Abstract
Subtilase cytotoxin (SubAB) is the prototype of a recently emerged family of AB5 cytotoxins produced by Shiga-toxigenic Escherichia coli (STEC). Its mechanism of action involves highly specific A-subunit-mediated proteolytic cleavage of the essential endoplasmic reticulum (ER) chaperone BiP. Our previous in vivo studies showed that intraperitoneal injection of purified SubAB causes a major redistribution of leukocytes and elevated leukocyte apoptosis in mice, as well as profound splenic atrophy. In the current study, we investigated selected chemokine and proinflammatory cytokine responses to treatment with SubAB, a nontoxic derivative (SubAA272B), or Shiga toxin 2 (Stx2) in human macrophage (U937), brain microvascular endothelial (HBMEC), and colonic epithelial (HCT-8) cell lines, at the levels of secreted protein, cell-associated protein, and gene expression. Stx2 treatment upregulated expression of chemokines and cytokines at both the protein and mRNA levels. In contrast, SubAB induced significant decreases in secreted interleukin-8 (IL-8) and monocyte chemoattractant protein 1 (MCP-1) in all three tested cell lines and a significant decrease in secreted IL-6 in HBMECs. The downregulation of secreted chemokines or cytokines was not observed in SubAA272B-treated cells, indicating a requirement for BiP cleavage. The downregulation of secreted chemokines and cytokines by SubAB was not reflected at the mRNA and cell-associated protein levels, suggesting a SubAB-induced export defect.
INTRODUCTION
Subtilase cytotoxin (SubAB) is the prototype of a family of AB5 cytotoxins produced by Shiga-toxigenic Escherichia coli (STEC) (1). SubAB was initially detected in a locus of enterocyte effacement-negative O113:H21 STEC strain responsible for a small outbreak of hemolytic-uremic syndrome (HUS) in South Australia (1, 2), but it is also produced by numerous other disease-causing STEC serotypes (3, 4). SubAB is extremely cytotoxic in vitro for a range of cell types, and it is more toxic for Vero cells than Shiga toxin (Stx); it is also lethal for mice when injected intraperitoneally (1).
SubAB acts by binding via its B-subunit pentamer to cell surface glycan receptors terminating in α2-3-linked N-glycolylneuraminic acid (5). This triggers internalization and retrograde transport to the endoplasmic reticulum (ER) (6), where the A subunit, a highly specific subtilase-like serine protease, cleaves the essential ER chaperone BiP (7). BiP is a highly conserved ER chaperone essential for the survival of eukaryotic cells. It also maintains the permeability barrier of the ER membrane and plays a crucial role in the unfolded-protein response (UPR), as the ER stress-signaling master regulator (8, 9). Indeed, we have shown that SubAB treatment rapidly triggers the three ER stress-signaling pathways (PERK, IRE1, and ATF6) (10). These three pathways are thought to be activated when accumulation of unfolded proteins in the ER lumen titrates BiP away from the respective membrane-spanning signaling molecule, collectively triggering a series of responses (UPR) designed to restore ER homeostasis or, if this cannot be accomplished, to promote apoptosis (11). The rapidity of the responses suggests that cleavage of BiP by SubAB may cause it to immediately dissociate from the signaling molecules, without the need for the accumulation of unfolded proteins in the ER lumen (10). This provides a mechanistic basis for the observation that SubAB triggers apoptosis in a Bax/Bak-dependent fashion (12, 13). This mechanism of action differs markedly from that of Stx, whose A subunit is an RNA N-glycosidase that cleaves a specific adenine base from 28S rRNA, thereby blocking protein synthesis (14). Despite its distinct mechanism of action, Stx can also activate the IRE1, PERK, and ATF6 pathways, and ER stress-induced upregulation of the transcription factor C/EBP homologous protein (CHOP) contributes to Stx-induced apoptosis (15, 16).
Our ongoing studies of SubAB have thrown significant light on its potential role in disease pathogenesis. Intraperitoneal injection of mice with purified SubAB causes microangiopathic hemolytic anemia, thrombocytopenia, and renal impairment—characteristics typical of Stx-induced HUS. Histological examination of organs also revealed extensive microvascular thrombosis and other histological damage in the brain, kidneys, and liver (17). The prothrombotic effects of the toxin include triggering release of tissue factor from macrophages and endothelial cells, initiating the coagulation cascade (18). The toxin also causes a major redistribution of leukocytes and elevated leukocyte apoptosis in vivo, as well as profound splenic atrophy (17, 19), suggesting that it has significant immunoregulatory effects.
Accordingly, in this study, we investigated the capacity of purified SubAB (or an active-site mutant thereof) to elicit chemokine and proinflammatory cytokine responses in human macrophage (U937), human brain microvascular endothelial (HBMEC), and human colonic epithelial (HCT-8) cell lines, at both the mRNA and protein levels. These effects were compared with those elicited by purified Stx2. The three cell lines examined in this study were selected to represent key cellular targets in the pathogenesis of STEC disease and HUS. Colonic epithelium is the first tissue to encounter toxins released from colonizing STEC, and local chemokine and cytokine responses may have a significant impact on gut inflammation. Macrophage responses in the gut and other target tissues may also play an important role in amplification of toxin-mediated injury; for example, release of tumor necrosis factor alpha (TNF-α) from macrophages is known to upregulate expression of Gb3, the receptor for Stx, in the glomerular microvasculature, sensitizing endothelial cells to Stx. Endothelial cells are also important targets for Stx, and endothelial insult underpins the development of Stx-induced HUS. The particular chemokines tested in the current study were interleukin-8 (IL-8), monocyte chemoattractant protein 1 (MCP-1) (strong chemoattractants for neutrophils and monocytes, respectively), and macrophage inflammatory protein 1β (MIP-1β), a chemoattractant for monocytes and natural killer cells. MIP-1β also activates human granulocytes, including neutrophils. The cytokines studied were TNF-α and IL-6, since they both are crucial proinflammatory cytokines. Indeed, TNF-α can trigger a series of inflammatory reactions that may result in increased levels of cytokine bathing and may ultimately lead to cell death and organ failure (20). Clinical studies have also demonstrated that HUS patients have elevated blood levels of TNF-α, IL-8, and IL-6 (21). Thus, these cytokines and chemokines collectively make a significant contribution to the inflammatory cascades induced by bacterial toxins. The capacity of the various toxins to elicit ER stress-induced UPR and their impact on viability of the various cell lines were also investigated.
MATERIALS AND METHODS
Purification of toxins.
SubAB and SubAA272B were purified as described previously (1, 22). SubAA272B is a SubAB active-site mutant with a Ser272-Ala mutation in the A subunit. Ser272 forms part of the critical catalytic triad of subtilase family serine proteases, and the mutation reduces both cytotoxicity for Vero cells and proteolytic activity by >99% (1, 7). Purification involved fusion of a His6 tag to the C terminus of the B subunit, which enabled purification by Ni-nitrilotriacetic acid (Ni-NTA) chromatography under nondenaturing conditions. Stx2 was purified using an identical approach.
Cell culture and toxin treatment.
All cells were grown at 37°C in 5% carbon dioxide in culture medium (RPMI 1640 medium [Gibco] and F-12 nutrient mixture [Gibco] [1:1] supplemented with 10 mM HEPES, 2 mM l-glutamine, 1 mM sodium pyruvate, 10% heat-inactivated fetal calf serum [FCS], 50 IU/ml penicillin, and 50 μg/ml streptomycin). For toxin treatment, cells were seeded into 24-well tissue culture plates at 1 million U937 cells per well or as confluent monolayers of HBMECs (2 × 105/well) or HCT-8 cells (5 × 105/well) and then exposed to the toxins at the indicated concentration in 300 μl of culture medium containing 1% FCS for the indicated times.
Measurement of secreted chemokines and cytokines.
The chemokines examined were IL-8 (also known as CXCL8; a strong chemoattractant for neutrophils), MCP-1 (also known as CCL2; a chemoattractant for monocytes, memory T cells, and macrophages), and MIP-1β (also known as CCL4; a chemoattractant for monocytes and natural killer cells). The examined proinflammatory cytokines were TNF-α and IL-6. Secreted chemokines and cytokines in cell culture supernatants were assayed using cytometric bead array kits (Bender Medsystems) according to the manufacturer's instructions, as described elsewhere (23). During incubation of the beads with culture supernatant, different analytes were captured by their corresponding beads. The bead mixtures were then mixed with biotin-conjugated chemokine/cytokine-detecting antibodies followed by streptavidin-phycoerythrin (streptavidin-PE) to form sandwich complexes. Following incubation and washing, fluorescence data were acquired with a BD FACSCanto or BD LSR II flow cytometer with a high-throughput sampler and BD FACSDiva software (version 5.0.3) and were analyzed with WEASLE v2.6 software. The fluorescence intensity of PE (which emits at 575 nm) is proportional to the amount of the respective analyte present in a test sample. The results were calculated and presented as percent changes in relative fluorescence intensity (RFI) relative to the levels in control cells. The data are reported as means ± standard errors of the means (SEM), and differences were analyzed using Student's t test.
Measurement of mRNA levels of chemokines and cytokines.
RNA was extracted from toxin-treated U937 cells, HBMECs, or HCT-8 cells by use of an RNeasy minikit (Qiagen) according to the manufacturer's instructions. RNasin RNase inhibitor (Promega) was then added to the samples. Contaminating DNA was digested with RNase-free DNase I (Roche Molecular Diagnostics), followed by DNase stop solution (Promega). The absence of DNA contamination in RNA preparations was confirmed by reverse transcription-PCR (RT-PCR) analysis using primers (Table 1) specific for the gene encoding the housekeeping enzyme glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The gene encoding GAPDH contains an intron such that the mRNA template directs amplification of a 239-bp product, whereas the chromosomal DNA template directs amplification of a 341-bp product.
TABLE 1.
Oligonucleotides used in this study
| Primer | Sequence (5′–3′) | Amplicon size (bp) | Reference |
|---|---|---|---|
| GAPDH F | TCCTTGGAGGCCATGTGGGCCAT | 239 | 33 |
| GAPDH R | TGATGACATCAAGAAGGTGGTGAAG | 239 | 33 |
| IL-8 F | GAAGGAACCATTCTCACTGTGTGTA | 327 | 33 |
| IL-8 R | TTATGAATTCTCAGCCCTCTTCAAAAAC | 327 | 33 |
| MCP-1 F | CCCCAGTCACCTGCTGTTAT | 171 | 35 |
| MCP-1 R | TGGAATCCTGAACCCACTTC | 171 | 35 |
| TNF-α F | GCCCATGTTGTAGCAAACCC | 227 | This study |
| TNF-α R | TCTGGTAGGAGACGGCGATG | 227 | This study |
| IL-6 F | ATGTAGCCGCCCCACACAGA | 190 | 36 |
| IL-6 R | GCATCCATCTTTTTCAGCCATC | 190 | 36 |
| XBP1 F | CTGGAAAGCAAGTGGTAGA | 424 (unspliced), 398 (spliced) | 10 |
| XBP1 R | CTGGGTCCTTCTGGGTAG | 424 (unspliced), 398 (spliced) | 10 |
| CHOP F | TGAGGAGAGAGTGTTCAAGAAG | 206 | 10 |
| CHOP R | TCCAGGAGGTGAAACATAGG | 206 | 10 |
Quantitative RT-PCR was performed using a one-step RT-PCR kit (Invitrogen) on a LightCycler 480 instrument (Roche) according to the manufacturers' instructions. It included the following steps: 15 min of reverse transcription at 50°C, followed by 2 min of denaturation at 95°C and then 40 cycles of amplification at 95°C for 15 s, 58°C for 30 s, and 72°C for 30 s. Each RNA sample was assayed in triplicate, using primers specific for the various chemokine or cytokine mRNAs (Table 1) or GAPDH mRNA, which was used as an internal control. Results were calculated using the comparative cycle threshold (2−ΔΔCT) method (user bulletin no. 2 [http://docs.appliedbiosystems.com/pebiodocs/04303859.pdf]; Applied Biosystems), in which the amount of target mRNA is normalized to a reference (untreated control cells) relative to an internal control (GAPDH mRNA). Results are expressed as relative changes in chemokine or cytokine mRNA levels in toxin-treated cells compared to that in untreated (medium-only) control cells. Standard deviations (SD) were initially determined according to the formula and the result was then applied to the formulas +SD = 2ΔΔCT − SD − 2ΔΔCT and −SD = 2ΔΔCT − 2ΔΔCT + SD. Fold changes of ≥2 and ≤0.5 were considered to indicate up- and downregulation, respectively, and the data were analyzed statistically (Student's unpaired, two-tailed t test).
Measurement of U937 cell-associated IL-8 and MCP-1.
Cell-associated IL-8 and MCP-1 were directly probed by incubating toxin-treated U937 cells (fixed with 1% paraformaldehyde and permeabilized with 0.05% Triton X-100 in phosphate-buffered saline [PBS]) with biotin-conjugated anti-IL-8 or anti-MCP-1 antibody (eBioscience), followed by streptavidin-PE. At the end of the experiments, after 3 PBS washes, the cells were analyzed with a BD FACSCanto flow cytometer, and the data were acquired with BD FACSDiva software (version 5.0.3) and analyzed with WEASLE v2.6. Data are presented as means ± standard errors (SE), and differences were analyzed using Student's t test.
RT-PCR analysis of CHOP induction and XBP1 mRNA splicing.
The changes in CHOP mRNA level were determined by quantitative RT-PCR as described above, using specific primers (Table 1). XBP1 mRNA splicing was assessed by RT-PCR, which was performed using a one-step Access RT-PCR system (Promega) according to the manufacturer's instructions. Each reaction was performed in a final volume of 20 ml containing 20 nmol of each oligonucleotide. The RT-PCR protocol included the following steps: 45 min of RT at 48°C, followed by 2 min of denaturation at 94°C and then 35 cycles of amplification at 94°C for 30 s, 58°C for 30 s, and 72°C for 45 s. Primer sequences are listed in Table 1. RT-PCR products were analyzed by agarose gel electrophoresis, and images were captured using Quantity One software (Bio-Rad) after staining with GelRed nucleic acid stain (Biotium).
Examination of cell viability and cell morphology changes.
Viability of U937 cells, HBMECs, or HCT-8 cells exposed to SubAB, SubAA272B, or Stx2 was analyzed using trypan blue staining and a Countess cell counter system (Invitrogen) according to the manufacturer's recommendations.
RESULTS
Toxin-induced changes in secreted chemokines and cytokines.
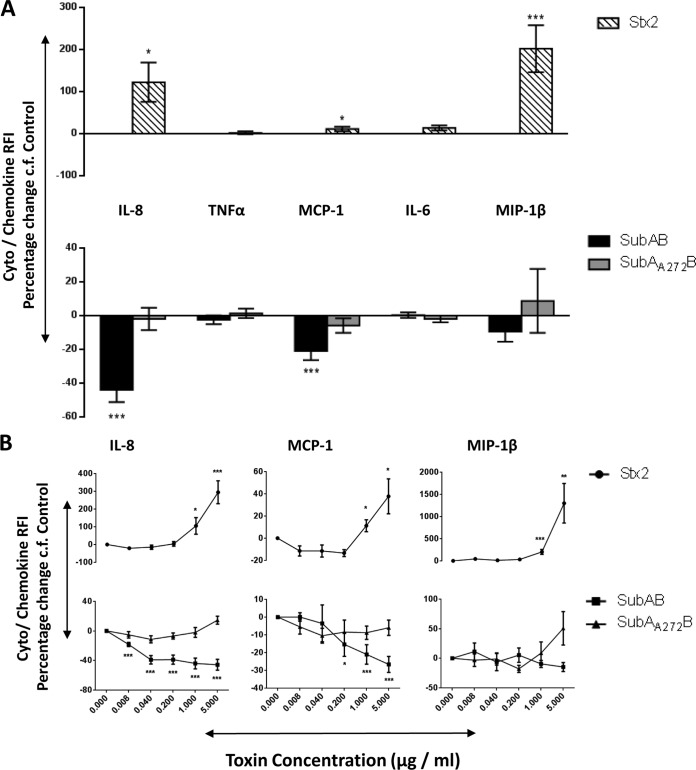
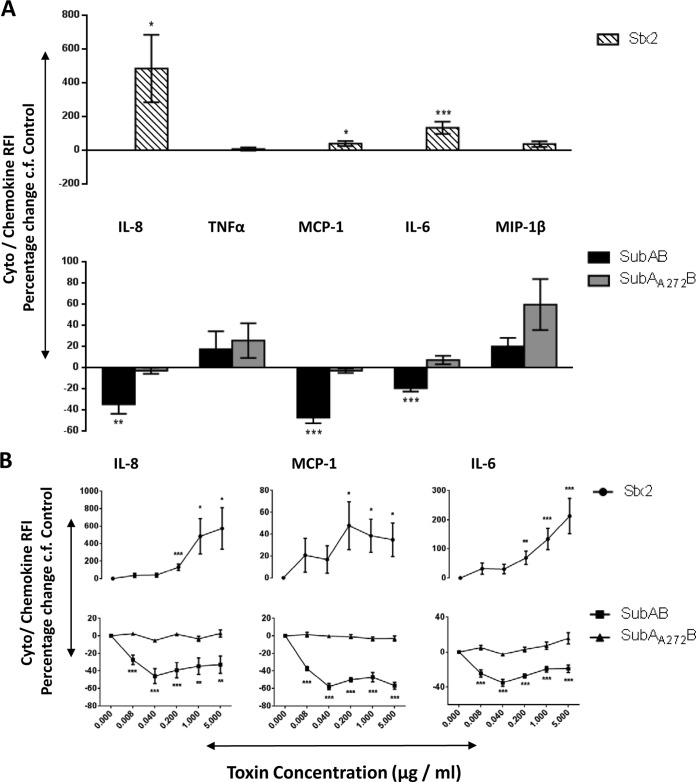
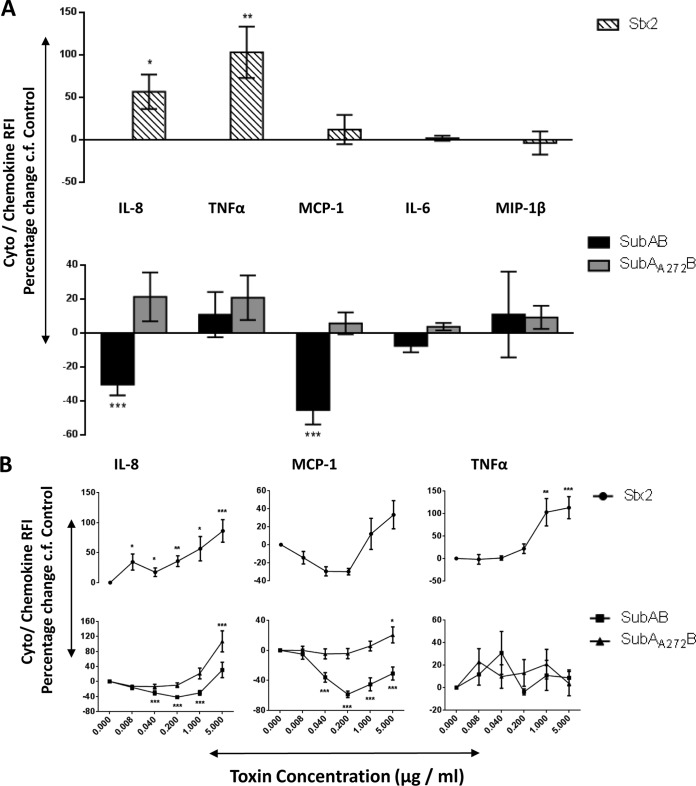
Changes in secreted chemokines and cytokines induced by toxin treatment of U937 cell, HBMEC, and HCT-8 cell lines were investigated using fluorescence-activated cell sorter (FACS) bead array assays. At 24 h, 1 μg/ml of SubAB induced significant decreases in secreted IL-8 and MCP-1 in all three tested cell lines (Fig. 1A, 2A, and 3A). In addition, in HBMECs only, this dose of SubAB also induced a significant decrease in secreted IL-6 (Fig. 2A). The decreases in secreted IL-8 and MCP-1 (Fig. 1B, 2B, and 3B) and in IL-6 (Fig. 2B) were SubAB dose dependent, with significant decreases evident at doses as low as 8 ng/ml. When the various cell lines were treated with 1 μg/ml of the nonproteolytic mutant toxin SubAA272B, there were no significant changes in secreted chemokines and cytokines (Fig. 1A, 2A, and 3A). However, at the highest dose tested (5 μg/ml), SubAA272B induced significant increases in secreted IL-8 and MCP in HCT-8 cells (Fig. 3B). In marked contrast to SubAB, 1 μg/ml Stx2 induced significant increases in secreted IL-8 in all three tested cell lines (Fig. 1A, 2A, and 3A); Stx2 also caused significant elevations of secreted MCP-1 and MIP-1β in U937 cells (Fig. 1A), MCP-1 and IL-6 in HBMECs (Fig. 2A), and TNF-α in HCT-8 cells (Fig. 3A). The increases in secreted chemokines or cytokines induced by Stx2 were dose dependent (Fig. 1B, 2B, and 3B). Interestingly, when U937 cells were treated with both SubAB and Stx2, the inhibitory effect of SubAB on IL-8 secretion was shown to be dominant over the stimulatory effect of Stx2. Indeed, the IL-8 level was significantly lower than that seen with SubAB alone (Fig. 4).
FIG 1.
Toxin-induced changes in secreted chemokines and cytokines in U937 cells. U937 cells were treated with 1 μg/ml (A) or the indicated doses (B) of SubAB, SubAA272B, or Stx2 for 24 h or were left untreated (control). Levels of secreted chemokines or cytokines (as indicated) were assayed using a cytometric bead array (see Materials and Methods). The results are presented as percent changes in RFI relative to levels in control cells. The data are means ± SEM from at least 8 samples from 3 independent experiments. ***, P < 0.001; **, P < 0.01; *, P < 0.05 relative to control cells (Student's unpaired, two-tailed t test).
FIG 2.
Toxin-induced changes in secreted chemokines and cytokines in HBMECs. HBMECs were treated with 1 μg/ml (A) or the indicated doses (B) of SubAB, SubAA272B, or Stx2 for 24 h or were left untreated (control). Levels of secreted chemokines or cytokines (as indicated) were assayed using a cytometric bead array (see Materials and Methods). The results are presented as percent changes in RFI relative to levels in control cells. The data are means ± SEM from at least 8 samples from 3 independent experiments. ***, P < 0.001; **, P < 0.01; *, P < 0.05 relative to control cells (Student's unpaired, two-tailed t test).
FIG 3.
Toxin-induced changes in secreted chemokines and cytokines in HCT-8 cells. HCT-8 cells were treated with 1 μg/ml (A) or the indicated doses (B) of SubAB, SubAA272B, or Stx2 for 24 h or were left untreated (control). Levels of secreted chemokines or cytokines (as indicated) were assayed using a cytometric bead array (see Materials and Methods). The results are presented as percent changes in RFI relative to levels in control cells. The data are means ± SEM from at least 7 samples from 3 independent experiments. ***, P < 0.001; **, P < 0.01; *, P < 0.05 relative to control cells (Student's unpaired, two-tailed t test).
FIG 4.
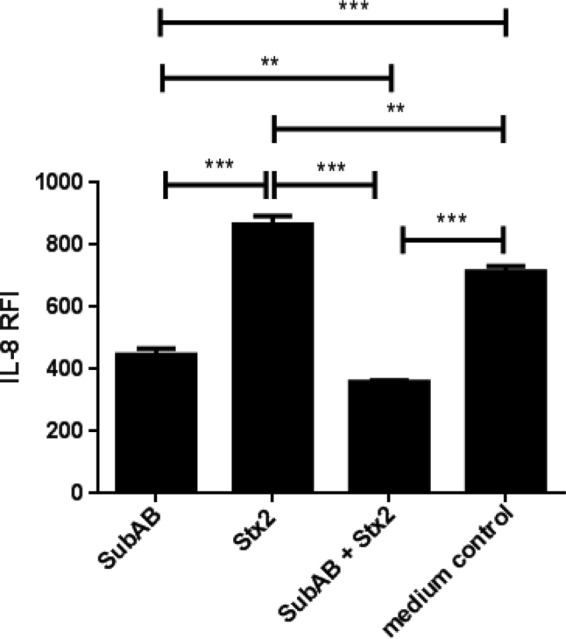
Effect of SubAB plus Stx2 on secretion of IL-8 in U937 cells. U937 cells were treated with 1 μg/ml SubAB or Stx2, or both combined, for 24 h or were left untreated (control). Levels of secreted IL-8 were assayed using a cytometric bead array (see Materials and Methods). The results are presented as IL-8 RFI. The data are means and SEM from at least 3 samples. ***, P < 0.001; **, P < 0.01 (Student's unpaired, two-tailed t test).
Transcriptional responses to SubAB and Stx2.
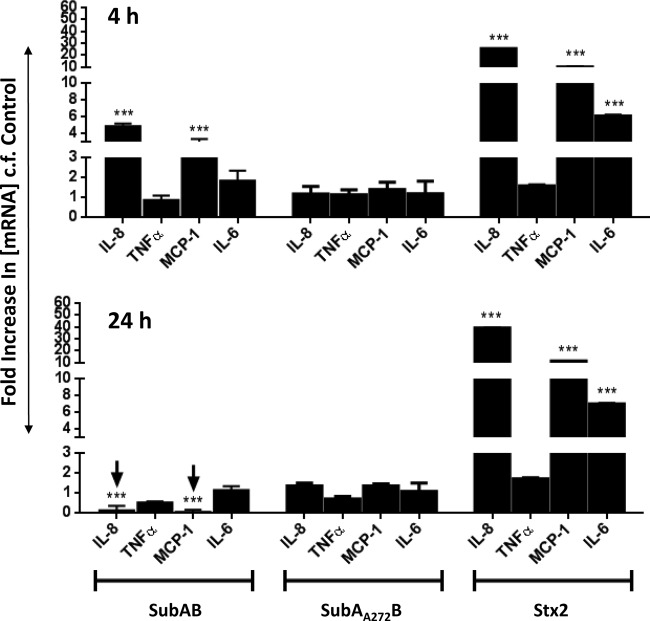
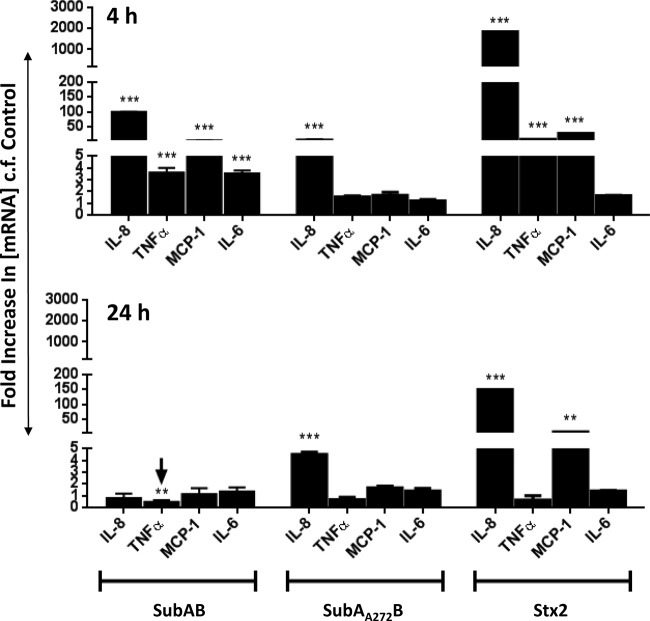
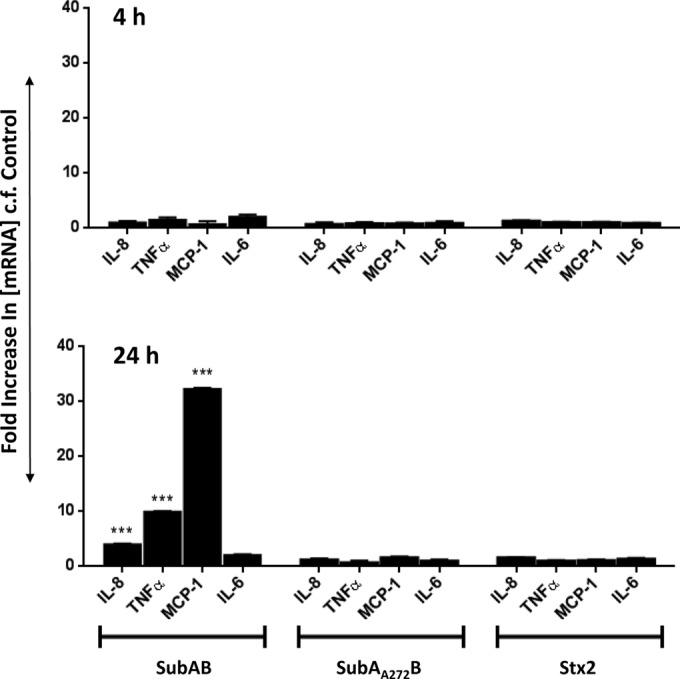
To determine whether the effects of SubAB and Stx2 on chemokine/cytokine expression at the protein level were also reflected at the transcriptional level, mRNA levels were measured by quantitative real-time RT-PCR 4 and 24 h after treatment of U937 cells (Fig. 5), HBMECs (Fig. 6), and HCT-8 cells (Fig. 7) with 1 μg/ml SubAB, SubAA272B, or Stx2. In general, U937 cells showed relatively late responses to SubAB (at 24 h only) (Fig. 5) and no response to Stx2, while HBMECs and HCT-8 cells showed earlier but transient responses to SubAB (at 4 h only) (Fig. 6 and 7) and earlier, sustained, and profound responses to Stx2 (at both 4 and 24 h) (Fig. 6 and 7).
FIG 5.
Toxin-induced changes in chemokine and cytokine mRNA levels in U937 cells. U937 cells were treated with 1 μg/ml SubAB, SubAA272B, or Stx2 or were left untreated (control) for 4 or 24 h. Total RNA was then extracted from the cells, and chemokine/cytokine mRNAs (as indicated) were quantitated by real-time RT-PCR (see Materials and Methods). The results are expressed as fold increases in concentrations of mRNAs relative to levels in control cells. The data are means and SEM from at least 8 samples from three independent experiments. ***, P < 0.001 relative to control cells (Student's unpaired, two-tailed t test).
FIG 6.
Toxin-induced changes in chemokine and cytokine mRNA levels in HBMECs. HBMECs were treated with 1 μg/ml SubAB, SubAA272B, or Stx2 or were left untreated (control) for 4 or 24 h. Total RNA was then extracted from the cells, and chemokine/cytokine mRNAs (as indicated) were quantitated by real-time RT-PCR (see Materials and Methods). The results are expressed as fold increases in concentrations of mRNAs relative to levels in control cells. The data are means and SEM from at least 8 samples from three independent experiments. ***, P < 0.001 relative to control cells (Student's unpaired, two-tailed t test).
FIG 7.
Toxin-induced changes in chemokine and cytokine mRNA levels in HCT-8 cells. HCT-8 cells were treated with 1 μg/ml SubAB, SubAA272B, or Stx2 or were left untreated (control) for 4 or 24 h. Total RNA was then extracted from the cells, and chemokine/cytokine mRNAs (as indicated) were quantitated by real-time RT-PCR (see Materials and Methods). The results are expressed as fold increases in concentrations of mRNAs relative to levels in control cells. The data are means and SEM from at least 8 samples from three independent experiments. ***, P < 0.001; **, P < 0.01 relative to control cells (Student's unpaired, two-tailed t test).
In SubAB-treated U937 cells (Fig. 5) at 24 h, the mRNA levels of IL-8, TNF-α, and MCP-1 were upregulated 3.9-fold, 9.9-fold, and 32-fold, respectively, relative to those in untreated control cells (all P values were <0.001). However, at 4 h, no significant upregulation was detected. Moreover, treatment of U937 cells with either SubAA272B or Stx2 did not significantly upregulate any of the chemokine/cytokine mRNAs at either time point (Fig. 5).
In SubAB-treated HBMECs (Fig. 6) at 4 h, the mRNA levels of IL-8 and MCP-1 were upregulated 4.9-fold and 2.9-fold, respectively, relative to those in untreated control cells (both P values were <0.001). However, this upregulation had subsided by 24 h, and the mRNA levels of IL-8 and MCP-1 in SubAB-treated HBMECs were actually significantly lower than those in untreated control cells (fold changes of 0.12-fold for IL-8 and 0.05-fold for MCP-1; both P values were <0.001). In marked contrast, the upregulation of IL-8, MCP-1, and IL-6 mRNAs in Stx2-treated HBMECs was sustained and was present at both 4 and 24 h. The increases relative to levels in untreated cells at 4 h and 24 h were 25-fold and 39-fold, respectively, for IL-8 (both P values were <0.001), 10-fold and 11-fold, respectively, for MCP-1 (both P values were <0.001), and 6.1-fold and 7-fold, respectively, for IL-6 (both P values were <0.001).
In SubAB-treated HCT-8 cells (Fig. 7), the mRNA levels of IL-8, MCP-1, TNF-α, and IL-6 were significantly upregulated at 4 h (99-fold, 3.6-fold, 5.9-fold, and 3.5-fold, respectively, relative to the levels in untreated control cells; all P values were <0.001). However, at 24 h, the upregulation had subsided, with a TNF-α mRNA level in SubAB-treated HCT-8 cells that was actually significantly lower than that in untreated control cells (0.46-fold; P < 0.01). SubAA272B also induced modest IL-8 mRNA responses at both the 4-h and 24-h time points (6.5-fold and 4.5-fold, respectively; both P values were <0.001) (Fig. 7). However, in Stx2-treated HCT-8 cells, the upregulation of mRNA levels of IL-8, TNF-α, and MCP-1 was much more pronounced at both time points. For example, the fold increases in IL-8 mRNA in Stx2-treated HCT-8 cells relative to untreated cells at 4 and 24 h were 1,800-fold and 150-fold, respectively (both P values were <0.001) (Fig. 7).
Toxin-induced changes in cell-associated IL-8 and MCP-1.
The above studies showed that in all three cell types, treatment with SubAB reduced the levels of IL-8 and MCP-1 secreted into the culture medium, yet the respective mRNAs were significantly upregulated. One possible explanation for this apparent incongruity is that effects of SubAB treatment on ER function through cleavage of BiP might impair export of the newly synthesized chemokines. Accordingly, levels of cell-associated IL-8 and MCP-1 were examined in U937 cells exposed to 1 μg/ml SubAB, SubAA272B, or Stx2 by using flow cytometry (Fig. 8). At 24 h, both SubAB- and Stx2-treated U937 cells showed elevated levels of cell-associated IL-8 and MCP-1 compared with those in untreated control cells (both P values were <0.001). SubAA272B-treated U937 cells also showed higher levels of cell-associated IL-8 and MCP-1 than those in untreated control cells, albeit to a lesser degree (P < 0.001). Furthermore, both SubAB- and Stx2-treated cells showed more cell-associated IL-8 and MCP-1 than those in SubAA272B-treated cells (both P values were <0.001).
FIG 8.
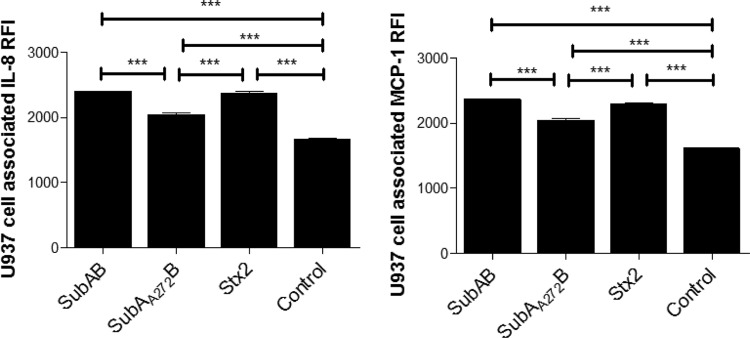
Toxin-induced changes in cell-associated IL-8 and MCP-1 in U937 cells. U937 cells were treated with 1 μg/ml SubAB, SubAA272B, or Stx2 for 24 h or were left untreated (control), after which cells were fixed and permeabilized. Cell-associated IL-8 and MCP-1 were measured by FACS analysis, using biotin-conjugated specific antibodies followed by streptavidin-PE, as described in Materials and Methods. For each sample, the mean chemokine RFI was acquired from 10,000 cells. The data are means and SEM from at least 4 samples. ***, P < 0.001 relative to control cells (Student's unpaired, two-tailed t test).
Toxin-induced CHOP induction and XBP1 mRNA splicing.
To confirm that SubAB was indeed eliciting ER stress-induced UPR in all three cell lines, we first examined CHOP gene induction by quantitative RT-PCR analysis at 4 h and 24 h (Fig. 9). SubAB significantly induced CHOP mRNA in all three tested cell lines at both time points. For U937 and HCT-8 cells, induction was greater at 4 h than at 24 h (26-fold versus 17-fold and 42-fold versus 2.3-fold, respectively), whereas in HBMECs, SubAB elicited 4.7-fold and 13-fold inductions of CHOP mRNA at 4 h and 24 h, respectively. Stx2 also significantly induced CHOP expression in HBMECs and HCT-8 cells, albeit to a lesser extent than that with SubAB. Like the case with SubAB, Stx2-mediated CHOP induction was greater at 4 h in HCT-8 cells and at 24 h in HBMECs. However, Stx2 did not induce CHOP mRNA in U937 cells. Likewise, SubAA272B did not induce CHOP mRNA in any of the tested cell lines at either time point (Fig. 9).
FIG 9.
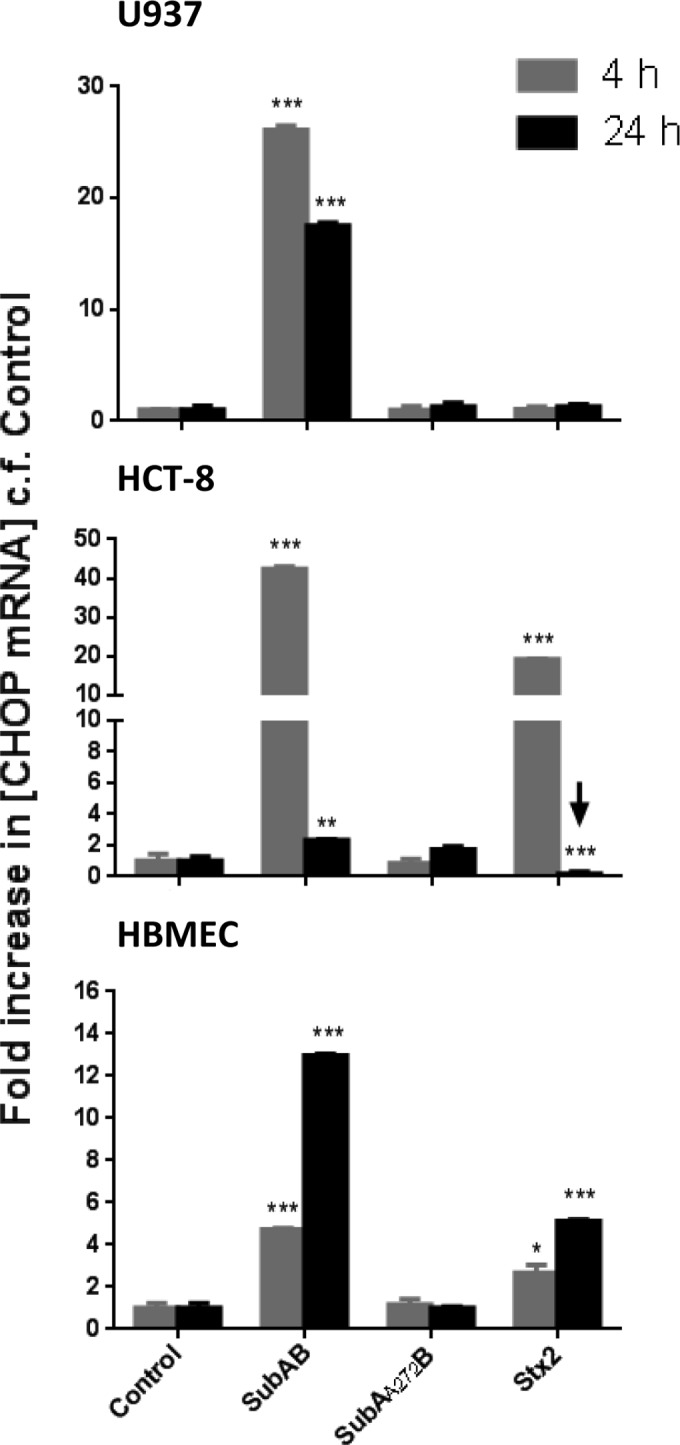
Toxin-induced CHOP mRNA induction. U937 cells, HBMECs, or HCT-8 cells were treated with 1 μg/ml SubAB, SubAA272B, or Stx2 or were left untreated (control) for 4 h or 24 h. Total RNA was extracted from the cells, and CHOP mRNA was quantitated by real-time RT-PCR using primers specific for CHOP (Table 1), as described in Materials and Methods. The results are expressed as fold increases in concentrations of mRNA relative to levels in control cells. The data are means and SEM from 3 samples. ***, P < 0.001; **, P < 0.01; *, P < 0.05 relative to control cells (Student's unpaired, two-tailed t test).
Activation of the IRE1 ER stress-signaling pathway by the various toxins was also examined by monitoring XBP1 mRNA splicing by RT-PCR (Fig. 10). The assay used generates amplicons of 398 and 424 bp for spliced (indicating IRE1 activation) and unspliced XBP1 mRNA variants, respectively (16). The assay can also generate a larger amplicon species (450 bp), which is a hybrid of the two products (16). Treatment with SubAB for 4 h elicited virtually complete splicing of XBP1 mRNA in all three tested cell lines, but there was negligible splicing in control cells and those treated with Stx2 or SubAA272B.
FIG 10.
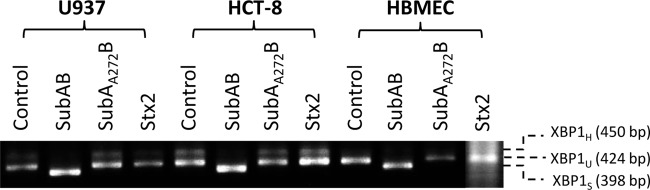
SubAB-induced XBP1 mRNA splicing. U937 cells, HBMECs, or HCT-8 cells were treated with 1 μg/ml SubAB, SubAA272B, or Stx2 or were left untreated (control) for 4 h. Total RNA was extracted from the cells, and XBP1 mRNA was analyzed by RT-PCR using primers (Table 1) specific for XBP1, as described in Materials and Methods. The expected mobilities of spliced (XBP1S), unspliced (XBP1U), and hybrid (XBP1H) amplicons are indicated.
Effect of toxin treatment on cell viability.
Viability of the three cell lines was monitored after 24 h of incubation with various doses of SubAB, SubAA272B, or Stx2 (Fig. 11). SubAA272B did not affect the viability of any of the cell lines. However, SubAB did reduce viability: at 1 μg/ml, the viability of U937 cells, HBMECs, and HCT-8 cells was reduced approximately 29%, 15%, and 6%, respectively, relative to that of untreated cells or cells treated with SubAA272B. Stx2 had a similar effect on cell viability for HBMECs and HCT-8 cells but did not affect U937 cell viability.
FIG 11.
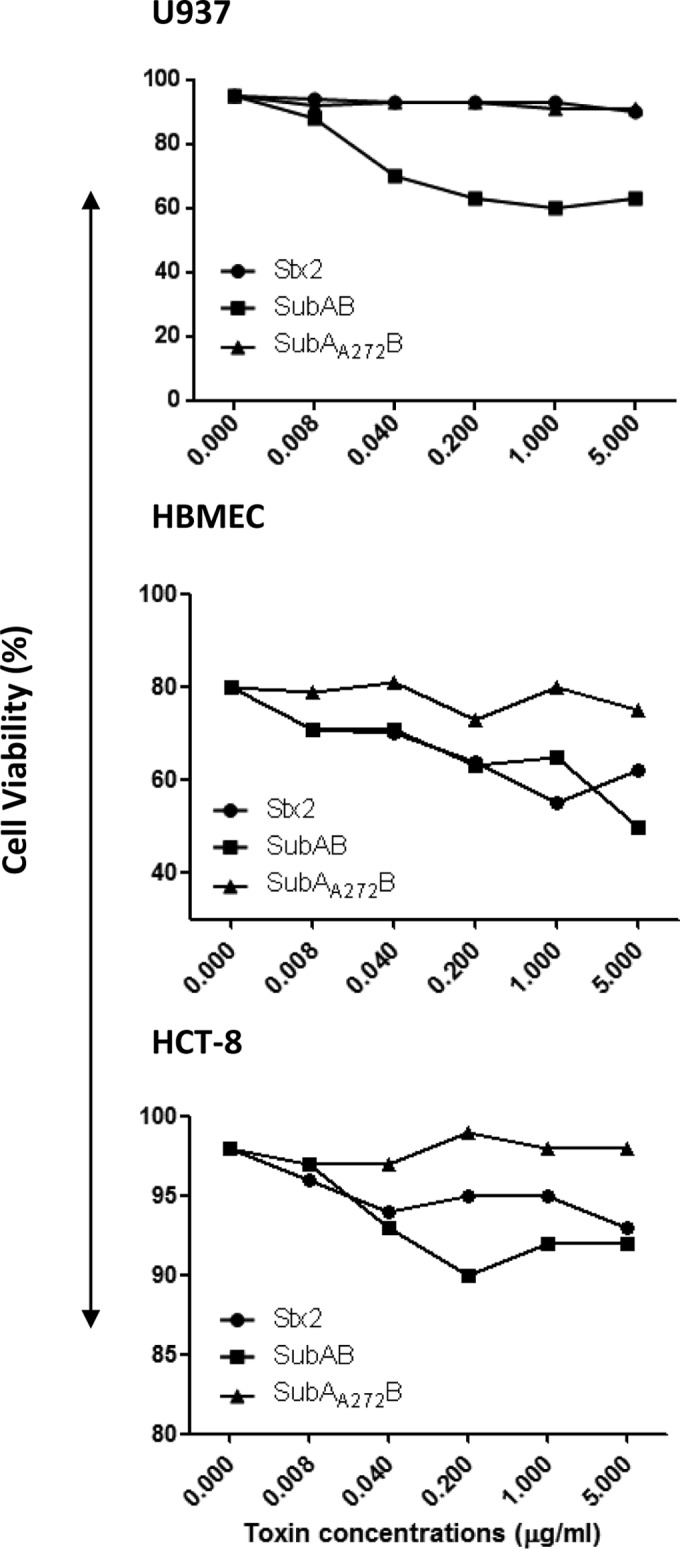
Effects of toxins on cell viability. U937 cells, HBMECs, or HCT-8 cells were treated with the indicated doses of SubAB, SubAA272B, or Stx2 for 24 h or were left untreated (control). The cells were then stained with trypan blue solution and counted with an automated differential cell counter.
DISCUSSION
Previous in vivo studies have shown that intraperitoneal injection of purified SubAB elicits profound effects on leukocyte populations in mice, including leukocytosis, multiorgan neutrophil infiltration, and dramatic splenic atrophy (17, 19). A previous study using a mouse macrophage cell line and murine peritoneal macrophages showed that treatment with SubAB decreased the basal level of secreted MCP-1 but also suppressed MCP-1 and TNF-α responses to lipopolysaccharide (24). In the current study, we investigated the effects of SubAB treatment on secretion of a larger set of chemokines and cytokines: IL-8, MCP-1, MIP-1β, TNF-α, and IL-6. Moreover, we investigated these effects in human macrophage, endothelial, and epithelial cell lines (U937 cells, HBMECs, and HCT-8 cells, respectively). These cell types may be involved in distinct stages of pathogenesis of STEC infections and development of HUS in humans. Moreover, the fact that they are human cell lines is important, because there are marked differences in expression of the receptor for SubAB (glycans terminating in α2,3-linked N-glycolylneuraminic acid) between mouse and human tissues (5). Thus, previous findings from mice may not necessarily be predictive of those in humans.
Changes in secreted leukocyte chemoattractants (IL-8, MCP-1, and MIP-1β) and secreted leukocyte activators (TNF-α and IL-6) and related gene expression (mRNA levels) were investigated in cells treated with SubAB compared with those treated with the nonproteolytic mutant derivative SubAA272B or Stx2. Treatment with Stx2 elicited significant increases in secretion of IL-8 in all three cell lines, as well as that of MCP-1 and MIP-1β in U937 cells, IL-6 and MCP-1 in HBMECs, and TNF-α in HCT-8 cells. However, SubAB treatment did not elicit any increases in the examined secreted chemokines and cytokines. Rather, it induced significant decreases in secreted IL-8 and MCP-1 in all three cell lines and a significant decrease in secreted IL-6 in HBMECs. This may reflect a general defect in chemokine and cytokine secretion in all three cell types, as in all other examples where SubAB was not shown to have significant inhibitory effects, total baseline cytokine or chemokine secretion levels for control cells were very low, precluding detection of any inhibitory effect of the toxin. The downregulation of secreted chemokines or cytokines was dependent on the cytotoxic (i.e., proteolytic) activity of SubAB, as it was not observed in SubAA272B-treated cells. Thus, SubAB-mediated suppression of secreted chemokines or cytokines is dependent on the toxin's capacity to cleave the ER chaperone BiP. The highly specific cleavage of BiP by SubAB (7) has been shown to rapidly activate ER stress-induced UPR (10), which in turn has been shown to suppress cytokine responses to inflammatory stimuli (25, 26). In the present study, SubAB, but not SubAA272B, induced CHOP expression and XBP1 mRNA splicing in all three cell lines, confirming the activation of ER stress-induced UPR. Interestingly, Stx2 also induced CHOP expression, albeit to a lesser extent than that with SubAB, but not XBP1 mRNA splicing, indicating that the weaker UPR emanating from Stx2 is independent of the IRE1 ER stress-signaling pathway. However, Stx2 has been reported to activate the PERK and ATF6 signaling pathways (15, 16), accounting for the upregulation of CHOP in the present study.
The decreased levels of cytokines and chemokines released into the culture supernatants of SubAB-treated cells were not matched by reductions in the respective cellular mRNA levels, except for IL-8 and MCP-1 mRNAs in HBMECs at 24 h. Indeed, in SubAB-treated HBMECs and HCT-8 cells, there were significant increases in IL-8 and MCP-1 mRNAs at 4 h. This contrasts with findings in Stx2-treated HBMECs and HCT-8 cells, which showed sustained elevated mRNA levels for IL-8 and MCP-1 at both 4 and 24 h. Yamazaki et al. reported that in mouse renal tubular epithelial (NRK-52E) cells, cleavage of BiP by SubAB leads to transient phosphorylation of Akt (also known as protein kinase B) and consequent activation of NF-κB through the ATF6 branch of the UPR. Transient activation of NF-κB induced transient upregulation of proinflammatory gene expression (27). These findings are consistent with those for HBMECs and HCT-8 cells in the current study. SubAB induced marked upregulation of IL-8, MCP-1, and IL-6 gene expression at 4 h, but this had subsided by 24 h. Indeed, the mRNA levels of IL-8 and MCP-1 in SubAB-treated HBMECs and the mRNA level of TNF-α in SubAB-treated HCT-8 cells at 24 h were significantly lower than those in untreated control cells. The possible mechanism for the delayed suppression of chemokine gene expression may involve UPR- and NF-κB-dependent, SubAB-induced expression of A20, an endogenous negative-feedback regulator of NF-κB (28).
The reduction in chemokine and cytokine secretion in SubAB-treated cells could be partly attributable to toxin-induced cell death, as modest reductions in viability were observed in all three cell lines at 24 h. However, significant decreases in some of the secreted chemokines or cytokines occurred at SubAB doses as low as 8 ng/ml, a concentration at which effects on viability were negligible. A further issue is that Stx2 treatment also reduced the viability of HBMECs and HCT-8 cells, to an extent similar to that with SubAB, yet it had the opposite effect on chemokine and cytokine secretion. Indeed, the stimulatory effect of Stx2 on secretion was greatest at the maximum toxin doses tested. Furthermore, the reductions in viability caused by treatment with either SubAB or Stx2 did not impair the capacity of the cells to upregulate cytokine and chemokine mRNA expression levels in response to the toxins.
The poor correlation between levels of secreted chemokines and the respective mRNAs in SubAB-treated cells may be attributable to two separate factors. First, SubAB-induced UPR is known to result in transient inhibition of translation through PERK-dependent phosphorylation of eIF2α (10). This component of the UPR is designed to slow the rate of arrival of newly translated proteins that require folding to the ER, while other arms of the UPR increase the protein-folding capacity in the hope that ER homeostasis can be reestablished (11). Thus, SubAB-induced stimulation of chemokine gene transcription may not translate into actual protein products. A second possibility is that perturbation of ER function by SubAB-mediated destruction of BiP may compromise the export of translated chemokines into the culture medium. In the present study, treatment of U937 cells with SubAB resulted in significant increases in the levels of cell-associated IL-8 and MCP-1, consistent with this hypothesis. SubAB was previously shown to block antibody secretion by mouse B lymphocytes (29). Cleavage of BiP by SubAB separates the N-terminal ATPase from the C-terminal protein-binding domain, such that in toxin-treated cells, newly synthesized immunoglobulin light chains remain bound to the C-terminal fragment and are retained in the ER compartment (29). Nevertheless, the precise mechanism whereby SubAB induces decreases in secreted chemokines and cytokines in the face of increased transcription needs to be investigated further.
Significantly increased levels of cell-associated IL-8 and MCP-1 were also seen in Stx2-treated U937 cells, but unlike the case for SubAB-treated cells, this paralleled increased levels of secreted chemokines. This may seem incongruous given the fact that Shiga toxins are potent protein synthesis inhibitors, but U937 cells have previously been shown to be relatively insensitive to Stx due to low expression of the toxin receptor Gb3 (30). Furthermore, Falguières et al. (31) showed that in highly susceptible cell lines (e.g., HeLa), Gb3-bound Stx associates with lipid rafts and is trafficked to the ER via early endosomes and the Golgi apparatus, circumventing the late endocytic pathway. However, this did not occur in cells derived from human monocytes, i.e., macrophages and dendritic cells, and although Stx was internalized in a receptor-dependent manner, lipid raft-mediated retrograde transport to the ER did not occur and the internalized toxin was degraded in lysosomes. This may also account for the findings in the present study showing that Stx2 treatment did not significantly increase chemokine or cytokine mRNA levels, CHOP induction, and cell death in U937 cells. In contrast to U937 cells, HBMECs and HCT-8 cells showed great sensitivity to Stx2. Stx2 induced profound and sustained increases in mRNAs of IL-8, MCP-1, and IL-6 in HBMECs and increases in mRNAs of IL-8, MCP-1, and TNF-α in HCT-8 cells, as well as significant increases in secreted IL-8, MCP-1, and IL-6 in HBMECs and secreted IL-8 and TNF-α in HCT-8 cells. Thus, Stx2 induced both leukocyte chemoattractants (IL-8 and MCP-1) and activators (TNF-α and IL-6) at both the protein and mRNA levels in human HBMECs and/or HCT-8 cells. This is consistent with previous observations in human umbilical vein and glomerular endothelial cells and human astrocytes (32, 33), as well as with clinical studies showing elevations of blood levels of TNF-α, IL-8, and IL-6 in HUS patients (21). At present, there is a paucity of information on the impact of SubAB on chemokine and cytokine responses in vivo. To the best of our knowledge, there are no published reports of cytokine profiles for patients with HUS caused by SubAB-expressing STEC strains. Furukawa et al. (34) reported elevated IL-6 expression in intestinal tissue of mice after intraperitoneal injection of purified SubAB, and this was associated with fatal hemorrhage. They did not observe changes in expression of any of the other cytokines tested (IL-1, IL-4, IL-10, IL-12, IL-17, IL-22, gamma interferon, TNF-α, or granulocyte-macrophage colony-stimulating factor [GM-CSF]). Notably, we did not observe SubAB-mediated increases in IL-6 secretion in any of the cell lines tested. Although IL-6 mRNA was elevated by SubAB in HCT-8 cells, this was not reflected at the protein level.
Further noteworthy observations in the current study were the modest but statistically significant increases in cell-associated IL-8 and MCP-1 in SubAA272B-treated U937 cells and the mild elevation in IL-8 mRNA in SubAA272B-treated HCT-8 cells. Statistically significant increases in secreted IL-8 and MCP-1 were also seen in HCT-8 cells treated with 5 μg/ml SubAA272B. This effect must be independent of BiP cleavage, because the mutant toxin has negligible proteolytic activity due to mutation of the critical active site Ser272 residue (1, 7). These findings are most likely attributable to signaling events triggered by interactions of the mutant toxin's B-subunit pentamer with receptors on the target cell surface. Indeed, we recently showed that ArtB, a homologue with ∼50% identity to SubB produced by Salmonella enterica serovar Typhi, upregulates a range of chemokines, cytokines, and adhesion molecules in the same cell lines used in the present study (23). Thus, the A and B subunits of SubAB may have distinct, perhaps even opposing effects on chemokine and cytokine responses during infection with SubAB-producing STEC. Superimposed on this is the potential for SubAB to modulate inflammatory responses elicited by Stx2. Indeed, we showed in the present study that treatment of U937 cells with both toxins abolished the stimulatory effect of Stx2 on IL-8 secretion. Furthermore, IL-8 secretion was significantly lower than that seen in cells treated with SubAB alone, suggesting that the two toxins may synergize. Such interplay may have important consequences for the pathogenesis of disease during infection with STEC strains that produce both these distinct, potent AB5 toxins.
ACKNOWLEDGMENTS
This work was supported by program grant 565526 and project grant 1002792 from the National Health and Medical Research Council of Australia (NHMRC) and by discovery grant DP120103178 from the Australian Research Council (ARC). A.W.P. is an ARC DORA Fellow. J.C.P. is an NHMRC Senior Principal Research Fellow.
Footnotes
Published ahead of print 9 June 2014
REFERENCES
- 1.Paton AW, Srimanote P, Talbot UM, Wang H, Paton JC. 2004. A new family of potent AB5 cytotoxins produced by Shiga toxigenic Escherichia coli. J. Exp. Med. 200:35–46. 10.1084/jem.20040392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paton AW, Woodrow MC, Doyle RM, Lanser JA, Paton JC. 1999. Molecular characterization of a Shiga toxigenic Escherichia coli O113:H21 strain lacking eae responsible for a cluster of cases of hemolytic-uremic syndrome. J. Clin. Microbiol. 37:3357–3361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paton AW, Paton JC. 2005. Multiplex PCR for direct detection of Shiga toxigenic Escherichia coli strains producing the novel subtilase cytotoxin. J. Clin. Microbiol. 43:2944–2947. 10.1128/JCM.43.6.2944-2947.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khaitan A, Jandhyala DM, Thorpe CM, Ritchie JM, Paton AW. 2007. The operon encoding SubAB, a novel cytotoxin, is present in Shiga toxin-producing Escherichia coli isolates from the United States. J. Clin. Microbiol. 45:1374–1375. 10.1128/JCM.00076-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Byres E, Paton AW, Paton JC, Löfling JC, Smith DF, Wilce MCJ, Talbot UM, Chong DC, Yu H, Huang S, Chen X, Varki NM, Varki A, Rossjohn J, Beddoe T. 2008. Incorporation of a non-human glycan mediates human susceptibility to a bacterial toxin. Nature 456:648–652. 10.1038/nature07428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chong DC, Paton JC, Thorpe CM, Paton AW. 2008. Clathrin-dependent trafficking of subtilase cytotoxin, a novel AB5 toxin that targets the ER chaperone BiP. Cell. Microbiol. 10:795–806. 10.1111/j.1462-5822.2007.01085.x [DOI] [PubMed] [Google Scholar]
- 7.Paton AW, Beddoe T, Thorpe CM, Whisstock JC, Wilce MC, Rossjohn J, Talbot UM, Paton JC. 2006. AB5 subtilase cytotoxin inactivates the endoplasmic reticulum chaperone BiP. Nature 443:548–552. 10.1038/nature05124 [DOI] [PubMed] [Google Scholar]
- 8.Gething MJ. 1999. Role and regulation of the ER chaperone BiP. Semin. Cell Dev. Biol. 10:465–472. 10.1006/scdb.1999.0318 [DOI] [PubMed] [Google Scholar]
- 9.Hendershot LM. 2004. The ER function BiP is a master regulator of ER function. Mt. Sinai J. Med. 71:289–297 [PubMed] [Google Scholar]
- 10.Wolfson JJ, May KL, Thorpe CM, Jandhyala DM, Paton JC, Paton AW. 2008. Subtilase cytotoxin activates PERK, IRE1 and ATF6 endoplasmic reticulum stress-signalling pathways. Cell. Microbiol. 10:1775–1786. 10.1111/j.1462-5822.2008.01164.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boyce M, Yuan J. 2006. Cellular response to endoplasmic reticulum stress: a matter of life or death. Cell Death Differ. 13:363–373. 10.1038/sj.cdd.4401817 [DOI] [PubMed] [Google Scholar]
- 12.May KL, Paton JC, Paton AW. 2010. Escherichia coli subtilase cytotoxin induces apoptosis regulated by host Bcl-2 family proteins, Bax/Bak. Infect. Immun. 78:4691–4696. 10.1128/IAI.00801-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yahiro K, Morinaga N, Moss J, Noda M. 2010. Subtilase cytotoxin induces apoptosis in HeLa cells by mitochondrial permeabilization via activation of Bax/Bak, independent of C/EBF-homologue protein (CHOP), Ire1alpha or JNK signaling. Microb. Pathog. 49:153–163. 10.1016/j.micpath.2010.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beddoe T, Paton AW, Le Nours J, Rossjohn J, Paton JC. 2010. Structure, biological functions and applications of the AB5 toxins. Trends Biochem. Sci. 35:411–418. 10.1016/j.tibs.2010.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee SY, Lee MS, Cherla RP, Tesh VL. 2008. Shiga toxin 1 induces apoptosis through the endoplasmic reticulum stress response in human monocytic cells. Cell. Microbiol. 10:770–780. 10.1111/j.1462-5822.2007.01083.x [DOI] [PubMed] [Google Scholar]
- 16.Fujii J, Wood K, Matsuda F, Carneiro-Filho BA, Schlegel KH, Yutsudo T, Binnington-Boyd B, Lingwood CA, Obata F, Kim KS, Yoshida SI, Obrig T. 2008. Shiga toxin 2 causes apoptosis in human brain microvascular endothelial cells via C/EBP homologous protein. Infect. Immun. 76:3679–3689. 10.1128/IAI.01581-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang H, Paton JC, Paton AW. 2007. Pathologic changes in mice induced by subtilase cytotoxin, a potent new Escherichia coli AB5 toxin that targets the endoplasmic reticulum. J. Infect. Dis. 196:1093–1101. 10.1086/521364 [DOI] [PubMed] [Google Scholar]
- 18.Wang H, Paton JC, Thorpe CM, Bonder CS, Sun WY, Paton AW. 2010. Tissue factor-dependent procoagulant activity of subtilase cytotoxin, a potent AB5 toxin produced by Shiga toxigenic Escherichia coli. J. Infect. Dis. 202:1415–1423. 10.1086/656534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang H, Paton AW, McColl SR, Paton JC. 2011. In vivo leukocyte changes induced by Escherichia coli subtilase cytotoxin. Infect. Immun. 79:1671–1679. 10.1128/IAI.01204-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Isogai E, Isogai H, Kimura K, Hayashi S, Kubota T, Fujii N, Takeshi K. 1998. Role of tumor necrosis factor alpha in gnotobiotic mice infected with an Escherichia coli O157:H7 strain. Infect. Immun. 66:197–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karpman D, Andreasson A, Thysell H, Kaplan BS, Svanborg C. 1995. Cytokines in childhood hemolytic uremic syndrome and thrombotic thrombocytopenic purpura. Pediatr. Nephrol. 9:694–699. 10.1007/BF00868714 [DOI] [PubMed] [Google Scholar]
- 22.Talbot UM, Paton JC, Paton AW. 2005. Protective immunization of mice with an active-site mutant of subtilase cytotoxin of Shiga toxin-producing Escherichia coli. Infect. Immun. 73:4432–4436. 10.1128/IAI.73.7.4432-4436.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang H, Paton JC, Herdman BP, Rogers TJ, Beddoe T, Paton AW. 2013. The B subunit of an AB5 toxin produced by Salmonella enterica serovar Typhi up-regulates chemokines, cytokines and adhesion molecules in human macrophage, colonic epithelial and brain microvascular endothelial cell lines. Infect. Immun. 81:673–683. 10.1128/IAI.01043-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harama D, Koyama K, Mukai M, Shimokawa N, Miyata M, Nakamura Y, Ohnuma Y, Ogawa H, Matsuoka S, Paton AW, Paton JC, Kitamura M, Nakao A. 2009. A subcytotoxic dose of subtilase cytotoxin prevents lipopolysaccharide-induced inflammatory responses, depending on its capacity to induce the unfolded protein response. J. Immunol. 183:1368–1374. 10.4049/jimmunol.0804066 [DOI] [PubMed] [Google Scholar]
- 25.Okamura M, Takano Y, Hiramatsu N, Hayakawa K, Yao J, Paton AW, Paton JC, Kitamura M. 2008. Suppression of cytokine responses by indomethacin in podocytes: a mechanism through induction of unfolded protein response. Am. J. Physiol. Renal Physiol. 295:F1495–F1503. 10.1152/ajprenal.00602.2007 [DOI] [PubMed] [Google Scholar]
- 26.Takano Y, Hiramatsu N, Okamura M, Hayakawa K, Shimada T, Kasai A, Yokouchi M, Shitamura A, Yao J, Paton AW, Paton JC, Kitamura M. 2007. Suppression of cytokine responses by GATA inhibitor K-7174: implication for unfolded protein response. Biochem. Biophys. Res. Commun. 360:470–475. 10.1016/j.bbrc.2007.06.082 [DOI] [PubMed] [Google Scholar]
- 27.Yamazaki H, Hiramatsu N, Hayakawa K, Okamura M, Huang T, Nakajima S, Yao J, Paton AW, Paton JC, Kitamura M. 2009. Activation of the Akt-NF-κB pathway by subtilase cytotoxin through the ATF6 branch of the unfolded protein response. J. Immunol. 183:1480–1487. 10.4049/jimmunol.0900017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakajima S, Saito Y, Takahashi S, Hiramatsu N, Kato H, Johno H, Yao J, Paton AW, Paton JC, Kitamura M. 2010. Anti-inflammatory subtilase cytotoxin up-regulates A20 through the unfolded protein response. Biochem. Biophys. Res. Commun. 397:176–180. 10.1016/j.bbrc.2010.05.069 [DOI] [PubMed] [Google Scholar]
- 29.Hu CA, Dougan SK, Winter SV, Paton AW, Paton JC, Ploegh HL. 2009. Subtilase cytotoxin cleaves newly synthesised BiP and blocks antibody secretion in B lymphocytes. J. Exp. Med. 206:2429–2440. 10.1084/jem.20090782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ramegowda B, Tesh VL. 1996. Differentiation-associated toxin receptor modulation, cytokine production, and sensitivity to Shiga-like toxins in human monocytes and monocytic cell lines. Infect. Immun. 64:1173–1180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Falguières T, Mallard F, Baron C, Hanau D, Lingwood C, Goud B, Salamero J, Johannes L. 2001. Targeting of Shiga toxin B-subunit to retrograde transport route in association with detergent-resistant membranes. Mol. Biol. Cell 12:2453–2468. 10.1091/mbc.12.8.2453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zoja C, Angioletti S, Donadelli R, Zanchi C, Tomasoni S, Binda E, Imberti B, te Loo M, Monnens L, Remuzzi G, Morigi M. 2002. Shiga toxin-2 triggers endothelial leukocyte adhesion and transmigration via NF-kappaB dependent up-regulation of IL-8 and MCP-1. Kidney Int. 62:846–856. 10.1046/j.1523-1755.2002.00503.x [DOI] [PubMed] [Google Scholar]
- 33.Rogers TJ, Paton AW, McColl SR, Paton JC. 2003. Enhanced CXC chemokine responses of human colonic epithelial cells to locus of enterocyte effacement-negative Shiga toxigenic Escherichia coli. Infect. Immun. 71:5623–5632. 10.1128/IAI.71.10.5623-5632.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Furukawa T, Yahiro K, Tsuji AB, Terasaki Y, Morinaga N, Miyazaki M, Fukuda Y, Saga T, Moss J, Noda M. 2011. Fatal hemorrhage induced by subtilase cytotoxin from Shiga-toxigenic Escherichia coli. Microb. Pathog. 50:159–167. 10.1016/j.micpath.2011.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oberbach A, Schlichting N, Blüher M, Kovacs P, Till H, Stolzenburg JU, Neuhaus J. 2010. Palmitate induced IL-6 and MCP-1 expression in human bladder smooth muscle cells provides a link between diabetes and urinary tract infections. PLoS One 5:e10882. 10.1371/journal.pone.0010882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Memoli B, Procino A, Calabrò P, Esposito P, Grandaliano G, Pertosa G, Prete MD, Andreucci M, Lillo SD, Ferulano G, Cillo C, Savastano S, Colao A, Guida B. 2007. Inflammation may modulate IL-6 and C-reactive protein gene expression in the adipose tissue: the role of IL-6 cell membrane receptor. Am. J. Physiol. Endocrinol. Metab. 293:E1030–E1035. 10.1152/ajpendo.00697.2006 [DOI] [PubMed] [Google Scholar]