 2004. The long pentraxin PTX3 up-regulates tissue factor in activated monocytes: another link between inflammation and clotting activation. J. Leukoc. Biol. 76:203–209. 10.1189/jlb.1003528 [DOI] [PubMed] [Google Scholar]
- 37.Napoleone E, Di Santo A, Bastone A, Peri G, Mantovani A, de Gaetano G, Donati MB, Lorenzet R. 2002. Long pentraxin PTX3 upregulates tissue factor expression in human endothelial cells: a novel link between vascular inflammation and clotting activation. Arterioscler. Thromb. Vasc. Biol. 22:782–787. 10.1161/01.ATV.0000012282.39306.64 [DOI] [PubMed] [Google Scholar]
- 38.Alles V, Bottazzi B, Peri G, Golay J, Introna M, Mantovani A. 1994. Inducible expression of PTX3, a new member of the pentraxin family, in human mononuclear phagocytes. Blood 84:3483–3493 [PubMed] [Google Scholar]
- 39.Manfredi AA, Rovere-Querini P, Bottazzi B, Garlanda C, Mantovani A. 2008. Pentraxins, humoral innate immunity and tissue injury. Curr. Opin. Immunol. 20:538–544. 10.1016/j.coi.2008.05.004 [DOI] [PubMed] [Google Scholar]
- 40.Dalle Lucca JJ, Li Y, Simovic MO, Slack JL, Cap A, Falabella MJ, Dubick M, Lebeda F, Tsokos GC. 2013. Decay-accelerating factor limits hemorrhage-instigated tissue injury and improves resuscitation clinical parameters. J. Surg. Res. 179:153–167. 10.1016/j.jss.2012.10.017 [DOI] [PubMed] [Google Scholar]
- 41.Dann SM, Spehlmann ME, Hammond DC, Iimura M, Hase K, Choi LJ, Hanson E, Eckmann L. 2008. IL-6-dependent mucosal protection prevents establishment of a microbial niche for attaching/effacing lesion-forming enteric bacterial pathogens. J. Immunol. 180:6816–6826. 10.4049/jimmunol.180.10.6816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maugeri N, Rovere-Querini P, Slavich M, Coppi G, Doni A, Bottazzi B, Garlanda C, Cianflone D, Maseri A, Mantovani A, Manfredi AA. 2011. Early and transient release of leukocyte pentraxin 3 during acute myocardial infarction. J. Immunol. 187:970–979. 10.4049/jimmunol.1100261 [DOI] [PubMed] [Google Scholar]
- 43.López-Rojas R, Docobo-Pérez F, Pachón-Ibáñez M, de la Torre B, Fernández-Reyes M, March C, Bengoechea J, Andreu D, Rivas L, Pachón J. 2011. Efficacy of cecropin A-melittin peptides on a sepsis model of infection by pan-resistant Acinetobacter baumannii. Eur. J. Clin. Microbiol. Infect. Dis 30:1391–1398. 10.1007/s10096-011-1233-y [DOI] [PubMed] [Google Scholar]
- 44.McConnell MJ, Domínguez-Herrera J, Smani Y, López-Rojas R, Docobo-Pérez F, Pachón J. 2011. Vaccination with outer membrane complexes elicits rapid protective immunity to multidrug-resistant Acinetobacter baumannii. Infect. Immun. 79:518–526. 10.1128/IAI.00741-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moffatt JH, Harper M, Mansell A, Crane B, Fitzsimons TC, Nation RL, Li J, Adler B, Boyce JD. 2013. Lipopolysaccharide-deficient Acinetobacter baumannii shows altered signaling through host toll-like receptors and increased susceptibility to the host antimicrobial peptide LL-37. Infect. Immun. 81:684–689. 10.1128/IAI.01362-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Siddiqi M, Garcia ZC, Stein DS, Denny TN, Spolarics Z. 2001. Relationship between oxidative burst activity and CD11b expression in neutrophils and monocytes from healthy individuals: effects of race and gender. Cytometry 46:243–246. 10.1002/cyto.1134 [DOI] [PubMed] [Google Scholar]
- 47.Hou J, Fu Y, Zhou J, Li W, Xie R, Cao F, Gilbert GE, Shi J. 2011. Lactadherin functions as a probe for phosphatidylserine exposure and as an anticoagulant in the study of stored platelets. Vox Sang. 100:187–195. 10.1111/j.1423-0410.2010.01375.x [DOI] [PubMed] [Google Scholar]
- 48.Sivula M, Pettilä V, Niemi TT, Varpula M, Kuitunen AH. 2009. Thromboelastometry in patients with severe sepsis and disseminated intravascular coagulation. Blood Coagul. Fibrinolysis 20:419–426. 10.1097/MBC.0b013e32832a76e1 [DOI] [PubMed] [Google Scholar]
- 49.Kang G, Hartzell JD, Howard R, Wood-Morris RN, Johnson MD, Fraser S, Weintrob A, Wortmann G. 2010. Mortality associated with Acinetobacter baumannii complex bacteremia among patients with war-related trauma. Infect. Control Hosp. Epidemiol. 31:92–94. 10.1086/649220 [DOI] [PubMed] [Google Scholar]
- 50.Hospenthal DR, Crouch HK, English JF, Leach F, Pool J, Conger NG, Whitman TJ, Wortmann GW, Robertson JL, Murray CK. 2011. Multidrug-resistant bacterial colonization of combat-injured personnel at admission to medical centers after evacuation from Afghanistan and Iraq. J. Trauma 71(Suppl 1):S52–S57. 10.1097/TA.0b013e31822118fb [DOI] [PubMed] [Google Scholar]
- 51.Tribble DR, Conger NG, Fraser S, Gleeson TD, Wilkins K, Antonille T, Weintrob A, Ganesan A, Gaskins LJ, Li P, Grandits G, Landrum ML, Hospenthal DR, Millar EV, Blackbourne LH, Dunne JR, Craft D, Mende K, Wortmann GW, Herlihy R, McDonald J, Murray CK. 2011. Infection-associated clinical outcomes in hospitalized medical evacuees after traumatic injury: trauma infectious disease outcome study. J. Trauma 71:S33–S42. 10.1097/TA.0b013e318221162e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Albrecht MA, Griffith ME, Murray CK, Chung KK, Horvath EE, Ward JA, Hospenthal DR, Holcomb JB, Wolf SE. 2006. Impact of Acinetobacter infection on the mortality of burn patients. J. Am. Coll. Surg. 203:546–550. 10.1016/j.jamcollsurg.2006.06.013 [DOI] [PubMed] [Google Scholar]
- 53.Cisneros JM, Rodríguez-Baño J. 2002. Nosocomial bacteremia due to Acinetobacter baumannii: epidemiology, clinical features and treatment. Clin. Microbiol. Infect. 8:687–693. 10.1046/j.1469-0691.2002.00487.x [DOI] [PubMed] [Google Scholar]
- 54.Corbella X, Pujol M, Ayats J, Sendra M, Ardanuy C, Domínguez MA, Liñares J, Ariza J, Gudiol F. 1996. Relevance of digestive tract colonization in the epidemiology of nosocomial infections due to multiresistant Acinetobacter baumannii. Clin. Infect. Dis. 23:329–334. 10.1093/clinids/23.2.329 [DOI] [PubMed] [Google Scholar]
- 55.Dy ME, Nord JA, LaBombardi VJ, Kislak JW. 1999. The emergence of resistant strains of Acinetobacter baumannii: clinical and infection control implications. Infect. Control Hosp. Epidemiol. 20:565–567. 10.1086/501673 [DOI] [PubMed] [Google Scholar]
- 56.Houang ET, Sormunen RT, Lai L, Chan CY, Leong AS. 1998. Effect of desiccation on the ultrastructural appearances of Acinetobacter baumannii and Acinetobacter lwoffii. J. Clin. Pathol. 51:786–788. 10.1136/jcp.51.10.786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jawad A, Seifert H, Snelling AM, Heritage J, Hawkey PM. 1998. Survival of Acinetobacter baumannii on dry surfaces: comparison of outbreak and sporadic isolates. J. Clin. Microbiol. 36:1938–1941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Koeleman JG, van der Bijl MW, Stoof J, Vandenbroucke-Grauls CM, Savelkoul PH. 2001. Antibiotic resistance is a major risk factor for epidemic behavior of Acinetobacter baumannii. Infect. Control Hosp. Epidemiol. 22:284–288. 10.1086/501901 [DOI] [PubMed] [Google Scholar]
- 59.Peleg AY, Seifert H, Paterson DL. 2008. Acinetobacter baumannii: emergence of a successful pathogen. Clin. Microbiol. Rev. 21:538–582. 10.1128/CMR.00058-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Simor AE, Lee MM, Vearncombe M, Jones-Paul L, Barry C, Gomez M, Fish JS, Cartotto RC, Palmer R, Louie M. 2002. An outbreak due to multiresistant Acinetobacter baumannii in a burn unit: risk factors for acquisition and management. Infect. Control Hosp. Epidemiol. 23:261–267. 10.1086/502046 [DOI] [PubMed] [Google Scholar]
- 61.Thom KA, Hsiao WWL, Harris AD, Stine OC, Rasko DA, Johnson JK. 2010. Patients with Acinetobacter baumannii bloodstream infections are colonized in the gastrointestinal tract with identical strains. Am. J. Infect. Control 38:751–753. 10.1016/j.ajic.2010.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Timsit J-F, Garrait V, Misset B, Goldstein FW, Renaud B, Carlet J. 1993. The digestive tract is a major site for Acinetobacter baumannii colonization in intensive care unit patients. J. Infect. Dis. 168:1336–1337. 10.1093/infdis/168.5.1336 [DOI] [PubMed] [Google Scholar]
- 63.Bottazzi B, Doni A, Garlanda C, Mantovani A. 2010. An integrated view of humoral innate immunity: pentraxins as a paradigm. Annu. Rev. Immunol. 28:157–183. 10.1146/annurev-immunol-030409-101305 [DOI] [PubMed] [Google Scholar]
- 64.Deban L, Bottazzi B, Garlanda C, de la Torre YM, Mantovani A. 2009. Pentraxins: multifunctional proteins at the interface of innate immunity and inflammation. BioFactors 35:138–145. 10.1002/biof.21 [DOI] [PubMed] [Google Scholar]
- 65.He X, Han B, Liu M. 2007. Long pentraxin 3 in pulmonary infection and acute lung injury. Am. J. Physiol Lung Cell. Mol. Physiol. 292:L1039–L1049. 10.1152/ajplung.00490.2006 [DOI] [PubMed] [Google Scholar]
- 66.Ortega-Hernandez O-D, Bassi N, Shoenfeld Y, Anaya J-M. 2009. The long pentraxin 3 and its role in autoimmunity. Semin. Arthritis Rheum. 39:38–54. 10.1016/j.semarthrit.2008.03.006 [DOI] [PubMed] [Google Scholar]
- 67.May L, Kuningas M, van Bodegom D, Meij HJ, Frolich M, Slagboom PE, Mantovani A, Westendorp RGJ. 2010. Genetic variation in pentraxin (PTX) 3 gene associates with PTX3 production and fertility in women. Biol. Reprod. 82:299–304. 10.1095/biolreprod.109.079111 [DOI] [PubMed] [Google Scholar]
- 68.Bottazzi B, Bastone A, Doni A, Garlanda C, Valentino S, Deban L, Maina V, Cotena A, Moalli F, Vago L, Salustri A, Romani L, Mantovani A. 2006. The long pentraxin PTX3 as a link among innate immunity, inflammation, and female fertility. J. Leukoc. Biol. 79:909–912. 10.1189/jlb.1005557 [DOI] [PubMed] [Google Scholar]
- 69.Gullo J, Bertotti M, Silva C, Schwarzbold M, Diaz A, Soares F, Freitas F, Nunes J, Pinheiro J, Morato E, Prediger R, Linhares M, Walz R. 2011. Hospital mortality of patients with severe traumatic brain injury is associated with serum PTX3 levels. Neurocrit. Care 14:194–199. 10.1007/s12028-010-9462-y [DOI] [PubMed] [Google Scholar]