Abstract
Despite the dramatic reduction in the number of leprosy cases worldwide in the 1990s, transmission of the causative agent, Mycobacterium leprae, is still occurring, and new cases continue to appear. New strategies are required in the pursuit of leprosy elimination. The cross-application of vaccines in development for tuberculosis may lead to tools applicable to elimination of leprosy. In this report, we demonstrate that the chimeric fusion proteins ID83 and ID93, developed as antigens for tuberculosis (TB) vaccine candidates, elicited gamma interferon (IFN-γ) responses from both TB and paucibacillary (PB) leprosy patients and from healthy household contacts of multibacillary (MB) patients (HHC) but not from nonexposed healthy controls. Immunization of mice with either protein formulated with a Toll-like receptor 4 ligand (TLR4L)-containing adjuvant (glucopyranosyl lipid adjuvant in a stable emulsion [GLA-SE]) stimulated antigen-specific IFN-γ secretion from pluripotent Th1 cells. When immunized mice were experimentally infected with M. leprae, both cellular infiltration into the local lymph node and bacterial growth at the site were reduced relative to those of unimmunized mice. Thus, the use of the Mycobacterium tuberculosis candidate vaccines ID83/GLA-SE and ID93/GLA-SE may confer cross-protection against M. leprae infection. Our data suggest these vaccines could potentially be used as an additional control measure for leprosy.
INTRODUCTION
Prevalence rates for leprosy have sharply declined over the last 20 years, with the major breakthrough being attributed to the provision of free-of-charge treatment to all diagnosed leprosy patients. The stalled decreases in both global prevalence and new case detection rates of leprosy over the last decade indicate that additional measures are likely required. The relative success of leprosy control, however, has prompted the integration of leprosy-specific programs into general health facilities and has also reduced the resources available to research (most notably specialized investigators and funds) (1, 2). During the same period, the World Health Organization (WHO) has declared tuberculosis (TB) a global public health emergency. Indeed, over 2 billion people are now believed to be infected with Mycobacterium tuberculosis, and multi- and extremely drug-resistant strains are rapidly emerging (3, 4). Numerous groups are actively engaged in developing replacement or supplementary vaccines as an alternative or additional control strategy for the TB epidemic (5). Defined antigens, delivered as plasmid DNA, vectored DNA, or as recombinant proteins in adjuvant, have proven effective in animal models, and at least nine subunit TB vaccines have entered clinical trials (6–11).
The Mycobacterium bovis bacillus Calmette-Guérin (BCG) vaccines represent an important component within TB control programs, since they provide at least partial protection against tuberculosis (12–14) and leprosy (15). In several countries, both TB and leprosy are endemic, and the contribution of mass BCG vaccination is often overlooked as a factor within leprosy control (16). The application of emerging TB vaccines to leprosy control programs could be logistically and economically beneficial as a public health intervention, but the capacity of such vaccine candidates to elicit protective responses against infection with Mycobacterium leprae (the causative agent of leprosy) has not been investigated.
As chimeric fusion proteins comprised of three and four M. tuberculosis proteins, the TB vaccine candidate antigens ID83 and ID93 each present M. tuberculosis proteins selected from various categories (17). When combined with the synthetic Toll-like receptor 4 ligand (TLR4L) glucopyranosyl lipid adjuvant (GLA) in a stable emulsion (SE), ID93 boosts the effects of BCG, protecting mice and guinea pigs against infection with M. tuberculosis (7, 18). The current study was designed to examine the potential of the ID83/GLA-SE and ID93/GLA-SE vaccines to protect against M. leprae infection.
MATERIALS AND METHODS
Subjects and samples.
Recently diagnosed and previously untreated leprosy patients and controls from an area to which leprosy is endemic (EC) were recruited at Centro de Referencia em Diagnostico e Terapeutica and Hospital Anuar Auad, Goiânia, Goiás State, Brazil. Leprosy patients were categorized as paucibacillary (PB) by clinical, bacilloscopic, and histological observations (bacterial index, skin lesions, nerve involvement, and histopathology) carried out by qualified personnel. Blood was obtained from tuberculosis patients (M. tuberculosis sputum-positive, HIV-negative individuals with clinically confirmed pulmonary tuberculosis) who were undergoing treatment. EC were healthy individuals who had never had tuberculosis, had no history of leprosy in the family, and were living in the area to which leprosy is endemic. All had previously been immunized with BCG, and all blood samples were obtained after informed consent and after local ethics committee approval.
Determining reactivity by 24-h WBA.
Whole-blood assays (WBA) were performed with venous undiluted heparinized whole blood (Greiner). Within 2 h of collection, blood was added to each well of a 24-well plate (450 μl/well; Sigma, St. Louis, MO) and incubated with antigens at 37°C, 5% CO2. For each assay, stimulations were conducted with 10 μg/ml of recombinant protein, 10 μg/ml M. leprae cell sonicate (provided by John Spencer, Colorado State University, Fort Collins, CO, under NIH contract N01 AI-25469), or 1 μg/ml phytohemagglutinin (PHA) (Sigma). After 24 h, plasma was collected and stored at −20°C. Gamma interferon (IFN-γ) content within the plasma was determined by enzyme-linked immunosorbent assay (ELISA), used according to the manufacturer's instructions (QuantiFERON CMI; Cellestis, Carnegie, Australia). The detection limit of the test was 0.05 IU/ml. For data interpretation, we assigned as a positive result a concentration above an arbitrary cutoff point of 0.5 IU/ml.
Mice and immunizations.
Wild-type C57BL/6 mice were purchased from Charles River Laboratories (Wilmington, MA). Mice were immunized with recombinant protein formulated with saline, SE, or GLA-SE to provide a final protein concentration of 100 μg/ml antigen and 200 μg/ml GLA-SE (19). Mice were immunized up to 3 times by subcutaneous (s.c.) injection of 0.1 ml vaccine at the base of the tail at 2-week intervals. Mice were maintained under specific-pathogen-free conditions, and all procedures were approved by the appropriate institutional animal care and use committees.
Antibody responses.
Mouse sera were prepared by collection of retroorbital blood into Microtainer serum collection tubes (VWR International, West Chester, PA), followed by centrifugation at 1,200 rpm for 5 min. Each serum sample was then analyzed by antibody capture ELISA. Briefly, ELISA plates (Nunc, Rochester, NY) were coated with 1 μg/ml recombinant antigen in 0.1 M bicarbonate buffer and blocked with 1% bovine serum albumin (BSA)-phosphate-buffered saline (PBS). Then, in consecutive order and following washes in PBS-Tween 20, serially diluted serum samples, anti-mouse IgG, IgG1, or IgG2c-horseradish peroxidase (HRP) (all from Southern Biotech, Birmingham, AL), and 2,2′-azinobis(3-ethylbenzthiazolinesulfonic acid) (ABTS)-H2O2 (Kirkegaard & Perry Laboratories, Gaithersburg, MD) were added to the plates. Plates were analyzed at 405 nm (ELX808; Bio-Tek Instruments Inc., Winooski, VT). The endpoint titer was determined as the last dilution to render a positive response, determined as 2 times the mean optical density of the replicates derived from sera from unimmunized mice in the Prism software program (GraphPad Software, La Jolla, CA).
Antigen stimulation and cytokine responses.
Single-cell suspensions were prepared by disrupting spleens between sterilized frosted slides. Red blood cells were removed by lysis in 1.66% NH4Cl solution, and then mononuclear cells were enumerated by ViaCount assay with a PCA system (Guava Technologies, Hayward, CA). Single-cell suspensions were cultured at 2 × 105 cells per well in duplicate in a 96-well plate (Corning Incorporated, Corning, NY) in RPMI 1640 supplemented with 5% heat-inactivated fetal calf serum (FCS) and 50,000 units penicillin-streptomycin (Invitrogen). Cells were cultured in the presence of 10 μg/ml antigen for 96 h, after which culture supernatants were collected and cytokine content was assessed. Cytokine concentrations within culture supernatants were determined by ELISA. ELISA kits for determination of mouse IFN-γ, interleukin 5 (IL-5), IL-13 and tumor necrosis factor alpha (TNF-α) were performed according to the manufacturer's instructions (eBioscience, San Diego, CA), and optical density was determined using an ELx808 plate reader (Bio-Tek Instruments Inc., Winooski, VT).
Alternatively, for the elucidation of intracellular cytokine expression, cells were cultured at 37°C for 16 h in the presence of 1 μg/ml phorbol myristate acetate (PMA)-ionomycin (Sigma, St. Louis, MO) or 10 μg/ml recombinant antigen and Golgi Stop (BD Biosciences, San Diego, CA). Cells were fixed and permeabilized in Cytofix/Cytoperm (BD Biosciences, San Diego, CA). To stain, cells were first incubated with the anti-FcγII/IIIR antibody 2.4G2 to block nonspecific binding, before addition of a cocktail of fluorescently conjugated antibodies to identify cytokine-producing antigen-experienced T helper cells (anti-CD4, anti-CD3ε, anti-CD44, anti-IL-2, anti-IFN-γ and anti-TNF [all from eBioscience]). Flow cytometry was performed using an LSR Vantage instrument (BD Biosciences), and the data were analyzed using the FlowJo software program (Treestar, Ashland, OR).
M. leprae-induced inflammation.
To assess M. leprae-induced inflammation, live M. leprae bacilli (Thai-53 strain) were purified from the footpads of nu/nu mice at National Hansen's Disease Programs and shipped overnight on ice to the Infectious Disease Research Institute (IDRI) for inoculations (20). Heat-killed M. leprae (HKML) bacteria were obtained by heating bacilli at 70°C for 1 h and then quenching on ice. Mice were inoculated with 1 × 106 bacilli in a volume of 10 μl by intradermal (i.d.) injection into the ear pinnae. Twelve weeks later, single-cell suspensions were prepared from the (auricular) draining lymph nodes (DLN), and cell numbers were determined by ViaCount assay with a PCA system (Guava Technologies).
Determination of bacterial burden.
To assess M. leprae growth, live M. leprae bacilli (Thai-53 strain) were purified from the footpads of nu/nu mice at National Institute of Infectious Diseases. Six C57BL/6 mice per group were s.c. vaccinated with a total of 5 μg/mouse of either the ID83 or ID93 fusion protein or GLA-SE (10 μg/mouse) as a negative control 3 times with an interval of 3 weeks between inoculations and a month later were challenged with 5 × 103 M. leprae bacilli by subcutaneous (s.c.) injection into each footpad. Footpads were harvested 7 months later, and the bacilli were enumerated by direct microscopic counting of acid-fast bacilli according to the method of Shepard and McRae, with a limit of detection of 3,700 bacilli (21). Animal studies were reviewed and approved by the Animal Research Committee of Experimental Animals of the National Institute of Infectious Diseases (Tokyo, Japan) and were conducted according to their guidelines.
Statistics.
For human data, the nonparametric Kruskal-Wallis analysis-of-variance test was used to compare the IFN-γ levels among all of the groups, and the Mann Whitney U test was applied for comparison between two groups. The P values for mouse studies were determined using the Student t test. Statistics were generated using the software program MS Excel (Microsoft Corporation, Redmond, WA) or Prism (GraphPad Software, Inc., La Jolla, CA). Statistical significance was considered when the P values were <0.05.
RESULTS
Recognition of ID83 and ID93 by leprosy patients.
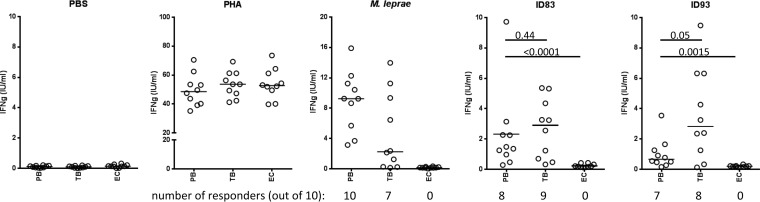
Replication and dissemination of M. leprae is limited in PB leprosy patients, suggesting that their potent cellular immune response is associated with limited or localized disease. Antigens that are recognized by immune cells of PB patients may therefore be the key to identifying an effective subunit vaccine against leprosy. We assessed the abilities of two TB vaccine candidate antigens we have developed, ID83 (a fusion of three M. tuberculosis proteins, Rv1813, Rv2608, and Rv3620) and ID93 (a fusion of four M. tuberculosis proteins, Rv1813, Rv2608, Rv3619, and Rv3620) (Table 1), to stimulate patient-specific IFN-γ recall responses in WBA. While none of the EC samples exhibited IFN-γ levels above 0.5 IU/ml, blood from TB patients reacted strongly to these proteins and secreted significant levels of IFN-γ (Fig. 1). IFN-γ levels were observed above this threshold in 8 of 10 TB patient samples stimulated with either protein. Although not as potently recognized as by blood from TB patients, ID83 and ID93 were also well recognized by PB leprosy patient blood (Fig. 1). IFN-γ levels above 0.5 IU/ml were observed in 8 of 10 PB patient samples stimulated with ID83 and 6 of 10 samples stimulated with ID93. Taken together, these data indicate that the TB vaccine candidate antigens ID83 and ID93 are also applicable to leprosy.
TABLE 1.
Homology of ID83 and ID93 components between mycobacteria
| Vaccine(s) | Component (M. tuberculosis protein)a | Homolog (% identity) from: |
|
|---|---|---|---|
| M. lepraeb | M. bovis | ||
| ID83 and ID93 | Rv1813 | Not found | Mb1843c (100) |
| Rv2608 | Not found | Mb2640 (99.8) | |
| Rv3620 | ML1055 (57.9 [in 95-aa overlap]) | ||
| ID93 only | Rv3619 | ML1056 (64.15 [in 92-aa overlap]) | |
M. tuberculosis H37Rv strain; homologs were searched for using Tuberculist.
M. leprae TN strain. aa, amino acid.
FIG 1.
The ID83 and ID93 fusion proteins are recognized by PB leprosy patients. Whole blood from the PB, TB, and EC groups was cultured for 24 h in the presence of antigen, and IFN-γ content in the plasma was measured by ELISA. Data from each individual is represented by a point, and the black bar indicates the median IFN-γ value. P values are indicated.
Impact of vaccine formulation on immune response.
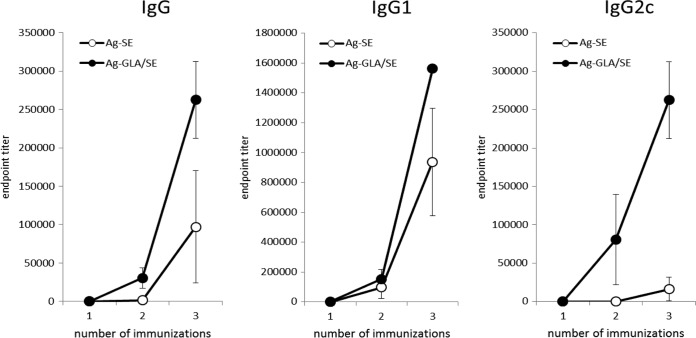
To examine this vaccine potential, mice were immunized with either ID83/SE or ID83/GLA-SE. Immunization with either formulation induced an antigen-specific IgG response (Fig. 2). However, although antigen-specific IgG1 responses were similar, GLA-SE induced a significantly greater anti-ID83 IgG2c response than SE (Fig. 2). These data indicate that immunization with the GLA-SE, but not the SE, formulation promotes a Th1-like response that could provide cross-protection against M. leprae infection.
FIG 2.
Immunization with ID83/GLA-SE but not ID83/SE promotes strong antigen-specific IgG2c responses. Mice were injected s.c. with ID83/SE and ID83/GLA-SE at biweekly intervals for a total of 3 immunizations. Serum was collected before each immunization and 1 month after the final immunization. Antigen-specific serum IgG, IgG1, and IgG2c endpoint titers were determined by antibody-capture ELISA.
Elicitation of pluripotent Th1 responses by TB vaccines.
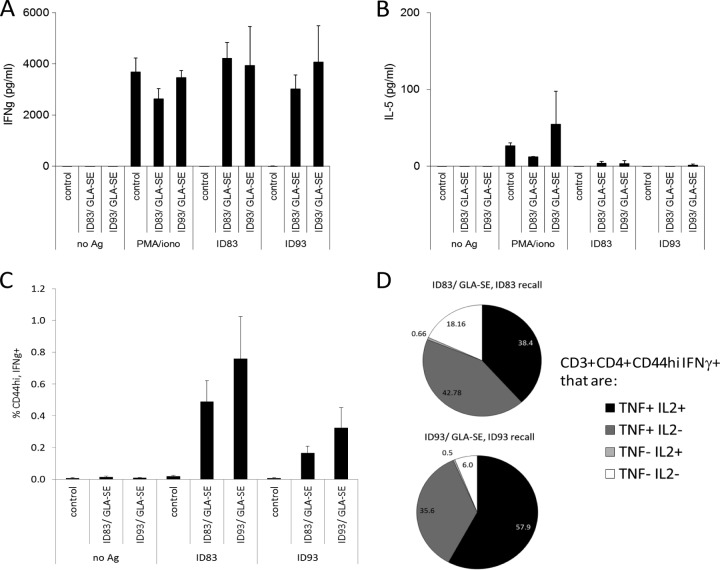
To further characterize this vaccine potential, mice were immunized with either ID83/GLA-SE or ID93/GLA-SE. Spleen cells from immunized mice responded to antigen stimulation by secreting large amounts of IFN-γ but very little IL-5, indicating the generation of a strong Th1 response (Fig. 3A and B). Furthermore, many of the antigen-specific IFN-γ-secreting cells also secreted both IL-2 and TNF, and the vast majority secreted at least one of these additional cytokines (Fig. 3C).These data indicate that immunization with either ID83/GLA-SE or ID93/GLA-SE promotes a Th1-like response that could be protective against M. leprae infection.
FIG 3.
Immunization with either ID83/GLA-SE or ID93/GLA-SE stimulates pluripotent Th1 antigen (Ag)-specific responses. Mice were injected s.c. with ID83/GLA-SE and ID93/GLA-SE at biweekly intervals for a total of 3 immunizations. Single-cell suspensions of spleen cells were prepared 1 month after the final immunization and cultured with 10 μg/ml protein. Culture supernatants were collected after 4 days, and IFN-γ (A) or IL-5 (B) content was determined by ELISA. Results are shown as means and SE; n = 3 per group. iono, ionomycin. Alternatively, cells were cultured with antigen and BD Golgi Stop for 16 h and then fixed and stained to determine the percentage (C) of CD3+ CD4+ CD44hi IFN-γ+ cells by flow cytometry. Results are shown as means and SE; n = 3 per group. The phenotype of each CD3+ CD4+ CD44hi IFN-γ+ cell was further delineated by costaining for IL-2 and/or TNF. In panel D, results are shown as percent CD3+ CD4+ CD44hi IFN-γ+ cells exhibiting each phenotype. Data are representative of at least 3 independent experiments.
M. leprae-induced inflammation is reduced in mice immunized with TB vaccines.
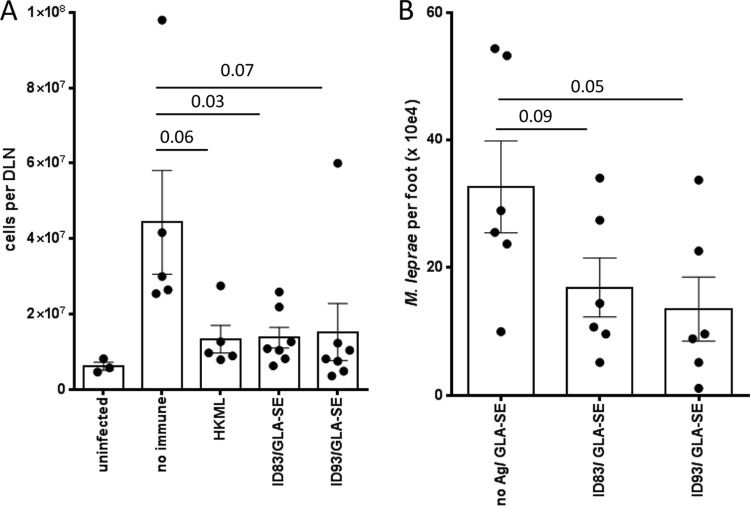
We previously demonstrated that M. leprae infection of the ear causes local inflammation that can be interrupted by drug treatment (22, 23). We hypothesized that appropriate vaccination with ID83 and ID93 would limit development of local inflammation. Mice were immunized by s.c. injection at the base of the tail and then infected in the ears with M. leprae. In agreement with our hypothesis, 12 weeks after infection, fewer cells were recovered from DLN of ID83/GLA-SE- or ID93/GLA-SE-immunized mice than from DLN of mock immunized mice (Fig. 4A). The DLN cell numbers of infected, immunized mice were similar to those of HKML-inoculated, infected mice (Fig. 4A). Taken together, these data indicate that the local inflammation observed following M. leprae ear infection can be limited by immunization with the candidate TB vaccines.
FIG 4.
Immunization with TB vaccines reduces lymphadenopathy induced by M. leprae infection and reduces M. leprae burden. Mice were injected s.c. with ID83/GLA-SE and ID93/GLA-SE at biweekly intervals, for a total of 3 immunizations. In panel A, 1 month after the last immunization, mice were infected with 1 × 106 M. leprae bacilli in each ear, and DLN cell numbers were determined 12 weeks later. Results are shown as means and SE (n = 5 per group), and data are representative of three independent experiments. Student's t test was used to calculate P values between each group. In panel B, 1 month after the last immunization, mice were infected with 5 × 103 M. leprae bacilli in each foot, and bacillus numbers were determined 7 months later. The Mann-Whitney test was used to calculate P values between each group, and results are shown as individual points for each animal, group mean, and SE (n = 6 per group).
Immunization with TB vaccines reduces M. leprae burden.
To investigate if the vaccines could limit growth of M. leprae, immunized mice were infected with M. leprae in the footpad and bacilli numbers were assessed 7 months later. Immunization with either vaccine decreased bacterial numbers compared with those of mice injected with adjuvant alone (Fig. 4B). Taken together, our experimental data indicate that defined subunit vaccines intended for TB could also be useful for leprosy.
DISCUSSION
Proteins that elicit IFN-γ responses from PB leprosy patients and healthy household contacts of multibacillary (MB) patients (HHC) are potential targets of the immune response that naturally limits M. leprae infection. Utilizing such proteins within subunit vaccines thus has the potential to disrupt disease development and bacterial dissemination. In this report, we demonstrate that the chimeric fusion proteins ID83 and ID93, each developed as antigen candidates within TB vaccines, are recognized by blood from PB leprosy patients. When either antigen was formulated with a TLR4L-containing adjuvant (GLA-SE), immunization stimulated strong, pluripotent Th1 responses and inhibited M. leprae-induced inflammation and bacterial growth in mice. Thus, the use of the M. tuberculosis candidate vaccines ID83/GLA-SE and ID93/GLA-SE may confer cross-protection against M. leprae infection and could potentially be used as a control measure for leprosy.
While many groups are attempting to develop vaccines for other neglected tropical diseases, difficulties inherent in leprosy research (e.g., our inability to culture M. leprae in vitro and the very long duration of experimental infections) have severely restricted the investigation and advancement of leprosy vaccines. There is an enormous effort, however, to develop vaccines against M. tuberculosis, and at least nine subunit vaccines have entered clinical trials (11). Taking advantage of TB research efforts could provide an efficient way to codevelop a vaccine for both leprosy and TB. As chimeric fusion proteins comprised of three and four M. tuberculosis proteins, ID83 and ID93 each present M. tuberculosis proteins selected from various categories (the PE/PPE family of proteins and the EsX family of virulence factors, associated with latent growth of M. tuberculosis and expressed during hypoxia, respectively). In this study, our original intent was to examine the T cell responsiveness of Brazilian TB patients to the ID83 and ID93 vaccine antigen candidates, using leprosy patients as a known mycobacterium-infected control group to determine specificity of the responses. Indeed, TB patients did respond strongly through the secretion of IFN-γ. In silico predictions revealed extremely low homology between the amino acid sequences of the individual proteins contained in ID83 and ID93 and the published M. leprae genome (24) (Table 1). It was therefore surprising that PB leprosy patients responded well to antigen stimulation. The response of leprosy patients was most likely due to M. leprae infection and, because EC did not respond, not due to previous BCG immunization or other factors, such as possible latent M. tuberculosis infection or exposure to other environmental mycobacteria. These data provide validation for the potential use of these vaccine candidates for the prevention of leprosy. It has previously been demonstrated that even though the identities between M. tuberculosis and M. leprae ESAT-6 and CFP-10 (36% and 40%, respectively) were very low and the heterologous proteins were not cross-reactive in terms of serum antibody responses in leprosy patients, there was a strong cross-reactive response at the T cell level in both TB and leprosy patients (25). Thus, the cell-mediated responses for low-homology proteins appear to be more promiscuous and are likely contained within similar T cell epitopes within the heterologous proteins.
When M. leprae infection manifests disease, leprosy can present across a diverse bacteriologic, clinical, immunologic, and pathological spectrum. The hallmark neuropathy associated with leprosy arises not only from the direct infection of peripheral nerves by M. leprae, a unique trait among bacteria, but also from the inflammatory and immunologic responses to the infection. Indeed, immune-inflammatory episodes known as leprosy reactions are the main cause of irreversible nerve damage and can be severe enough to require hospitalization. Thus, the promotion of strong immune responses could theoretically precipitate immune pathology, and it is therefore of paramount importance to ensure the safety of a vaccine for leprosy. ID83 subunit vaccines containing synthetic TLR4 or TLR9 agonists generated a Th1 immune response and protected mice against challenge with M. tuberculosis (19). Experimental infection of mice with M. leprae does not precipitate the nerve damage that is a common feature in leprosy patients (21), but it is noteworthy that vaccination with the TB vaccines reduced the M. leprae-induced lymphadenopathy. This observation implies that the vaccines, at least at the DLN level, do not elicit strong infection-site inflammation that causes immune pathology. Further exploration in armadillos that do develop neuropathy following M. leprae infection appears prudent (26–28).
Currently the Leprosy Control Program in Brazil recommends BCG vaccination for all intradomiciliary contacts of both MB and PB leprosy that already have a BCG scar or the ones that haven't been previously vaccinated. In this regard, a new TB vaccine that could also protect against leprosy could also provide additional protection for exposed contacts of leprosy patients. The majority of research directed toward a vaccine for leprosy dates back a few decades and has focused on the use of related whole mycobacteria or purification of protein fractions from M. leprae. Purified and/or recombinant 10-kDa, 25-kDa, and 65-kDa proteins provided protection when administered with Freund's adjuvant (29). Modern vaccine standards, regulations, and safety concerns suggest that more refined products should be developed. The development, production, and advancement of vaccines through the necessary regulatory processes is not trivial or inexpensive, however, and it is therefore noteworthy that at least nine subunit TB vaccines have already passed such scrutiny and have entered clinical trials (11). Our strongest indication that any of these TB vaccines could also contribute toward leprosy control is the demonstration of reduced M. leprae burdens in ID83- and ID93-immunized mice following experimental infection.
In summary, despite the positive impact that the widespread provision of multidrug therapy has had on the global prevalence of leprosy, there are indications that further effort and additional strategies are required in the push for elimination. It is our strong opinion that this effort should include an effective vaccine and that perhaps the smoothest path toward this would be the use of TB vaccines that also protect against leprosy. Our data suggest that new TB vaccine initiatives that are advancing the ID83- and ID93-based vaccine candidates could also be highly beneficial for the sustained control of leprosy.
ACKNOWLEDGMENTS
We are extremely grateful to the patients and staff of the Centro de Referência em Diagnóstico e Terapêutica, Goiânia/Goiás for their cooperation and support.
This work was conducted with support from American Leprosy Missions, UNICEF/UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases (TDR) grant no. A20509, The Heiser Program for Research in Leprosy and Tuberculosis of The New York Community Trust, National Institutes of Health grant AI-078054 and contract HHSN272200800045C, and the Bill and Melinda Gates Foundation (grant 42387). M.M.A.S. is a recipient of a fellowship from National Counsel of Technological and Scientific Development (CNPq/Brazil) (grant no. 304869/2008-2), and R.M.O. was supported by a scholarship awarded by the Brazilian Federal Agency for the Support and Evaluation of Graduate Education (CAPES). This work was also partly supported by a Grant-in-Aid for Research on Emerging and Re-emerging Infectious Diseases (grant no. H21-Shinko-Ippan-007, to M. Makino and Y. Maeda) from the Ministry of Health, Labor and Welfare of Japan.
Footnotes
Published ahead of print 14 July 2014
REFERENCES
- 1.Pandey A, Rathod H. 2010. Integration of leprosy into GHS in India: a follow up study (2006-2007). Lepr. Rev. 81:306–317 [PubMed] [Google Scholar]
- 2.Siddiqui MR, Velidi NR, Pati S, Rath N, Kanungo AK, Bhanjadeo AK, Rao BB, Ojha BM, Krishna Moorthy K, Soutar D, Porter JD, Ranganadha Rao PV. 2009. Integration of leprosy elimination into primary health care in Orissa, India. PLoS One 4:e8351. 10.1371/journal.pone.0008351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Young DB, Gideon HP, Wilkinson RJ. 2009. Eliminating latent tuberculosis. Trends Microbiol. 17:183–188. 10.1016/j.tim.2009.02.005 [DOI] [PubMed] [Google Scholar]
- 4.Zumla A, Atun R, Maeurer M, Mwaba P, Ma Z, O'Grady J, Bates M, Dheda K, Hoelscher M, Grange J. 2011. Viewpoint: scientific dogmas, paradoxes and mysteries of latent Mycobacterium tuberculosis infection. Trop. Med. Int. Health 16:79–83. 10.1111/j.1365-3156.2010.02665.x [DOI] [PubMed] [Google Scholar]
- 5.Kaufmann SH. 2013. Tuberculosis vaccines: time to think about the next generation. Semin. Immunol. 25:172–181. 10.1016/j.smim.2013.04.006 [DOI] [PubMed] [Google Scholar]
- 6.Coler RN, Campos-Neto A, Ovendale P, Day FH, Fling SP, Zhu L, Serbina N, Flynn JL, Reed SG, Alderson MR. 2001. Vaccination with the T cell antigen Mtb 8.4 protects against challenge with Mycobacterium tuberculosis. J. Immunol. 166:6227–6235. 10.4049/jimmunol.166.10.6227 [DOI] [PubMed] [Google Scholar]
- 7.Bertholet S, Ireton GC, Ordway DJ, Windish HP, Pine SO, Kahn M, Phan T, Orme IM, Vedvick TS, Baldwin SL, Coler RN, Reed SG. 2010. A defined tuberculosis vaccine candidate boosts BCG and protects against multidrug-resistant Mycobacterium tuberculosis. Sci. Transl. Med. 2:53ra74. 10.1126/scitranslmed.3001094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dietrich J, Aagaard C, Leah R, Olsen AW, Stryhn A, Doherty TM, Andersen P. 2005. Exchanging ESAT6 with TB10.4 in an Ag85B fusion molecule-based tuberculosis subunit vaccine: efficient protection and ESAT6-based sensitive monitoring of vaccine efficacy. J. Immunol. 174:6332–6339. 10.4049/jimmunol.174.10.6332 [DOI] [PubMed] [Google Scholar]
- 9.Reed SG, Coler RN, Dalemans W, Tan EV, DeLa Cruz EC, Basaraba RJ, Orme IM, Skeiky YA, Alderson MR, Cowgill KD, Prieels JP, Abalos RM, Dubois MC, Cohen J, Mettens P, Lobet Y. 2009. Defined tuberculosis vaccine, Mtb72F/AS02A, evidence of protection in cynomolgus monkeys. Proc. Natl. Acad. Sci. U. S. A. 106:2301–2306. 10.1073/pnas.0712077106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abel B, Tameris M, Mansoor N, Gelderbloem S, Hughes J, Abrahams D, Makhethe L, Erasmus M, de Kock M, van der Merwe L, Hawkridge A, Veldsman A, Hatherill M, Schirru G, Pau MG, Hendriks J, Weverling GJ, Goudsmit J, Sizemore D, McClain JB, Goetz M, Gearhart J, Mahomed H, Hussey GD, Sadoff JC, Hanekom WA. 2010. The novel tuberculosis vaccine, AERAS-402, induces robust and polyfunctional CD4+ and CD8+ T cells in adults. Am. J. Respir. Crit. Care Med. 181:1407–1417. 10.1164/rccm.200910-1484OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parida SK, Kaufmann SH. 2010. Novel tuberculosis vaccines on the horizon. Curr. Opin. Immunol. 22:374–384. 10.1016/j.coi.2010.04.006 [DOI] [PubMed] [Google Scholar]
- 12.Brewer TF. 2000. Preventing tuberculosis with bacillus Calmette-Guerin vaccine: a meta-analysis of the literature. Clin. Infect. Dis. 31(Suppl 3):S64–S67. 10.1086/314072 [DOI] [PubMed] [Google Scholar]
- 13.Merle CS, Cunha SS, Rodrigues LC. 2010. BCG vaccination and leprosy protection: review of current evidence and status of BCG in leprosy control. Exp. Rev. Vaccines 9:209–222. 10.1586/erv.09.161 [DOI] [PubMed] [Google Scholar]
- 14.Duthie MS, Gillis TP, Reed SG. 2011. Advances and hurdles on the way toward a leprosy vaccine. Hum. Vaccin. 7:1172–1183. 10.4161/hv.7.11.16848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Setia MS, Steinmaus C, Ho CS, Rutherford GW. 2006. The role of BCG in prevention of leprosy: a meta-analysis. Lancet Infect. Dis. 6:162–170. 10.1016/S1473-3099(06)70412-1 [DOI] [PubMed] [Google Scholar]
- 16.Meima A, Smith WC, van Oortmarssen GJ, Richardus JH, Habbema JD. 2004. The future incidence of leprosy: a scenario analysis. Bull. World Health Organ. 82:373–380 [PMC free article] [PubMed] [Google Scholar]
- 17.Bertholet S, Ireton GC, Kahn M, Guderian J, Mohamath R, Stride N, Laughlin EM, Baldwin SL, Vedvick TS, Coler RN, Reed SG. 2008. Identification of human T cell antigens for the development of vaccines against Mycobacterium tuberculosis. J. Immunol. 181:7948–7957. 10.4049/jimmunol.181.11.7948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baldwin SL, Bertholet S, Reese VA, Ching LK, Reed SG, Coler RN. 2012. The importance of adjuvant formulation in the development of a tuberculosis vaccine. J. Immunol. 188:2189–2197. 10.4049/jimmunol.1102696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baldwin SL, Bertholet S, Kahn M, Zharkikh I, Ireton GC, Vedvick TS, Reed SG, Coler RN. 2009. Intradermal immunization improves protective efficacy of a novel TB vaccine candidate. Vaccine 27:3063–3071. 10.1016/j.vaccine.2009.03.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Truman RW, Krahenbuhl JL. 2001. Viable M. leprae as a research reagent. Int. J. Lepr. Other Mycobact. Dis. 69:1–12 [PubMed] [Google Scholar]
- 21.Shepard CC. 1960. The experimental disease that follows the injection of human leprosy bacilli into foot-pads of mice. J. Exp. Med. 112:445–454. 10.1084/jem.112.3.445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duthie MS, Reece ST, Lahiri R, Goto W, Raman VS, Kaplan J, Ireton GC, Bertholet S, Gillis TP, Krahenbuhl JL, Reed SG. 2007. Antigen-specific cellular and humoral responses are induced by intradermal Mycobacterium leprae infection of the mouse ear. Infect. Immun. 75:5290–5297. 10.1128/IAI.00564-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raman VS, O'Donnell J, Bailor HR, Goto W, Lahiri R, Gillis TP, Reed SG, Duthie MS. 2009. Vaccination with the ML0276 antigen reduces local inflammation but not bacterial burden during experimental Mycobacterium leprae infection. Infect. Immun. 77:5623–5630. 10.1128/IAI.00508-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cole ST, Eiglmeier K, Parkhill J, James KD, Thomson NR, Wheeler PR, Honore N, Garnier T, Churcher C, Harris D, Mungall K, Basham D, Brown D, Chillingworth T, Connor R, Davies RM, Devlin K, Duthoy S, Feltwell T, Fraser A, Hamlin N, Holroyd S, Hornsby T, Jagels K, Lacroix C, Maclean J, Moule S, Murphy L, Oliver K, Quail MA, Rajandream MA, Rutherford KM, Rutter S, Seeger K, Simon S, Simmonds M, Skelton J, Squares R, Squares S, Stevens K, Taylor K, Whitehead S, Woodward JR, Barrell BG. 2001. Massive gene decay in the leprosy bacillus. Nature 409:1007–1011. 10.1038/35059006 [DOI] [PubMed] [Google Scholar]
- 25.Geluk A, van Meijgaarden KE, Franken KL, Wieles B, Arend SM, Faber WR, Naafs B, Ottenhoff TH. 2004. Immunological crossreactivity of the Mycobacterium leprae CFP-10 with its homologue in Mycobacterium tuberculosis. Scand. J. Immunol. 59:66–70. 10.1111/j.0300-9475.2004.01358.x [DOI] [PubMed] [Google Scholar]
- 26.Job CK, Sanchez RM, Hunt R, Truman RW, Hastings RC. 1993. Armadillos (Dasypus novemcinctus) as a model to test antileprosy vaccines; a preliminary report. Int. J. Lepr. Other Mycobact. Dis. 61:394–397 [PubMed] [Google Scholar]
- 27.Scollard DS, Truman RW. 1999. Armadillos as animal models for lepromatous neuropathy, p 330–336 In Animal models for biomedical research. Academic Press, New York, NY [Google Scholar]
- 28.Truman RW, Sanchez RM. 1993. Armadillos: models for leprosy. Lab Anim. 22:28–32 [Google Scholar]
- 29.Gelber RH, Mehra V, Bloom B, Murray LP, Siu P, Tsang M, Brennan PJ. 1994. Vaccination with pure Mycobacterium leprae proteins inhibits M. leprae multiplication in mouse footpads. Infect. Immun. 62:4250–4255 [DOI] [PMC free article] [PubMed] [Google Scholar]