Abstract
Salmonella enterica serovar Typhimurium is a Gram-negative food-borne pathogen that is a major cause of acute gastroenteritis in humans. The ability of the host to control such bacterial pathogens may be influenced by host immune status and by concurrent infections. Helminth parasites are of particular interest in this context because of their ability to modulate host immune responses and because their geographic distribution coincides with those parts of the world where infectious gastroenteritis is most problematic. To test the hypothesis that helminth infection may negatively regulate host mucosal innate immunity against bacterial enteropathogens, a murine coinfection model was established by using the intestinal nematode Heligmosomoides polygyrus and S. Typhimurium. We found that mice coinfected with S. Typhimurium and H. polygyrus developed more severe intestinal inflammation than animals infected with S. Typhimurium alone. The enhanced susceptibility to Salmonella-induced intestinal injury in coinfected mice was found to be associated with diminished neutrophil recruitment to the site of bacterial infection that correlated with decreased expression of the chemoattractants CXCL2/macrophage inflammatory protein 2 (MIP-2) and CXCL1/keratinocyte-derived chemokine (KC), poor control of bacterial replication, and exacerbated intestinal inflammation. The mechanism of helminth-induced inhibition of MIP-2 and KC expression involved interleukin-10 (IL-10) and, to a lesser extent, IL-4 and IL-13. Ly6G antibody-mediated depletion of neutrophils reproduced the adverse effects of H. polygyrus on Salmonella infection. Our results suggest that impaired neutrophil recruitment is an important contributor to the enhanced severity of Salmonella enterocolitis associated with helminth coinfection.
INTRODUCTION
Salmonella enterica serovar Typhimurium is a Gram-negative food-borne pathogen that is frequently associated with disease in various host species, including humans, livestock, domestic fowl, and rodents (1). In humans, S. Typhimurium infection, which is typically transmitted via contaminated food or water, results in a diarrheal illness that is responsible for approximately 40,000 cases of acute gastroenteritis annually in the United States (1). To establish infection in the host, this bacterial pathogen must exit the intestinal lumen by crossing the epithelial barrier. During this process, the innate immune system can be activated through recognition of pathogen-associated molecular patterns by evolutionarily conserved pattern recognition receptors such as the Toll-like receptors (TLRs) on intestinal epithelial and subepithelial cells (2). Activation of TLRs initiates a rapid response to infection, including the secretion of chemoattractant molecules and cytokines such as tumor necrosis factor alpha (TNF-α), keratinocyte-derived chemokine (KC), and macrophage inflammatory protein 2 (MIP-2) (3). The local production of these molecules leads to the recruitment of various cell populations, including neutrophils, monocytes, dendritic cells, and lymphocytes (4–8) and the consequent control of bacterial infection. A more detailed understanding of this response, as well as the factors that influence it, will provide insights into the pathogenesis of Salmonella gastroenteritis and may suggest new approaches to preventing and treating this important public health problem.
The ability of the host to control bacterial pathogens may be influenced by host immune status and by concurrent infections. Helminth parasites are of particular interest in this context because of their ability to modulate host immune responses and because their geographic distribution coincides with those parts of the world where infectious gastroenteritis is most problematic. Parasitic infections are common in countries with poor hygienic conditions, where a lack of sanitation and health care facilitates the transmission and spread of helminths like Ascaris lumbricoides, Schistosoma spp., hookworms, and protozoan-like Entamoeba histolytica/dispar (9). Coinfection of individual hosts by multiple pathogens can be very commonly observed under such circumstances. The major importance of helminth infections includes not only the direct pathogenic effect of the worms but also the modulatory role of the parasite on the host immune system, which may alter the response to other antigens and cause additional immunopathology (10, 11). Although much is known about the potential role of helminth-stimulated T cells, typically Th2 and Treg, in altering host protection against the bacterial infection, the impact of helminth infection on the innate immune response to enteric bacterial pathogens is less well understood. To shed light on this issue, we determined whether and how an ongoing intestinal helminth parasite infection influences the early phase of the host innate immune response against S. Typhimurium. To investigate this issue, we used the well-established murine model of Salmonella infection, an experimental system that can be used for the analysis of intestinal inflammation, as well as systemic bacterial dissemination.
MATERIALS AND METHODS
Mice.
Six- to eight-week-old female C57BL/6 mice were purchased from The Jackson Laboratory (Bar Harbor, ME), fed autoclaved food and water, and maintained in a specific-pathogen-free facility at Massachusetts General Hospital. Animal care was provided in accordance with protocols approved by the Institutional Animal Care and Use Committee of Massachusetts General Hospital.
Heligmosomoides polygyrus and S. Typhimurium infection.
H. polygyrus was propagated as previously described and stored at 4°C until use (12). Mice were inoculated orally with 200 third-stage larvae. At 2 weeks after parasitic infection, subsets of H. polygyrus-infected and uninfected mice were infected orally with 108 CFU of a naturally streptomycin-resistant SL1344 strain of S. Typhimurium. The mice were sacrificed 48 h after infection. The bacterial loads in the spleen and liver were determined. To study whether and how helminth coinfection affects the early mucosal innate immune and inflammatory responses, we utilized the streptomycin pretreatment model of Salmonella infection, a model that has been widely used in the field to analyze Salmonella-induced intestinal inflammation (13). It was shown previously that pretreating mice with a single oral dose of streptomycin, followed 24 h later by oral infection with a naturally streptomycin-resistant strain of S. Typhimurium (SL1344) allows colonization of the cecum to high numbers and the resultant development of an acute inflammatory response in the intestine (13). In our study, subsets of H. polygyrus-infected (2 weeks postinfection) and uninfected mice were each given 20 mg of streptomycin in phosphate-buffered saline (PBS) by gavage with a 21-gauge feeding needle. After 24 h, the mice were infected orally with 108 CFU of S. Typhimurium SL1344 strain. The mice were sacrificed 48 h after infection. Intestinal inflammation was assessed at necropsy based on gross appearance and histopathology.
Worm burden.
At necropsy, small intestines were removed and opened. The worm burden was determined by counting the total number of worms present in the lumen of the intestine as visualized under a dissecting microscope.
Histopathological examinations.
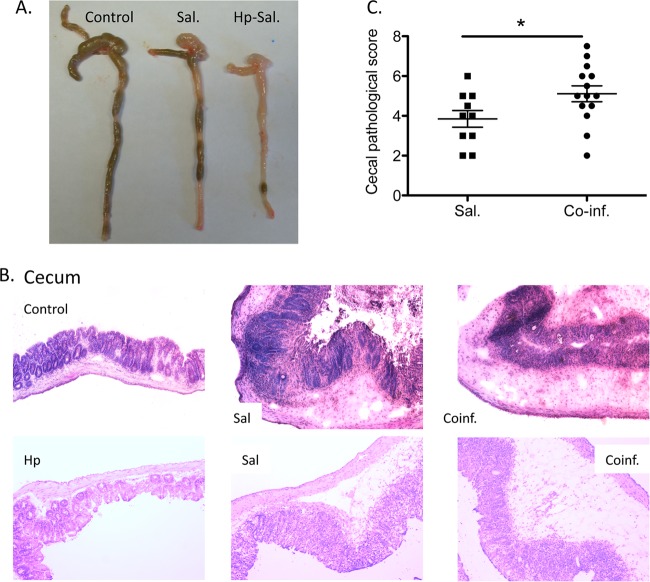
At necropsy, cecum and colonic tissues were collected, frozen in Tissue Tek OCT compound (Miles, Inc., Elkhart, IN), and then stored at −80°C. Then, 5-μm sections were cut on a Leica CM1850 Cryostat (Leica Biosystems) and were stained with hematoxylin and eosin. Intestinal pathology was scored using a modified histology scoring system based on previously published methods (14, 15). The scoring system consists of two parts. Part 1 is the determination of the infiltration of inflammatory cells in the tissue, with scores ranging from 0 to 4 (0, normal cell pattern; 1, scattered inflammatory cells in the lamina propria; 2, increased numbers of inflammatory cells in the lamina propria; 3, confluence of inflammatory cells extending into the submucosa; and 4, transmural extension of the infiltrative inflammatory cells). Part 2 is the evaluation of the tissue damage, with scores that also range from 0 to 4 (0, normal tissue pattern; 1, minimal inflammation and cecum crypt hyperplasia; 2, mild crypt hyperplasia with or without focal invasion of epithelium; 3, obvious cecum crypt hyperplasia and invasion of epithelium; and 4, extensive mucosal damage and extension through deeper structures of the bowel wall). The total tissue pathology score equals the inflammatory cell score plus the tissue damage score (Fig. 1C).
FIG 1.
Helminth coinfection exacerbates Salmonella-induced mucosal injury and mortality in mice. C57BL/6 mice were infected with H. polygyrus (200 L3), treated with streptomycin, and inoculated with 108 CFU of S. Typhimurium (SL1344 strain) 7 days later. (A) Macroscopic examination of cecum and colon tissues of mice from different groups. (B) Ceca were removed from uninfected mice or from mice infected with H. polygyrus, Salmonella, or both at 48 h after bacterial infection and frozen in Tissue Tek OCT compound, and the sections were stained with hematoxylin and eosin. Magnification, ×100. Duplicate samples are presented from the Salmonella-infected and coinfected groups. (C) Histopathological score of cecal inflammation in mice infected with Salmonella or both. The scores were assessed by determination of infiltration of inflammatory cells (score range, 0 to 4), together with the evaluation of cecal tissue damage (score range, 0 to 4). The data shown are pooled from three independent experiments with total (n = 9 to 15 per group). *, P < 0.05.
Immunofluorescence microscopy.
Tissue sections were fixed in ice-cold acetone, washed, and then blocked with avidin/biotin agent (Vector Laboratories). To examine intestinal polymorphonuclear neutrophils (PMNs), the sections were stained with fluorescein isothiocyanate (FITC)-labeled anti-mouse Mac1 (CD11b; BD Pharmingen) or Cy3-labeled anti-mouse GR1 (BD Pharmingen). Sections were analyzed by immunofluorescence microscopy (16).
Determination of Salmonella translocation.
To examine whether coinfection with helminth parasites resulted in enhanced Salmonella translocation into both the mucosal and the systemic compartments, mice were infected with H. polygyrus, and 2 weeks later they were infected with DS Red-expressing S. Typhimurium (following streptomycin pretreatment). Separate groups of mice were infected with DS Red-expressing salmonellae only or not infected. The mice were sacrificed at 24 h after bacterial infection. The appearance and distribution of DS Red-Salmonella was examined using immunofluorescence microscopy. To further determine the impact of helminth coinfection on tissue bacterial loads, spleens and livers were collected from Salmonella-infected and helminth-coinfected groups (with or without streptomycin treatment), homogenized and plated on Luria-Bertani (LB) plates. The CFU were determined.
Intraperitoneal infection of mice.
To study the impact of helminth infection on the recruitment of neutrophils, both H. polygyrus-infected (at 2 weeks postinfection) and uninfected mice were injected intraperitoneally (i.p.) with approximately 5,000 CFU of S. Typhimurium. Mice were sacrificed 24 h later. Cells from peripheral blood, spleen, mesenteric lymph nodes (MLN), and peritoneal cavity were collected, stained and used for fluorescence-activated cell sorting (FACS) analysis. The GR1+ neutrophil population from different groups was compared.
Depletion of GR1+ neutrophils in vivo.
Mice were treated with Ly6G-specific monoclonal antibody (MAb) (17), anti-mLy6G (BioXCell, West Lebanon, NH), or isotype control (rat IgG2b; BioXCell) on 1 day before, on the same day, and 1 day after Salmonella infection (300 μg/mouse) i.p. Both Ly6G-specific MAb-treated and untreated mice were infected with Salmonella and sacrificed 48 h after the bacterial infection. The fecal bacterial load was determined and compared. Neutrophil depletion was confirmed by FACS.
FACS analysis.
Cellular populations of various compartments (i.e., the peripheral blood, spleen, MLN, and peritoneal cavity) from different groups were isolated after bacterial infection. In each of the tissue preparations the neutrophils were identified with anti-GR1 MAb, macrophages were identified with anti-CD11b or F4/80 antibodies. The stained samples were analyzed with an Accuri C6 FACS machine. Dead cells and debris were excluded from analysis by gates set on forward and side angle light scatter.
Quantitative detection of chemokine expression in cecal tissues.
Total RNA was prepared from cecal tissue using TRIzol reagent (Invitrogen Life Technologies) according to the manufacturer's recommendations and reverse transcribed into cDNA using the Superscript first-strand synthesis system (Invitrogen Life Technologies). The cDNA samples were then tested for the expression of cytokines (interleukin-22 [IL-22], IL-23-p19, IL-17, IL-10, gamma interferon [IFN-γ], and TNF-α), chemoattractants (KC and MIP-2), and antimicrobial peptides (RegIIIβ and RegIIIγ) by real-time quantitative reverse transcription-PCR (RT-PCR) using SYBR green PCR master mix (Applied Biosystems) on a StepOne Plus real-time PCR system (Applied Biosystems). Samples were run in triplicate. GAPDH was used as the housekeeping control. The sequences for the sense and antisense primers used to quantify mRNA are listed in Table 1.
TABLE 1.
Primers used in this study
| Primer | Orientation | Sequence (5′–3′) |
|---|---|---|
| GAPDH | F | TGGAATCCTGTGGCATCCATGAAAC |
| R | TAAAACGCAGCTCAGTAACAGTCCG | |
| IFN-γ | F | TCAAGTGGCATAGATGTGGAAGAA |
| R | TGGCTCTGCAGGATTTTCATG | |
| IL-10 | F | CCACAAAGCAGCCTTGCA |
| R | AGTAAGAGCAGGCAGCATAGCA | |
| IL-22 | F | TCCGAGGAGTCAGTGCTAAA |
| R | AGAACGTCTTCCAGGGTGAA | |
| IL-23p19 | F | TGCTGGATTGCAGAGCAGTAA |
| R | GCATGCAGAATTCCGAAGA | |
| IL-17 | F | CCACGTCACCCTGGACTCTC |
| R | CTCCGCATTGACACAGCG | |
| KC | F | CCGAAGTCATAGCCACACTCAA |
| R | GCAGTCTGTCTTCTTTCTCCGTTAC | |
| MIP-2 | F | CCAACCACCAGGCTACAGG |
| R | GCGTCACACTCAAGCTCTG | |
| TNF-α | F | CCCTCACACTCAGATCATCTTCT |
| R | GCTACGACGTGGGCTACAG | |
| RegIIIγ | F | TTCCTGTCCTCCATGATCAAAA |
| R | CATCCACCTCTGTTGGGTTCA | |
| RegIIIβ | F | ATGGCTCCTACTGCTATGCC |
| R | GTGTCCTCCAGGCCTCTTT |
In vitro stimulation of peritoneal macrophages.
To demonstrate the impact of the helminth-associated Th2 response on the bacterium-induced chemokine expression by innate immune cells, peritoneal macrophages were isolated from normal and helminth-infected mice (14 to 21 days postinfection) by incubating total peritoneal cells in complete Dulbecco modified Eagle medium at 37°C for 2 h. Nonadherent cells were removed by washing with PBS, and the adherent macrophages were treated with rIL-4/rIL-3 (3 ng/ml, for cells from normal mice) or with anti-IL-10 (20 μg/ml) or anti-IL-4/IL-13 (20 μg/ml, for cells derived from helminth-infected hosts) for 24 h before Salmonella infection (cell/bacterium ratio, 1:2). After 1 h, RNA was isolated tested for the expression of KC, MIP-2, TNF-α, and IL-10 by real-time RT-PCR.
Statistical analysis.
All results are expressed as means and standard errors of the mean (SEM). “N” refers to the number of mice used. Statistical differences were determined by using one-way analysis of variance (ANOVA) test (Tukey's multiple-comparison test) with GraphPad Prism or a two-tailed Student t test with StatView software (Abacus Concepts, Berkeley, CA) when the differences were tested between two groups (histology score, fecal bacterial loads, and neutrophil infiltration in Salmonella-infected and coinfected groups). To compare gene expression of cells that were isolated from individual mouse before and after in vitro cytokine treatment, a paired t test was used. A P value of <0.05 was considered significant.
RESULTS
Helminth coinfection exacerbates Salmonella-induced mucosal injury in mice.
Salmonella infection causes acute gastroenteritis (4, 13). To examine whether an intestinal helminth infection can affect the susceptibility of the host to Salmonella infection, wild-type C57BL/6 mice were infected with H. polygyrus and inoculated with Salmonella 2 weeks later. As expected, mice infected only with Salmonella showed signs of disease, such as soft stool, a hunched posture, disturbed body hair, and body weight loss soon after bacterial inoculation. A significant impact of H. polygyrus on mice with Salmonella infection was demonstrated by a 20 to 33% mortality in coinfected animals that occurred 24 to 48 h after the bacterial infection, compared to a 10% mortality in mice infected with Salmonella alone (data not shown). At necropsy, the ceca and colons from the different groups (Salmonella alone, H. polygyrus alone, coinfection, and noninfected controls) were examined both macroscopically and microscopically. Salmonella infection induced thickening and shortening of the ceca (Fig. 1A), in agreement with previous reports (13, 14). In the coinfected mice, these changes were more pronounced and also extended to the colon (Fig. 1A).
In mice infected with Salmonella alone, histological examination of the cecum showed typical pathological changes, including thickening of the wall of the cecum, edema, disruption of epithelial architecture and marked infiltration by PMNs (Fig. 1B). The ceca of coinfected mice showed more severe pathology, with increased epithelial erosions, thickening of the gut wall, and more pronounced edema of the intestinal tissue (Fig. 1B). Moreover, the enhanced intestinal tissue injury extended to the colon tissue in mice with helminth coinfection (data not shown), whereas prominent gut inflammation was restricted to the cecal tissue in mice with Salmonella infection alone (Fig. 1A and B). The pathology scores for inflammation and intestinal damage were significantly higher in coinfected mice than in mice with Salmonella infection alone (Fig. 1C). These results demonstrate that concurrent intestinal helminth infection promotes Salmonella-induced intestinal injury, leading to the development of more severe intestinal inflammation.
Helminth infection results in increased tissue Salmonella numbers.
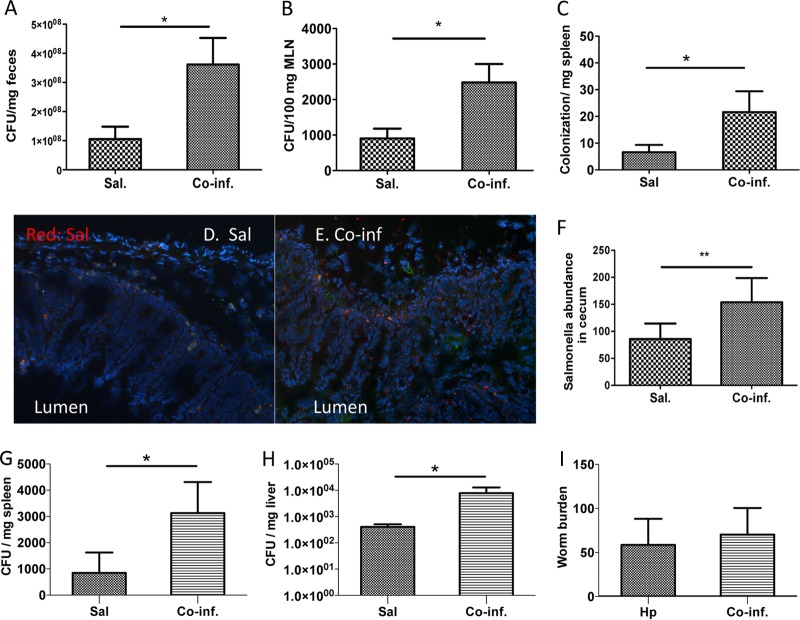
We questioned whether the more severe intestinal inflammation in the mice coinfected with H. polygyrus and Salmonella was related to poor control of bacterial replication. H. polygyrus-infected and uninfected mice were inoculated with S. Typhimurium after streptomycin pretreatment and sacrificed 48 h later. There was a significant increase in fecal bacterial output from the helminth coinfected mice compared to the mice infected with Salmonella alone (Fig. 2A). At the peak of inflammation (48 h after Salmonella-infection), we collected the MLN and spleens. The tissues were homogenized and plated on LB plates. The bacterial load in the MLN (Fig. 2B), as well as the spleen (Fig. 2C), was found to be significantly higher in mice with helminth coinfection. Furthermore, immunofluorescence microscopic analysis of cecal tissues revealed increased number of Salmonella in the lamina propriae of coinfected mice (Fig. 2D and E). To further examine the impact of helminth on Salmonella translocation after oral infection, we infected helminth-infected and uninfected mice with Salmonella in the absence of streptomycin treatment and then determined the bacterial loads in the spleen and livers. Our data showed that the bacterial loads in both the spleen and the liver was significantly higher in mice with helminth coinfections (Fig. 2G and H) compared to mice infected with Salmonella alone. The numbers of worms that were recovered at 3 weeks after helminth infection did not differ significantly between mice infected with H. polygyrus alone and mice coinfected with Salmonella (Fig. 2I).
FIG 2.
Helminth infection results in enhanced Salmonella output in the fecal pellets and results in increased tissue Salmonella numbers. (A) Numbers of bacteria recovered from fecal samples of Salmonella-infected and coinfected mice at 48 h postinfection. The data shown are represented as means ± the SEM (n = 6 to 7 mice). *, P < 0.05. (B and C) Numbers of bacteria recovered from MLN (B) and spleen (C) homogenates of Salmonella-infected and coinfected mice at 48 h postinfection. The data shown are represented as the means ± the SEM of the bacterial load in the MLN (n = 5 to 8). *, P < 0.05. (D and E) Immunofluorescence microscopy data show the bacteria (in red) in cecal tissues of Salmonella-infected (D) and H. polygyrus-coinfected (E) mice. (F) The number of bacteria (in red) detected per high-power field (×100) by counting five fields from each sample (three mice from each group were counted). **, P < 0.01. (G and H) Numbers of bacteria recovered from spleen (G) and liver (H) homogenates of Salmonella-infected and coinfected mice at 48 h postinfection. The data shown are represented as the mean ± the SEM of the bacterial load in the spleen and liver (n = 5 to 8). (I) Worm burden recovered at 3 weeks postinfection. *, P < 0.05.
Helminth coinfection negatively regulates the IL-23/IL-17 response to Salmonella infection of the cecum.
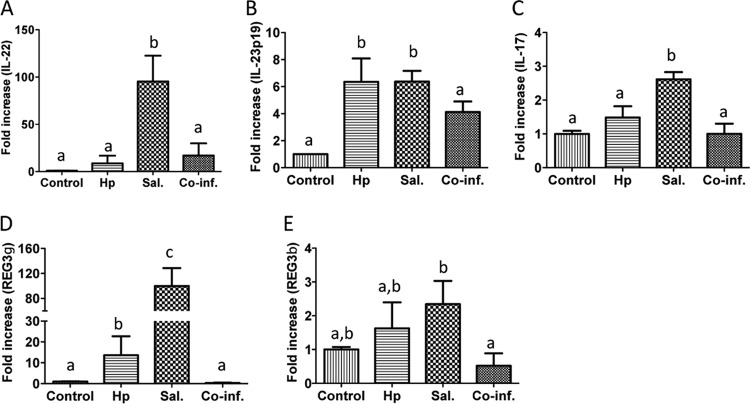
It has been demonstrated that IL-23 plays an important role in the induction of intestinal mucosal IL-17 and IL-22 responses to S. Typhimurium infection (18). IL-17 and IL-22 amplify inflammatory responses and increase the expression of epithelial antimicrobial peptides (18–20). To investigate the impact of helminth coinfection on the Salmonella-induced intestinal cytokine response, we measured the expression of IL-23, IL-22, and IL-17 in the ceca of various groups of mice using quantitative real-time RT-PCR. Our results show that Salmonella infection induced a significant upregulation of all three of these cytokines in the cecal tissue (Fig. 3A to C). Helminth coinfection induced a marked reduction of Salmonella-associated expression of these cytokines in the cecal tissue (Fig. 3). These data demonstrate that intestinal helminth parasites can negatively regulate host intestinal mucosal IL-22, IL-23, and IL-17 responses to enteric bacterial pathogens.
FIG 3.
Coinfection with H. polygyrus results in downregulation of Salmonella-induced cecal cytokines and antimicrobial peptides. Cecal tissues were collected from control, H. polygyrus-infected, Salmonella-infected, and helminth-coinfected mice (48 h after Salmonella infection). Total RNA was isolated from the cecal tissues. IL-22, IL-23, IL-17, Reg3γ, and Reg3β expression was determined using quantitative RT-PCR. Values are the fold increase compared to the baseline obtained from uninfected mice. The data shown are means ± the SEM (n = 5 mice/group) from one of three experiments performed showing similar results. Different letters indicate the significant differences among groups, based on one-way ANOVA. *, P < 0.05.
To further examine the impact of helminth coinfection on the mucosal innate immune response to Salmonella, we examined the expression of the antimicrobial peptides “regenerating islet-derived 3γ” (Reg3γ) and Reg3β, which are induced by IL-17 and IL-22. Recent reports have demonstrated the potential protective role for Reg3 γ (21) and Reg3β (15) in host protection against Salmonella infection in mice. Our results show that Salmonella-infection induces the expression of both Reg3γ and, to a lesser extent, Reg3β in cecal tissue (Fig. 3D and E). In contrast, the cecal tissues from the coinfected mice displayed a clear downregulation of Reg3γ and Reg3β (Fig. 3D and E).
Helminth coinfection impairs neutrophil recruitment.
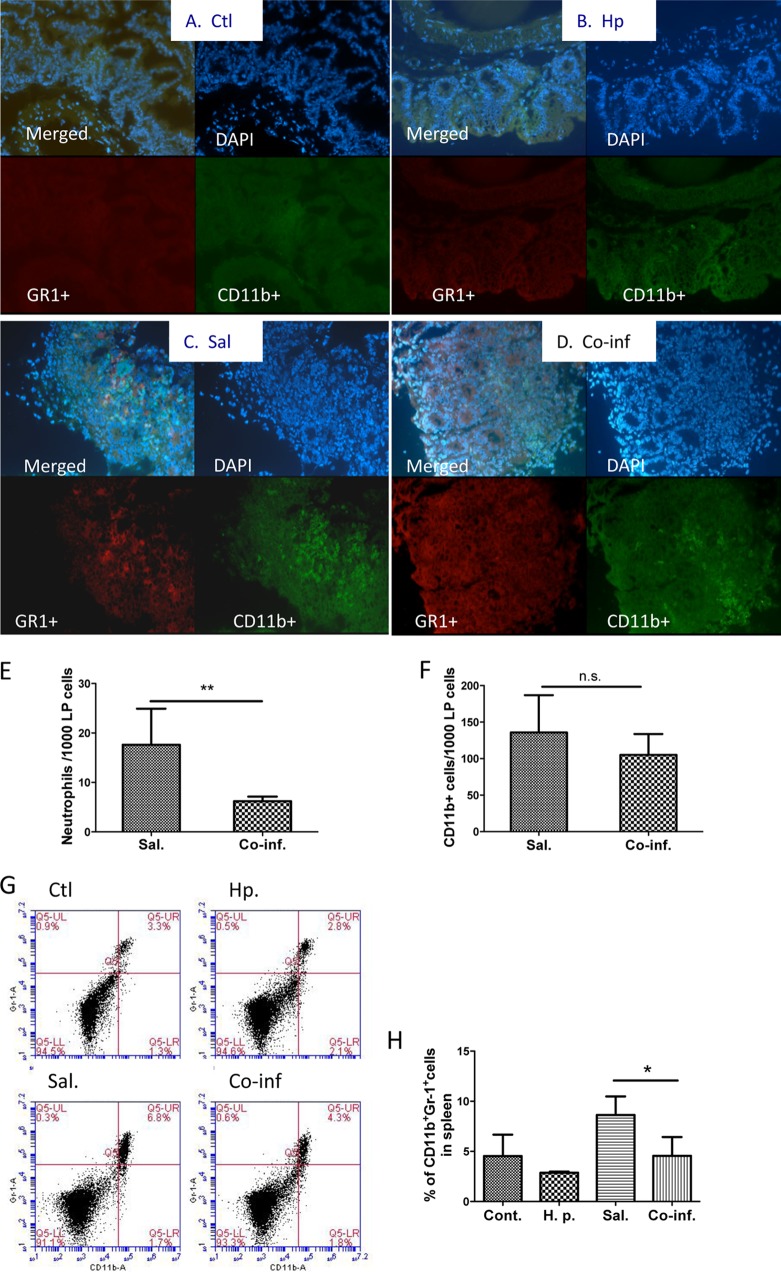
An important component of the antimicrobial response induced by the IL-23/IL-17 axis has been suggested to be the recruitment of neutrophils (18, 20), which are phagocytic innate immune cells that provide a first line of defense against bacterial infection (reviewed in reference 22). Transmigration of neutrophils into the infected mucosa during bacterial infection is necessary for mucosal defense to prevent S. Typhimurium dissemination and to eradicate the invaded bacterial pathogens. To study the potential influence of concurrent helminth infection on this innate immune response to enteric bacterial pathogens, we determined the distribution and frequency of neutrophils in cecal mucosa by immunofluorescence microscopy. As shown in Fig. 4C and E, a clear infiltration of GR1+ neutrophils in the cecum was evident in mice with Salmonella infection alone. In contrast, the frequency of GR1+ neutrophils found in the cecal tissue of mice with helminth coinfection was significantly lower (Fig. 4D and E). In both Salmonella-infected and helminth-coinfected mice, an increase in CD11b+ macrophage numbers was detected (Fig. 4C and D), and the frequency of these cells in the cecal tissues did not differ significantly between mice infected with Salmonella alone and mice coinfected with the helminth parasite (Fig. 4F). In line with this observations, our FACS analysis of spleen cells revealed that Salmonella infection led to an increase in the frequency of GR1+ cells (Fig. 4G and H) and that this Salmonella-induced splenic neutrophil response was significantly inhibited by coinfection with H. polygyrus (Fig. 4G and H). These results suggest that intestinal helminth infection downregulates neutrophil recruitment to the site of bacterial infection.
FIG 4.
Helminth coinfection results in decreased number of Salmonella-induced neutrophils in cecal lamina propria. (A to D) C57BL/6 mice were infected with H. polygyrus and inoculated with Salmonella orally 2 weeks later. Uninfected control (Ctl) mice (A) and mice infected with H. polygyrus (Hp) (B), Salmonella (Sal) (C), or both (Co-inf) (D) were sacrificed 48 h after bacterial infection. Histological sections of the ceca were stained with anti-CD11b-FITC (green) and anti-GR1-Cy3 (red) and analyzed by immunofluorescence microscopy. Magnification, ×100. All images were digitized and cropped in Adobe Photoshop LE 5.0 (Adobe Systems). (E and F) The mean number of GR1-positive cells (C) or CD11b+ cells (D) detected in each high power field was determined by counting 5 fields from each sample (samples from three mice per group were counted). **, P < 0.01. (G) FACS data show that helminth coinfection reduces the GR1+ neutrophils in the spleen. Spleen cells were collected from noninfected and infected mice and stained for neutrophils with anti-GR1 and anti-CD11b antibodies. (H) Data shown are the percentages of neutrophils (GR1+ CD11b+) detected in the spleen (means ± the SEM, n = 5 per group). *, P value of <0.05.
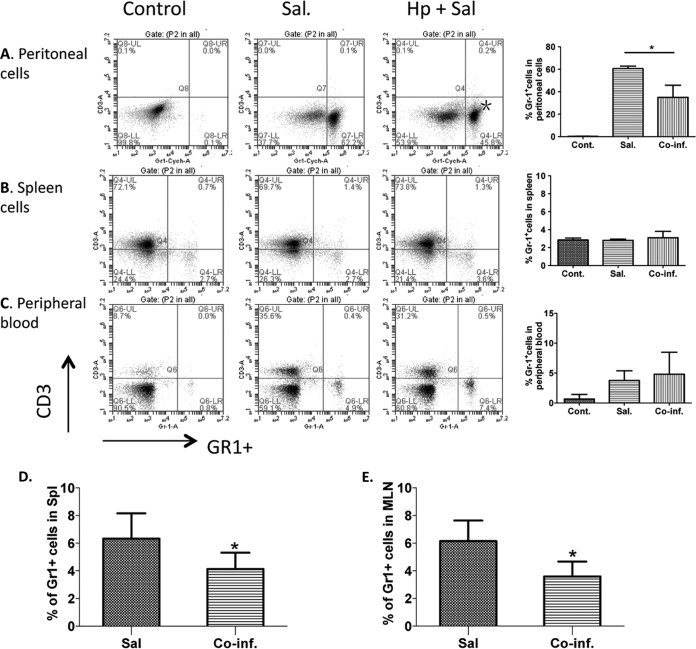
To further test this idea and to examine whether the reduced neutrophil numbers in the intestinal tissue was due to a decrease in circulating neutrophils or because of reduced recruitment to the infected sites, we assessed the systemic response of neutrophils to Salmonella infection. To this end, we infected both helminth preinfected and normal control mice with Salmonella systemically via i.p. injection and sacrificed the mice 24 h later. The percentage of GR1+ neutrophil in peripheral blood, spleen and peritoneal cellular compartments was examined using FACS. Our results show that the neutrophil recruitment to the peritoneal cavity, i.e., the infection site, is induced dramatically after Salmonella inoculation through the i.p. route (Fig. 5A). Such recruitment of GR1+ neutrophils to the bacterial infecting site was reduced significantly in mice that are coinfected with H. polygyrus (Fig. 5A). No significant difference in splenic neutrophil numbers was detected between mice with Salmonella alone and helminth coinfection after i.p. infection (Fig. 5B). Mice infected with Salmonella only, as well as mice coinfected with both pathogens, showed a similar elevated level of circulating neutrophils in peripheral blood compared to uninfected control mice, suggesting unchanged generation and differentiation of GR1+ neutrophils in response to the bacterial pathogen in the presence or absence of helminth coexposure (Fig. 5C). We further examined the helminth-induced downregulation of neutrophil recruitment to the site of bacterial infection in mice that have not received antibiotic treatment by infecting both helminth-infected and uninfected mice with Salmonella and determining the frequency of GR1+ neutrophils in the spleen of these mice. Our results showed that there was a clear increase in the frequency of GR1+ neutrophils in the spleen (Fig. 5D and E) and MLN (Fig. 3F) after oral infection with Salmonella (48 h after infection) and that this increase in Salmonella-induced neutrophil recruitment was significantly reduced in mice with helminth coinfection (Fig. 5D and E). The decreased neutrophil recruitment detected in coinfected mice was correlated with increased bacterial loads in spleen and liver (Fig. 2). These data further support our hypothesis that intestinal helminth parasite coinfection impairs neutrophil recruitment.
FIG 5.
Helminth coinfection results in reduced neutrophil recruitment to the site of bacterial infection in the peritoneum. H. polygyrus-infected and uninfected mice were inoculated i.p. (A to C) or orally (D and E) with Salmonella and sacrificed 24 h (i.p.) or 48 h (oral infection) later. Peritoneal cells (A), spleens (B), and peripheral blood (C) were collected from uninfected (control), Salmonella-infected (Sal), or coinfected (Hp+Sal) mice stained with anti-GR1 and anti-CD3 antibodies and analyzed by FACS. The percentages of GR1+ cells in each compartment are quantified on the right (means ± the SEM, n = 5 per group). *, P < 0.05. (D and E) Percentages of GR1+ cells in spleen and MLN after oral infection with Salmonella without antibiotic treatment. *, P < 0.05.
Helminth coinfection results in downregulation of Salmonella-induced neutrophil chemoattractant expression and proinflammatory cytokine responses.
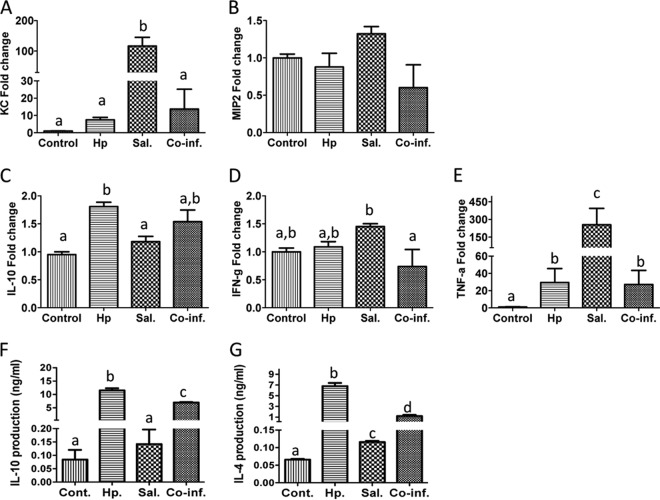
Chemokines play an important role in the movement and localization of inflammatory cells in disease (23). The recruitment of neutrophils to the sites of infection can be expected to play a significant part in host protection against infection. Since neutrophils do not appear to proliferate at extramedullary sites, we tested the hypothesis that helminths may exert their inhibitory effect on bacterium-induced neutrophil recruitment by regulating chemoattractant expression. To this end, we first determined the expression of the two major chemoattractants for recruiting neutrophils: KC (CXCL1) and MIP-2 (CXCL2) in the cecal tissue. Our real-time quantitative RT-PCR analysis showed that Salmonella infection induced an upregulation of the expression of the two chemokines (Fig. 6A and B). The upregulation of KC and MIP-2 expression correlated with the increase in neutrophil transmigration into the infected mucosa. In addition, our results showed that coinfection with H. polygyrus significantly suppressed the Salmonella-induced upregulation of KC and, to a lesser extent, MIP-2 (Fig. 6A and B).
FIG 6.
Helminth coinfection results in downregulation of Salmonella-induced neutrophil chemoattractants and proinflammatory cytokines and upregulation of IL-10 in the cecum and MLN. (A to E) Cecal tissue was collected from control, Salmonella-infected, and coinfected mice 48 h after Salmonella infection. Total RNA was prepared and used for quantitative RT-PCR determination of the neutrophil chemoattractants KC and MIP-2, the proinflammatory cytokines (TNF-α and IFN-γ), and IL-10. Values represent the fold increase compared to baseline obtained from uninfected control mice. (F and G) MLN cells were collected from various groups and stimulated in vitro with surface-bound anti-CD3 MAb. Culture supernatants were collected 48 h later. Cytokine (IL-10 and IL-4) secretion into the culture supernatants was determined by ELISA. The data shown are means ± the SEM (n = 5 mice per group) from one of three experiments performed showing similar results. Different letters indicate the significant differences among groups, based on one-way ANOVA. *, P < 0.05.
Analysis of systemic antibody levels in the various groups of mice revealed that both helminth-infected and coinfected mice developed strong Th2-biased IgG1 responses (data not shown). Further quantitative RT-PCR analysis of infected cecal tissues showed the induction of Th2/Treg responses in both helminth-infected and helminth/Salmonella-coinfected mice, with increased expression of IL-10 (Fig. 6C). This is supported by the cytokine enzyme-linked immunosorbent assay (ELISA) data showing increased IL-10 and IL-4 production by MLN cells from helminth-infected or coinfected mice after in vitro stimulation with anti-CD3 (Fig. 6F and G). Salmonella infection, on the other hand, induced cecal IFN-γ and TNF-α expression (Fig. 6D and E), both of which were found to be inhibited by helminth coinfection.
Impairment of Salmonella-induced chemoattractant expression in the coinfected host is mediated by a helminth infection-associated cytokine response.
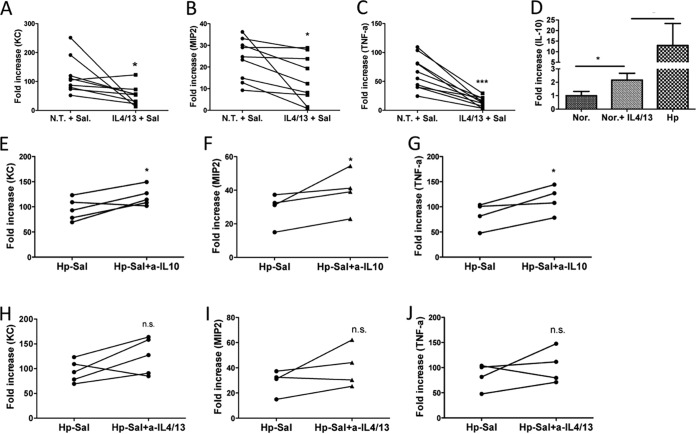
Recent evidence suggests that TLR signaling in tissue macrophages directly controls the synthesis of neutrophil-attracting chemokines that are essential for the earliest recruitment step in the innate immune response to microbial challenge (24). To directly test the hypothesis that helminth infection may affect the bacteria-induced neutrophil recruiting chemokine response by Th2 cytokine IL-4/IL-13 and/or IL-10, we took an in vitro approach by isolating peritoneal macrophages from control and helminth-infected mice and infecting these cells with Salmonella. Our results showed that Salmonella infection of the macrophages from the control mice resulted in a marked upregulation of KC and MIP-2 (Fig. 7A and B). To determine the potential role of the helminth-induced Th2 response in regulating bacterium-induced KC and MIP-2 expression, the control macrophages were treated with recombinant IL-4/IL-13 overnight before Salmonella infection. Our quantitative RT-PCR results showed that IL-4/IL-13 pretreatment led to a significant inhibition of KC and MIP-2 expression (Fig. 7A and B) in association with marked reduction of TNF-α (Fig. 7C) and upregulation of IL-10 in these cells (Fig. 7D). IL-10 is an anti-inflammatory cytokine produced by macrophages and T and B cells and may regulate changes in macrophage phenotype that influence the functional capacity of the cells. To test the possibility that helminth infection may exert its influence on host KC and MIP-2 expression through the induction of IL-10 and/or IL-4/IL-13, we isolated peritoneal macrophages from helminth-infected mice and pretreated the cells with anti-IL-10 or anti-IL-4/-13 before Salmonella infection. Quantitative RT-PCR results indicated that pretreatment of macrophages isolated from helminth-infected mice with anti-IL-10 significantly attenuated the inhibition of Salmonella-induced expression of KC and MIP-2 (Fig. 7E and F). Although Th2 cytokine (IL-4 and IL-13) treatment showed the same trend, the effect was not statistically significant (Fig. 7H to J). These results suggest that helminth infection stimulates macrophage expression of IL-10, which inhibits MIP-2 and KC expression and thus reduces neutrophil recruitment.
FIG 7.
Coinfection-dependent impairment of neutrophil chemoattractant expression is mediated by helminth-induced cytokines. (A to C) Peritoneal macrophages from normal control mice were pretreated with Th2 cytokines (rIL-4/IL-13) or not treated (N.T.) and then exposed to Salmonella in vitro. Total RNA was isolated from the macrophages, and KC, MIP-2, and TNF-α expression was determined using quantitative RT-PCR. The data shown represent the fold changes of various gene expression compared to baseline obtained from untreated/uninfected control mice. *, P < 0.05; ***, P < 0.001 (based on a paired t test). (D) IL-4/IL-13-treated normal macrophages or macrophages from helminth-infected mice showed increased expression of IL-10. *, P < 0.05 (n = 3 to 5 mice per group). (E to J) Peritoneal macrophages from H. polygyrus-infected mice were treated with anti-IL-10 (E to G) or IL-4/IL-13 (F to J) and then exposed to Salmonella in vitro. The data shown represent the fold changes of various gene expression compared to the baseline obtained from untreated/uninfected control mice. *, P < 0.05 based on a paired t test.
Neutrophil depletion results in enhanced infection and intestinal injury during Salmonella infection.
To determine the functional relevance of the helminth-induced impairment of neutrophil recruitment during Salmonella infection, we administered Ly6G (anti-Gr1) antibody to deplete this cell type. The effectiveness of the depletion was confirmed by FACS analysis, which demonstrated a marked reduction of GR1+ cells in the spleen (Fig. 8A) and other immune compartments (peripheral blood and MLN [data not shown]). After Salmonella infection, mice depleted of neutrophils had significantly elevated pathogen numbers in the stool (Fig. 8B) and increased tissue damage (Fig. 8C). Moreover, the Ly6G-treated animals had severe bleeding into the small intestine as a result of the Salmonella infection (data not shown). These results are consistent with the idea that the helminth-induced impairment of neutrophil recruitment is likely to be an important factor in the increased severity of enterocolitis caused by Salmonella infection.
FIG 8.
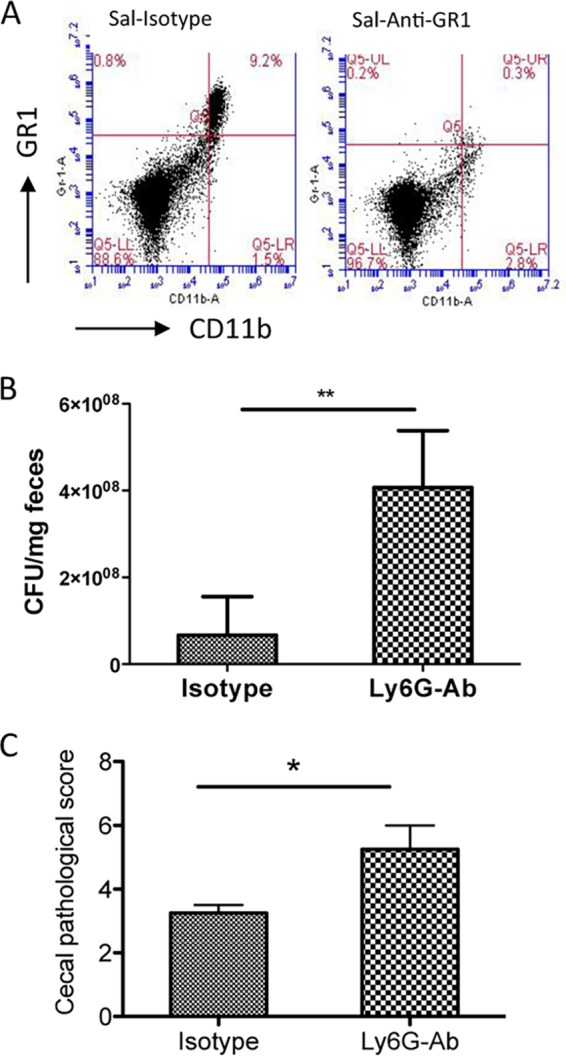
Neutrophil depletion results in enhanced Salmonella-colitis and fecal output. (A) FACS analysis shows the efficacy of neutrophil depletion by Ly6G antibody treatment in vivo. (B) Fecal samples were collected from Salmonella-infected mice that were treated with isotype control antibody or Ly6G antibody, and the numbers of Salmonella recovered from fecal samples were determined at 48 h postinfection. **, P < 0.01 (n = 4 to 5 mice per group). (C) Histopathological score of cecal inflammation in mice infected with Salmonella after treatment with isotype control antibody or anti-Ly6G antibody. *, P < 0.05.
DISCUSSION
Salmonella infection is a major cause of human food-borne gastroenteritis worldwide. To effectively control the infection, molecules that can enhance the microbicidal capacity of phagocytes, such as IL-12, IFN-γ, and TNF-α, are required. Mice lacking any of these cytokines become more susceptible to the infection (25). In many parts of the world, enteric bacterial infections often occur in individuals who are also infected with parasitic worms. In the present study, we tested the hypothesis that coinfection with an intestinal helminth parasite renders mice more susceptible to infection by S. Typhimurium, resulting in increased dissemination of the bacteria and enhanced lethality. Specifically, we examined the effect of an ongoing Th2 polarizing helminth parasite infection on the pathogenesis and acute innate immune response in Salmonella infection.
Our results show that C57BL/6 mice coinfected with S. Typhimurium and the intestinal nematode H. polygyrus show an increase in morbidity and mortality compared to mice infected with Salmonella alone. The enhanced susceptibility to S. Typhimurium-induced intestinal injury in the coinfected mice was found to be associated with dysregulated innate immune responses to Salmonella infection, as evidenced by the downregulation of the IL-23/IL-17 axis, inhibition of antimicrobial peptide expression, the reduction of Salmonella-induced expression of MIP-2 and KC (Fig. 6), and a decrease in neutrophil recruitment to the site of bacterial infection (Fig. 4 and 5). The impaired recruitment of neutrophils is likely to be an important factor in our experimental model since neutrophil depletion mimicked the effects of helminth infection (Fig. 8). Earlier work from other investigators has also demonstrated the importance of neutrophils in the response to S. Typhimurium infection (26). However, to our knowledge, the present study is the first to demonstrate that an intestinal nematode infection impairs host innate immunity against Salmonella infection and enhances Salmonella-induced intestinal pathology through a mechanism that involves inhibition of neutrophil recruitment.
Recruitment of neutrophils plays a vital role in controlling infections. These cells are able to deploy a number of key antimicrobial mechanisms, including phagocytosis, the generation of reactive oxygen species, and the release of neutrophil extracellular traps (27–30). In the streptomycin model of Salmonella colitis, a marked intestinal neutrophil infiltration is evident (13). The importance of neutrophils in defense against intestinal bacterial pathogens is illustrated by the observation that diminished neutrophil infiltration into the lamina propria in TLR4- or MyD88-deficient mice is associated with impaired bacterial clearance (27). The mechanism of this effect involves decreased MIP-2 expression in the knockout animals (31). Conversely, increased KC expression in KC transgenic mice leads to a marked infiltration of neutrophils at the sites of transgene expression (28). These results, together with our findings reported here, support the notion that local production of MIP-2 and KC is essential for neutrophil recruitment and bacterial clearance (24, 29, 30). Our data further demonstrate that this innate defense mechanism against bacterial enteropathogens can be negatively regulated by concurrent helminth infection.
Based on our in vitro experiments, the mechanism by which helminth infection inhibits macrophage MIP-2 and KC expression appears to involve IL-10 and, to a lesser extent, IL-4 and IL-13. In line with our observations, an early study using the RAW 264.7 macrophage cell line showed that IL-10 inhibited production of MIP-1 and MIP-2 after lipopolysaccharide stimulation (31). The impact of IL-10 on neutrophil recruitment was also studied by using a lung-specific, tetracycline-inducible IL-10-overexpressing transgenic mouse (32). It was found that during the early phase of Pseudomonas aeruginosa infection, neutrophil recruitment and cytokine (TNF-α) and chemokine (KC) expression were significantly decreased in the IL-10 transgenic mice, which resulted in impaired clearance of P. aeruginosa (32). It has been shown previously that IL-10 reduces the stability of a number of mRNAs, including KC, TNF-α, and MIP-1 (33, 34). The molecular events responsible for IL-10-mediated downregulation of MIP2 are less clear. One potential mechanism is the IL-10-induced inhibition of NF-κB nuclear translocation, since the MIP-2 promoter contains a consensus NF-κB binding site (35). The role of IL-4 and IL-13 is supported by a recent study showing that these cytokines suppress neutrophil infiltration and proinflammatory cytokine responses during Schistosomiasis japonica infection in mice (33).
In addition to inhibiting the expression of neutrophil recruiting chemokines, our results suggest that helminth coinfection may also adversely affect the host response to Salmonella by reducing the expression of antimicrobial peptides such as Reg3γ and Reg3β. Recent evidence suggests that Reg3β plays a protective role against intestinal translocation of Salmonella in mice (34). Reg3γ has been thought to have antimicrobial activity against Gram-positive bacteria (36). However, a recent study using mice that are deficient in Atg16L1 in intestinal epithelial cells showed that the mutant mice had reduced expression of several antimicrobial peptides, including Reg3γ, an abnormality that correlated with increased Salmonella-mediated inflammation and systemic translocation of the bacteria (15).
Helminth infection may regulate the innate immune response to concurrent bacterial pathogens by simultaneously stimulating regulatory cytokines, such as IL-10, and antagonizing proinflammatory factors, such as MIP-2 and KC. Studies have suggested that helminth infection induces the development of alternatively activated macrophages (AAM) (16, 37), and one of the mechanisms by which AAM may exert their suppressive effect on host innate immunity involves IL-10 release by these cells (16, 35). During helminth infection, IL-10 can play a critical role in the regulation of the intensity of host immune response and in minimizing immunopathology (38, 39), which may be in the best interests of the host.
Our observations support the hypothesis that helminth infection increases susceptibility to bacterial enteropathogens. These findings could have significant implications for the use of Salmonella-based vaccines in countries with a high prevalence of helminth infections. Attenuated Salmonella strains are attractive live vectors for vaccine development because they elicit mucosal immunity. Based on our data it can be speculated that helminth infection may markedly alter immune responses to Salmonella-based vaccines. Clearly, a more comprehensive understanding of helminth-induced immunoregulation in the intestinal mucosa is needed.
ACKNOWLEDGMENTS
This study was supported by grants from the National Institutes of Health (DK082427 to H.N.S. and AI089700 to B.J.C.) and from the Nutrition Obesity Research Center at Harvard (P30 DK040561).
Footnotes
Published ahead of print 30 June 2014
REFERENCES
- 1.Rabsch W, Andrews H, Kingsley R, Prager R, Tschäpe H, Adams L, A Bäumler. 2002. Salmonella enterica serotype Typhimurium and its host-adapted variants. Infect. Immun. 70:2249–2255. 10.1128/IAI.70.5.2249-2255.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prost L, Sanowar S, Miller S. 2007. Salmonella sensing of anti-microbial mechanisms to promote survival within macrophages. Immunol. Rev. 219:55–65. 10.1111/j.1600-065X.2007.00557.x [DOI] [PubMed] [Google Scholar]
- 3.Ohtsuka YLJ, Stamm D, Sanderson I. 2001. Mip-2 secreted by epithelial cells increases neutrophil and lymphocyte recruitment in the mouse intestine. Gut 49:526–533. 10.1136/gut.49.4.526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Srikanth C, Cherayil B. 2007. Intestinal innate immunity and the pathogenesis of Salmonella enteritis. Immunol. Res. 37:61–78. 10.1007/BF02686090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harrington L, Srikanth C, Antony R, Shi H, Cherayil B. 2007. A role for natural killer cells in intestinal inflammation caused by infection with Salmonella enterica serovar Typhimurium. FEMS Immunol. Med. Microbiol. 51:372–380. 10.1111/j.1574-695X.2007.00313.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maldonado-Contreras A, McCormick B. 2011. Intestinal epithelial cells and their role in innate mucosal immunity. Cell Tissue Res. 343:5–12. 10.1007/s00441-010-1082-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Srikanth C, Mercado-Lubo R, Hallstrom K, McCormick B. 2011. Salmonella effector proteins and host-cell responses. Cell. Mol. Life Sci. 68:3687–3697. 10.1007/s00018-011-0841-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Szabady R, McCormick B. 2013. Control of neutrophil inflammation at mucosal surfaces by secreted epithelial products. Front. Immunol. 4:220. 10.3389/fimmu.2013.00220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Supali T, Verweij J, Wiria A, Djuardi Y, Hamid F, Kaisar MM, Wammes L, L van Lieshout Luty A, Sartono E, Yazdanbakhsh M. 2010. Polyparasitism and its impact on the immune system. Int. J. Parasitol. 40:1171–1176. 10.1016/j.ijpara.2010.05.003 [DOI] [PubMed] [Google Scholar]
- 10.Chen C-C, Louie S, McCormick B, Walker W, Shi H. 2005. Concurrent infection with an intestinal helminth parasite impairs host resistance to enteric Citrobacter rodentium and enhances Citrobacter-induced colitis in mice. Infect. Immun. 73:5468–5481. 10.1128/IAI.73.9.5468-5481.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen C-C, Louie S, McCormick B, Walker W, Shi H. 2006. Helminth-primed dendritic cells alter the host response to enteric bacterial infection. J. Immunol. 176:472–483. 10.4049/jimmunol.176.1.472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shi HN SM, Stevenson MM, Koski KG. 1994. Zinc deficiency impairs T cell function in mice with primary infection of Heligmosomoides polygyrus (Nematoda). Parasite Immunol. 16:339–350. 10.1111/j.1365-3024.1994.tb00359.x [DOI] [PubMed] [Google Scholar]
- 13.Barthel M, Hapfelmeier S, Quintanilla-Martinez L, Kremer M, Rohde M, Hogardt M, Pfeffer K, Russmann H, Hardt WD. 2003. Pretreatment of mice with streptomycin provides a Salmonella enterica serovar Typhimurium colitis model that allows analysis of both pathogen and host. Infect. Immun. 71:2839–2858. 10.1128/IAI.71.5.2839-2858.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Srikanth C, Wall D, Maldonado-Contreras A, Shi H, Zhou D, Demma Z, Mumy K, McCormick B. 2010. Salmonella pathogenesis and processing of secreted effectors by caspase-3. Science 330:390–393. 10.1126/science.1194598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Conway K, Kuballa P, Song J-H, Patel K, Castoreno A, Yilmaz O, Jijon H, Zhang M, Aldrich L, Villablanca E, Peloquin J, Goel G, Lee I-A, Mizoguchi E, Shi H, Bhan A, Shaw S, Schreiber S, Virgin H, Shamji A, Stappenbeck T, Reinecker H-C, Xavier R. 2013. Atg16l1 is required for autophagy in intestinal epithelial cells and protection of mice from Salmonella infection. Gastroenterology 145:1347–1357. 10.1053/j.gastro.2013.08.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weng M, Huntley D, Huang IF, Foye-Jackson O, Wang L, Sarkissian A, Zhou Q, Walker W, Cherayil B, Shi H. 2007. Alternatively activated macrophages in intestinal helminth infection: effects on concurrent bacterial colitis. J. Immunol. 179:4721–4731. 10.4049/jimmunol.179.7.4721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Daley J, Thomay A, Connolly M, Reichner J, Albina J. 2008. Use of Ly6g-specific monoclonal antibody to deplete neutrophils in mice. J. Leukoc. Biol. 83:64–70. 10.1189/jlb.0407247 [DOI] [PubMed] [Google Scholar]
- 18.Godinez I, Raffatellu M, Chu H, Paixão T, Haneda T, Santos R, Bevins C, Tsolis R, A Bäumler. 2009. Interleukin-23 orchestrates mucosal responses to Salmonella enterica serotype Typhimurium in the intestine. Infect. Immun. 77:387–398. 10.1128/IAI.00933-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raffatellu M, Santos R, Chessa D, Wilson R, Winter S, Rossetti C, Lawhon S, Chu H, Lau T, Bevins C, Adams L, A Bäumler. 2007. The capsule encoding the Viab locus reduces interleukin-17 expression and mucosal innate responses in the bovine intestinal mucosa during infection with Salmonella enterica serotype Typhi. Infect. Immun. 75:4342–4350. 10.1128/IAI.01571-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Raffatellu MSR, Verhoeven D, George M, Wilson R, Winter S, Godinez I, Sankaran S, Paixao T, Gordon M, Kolls J, Dandekar S, Bäumler A. 2008. Simian immunodeficiency virus-induced mucosal interleukin-17 deficiency promotes Salmonella dissemination from the gut. Nat. Med. 14:421–428. 10.1038/nm1743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Ampting M, Loonen L, Schonewille A, Konings I, Vink C, Iovanna J, Chamaillard M, Dekker J, R van der Meer Wells J, Bovee-Oudenhoven I. 2012. Intestinally secreted C-type lectin Reg3b attenuates salmonellosis but not listeriosis in mice. Infect. Immun. 80:1115–1120. 10.1128/IAI.06165-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Urban C, Lourido S, Zychlinsky A. 2006. How do microbes evade neutrophil killing? Cell. Microbiol. 8:1687–1696. 10.1111/j.1462-5822.2006.00792.x [DOI] [PubMed] [Google Scholar]
- 23.Schmitz J, McCracken V, Dimmitt R, Lorenz R. 2007. Expression of Cxcl15 (Lungkine) in murine gastrointestinal, urogenital, and endocrine organs. J. Histochem. Cytochem. 55:515–524. 10.1369/jhc.6A7121.2007 [DOI] [PubMed] [Google Scholar]
- 24.De Filippo K, Henderson R, Laschinger M, Hogg N. 2008. Neutrophil chemokines Kc and macrophage-inflammatory protein-2 are newly synthesized by tissue macrophages using distinct TLR signaling pathways. J. Immunol. 180:4308–4315. 10.4049/jimmunol.180.6.4308 [DOI] [PubMed] [Google Scholar]
- 25.Dougan G, John V, Palmer S, Mastroeni P. 2011. Immunity to salmonellosis. Immunol. Rev. 240:196–210. 10.1111/j.1600-065X.2010.00999.x [DOI] [PubMed] [Google Scholar]
- 26.Cheminay C, Chakravortty D, Hensel M. 2004. Role of neutrophils in murine salmonellosis. Infect. Immun. 72:468–477. 10.1128/IAI.72.1.468-477.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fukata M, Michelsen K, Eri R, Thomas L, Hu B, Lukasek K, Nast C, Lechago J, Xu R, Naiki Y, Soliman A, Arditi M, Abreu M. 2005. Toll-like receptor-4 is required for intestinal response to epithelial injury and limiting bacterial translocation in a murine model of acute colitis. Am. J. Physiol. 288:G1055–G1065. 10.1152/ajpgi.00328.2004 [DOI] [PubMed] [Google Scholar]
- 28.Lira S, Zalamea P, Heinrich J, Fuentes M, Carrasco D, Lewin A, Barton D, Durham S, Bravo R. 1994. Expression of the chemokine N51/Kc in the thymus and epidermis of transgenic mice results in marked infiltration of a single class of inflammatory cells. J. Exp. Med. 180:2039–2048. 10.1084/jem.180.6.2039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ritzman A, Hughes-Hanks J, Blaho V, Wax L, Mitchell W, Brown C. 2010. The chemokine receptor Cxcr2 ligand Kc (Cxcl1) mediates neutrophil recruitment and is critical for development of experimental Lyme arthritis and carditis. Infect. Immun. 78:4593–4600. 10.1128/IAI.00798-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Armstrong D, Major J, Chudyk A, Hamilton T. 2004. Neutrophil chemoattractant genes Kc and Mip-2 are expressed in different cell populations at sites of surgical injury. J. Leukoc. Biol. 75:641–648. 10.1189/jlb.0803370 [DOI] [PubMed] [Google Scholar]
- 31.Shanley T, Vasi N, Denenberg A. 2000. Regulation of chemokine expression by IL-10 in lung inflammation. Cytokine 12:1054–1064. 10.1006/cyto.1999.0655 [DOI] [PubMed] [Google Scholar]
- 32.Sun L, Guo R-F, Newstead M, Standiford T, Macariola D, Shanley T. 2009. Effect of IL-10 on neutrophil recruitment and survival after Pseudomonas aeruginosa challenge. Am. J. Respir. Cell Mol. Biol. 41:76–84. 10.1165/rcmb.2008-0202OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seki T, Kumagai T, Kwansa-Bentum B, Furushima-Shimogawara R, Anyan W, Miyazawa Y, Iwakura Y, Ohta N. 2012. Interleukin-4 (Il-4) and IL-13 suppress excessive neutrophil infiltration and hepatocyte damage during acute murine schistosomiasis japonica. Infect. Immun. 80:159–168. 10.1128/IAI.05581-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reinés M, Llobet E, Llompart C, Moranta D, C Pérez-Gutiérrez Bengoechea J. 2012. Molecular basis of Yersinia enterocolitica temperature-dependent resistance to antimicrobial peptides. J. Bacteriol. 194:3173–3188. 10.1128/JB.00308-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Araki A, Kanai T, Ishikura T, Makita S, Uraushihara K, Iiyama R, Totsuka T, Takeda K, Akira S, Watanabe M. 2005. Myd88-deficient mice develop severe intestinal inflammation in dextran sodium sulfate colitis. J. Gastroenterology 40:16–23. 10.1007/s00535-004-1492-9 [DOI] [PubMed] [Google Scholar]
- 36.Cash H, Whitham C, Behrendt C, Hooper L. 2006. Symbiotic bacteria direct expression of an intestinal bactericidal lectin. Science 313:1126–1130. 10.1126/science.1127119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martinez F, Sica A, Mantovani A, Locati M. 2008. Macrophage activation and polarization. Front. Biosci. 13:453–461. 10.2741/2692 [DOI] [PubMed] [Google Scholar]
- 38.MacDonald A, Araujo M, Pearce E. 2002. Immunology of parasitic helminth infections. Infect. Immun. 70:427–433. 10.1128/IAI.70.2.427-433.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Taylor M, Harris A, Nair M, Maizeis R, Allen J. 2006. F4/80+ alternatively activated macrophages control Cd4+ T cell hyporesponsiveness at sites peripheral to filarial infection. J. Immunol. 176:6918–6927. 10.4049/jimmunol.176.11.6918 [DOI] [PubMed] [Google Scholar]