Abstract
Using flow cytometry, we evaluated the frequencies of CD4+ and CD8+ T cells and Foxp3+ regulatory T cells (Tregs) in mononuclear cells in the jejunum, colon, and cervical and mesenteric lymph nodes of dogs naturally infected with Leishmania infantum and in uninfected controls. All infected dogs showed chronic lymphadenitis and enteritis. Despite persistent parasite loads, no erosion or ulcers were evident in the epithelial mucosa. The colon harbored more parasites than the jejunum. Frequencies of total CD4+, total Foxp3, and CD4+ Foxp3+ cells were higher in the jejunum than in the colon. Despite negative enzyme-linked immunosorbent assay (ELISA) serum results for cytokines, levels of interleukin-10 (IL-10), gamma interferon (IFN-γ), transforming growth factor beta (TGF-β), and tumor necrosis factor alpha (TNF-α) were higher in the jejunum than in the colon for infected dogs. However, IL-4 levels were higher in the colon than in the jejunum for infected dogs. There was no observed correlation between clinical signs and histopathological changes or immunological and parasitological findings in the gastrointestinal tract (GIT) of canines with visceral leishmaniasis. However, distinct segments of the GIT presented different immunological and parasitological responses. The jejunum showed a lower parasite load, with increased frequencies and expression of CD4, Foxp3, and CD8 receptors and IL-10, TGF-β, IFN-γ, and TNF-α cytokines. The colon showed a higher parasite load, with increasing expression of IL-4. Leishmania infantum infection increased expression of CD4, Foxp3, IL-10, TGF-β, IFN-γ, and TNF-α and reduced CD8 and IL-4 expression in both the jejunum and the colon.
INTRODUCTION
In Latin America, Leishmania infantum is the causative agent of visceral leishmaniasis (VL), a potentially fatal disease in humans. Dogs are the main reservoir, and when the female sandfly sucks blood from an infected dog, it can become infected and subsequently be capable of transmitting the parasite to a new vertebrate host. After migrating to the viscera, the parasite reaches the liver, spleen, bone marrow, gut, lymph nodes, and skin (1, 2).
Little is known of the specific immune response occurring in these organs in either human or canine leishmaniasis. Based on the similarity of clinical signs, canine VL (CVL) has been suggested as a good model for understanding the pathogenesis of the human disease (3, 4).
Few studies have described the association of disease progression with cell-mediated immunity in dogs. Several investigators have shown the lack of specific cell-mediated immunity in infected dogs, characterized by the presence of parasites and reduced frequencies of CD4+ T cells and CD8+ T cells (5–9).
The gastrointestinal lymphoid tissue contains approximately 50% of the lymphocytes in the body and is believed to play a major role in both local and systemic immunity, including modulation of the immune response. Major components of the gastrointestinal immune system in humans include T and B lymphocytes, dendritic cells in the lamina propria, and intraepithelial lymphocytes. T cells may be particularly important to local immune regulation (10).
The ability of CD4+ regulatory T cells (Tregs) to resolve established inflammation raises the possibility that these cells may be useful as therapeutic agents for chronic inflammatory diseases in humans (11). It has been shown that during recovery from experimentally induced colitis, CD4+ T cells proliferate and accumulate in the mesenteric lymph nodes and in the colonic lamina propria. At both sites, the progeny of CD4+ Tregs are in direct contact with dendritic cells in the draining lymph node as well as at the site of inflammation. Dendritic cells are essential in antigen (Ag) presentation and play an important role in the generation of Tregs in the gut mucosa (12). The most specific Treg marker known is the forkhead box transcription factor Foxp3, which acts as a master switch in the regulation of development and function of these Tregs (13). Foxp3 has been used as a specific marker of Tregs, which is why many reports have used only two markers for characterization: CD4 and Foxp3. We elected to use this approach for precisely this reason. Foxp3 is necessary and sufficient to characterize this type of regulatory CD4+ T cell, and there are reports showing that depletion of Tregs via anti-CD25 completely eliminates CD4+ Foxp3+ Tregs (14).
Gut-induced CD4+ Foxp3+ Tregs are known to play an important role in intestinal homeostasis, as they are involved in tolerance to food and microbiota antigens (15–17). Although most studies involving Tregs focus on thymically imprinted natural Tregs, there is evidence that gut-associated lymphoid tissue is a site of induction of CD4+ Foxp3+ Tregs from naive precursors (11). Functionally specialized intestinal dendritic cells that express the integrin CD103 have been linked to Treg development and are enriched in the colon and in the mesenteric lymph nodes (18, 19). This may represent a mechanism for generating specific regulation in response to local, nonthymically expressed antigens. However, it does not necessarily mean that only peripherally induced Foxp3+ cells can control intestinal inflammation (20). Clearly, much remains to be learned about the roles of thymic and peripherally induced Tregs in tolerance to foreign antigens, such as Leishmania.
Using the T cell transfer model of colitis, Uhlig et al. (11) found that Tregs can reverse an established T cell-mediated inflammatory response in the intestinal mucosa and restore normal intestinal architecture. It is likely that the mechanisms by which Tregs prevent and reverse colitis differ. For colitis prevention, Tregs must control activation of a predominantly naive population of T cells, whereas, for a cure, they must act on an ongoing inflammatory response driven by Ag-experienced T cells.
We previously reported (21) that with CVL, the jejunum shows a lower parasite load, with increased frequencies and expression of CD11b, Toll-like receptor 9 (TLR9), CD14/CD11b/TLR9 receptors, and the cytokines interleukin-10 (IL-10) and tumor necrosis factor alpha (TNF-α). Conversely, the colon shows a higher parasite load, with increased frequencies and expression of TLR2, CD11c receptors, and IL-4. In the present study, we investigated CD4+ Foxp3+ T cells in the jejunum and colon as well as in mesenteric and cervical lymph nodes of naturally infected dogs from areas where leishmaniasis is endemic. We also examined the inflammatory reactions and parasite loads in the esophagi, stomachs, duodena, jejuna, ilea, ceca, colons, and rectums of these dogs.
MATERIALS AND METHODS
Animals.
Animal care protocols followed the guidelines of the Brazilian Animal Experimental College (COBEA) and were approved by CETEA (Comitê de Ética em Experimentação Animal)-UFMG (approval number 257/2008).
Naturally infected dogs and clinical examination.
Twenty-four mixed-breed dogs of both sexes that were naturally infected with Leishmania infantum were obtained from the Zoonosis Center of the metropolitan area of Belo Horizonte (Municipality of Ribeirão das Neves, Minas Gerais State, Brazil). Dogs were older than 2 years and weighed 8 to 15 kg. Dogs were treated therapeutically and prophylactically for ecto- and metazoan endoparasites. Any dog found to harbor intestinal metazoan parasites upon necropsy (macroscopic analysis) or histological examination was excluded from the analysis. Serological tests to confirm infection included an immunofluorescent-antibody test (IFAT) (titer of >40) and enzyme-linked immunosorbent assay (ELISA) with an optical density of >100 (1:400 dilution). PCR and immunohistochemistry (IHC) using streptavidin-peroxidase and Giemsa stain were conducted for parasitological assessment. The dogs tested positive in at least one fragment of skin (ear), liver, or spleen in all procedures. Infected dogs were designated asymptomatic, without classical signs of leishmaniasis (n = 12), or symptomatic, with signs of CVL, including lymphadenopathy, cutaneous alterations (alopecia, dry exfoliative dermatitis, and ulcers), onychogryphosis, keratoconjunctivitis, and cachexia (n = 12).
Uninfected dogs.
Six dogs of unknown age, obtained from the Control Zoonosis Center of the Municipality of Carandaí, Minas Gerais State, Brazil, and negative for Leishmania infection by all tests, were used as controls. Dogs were confirmed to be Leishmania-free by immunohistochemistry (21–25). This protocol received the approval of the CETEA-UFMG (protocol 139/2012).
IFAT.
IFAT was used to detect Leishmania antibodies. Antigen was prepared from L. infantum MHOM/BR/1967/BH46 promastigotes and fixed on slides. Serum samples were diluted 1:40 in phosphate-buffered saline (PBS), and 25 ml was placed on demarcated regions of the slide. The slide was incubated in a humid chamber at 37°C for 30 min, washed with PBS, and dried at room temperature. Twenty-five milliliters of a commercially available fluorescein-conjugated anti-dog IgG (Bethyl Laboratories, Montgomery, TX), diluted 1:1,500 in PBS containing 2% Tween 80 (Merck, Germany), was added to each demarcated region of the slide, followed by further incubation, washing, and drying. Samples presenting fluorescence at a 1:40 dilution were considered positive (24).
ELISA.
Detection of anti-Leishmania IgG was carried out using standard ELISA. Leishmania soluble antigen (LSA) was derived from L. infantum strain MHOM/BR/1967/BH46 promastigotes that were ruptured ultrasonically. Aliquots (100 μl) of LSA dissolved in 0.05 M carbonate buffer (pH 9.6) to a final concentration of 2 mg/ml were transferred to wells of a 96-well microplate and incubated for 16 h at 4°C. Coated wells were washed five times with PBS containing 0.2% Tween 20, and antigenic sites were saturated with 150 μl PBS containing 0.2% Tween 20 and 2% casein (Sigma, St. Louis, MO) for 30 min at 37°C and washed three times with PBS. Next, a 100-μl aliquot of serum sample, diluted 1:400, was placed into each well. Plates were incubated for 45 min at 37°C and washed five times with PBS, and a 100-μl aliquot of diluted enzyme-labeled immunoglobulin was added to each well. The titer of rabbit anti-canine IgG conjugate (Sigma) was 1:10,000. Following incubation for 45 min at 37°C, plates were washed five times with PBS, and a 100-μl aliquot of 4% (wt/vol) ortho-phenylenediamine in phosphate-citrate buffer (pH 5) containing 4 μl of 30% (vol/vol) hydrogen peroxide was added to each well. The reaction mixture was incubated at 37°C for 10 min. The reaction was stopped by addition of 25 μl 2 M sulfuric acid to the well, and absorbance was measured at 492 nm, using a Bio-Rad (São Paulo, Brazil) model 550 ELISA reader. The cutoff was the mean absorbance reading of the VL-negative controls plus twice the standard deviation (24).
PCR.
Specific PCR carried out on ear skin samples confirmed that animals were positive for L. infantum. DNAs were extracted from 25-mg ear skin samples by use of a DNeasy H Blood & Tissue kit (Qiagen Inc., Valencia, CA) according to the manufacturer's instructions. Oligonucleotide primers LV1 (5′-ACGAGGTCAGCTCCACTCC-3′) and LV2 (5′-CTGCAACGCCTGTGTCTACG-3′) were specific for a repetitive DNA sequence of L. infantum. Amplified PCR products were analyzed on 5% silver-stained polyacrylamide gels (4, 21). All dogs were positive.
IHC.
In addition to being a basis for diagnosis, immunohistochemistry was used to quantify the parasite loads in the studied tissues. Samples of ear skin, spleen, liver, cervical and mesenteric lymph nodes, esophagus, stomach, duodenum, jejunum, ileum, cecum, colon, and rectum were collected and fixed in 10% neutral buffered formalin. Samples were dehydrated, cleared, embedded in paraffin, and cut into 4-μm sections for measuring parasite loads. Deparaffinized slides were hydrated and incubated with 4% hydrogen peroxide (30% [vol/vol]) in 0.01 M PBS, pH 7.2, followed by incubation with normal goat serum (diluted 1:50). Heterologous immune sera from dogs naturally infected with L. infantum (diluted 1:100 in 0.01 M PBS) were used as cross-reactive primary antibodies, as described by Tafuri and colleagues (23). Slides were incubated for 18 to 22 h at 4°C in a humid chamber, washed in PBS, incubated with biotinylated goat anti-mouse and anti-rabbit antibodies (LSAB2 kit; Dako), washed with PBS, and incubated with streptavidin-peroxidase complex (LSAB2 kit; Dako) for 20 min at room temperature. The reaction was developed with 0.024% diaminobenzidine (DAB; Sigma, St. Louis, MO) and 0.16% hydrogen peroxide (40% [vol/vol]). Slides were counterstained with Harris's hematoxylin, dehydrated, cleared, and mounted with coverslips.
Histopathology.
After confirmation of positivity, dogs were euthanized using 2.5% (1.0 ml/kg of body weight) thiopental (intravenous) and T61 (0.3 ml/kg) for necropsy. Samples of ear skin, spleen, liver, cervical and mesenteric lymph nodes, esophagus, stomach, duodenum, jejunum, ileum, cecum, colon, and rectum were collected and fixed in 10% neutral buffered formalin. Samples were dehydrated, cleared, embedded in paraffin, cut into 4-μm sections, and stained with hematoxylin and eosin (HE) for histology (21).
Histology for semiquantitative and quantitative (morphometric analysis) analyses of inflammatory reactions in canine tissues.
For assessment of chronic inflammatory reactions in the jejunum and colon, semiquantitative and quantitative analyses were carried out as follows. The inflammatory reactions in the cervical and mesenteric lymph nodes, esophagus, stomach, duodenum, jejunum, ileum, cecum, colon, and rectum were evaluated by light microscopy. Mononuclear cells were counted in 20 randomly chosen images (20 fields photographed at a magnification of ×40) of each intestinal segment of each animal. For the semiquantitative analysis, the inflammatory mononuclear cells in the organ samples were evaluated using the following scores: slight, 1 to 9 cells per field over 20 fields; moderate, 10 to 30 cells per field over 20 fields; and intense, >30 cells per field over 20 fields. For quantitative analysis, the images were analyzed with Zeiss KS300 software, viewed on video, and downloaded to a computer-assisted image analysis system (Kontron Elektronik/Carl Zeiss, Germany) (25).
IHC studies for quantitative analysis (morphometric analysis) of the parasite loads of examined tissues.
Light microscopy and morphometric analysis of the organ tissues were used for quantitative study. For the histomorphometric study, the immunolabeled amastigotes were counted in 20 randomly chosen images of tissue slides (horizontal and vertical microscope stage micrometer, with x-y translation to avoid overlapping fields). A Kontron Elektronik/Carl Zeiss image analyzer (KS300 software) was utilized as described by Figueiredo et al. (21), with an Axiolab light microscope (Zeiss) at a resolution of ×440.
Flow cytometry.
To isolate normal lamina propria cells, the jejunum and colon were opened, washed with PBS, and cut into small pieces (40 mg per fragment). The dissected mucosa was incubated with Ca2+- and Mg2+-free Hanks' buffered salt solution for 60 min under slow agitation, with exchanges every 10 min. Fragments were incubated in Ca2+- and Mg2+-free Hanks' solution containing 1 mM dl-dithiothreitol (DTT; Sigma-Aldrich) twice for 30 min each, with gentle shaking at 37°C to remove mucus, and then treated with 0.11 mg/ml collagenase II for 4 h with gentle shaking at 37°C, with the supernatant collected every 45 min. Supernatants were centrifuged at 1,300 rpm for 10 min, subjected to erythrocyte lysis with 9 ml distilled water for 30 s and then 1 ml concentrated PBS to stop lysis, and centrifuged for 10 min at 1,300 rpm at 4°C. Pellets were resuspended in 950 μl RPMI medium. Final volumes were adjusted to 1 ml, and cell concentrations were adjusted to 1 × 107 cells/ml. To isolate cells from cervical and mesenteric lymph nodes, tissue was macerated in 2 ml of RPMI medium and centrifuged for 10 min at 1,300 rpm at 4°C. Macerated tissue was resuspended in 9 ml distilled water and 1 ml PBS concentrate and centrifuged for 10 min at 1,300 rpm at 4°C. This procedure was performed twice to lyse erythrocytes. Pellets were resuspended in 950 μl complete RPMI medium. The final volume was adjusted to 1 ml, and the concentration was adjusted to 1 × 107 cells/ml, as described by Figueiredo et al. (21, 22). A 20-μl cell suspension was incubated with 20 μl of a solution containing monoclonal antibodies (MAbs) in 96-well U-bottom plates (Limbro Biomedicals, Aurora, OH) at 4°C for 30 min in darkness. We diluted antibodies against the cell surface in PBS plus fetal bovine serum (0.1%), and antibodies against intracellular markers were diluted in wash buffer as described by Figueiredo et al. (21, 22). Nonconjugated, purified antibodies were conjugated using a Zenon tricolor kit (Molecular Probes) as described by the manufacturer. After staining, samples were washed with 0.1% Na3N in PBS, fixed with 200 μl 2% formaldehyde in PBS, and held at 4°C until analysis by flow cytometry (FACSVantage; Becton, Dickinson, San Jose, CA). Intracytoplasmic marking followed the protocol described by Figueiredo et al. (21, 22). An isotype control was used to define nonspecific markings. Cells were analyzed with an analytical flow cytometer equipped with a laser emitting at 488 nm (FACSVantage; Becton, Dickinson). Whole cells were distinguished from fragments by gating based on the forward and side scatter signals, with a minimum of 40,000 events for each sample. Data were analyzed using the program FlowJo (Tree Star Inc., Ashland, OR). The phenotypic aspects of lamina propria cells were expressed as the percentage of cells expressing a given phenotypic marker, using bimodally distributed cell and isotype control cutoffs. Fluorochrome-labeled antibodies used for staining are described in Table 1.
TABLE 1.
Superficial and intracellular antibodies used in a flow cytometric assay
| Antibody | Clone | Fluorochromea | Manufacturer | Dilution |
|---|---|---|---|---|
| Rat anti-canine CD4 | MCA1038 | FITC | Serotec | 1:40 |
| Mouse anti-canine CD8 | MCA1039F | Cy | Serotec | 1:40 |
| Goat anti-dog (IgG1) | A40-12OP8 | FITC | Imgenex | 1:20 |
| Mouse/rat anti-canine Foxp3 | FJK-16S | PE | eBioscience | 1:20 |
| Sheep anti-dog (IgG2a) | HOPC-1 | PE | SouthernBiotech | 1:20 |
FITC, fluorescein isothiocyanate; PE, phycoerythrin.
ELISA of sera and of jejunum and colon extracts.
Sera and extracted tissue samples of the jejunum and colon were collected for all dogs. For quantitative analysis by capture ELISA, 100 mg of tissue for measurement of gamma interferon (IFN-γ), IL-4, transforming growth factor beta (TGF-β), and TNF-α cytokines, or 300 mg of tissue for measurement of IL-10, was processed in a tissue homogenizer (T10 Basic Ultra Turrax disperser; Ika) for approximately 5 min in 2 ml of RPMI 1640 medium (Sigma, St. Louis, MO), pH 7.2. The resulting homogenate was centrifuged at 10,000 rpm for 15 min at 4°C (Eppendorf 5810R centrifuge; Eppendorf, Hamburg, Germany). A DuoSet ELISA development kit (R&D Systems) was used for quantitative analysis of IFN-γ, IL-4, IL-10, TGF-β, and TNF-α by using anti-canine IL-4 (mouse IgG1; R&D Systems), anti-canine IL-10 (mouse IgG1; R&D Systems), anti-canine TNF-α (mouse IgG1; R&D systems), anti-canine TGF-β (mouse IgG1; R&D Systems), and anti-canine IFN-γ (mouse IgG1; R&D Systems) MAbs.
Statistical analysis.
The Friedman test was used to compare each segment of the gastrointestinal tract (GIT) within each group of dogs, and the Mann-Whitney test was used to compare the GIT segments of asymptomatic and symptomatic dogs, using Graph Pad InStat and Prism 5.0. In all cases, the differences were considered significant when the P values were <0.05.
RESULTS
Clinical physical examination revealed primarily superficial cervical lymph node enlargement (90%), conjunctivitis (78%), popliteal lymph node enlargement (55%), onychogryphosis (55%), alopecia (55%), nasal hyperkeratosis (55%), dry seborrhea (55%), tail erosive-ulcerative dermatitis (45%), cachexy (45%), and ulcerated lesions, mainly in the ear extremity (20%).
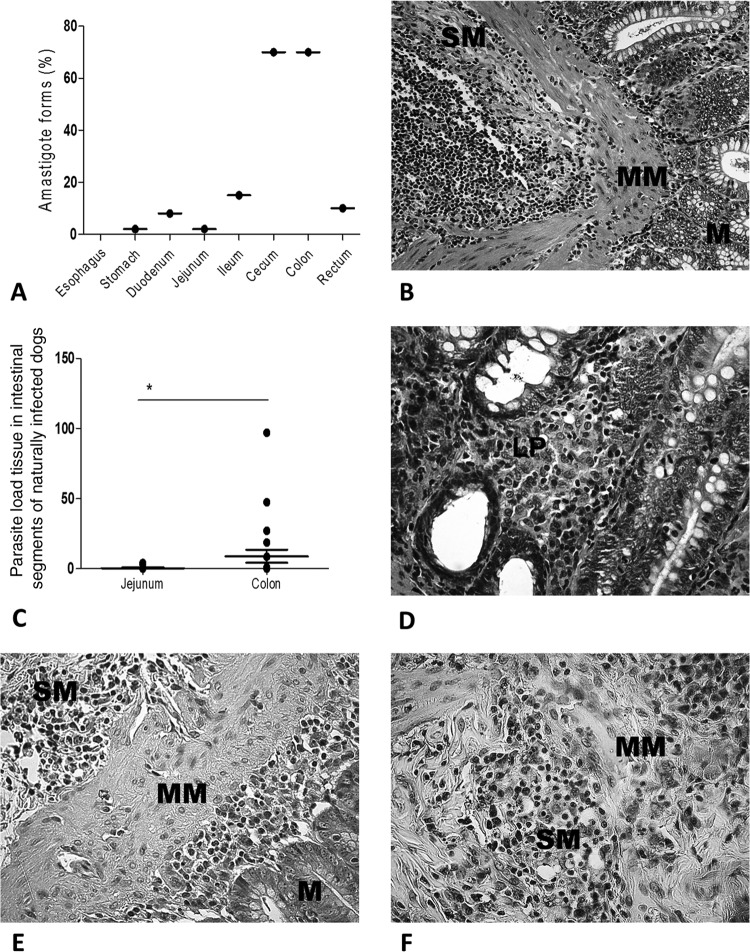
A chronic inflammatory reaction was observed throughout the GIT of all infected animals, characterized by a mononuclear cellular exudate composed of lymphocytes, plasma cells, and macrophages. Rare polymorphonuclear neutrophils and eosinophils were observed. In general, the cellular inflammatory exudate was diffuse in the mucosal and submucosal layers. Macrophages infected by many intracellular Leishmania amastigotes were readily observed in the lamina propria of the mucosae in the majority of cases. Also, parasitized macrophages were found, in some cases, in the muscularis mucosa and submucosa. In the lamina propria of the mucosae, these macrophages frequently showed an atypical morphology characterized by pale and abundant cytoplasm and a less dense nucleus than expected (epithelioid cells). In 13% of cases, multinucleated giant cells and epithelioid cells with granuloma formations were associated with areas in which mononuclear cells were concentrated. In the submucosal layer, chronic inflammatory infiltrates, both diffuse and focal, were observed, mainly around vessels. In 39% of cases, the intense inflammatory infiltrate resulted in discontinuity of the muscle mucosa, coalescing with the cells of the lamina propria. The only anomaly observed in the epithelial layer was atrophy in 8% of infected dogs. Despite the parasitism, we did not find erosion or ulcers in the epithelial mucosa layers or glands. Regardless of the clinical status of the animal, microscopic analysis revealed the presence of amastigotes of Leishmania in all GIT layers, especially in lamina propria macrophages (Fig. 1A and D).
FIG 1.
Parasite loads in dogs naturally infected with Leishmania infantum. (A) Frequencies of L. infantum amastigotes from the esophagus to the rectum. The cecum and colon harbored larger numbers of parasites than the other intestinal segments did. (B) Colonic tissue of an asymptomatic dog. The panoramic view shows the mucosa (M), muscularis mucosa (MM), and submucosa (SM). Note the intense and diffuse mononuclear cell infiltrate. Staining was done with HE. (C) Higher parasite loads in the colon than in the jejunum. (D) Higher-magnification view of panel B. Plasma cells predominate over lymphocytes and macrophages in the lamina propria (LP), without epithelial disruption. Staining was done with HE. (E) Immunolabeled amastigote forms of Leishmania throughout the mucosal (M), muscularis mucosal (MM), and submucosal (SM) layers (cecum-colon histological segment transition). (F) Higher-magnification view showing numerous immunolabeled parasites. Staining was done with streptavidin-peroxidase. *, P < 0.05.
Skin contained parasites in 100% of infected dogs by PCR and immunohistochemical (IHQ) analysis. Spleens and livers were positive in 70% and 34% of naturally infected dogs, respectively, by only IHQ parasitological analysis. Immunohistochemistry revealed parasites throughout the GIT in 83% of infected dogs, and in 70% of cases, parasites were found in the greatest proportions in the cecum and colon. This was confirmed by quantitative assessment, with the large intestine having a significantly larger number of parasites than those in the other segments of the GIT (P = 0.001). Parasites were found in the duodenum and ileum in 40% of infected dogs, whereas only one showed labeling of amastigotes in the jejunum (2%), which was also the case for the stomach (Fig. 1A). Ten percent of the dogs had parasites in the rectum. No parasites were found in the esophagus. The large intestine had a larger number of parasites than the other GIT segments did (P = 0.001), and the colon showed significantly higher parasite loads than those in the jejunum (P < 0.0378) (Fig. 1C to F).
In the large intestine, most parasites were found near the muscularis mucosa and distant from the intestinal lumen (88%). However, in the small intestine, parasites were present in larger numbers near the intestinal lumen, in the villi below the epithelium. Otherwise, under immunohistochemical analysis, skin and spleen harbored more parasites than other tissues (P < 0.009).
Analysis of the relationship of parasite load to the levels of inflammation in several GIT segments showed a positive correlation in the jejunum (r2 = 0.7075; P < 0.005) and the colon (r2 = 0.2739; P < 0.005). On the other hand, this analysis of the lymph nodes showed few Leishmania parasites, and the correlation with inflammation was negative in cervical (r2 = −0.3466) and mesenteric (r2 = −0.1598) (P < 0.005) lymph nodes. In other words, the number of inflammatory cells in lymph nodes was inversely proportional to the parasite load.
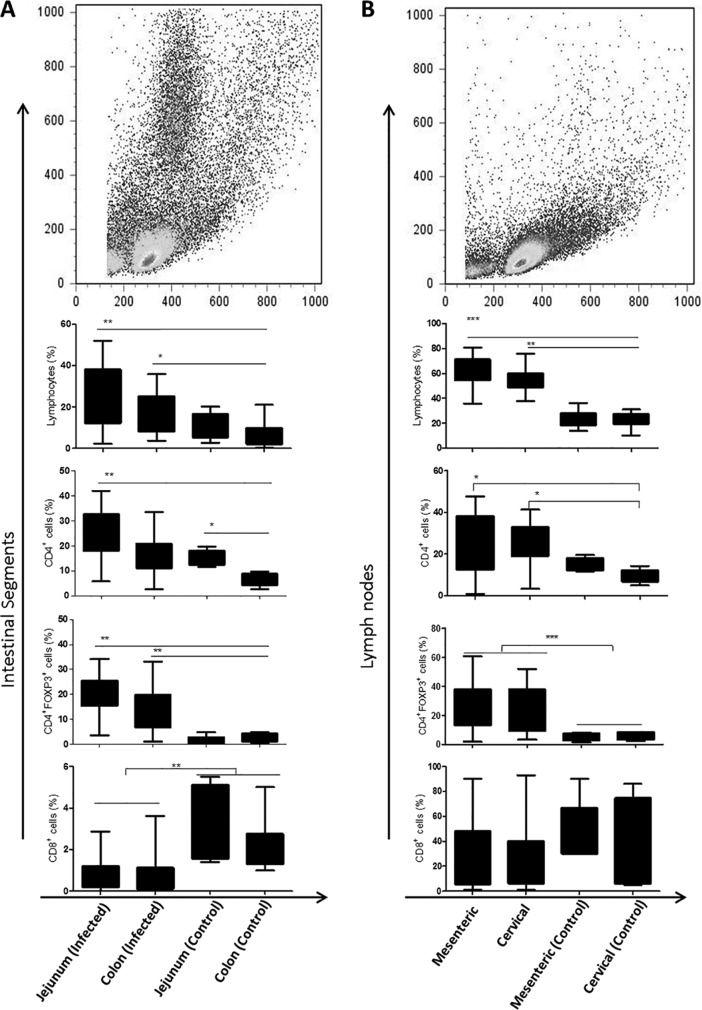
We evaluated the frequencies of CD4+ and CD8+ T cells and CD4+ Foxp3+ Tregs, as well as the mean fluorescence intensities of CD4+, CD8+, and Foxp3+ cells, from the lymph nodes, colons, and jejuna of dogs naturally infected with L. infantum, using flow cytometry. There were higher frequencies and expression levels of CD4+ T cells and CD4+ Foxp3+ Tregs in the jejunum than in the colon (P < 0.0067 and P = 0.0054, respectively) (Fig. 2). We did not find a correlation of these molecules with parasite loads in mesenteric and cervical lymph nodes. Infected animals showed more CD4 and Foxp3 expression than that of controls in both types of lymph nodes, the jejunum, and the colon. Infected animals had reduced CD8 expression in the jejunum and colon compared to controls. Our results revealed a predominance of CD4+ T cells over CD8+ T cells in the jejunum, colon, and both lymph node types (P < 0.0001). Differences in this marker were not observed in the jejunum, colon, and mesenteric and cervical lymph nodes in infected dogs (Fig. 2A and B).
FIG 2.
Leishmania interferes with expression of CD4, CD8, and Foxp3 receptors in the intestine and lymph nodes in dogs naturally infected with Leishmania infantum. (A) Gating strategies demonstrating fluorescence profiles of colonic lamina propria cells. The bar charts show frequencies of lymphocytes and CD4+, CD4+ Foxp3+, and CD8+ cells in intestinal tissue. (B) Gating strategies demonstrating fluorescence profiles of mesenteric and cervical lymph node cells. The bar charts show frequencies of lymphocytes and CD4+, CD4+ Foxp3+, and CD8+ cells in intestinal tissue. *, P < 0.05; **, P < 0.005; ***, P < 0.0005.
We observed a higher frequency of CD4+ T cells (P < 0.005) in mesenteric lymph nodes of symptomatic dogs, with more expression of Foxp3 in CD4+ T cells of asymptomatic dogs (P < 0.05). Significant differences between asymptomatic and symptomatic dogs were not observed in the jejunum and colon.
Positive correlations were found between parasite load and the number of CD4+ T cells in the colon (r2 = 0.2858) and between parasite load and the number of CD8+ T cells in cervical lymph nodes (r2 = 0.2024). The correlation of parasite load with the number of CD4+ Foxp3+ Tregs (r2 = 0.2672) was higher in the jejunum.
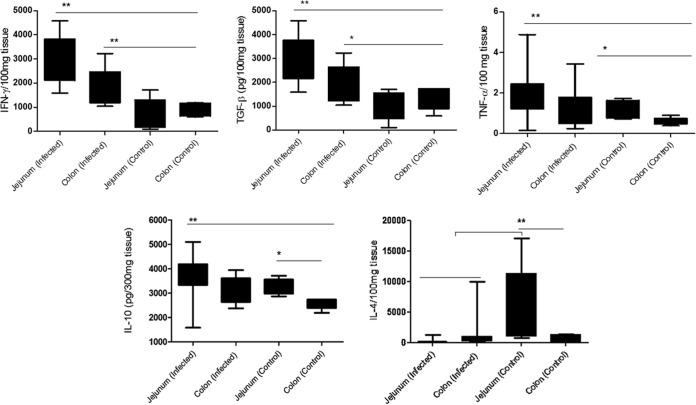
Despite the negative ELISA serum results for IL-4, IL-10, IFN-γ, TGB-β, and TNF-α, these cytokines were detected in the jejuna and colons of all dogs. IL-10, IFN-γ, TGB-β, and TNF-α levels were higher in the jejunum than in the colon (P = 0.003, 0.003, 0.002, and 0.001, respectively). However, IL-4 levels were higher in the colon than in the jejunum (P = 0.004) (Fig. 3).
FIG 3.
Expression of cytokines in intestinal tissue of dogs naturally infected with Leishmania infantum and in controls. The graphs depict jejunal and colonic cytokine expression as follows. IFN-γ, TGF-β, TNF-α, and IL-10 were expressed more in the jejunum than in the colon and in controls. Infected animals showed lower levels of IL-4 than those of controls, with greater reductions in the jejunum. *, P < 0.05; **, P < 0.005.
DISCUSSION
Canine leishmaniasis is a severe, often fatal systemic disease with various clinical profiles and almost every organ system involved (2). In infected dogs, lymphadenopathy was the most frequent clinical symptom observed, as cervical lymph nodes were the primary infection site, while no changes were found in the control dogs. Lymphadenopathy is usually defined as an enlargement of lymph nodes, which can appear as a regional or generalized alteration (26), as discussed by Lima et al. (27) and Costa et al. (28) in Brazil and Ciaramella et al. (29) in the Mediterranean. These authors suggest that cervical lymph nodes are more reactive in CVL because of their anatomical position. As reported by Lima et al. (27), and also in this study, despite chronic lymphadenitis in all infected dogs, the overall architecture of the lymph nodes was preserved.
Microscopic analysis revealed classical CVL pathology (2). Histological analysis of segments of the guts of all infected animals showed a marked increase in cellularity, often with inflammatory infiltrates, in the lamina propria and submucosa of the GIT segments (except for the esophagus), and this was not observed in control animals.
A mononuclear exudate composed of lymphocytes, plasma cells, and macrophages was observed in all infected dogs, independent of clinical status. Epithelioid macrophages were present, with multinuclear cells, although they lacked a typical granulomatous formation in the majority of the cases. The significance of granuloma, according to some authors, is that it is directly related to the lowest parasite tissue burden (27, 30). We observed parasites associated with the inflammatory response throughout the mucosa, but without inducing severe pathologies, such as ulcers or necrosis. The immunohistochemical morphometric analysis revealed higher parasite loads in the colon than in the jejunum, as previously reported by our group (25) and in accordance with other reports (31–36).
The expression of CD4+ lymphocytes was significantly higher in the jejunum than in the colon (P < 0.0116) in infected and control dogs. The functions of mucosal T cells are largely unknown, but cells with a “memory” phenotype predominate in both the epithelium and lamina propria, indicating that they have been exposed to antibodies (37). CD4+ T cells may be of particular importance to local immune regulation. Makita et al. (10) demonstrated that several fractions of CD4+ T cells could suppress the development of murine colitis.
The correlation of inflammatory cells with CD4+ Foxp3+ cells was weakly positive in the jejunum, while in the colon and cervical lymph nodes, this correlation was negative. There was a positive correlation of parasite load with CD4+ Foxp3+ cells in cervical lymph nodes, while the correlation was negative in the colon. Reports have confirmed that during recovery of animals from experimental colitis, CD4+ T cells proliferate and accumulate in mesenteric lymph nodes and colonic lamina propria. At both sites, the progeny of CD4+ T cells are in direct contact with CD11c+ dendritic cells, as well as effector T cells. These findings suggest that regulation of an active immune response by CD4+ T cells occurs in the draining lymph node as well as at the site of inflammation (11). The site of CD4+ Foxp3+ cell expression during suppression of the development of colitis remains unclear. Current knowledge suggests that mechanisms of induction, maintenance, and suppression of colitis may be controlled centrally by CD4+ Foxp3+ cells in the inductive sites. The results of the present study raise questions about whether these sites are solely involved in the induction and suppression of intestinal inflammation. In agreement with our previous findings (22), CD4+ Foxp3+ cells were recently reported in inflamed mucosae of colitic mice and in skin lesions in mice infected with L. major (37, 38). There is compelling evidence that Tregs play a fundamental role in controlling immune responses (39), and our knowledge of established and potentially novel subsets of Tregs and their immunoregulatory mechanisms is expanding rapidly (40). In the colonic mucosa, a variety of distinct T cell subsets with regulatory functions have been identified.
Mucosal inflammation is almost always mediated by one of two pathways: an excessive Th1 cell response associated with increased secretion of IL-12, IFN-γ, and TNF or an excessive Th2 cell response associated with increased secretion of IL-4, IL-5, and IL-13 (41). The immune response to leishmaniasis is currently considered to be mediated by activation of lamina propria macrophages and dendritic cells, leading to lymphocyte proliferation. In the normal gut, inappropriate immune responses are tightly controlled by regulatory T cells (Tr1 or Th3 cells) producing anti-inflammatory cytokines (IL-10 and TGF-β) (41). In this work, we did not observe significant differences in CD4+ Foxp3+ levels in mesenteric and cervical lymph nodes, demonstrating that lymphadenopathy was not due to an increase of one cell type relative to others but rather to a general expansion of all the lymphocyte subpopulations. Analysis of the mean florescence intensity of CD4+ Foxp3+ cells was not likely to demonstrate significant differences among the organs examined, suggesting that chronic intestinal inflammation in dogs infected with leishmaniasis is not simply a consequence of a lack of Tregs at the inflammatory site. We infer that leishmaniasis involves more expression of Foxp3+ Tregs in lymph nodes and in the intestinal mucosa lamina propria in infected dogs. In addition, it seems that the normal intestinal architecture may include an IL-10-dependent mechanism and that Foxp3+ cells are able to secrete IL-10. Foxp3+ was also found in the colon under normal physiological conditions, suggesting an in situ role for Tregs in intestinal homeostasis.
No correlation was found between clinical signs and pathological, immunological, and parasitological findings in GIT for canine visceral leishmaniasis. However, distinct segments of GIT presented different immunological and parasitological responses. The jejunum contained a lower parasite load and increasing frequencies and expression of CD4, Foxp3, and CD8 receptors and IL-10, TGF-β, IFN-γ, and TNF-α cytokines. The colon contained a higher parasite load and increasing expression of IL-4. Leishmania infantum was able to interfere in jejunal and colonic expression of CD4, Foxp3, IL-10, TGF-β, IFN-γ, and TNF-α and to reduce CD8 and IL-4 expression.
ACKNOWLEDGMENTS
We acknowledge the support of the Control Zoonosis Center of the Municipality of Carandaí-Minas Gerais State, Fundação de Amparo e Pesquisa do Estado de Minas Gerais (FAPEMIG) (protocol CDS-AQP 00068-08), Programa Nacional da Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (PNPD/CAPES) (protocol 2258/2011), and Pro-Reitoria de Pesquisa (PRPq-Edital 07/2010), Universidade Federal de Minas Gerais, Minas Gerais, Brazil.
Footnotes
Published ahead of print 16 June 2014
REFERENCES
- 1.Deane LM, Deane MP, Alencar JE. 1955. Control of Phlebotomus longipalpis by DDT house spraying endemic foci of kala-azar in Ceará. Rev. Bras. Malariol. Doencas Trop. 7:131–141 [PubMed] [Google Scholar]
- 2.Alvar J, Canavate C, Molina R, Moreno J, Nieto J. 2004. Canine leishmaniasis. Adv. Parasitol. 57:1–88. 10.1016/S0065-308X(04)57001-X [DOI] [PubMed] [Google Scholar]
- 3.Barbieri CL. 2006. Immunology of canine leishmaniasis. Parasite Immunol. 28:329–337. 10.1111/j.1365-3024.2006.00840.x [DOI] [PubMed] [Google Scholar]
- 4.da Silva SM, Amorim IF, Ribeiro RR, Azevedo EG, Demicheli C, Melo MN, Tafuri WL, Gontijo NF, Michalick MS, Frezard F. 2012. Efficacy of combined therapy with liposome-encapsulated meglumine antimoniate and allopurinol in treatment of canine visceral leishmaniasis. Antimicrob. Agents Chemother. 56:2858–2867. 10.1128/AAC.00208-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moreno J, Nieto J, Chamizo C, Gonzalez F, Blanco F, Barker DC, Alva J. 1999. The immune response and PBMC subsets in canine visceral leishmaniasis before, and after, chemotherapy. Vet. Immunol. Immunopathol. 71:181–195. 10.1016/S0165-2427(99)00096-3 [DOI] [PubMed] [Google Scholar]
- 6.Pinelli E, Rutten VP, Bruysters M, Moore PF, Ruitenberg EJ. 1999. Compensation for decreased expression of B7 molecules on Leishmania infantum-infected canine macrophages results in restoration of parasite-specific T-cell proliferation and gamma interferon production. Infect. Immun. 67:237–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Solano-Gallego L, Llull J, Ramos G, Riera C, Arboix M, Alberola J, Ferrer L. 2000. The Ibizian hound presents a predominantly cellular immune response against natural Leishmania infection. Vet. Parasitol. 90:37–45. 10.1016/S0304-4017(00)00223-5 [DOI] [PubMed] [Google Scholar]
- 8.Reis AB, Martins-Filho OA, Teixeira-Carvalho A, Giunchetti RC, Carneiro CM, Mayrink W, Tafuri WL, Correa-Oliveira R. 2009. Systemic and compartmentalized immune response in canine visceral leishmaniasis. Vet. Immunol. Immunopathol. 128:87–95. 10.1016/j.vetimm.2008.10.307 [DOI] [PubMed] [Google Scholar]
- 9.Reis AB, Teixeira-Carvalho A, Giunchetti RC, Roatt BM, Coura-Vital W, Nicolato Rde C, Silveira-Lemos D, Correa-Oliveira R, Martins-Filho Ode A. 2014. Cellular immunophenotypic profile in the splenic compartment during canine visceral leishmaniasis. Vet. Immunol. Immunopathol. 157:190–196. 10.1016/j.vetimm.2013.11.005 [DOI] [PubMed] [Google Scholar]
- 10.Makita S, Kanai T, Oshima S, Uraushihara K, Totsuka T, Sawada T, Nakamura T, Koganei K, Fukushima T, Watanabe M. 2004. CD4+CD25bright T cells in human intestinal lamina propria as regulatory cells. J. Immunol. 173:3119–3130. 10.4049/jimmunol.173.5.3119 [DOI] [PubMed] [Google Scholar]
- 11.Uhlig HH, Coombes J, Mottet C, Izcue A, Thompson C, Fanger A, Tannapfel A, Fontenot JD, Ramsdell F, Powrie F. 2006. Characterization of Foxp3+CD4+CD25+ and IL-10-secreting CD4+CD25+ T cells during cure of colitis. J. Immunol. 177:5852–5860. 10.4049/jimmunol.177.9.5852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kretschmer K, Apostolou I, Hawiger D, Khazaie K, Nussenzweig MC, von Boehmer H. 2005. Inducing and expanding regulatory T cell populations by foreign antigen. Nat. Immunol. 6:1219–1227. 10.1038/ni1265 [DOI] [PubMed] [Google Scholar]
- 13.Fontenot JD, Rudensky AY. 2005. A well adapted regulatory contrivance: regulatory T cell development and the forkhead family transcription factor Foxp3. Nat. Immunol. 6:331–337. 10.1038/ni1179 [DOI] [PubMed] [Google Scholar]
- 14.Tiwananthagorn S, Iwabuchi K, Ato M, Sakurai T, Kato H, Katakura K. 2012. Involvement of CD4(+) Foxp3(+) regulatory T cells in persistence of Leishmania donovani in the liver of alymphoplastic aly/aly mice. PLoS Negl. Trop. Dis. 6:e1798. 10.1371/journal.pntd.0001798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Macpherson AJ, Harris NL. 2004. Interactions between commensal intestinal bacteria and the immune system. Nat. Rev. Immunol. 4:478–485. 10.1038/nri1373 [DOI] [PubMed] [Google Scholar]
- 16.Fantry L. 2002. Gastrointestinal infections in the immunocompromised host. Curr. Opin. Gastroenterol. 18:34–39. 10.1097/00001574-200201000-00006 [DOI] [PubMed] [Google Scholar]
- 17.Faria AM, Weiner HL. 2006. Oral tolerance: therapeutic implications for autoimmune diseases. Clin. Dev. Immunol. 13:143–157. 10.1080/17402520600876804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Annacker O, Coombes JL, Malmstrom V, Uhlig HH, Bourne T, Johansson-Lindbom B, Agace WW, Parker CM, Powrie F. 2005. Essential role for CD103 in the T cell-mediated regulation of experimental colitis. J. Exp. Med. 202:1051–1061. 10.1084/jem.20040662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johansson-Lindbom B, Svensson M, Pabst O, Palmqvist C, Marquez G, Forster R, Agace WW. 2005. Functional specialization of gut CD103+ dendritic cells in the regulation of tissue-selective T cell homing. J. Exp. Med. 202:1063–1073. 10.1084/jem.20051100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singh B, Read S, Asseman C, Malmstrom V, Mottet C, Stephens LA, Stepankova R, Tlaskalova H, Powrie F. 2001. Control of intestinal inflammation by regulatory T cells. Immunol. Rev. 182:190–200. 10.1034/j.1600-065X.2001.1820115.x [DOI] [PubMed] [Google Scholar]
- 21.Figueiredo MM, Amorim IF, Pinto AJ, Barbosa VS, de Jesus Pinheiro L, Deoti B, Faria AM, Tafuri WL. 2013. Expression of Toll-like receptors 2 and 9 in cells of dog jejunum and colon naturally infected with Leishmania infantum. BMC Immunol. 14:22. 10.1186/1471-2172-14-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Figueiredo MM, Barbosa VS, de Amorim IFG, Deotti B, Michalick MSM, Faria AC, Tafuri WL. 2011. Obtaining cells from colon of dog with leishmaniasis for flow cytometric analysis. Protoc. Exch. 1038:273. 10.1038/protex.2011.273 [DOI] [Google Scholar]
- 23.Tafuri WL, Santos RL, Arantes RM, Goncalves R, de Melo MN, Michalick MS. 2004. An alternative immunohistochemical method for detecting Leishmania amastigotes in paraffin-embedded canine tissues. J. Immunol. Methods 292:17–23. 10.1016/j.jim.2004.05.009 [DOI] [PubMed] [Google Scholar]
- 24.Amorim IF, Silva SM, Figueiredo MM, Moura EP, Castro RS, Lima TK, Gontijo Nde F, Michalick MS, Gollob KJ, Tafuri WL. 2011. Toll receptors type-2 and CR3 expression of canine monocytes and its correlation with immunohistochemistry and xenodiagnosis in visceral leishmaniasis. PLoS One 6:e27679. 10.1371/journal.pone.0027679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pinto AJ, Figueiredo MM, Silva FL, Martins T, Michalick MS, Tafuri WL. 2011. Histopathological and parasitological study of the gastrointestinal tract of dogs naturally infected with Leishmania infantum. Acta Vet. Scand. 53:67. 10.1186/1751-0147-53-67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Giunchetti RC, Martins-Filho OA, Carneiro CM, Mayrink W, Marques MJ, Tafuri WL, Correa-Oliveira R, Reis AB. 2008. Histopathology, parasite density and cell phenotypes of the popliteal lymph node in canine visceral leishmaniasis. Vet. Immunol. Immunopathol. 121:23–33. 10.1016/j.vetimm.2007.07.009 [DOI] [PubMed] [Google Scholar]
- 27.Lima WG, Michalick MS, de Melo MN, Luiz Tafuri W. 2004. Canine visceral leishmaniasis: a histopathological study of lymph nodes. Acta Trop. 92:43–53. 10.1016/j.actatropica.2004.04.007 [DOI] [PubMed] [Google Scholar]
- 28.Costa MM, Lima WG, Figueiredo MM, Michalick MS, Tafuri WL. 2008. Cervical, mandibular, and parotid lymph nodes of dogs naturally infected with Leishmania infantum: a histopathologic and immunohistochemistry study and its correlation with facial skin lesions. Vet. Pathol. 45:613–616. 10.1354/vp.45-5-613 [DOI] [PubMed] [Google Scholar]
- 29.Ciaramella P, Oliva G, Luna RD, Gradoni L, Ambrosio R, Cortese L, Scalone A, Persechino A. 1997. A retrospective clinical study of canine leishmaniasis in 150 dogs naturally infected by Leishmania infantum. Vet. Rec. 141:539–543. 10.1136/vr.141.21.539 [DOI] [PubMed] [Google Scholar]
- 30.dos-Santos WL, David J, Badaro R, de-Freitas LA. 2004. Association between skin parasitism and a granulomatous inflammatory pattern in canine visceral leishmaniosis. Parasitol. Res. 92:89–94. 10.1007/s00436-003-1016-1 [DOI] [PubMed] [Google Scholar]
- 31.Anderson DC, Buckner RG, Glenn BL, MacVean DW. 1980. Endemic canine leishmaniasis. Vet. Pathol. 17:94–96 [DOI] [PubMed] [Google Scholar]
- 32.Ferrer L, Juanola B, Ramos JA, Ramis A. 1991. Chronic colitis due to Leishmania infection in two dogs. Vet. Pathol. 28:342–343. 10.1177/030098589102800414 [DOI] [PubMed] [Google Scholar]
- 33.Gonzalez JL, Fermin ML, Garcia P, Rollan E, Castano M. 1990. Erosive colitis in experimental canine leishmaniasis. Zentralbl. Veterinarmed. B 37:377–382 [DOI] [PubMed] [Google Scholar]
- 34.Silva FL, Tafuri WL, Oliveira MR, Tafuri WL. 2002. Histopathological and immunohistochemical study of the gastrointestinal tract from a dog naturally infected with Leishmania (Leishmania) chagasi: a case report. Arq. Bras. Med. Vet. Zootec. 54:4. 10.1590/S0102-09352002000400002 [DOI] [Google Scholar]
- 35.Longstaffe JA, Guy MW. 1985. Leishmaniasis in dogs. Vet. Annu. 25:358–367 [Google Scholar]
- 36.Keenan CM, Hendricks LD, Lightner L, Johnson AJ. 1984. Visceral leishmaniasis in the German shepherd dog. II. Pathol. Vet. Pathol. 21:80–86 [DOI] [PubMed] [Google Scholar]
- 37.Denning TL, Kim G, Kronenberg M. 2005. Cutting edge: CD4+CD25+ regulatory T cells impaired for intestinal homing can prevent colitis. J. Immunol. 174:7487–7491. 10.4049/jimmunol.174.12.7487 [DOI] [PubMed] [Google Scholar]
- 38.Belkaid Y, Piccirillo CA, Mendez S, Shevach EM, Sacks DL. 2002. CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature 420:502–507. 10.1038/nature01152 [DOI] [PubMed] [Google Scholar]
- 39.Sakaguchi S. 2004. Naturally arising CD4+ regulatory T cells for immunologic self-tolerance and negative control of immune responses. Annu. Rev. Immunol. 22:531–562. 10.1146/annurev.immunol.21.120601.141122 [DOI] [PubMed] [Google Scholar]
- 40.Brimnes J, Allez M, Dotan I, Shao L, Nakazawa A, Mayer L. 2005. Defects in CD8+ regulatory T cells in the lamina propria of patients with inflammatory bowel disease. J. Immunol. 174:5814–5822. 10.4049/jimmunol.174.9.5814 [DOI] [PubMed] [Google Scholar]
- 41.Playford RJ, Ghosh S. 2005. Cytokines and growth factor modulators in intestinal inflammation and repair. J. Pathol. 205:417–425. 10.1002/path.1722 [DOI] [PubMed] [Google Scholar]