Abstract
Humans commonly carry pathogenic bacteria asymptomatically, but despite decades of study, the underlying molecular contributors remain poorly understood. Here, we show that a group A streptococcus carriage strain contains a frameshift mutation in the hasA gene resulting in loss of hyaluronic acid capsule biosynthesis. This mutation was repaired by allelic replacement, resulting in restoration of capsule production in the isogenic derivative strain. The “repaired” isogenic strain was significantly more virulent than the carriage strain in a mouse model of necrotizing fasciitis and had enhanced growth ex vivo in human blood. Importantly, the repaired isogenic strain colonized the mouse oropharynx with significantly greater bacterial burden and had significantly reduced ability to internalize into cultured epithelial cells than the acapsular carriage strain. We conducted full-genome sequencing of 81 strains cultured serially from 19 epidemiologically unrelated human subjects and discovered the common theme that mutations negatively affecting capsule biosynthesis arise in vivo in the has operon. The significantly decreased capsule production is a key factor contributing to the molecular détente between pathogen and host. Our discoveries suggest a general model for bacterial pathogens in which mutations that downregulate or ablate virulence factor production contribute to carriage.
INTRODUCTION
Asymptomatic carriage is a common but poorly understood phenomenon that occurs for many human bacterial pathogens, including Neisseria meningitidis, Streptococcus pneumoniae, Staphylococcus aureus, and Streptococcus pyogenes (group A streptococcus [GAS]) (1–4). The fact of the matter is that these and other frank pathogens spend the majority of their life cycle peacefully coexisting with their host, rarely causing clinical symptoms. The underlying molecular mechanisms favoring carriage over disease are unknown, but a combination of pathogen and host factors is believed to be involved. However, in spite of a relatively sophisticated understanding of many bacterial pathogenesis processes, we understand little about the molecular basis by which major bacterial pathogens are able to colonize humans asymptomatically for prolonged periods.
GAS is an ideal model organism to study asymptomatic carriage mechanisms for several reasons. This organism is responsible for a variety of human infections, ranging from the severe necrotizing fasciitis and streptococcal toxic shock syndrome to the relatively benign impetigo and pharyngitis. However, in most individuals, GAS primarily exists as a naso- and oropharyngeal mucosal colonizer. Individuals can carry GAS in the oropharynx or nose for many months following resolution of clinical disease or may carry GAS asymptomatically with no antecedent history of clinical symptoms (5). Depending on the population studied, GAS carriage rates range from 5 to 15% in children (3), a rate far exceeding that of any disease caused by GAS. For example, rates of invasive infection in well-studied populations generally range from 1 to 3 cases per 100,000 (6). Thus, asymptomatic carriage represents the numerically dominant state of interaction with the human host, but little work has investigated the molecular mechanisms contributing to this common phenomenon.
Very early studies found that GAS strains isolated during carriage produce less hyaluronic acid capsule than strains isolated from infection sites (7). Strains recovered during carriage also have other decreased phenotypes, such as M protein production, hemolysis, and dissemination to other individuals (8–10). Unencapsulated strains of N. meningitidis have been isolated from carriers (11), suggesting that lack of capsule production is linked to carriage. Recently, a comparative genomic analysis showed that hyaluronic acid capsule biosynthesis in GAS is under strong selective pressure depending on the host environment (12). In that study, strains sequentially isolated from nonhuman primates contained mutations that negatively affected capsule production. However, the contribution and molecular underpinnings of decreased capsule production in carriage of GAS in humans remain undefined.
In a study designed to provide new molecular information about GAS carriage, Beres et al. (13) performed genome-wide polymorphism identification of four epidemiologically independent carriage strains and studied their virulence in an animal model of invasive GAS infection. Compared to strains cultured from patients with invasive infections, GAS carriage strains were significantly less virulent for mice as assessed by intraperitoneal inoculation. Genome sequencing identified several candidate polymorphisms that may contribute to the phenotypes of decreased virulence and carriage. However, definitive studies demonstrating a causal role of these and other genetic polymorphisms to carriage have not been conducted.
Here, we report the results of studies designed to test the hypothesis that small genetic changes contribute to the carrier phenotype and decreased virulence. We discovered a single-nucleotide insertion mutation in a carriage strain that eliminated hyaluronic acid capsule production. Our functional analyses conclusively show that mutations negatively affecting capsule synthesis contribute to the phenotypic differences observed between carriage and invasive strains. Using whole-genome sequencing of 81 additional GAS strains serially cultured from 19 subjects with persistent carriage, we also demonstrate that mutations that abrogate capsule synthesis arise in the human upper respiratory tract. This common theme provides new information about the molecular basis of asymptomatic colonization, a critical but very poorly understood aspect of bacterial host-pathogen interaction.
MATERIALS AND METHODS
Bacterial strains.
Carriage strain MGAS12503 was isolated in a population-based study (14) from a healthy individual with no recent history of pharyngitis; its genome has been sequenced (13). Serotype M3 strain MGAS315 was isolated in the late 1980s from a patient with streptococcal toxic shock-like syndrome (15), and the complete genome sequence is available (GenBank accession number NC_004070). Bacteria were grown on Trypticase soy agar containing 5% sheep blood agar (SBA) (Becton, Dickinson, Cockeysville, MD), in Todd-Hewitt broth containing 0.2% (wt/vol) yeast extract (THY) (Becton, Dickinson), or on THY agar. When needed, GAS medium was supplemented with chloramphenicol (Sigma-Aldrich, St. Louis, MO) at 10 μg/ml. For cloning experiments, we used Escherichia coli DH5α or TOP10 (Invitrogen) grown in Luria-Bertani (LB) broth or on LB agar (Becton, Dickinson) and supplemented with ampicillin (Sigma-Aldrich) at 100 μg/ml or chloramphenicol (Sigma-Aldrich) at 20 μg/ml when appropriate.
Allelic exchange in carriage strain MGAS12503.
Temperature-sensitive E. coli Gram-positive shuttle vector pJL1055 was used for allelic replacement (16, 17). To repair hasA in MGAS12503, a 1.5-kb fragment containing the wild-type hasA from MGAS315 was amplified using primer pair MSP107 (5′-TAATCTATTAACGCGACTTA-3′) and MSP114 (5′-TGGGTTATTATAATGCATTC-3′), cloned into pCR2.1-TOPO (Invitrogen) to generate pJSF21, excised using BamHI and XhoI, and ligated into the same sites of pJL1055 to generate pJSF26. Electrocompetent cells of the appropriate GAS strains were transformed with pJSF6 and allelic replacement carried out as previously described (18). Isoallelic “repaired” mutants were confirmed using DNA cycle sequencing (BigDye Terminator v3.1 cycle sequencing kit; Applied Biosystems).
Animal virulence experiments.
Immunocompetent female CD1 mice (Harlan Laboratories) were used for virulence studies (19). Mice were randomly assigned to treatment groups and inoculated in the right hind limb with 1 × 107 CFU of GAS in 100 μl phosphate-buffered saline (PBS). Near-mortality was determined by observation using predefined criteria (19). All mouse experiments were approved by the Institutional Animal Care and Use Committee of the Houston Methodist Research Institute.
Adult cynomolgus macaques (Macaca fascicularis) (Charles River BRF) were used for the nonhuman primate experiments. All monkey experiments were performed as previously described (19). Each animal was inoculated (n = 3 per strain treatment group) with 1 × 108 CFU/kg of body weight of either strain MGAS315 or MGAS12503. Each animal was anesthetized, outfitted with a transdermal fentanyl patch, and inoculated intramuscularly in the anterior thigh to a uniform depth. Animals were observed continuously, sacrificed in matched pairs, and necropsied. Infected tissue collected at necropsy was examined by one or more pathologists and veterinarians. Tissue was fixed in 10% phosphate-buffered formalin, serially sectioned, and embedded in paraffin using automated standard instruments. Microscopic pathology was scored in a blinded fashion by a pathologist using a previously validated scoring system for necrotizing fasciitis (NF) in cynomolgus macaques (19). Hematoxylin and eosin-stained and Gram-stained sections were examined with a BX5 microscope and photographed with a DP70 camera (Olympus). The study protocol was approved by the Institutional Animal Care and Use Committee of the University of Houston.
Mouse nasopharyngeal colonization.
Experiments involving mouse colonization were approved by the Institutional Animal Care and Use Committee of the Houston Methodist Research Institute and were carried out as previously described (20, 21). Female CD1 mice (Harlan Laboratories) were inoculated intranasally with either 5 × 107 CFU (MGAS12503 or MGAS12503hasAwt) or 1 × 106 CFU (MGAS315) in 50 μl PBS. Mouse throats were swabbed prior to inoculation, to document absence of beta-hemolytic bacteria, and daily thereafter. Bacteria were released from swabs by suspending them in 300 μl PBS, serial dilutions were performed for quantitative CFU, and bacteria were plated on SBA. Beta-hemolytic colonies were counted after overnight incubation at 37°C with 5% CO2 and tested for the presence of GAS carbohydrate antigen using latex agglutination (BD Biosciences).
Human epithelial cell internalization assays.
GAS internalization to cultured epithelial cells was carried out as previously described by LaPenta et al. (22). Briefly, HaCaT cells were seeded at a density of 6 × 105 in 2 ml high-glucose Dulbecco's modified Eagle's medium (DMEM) with l-glutamine and 10% fetal bovine serum (FBS) in a 12-well plate. Cells were incubated in a dedicated incubator overnight at 38.5°C. Indicated GAS strains were grown to mid-exponential phase in THY, washed with sterile PBS, and resuspended in an equal volume of PBS. Approximately 1 ×107 CFU GAS was added to 8 replicate wells, rocked briefly, and then incubated for 1 h at 37°C. Each well was then washed twice with 1 ml PBS, twice with 2 ml PBS, and once with 1 ml PBS. Gentamicin was then added to each well at a concentration of 100 μg/ml and incubated for 1 h at 37°C to kill extracellular GAS. Each well was then washed 3 times with 1 ml PBS to remove antibiotics. HaCaT cells were released from the wells using 0.25% trypsin-1 M EDTA. Cells were lysed using sterile water, serially diluted, and plated on SBA to enumerate GAS. Percentage adherence was calculated by dividing the recovered CFU by the original inoculum.
Growth in human blood.
Experiments assessing the ability of GAS to grow in human blood were conducted under a human subject protocol approved by Houston Methodist Research Institute Institutional Review Board. Experiments were carried out as described by Lancefield (23). A minimum of three healthy, nonimmune, adult donors were used for each experiment. Briefly, cultures of strains were grown overnight in THY at 37°C supplemented with 5% CO2 and were used to inoculate fresh, prewarmed THY. Cultures were grown to mid-exponential phase, and bacteria were pelleted and suspended in an equal volume of PBS. Each strain was subsequently diluted to approximately 1 × 103 CFU from which 10 to 100 CFU of GAS was used to inoculate 300 μl of fresh human blood. Samples were incubated at 37°C with 5% CO2 with gentle rotation for 3 h and then serially diluted in PBS and immediately plated on SBA for CFU enumeration. The multiplication factor was calculated by dividing the resulting CFU/ml after 3 h of incubation by the starting inoculum.
Genome sequencing of sequentially cultured strains.
Strains were isolated from individuals enrolled in a study to investigate the human immune response to GAS infection (24). Genome sequencing and data processing were performed as previously described (12). Genome sequences have been deposited in the Short Read Archive (SRA) under BioProject accession number PRJNA255899. All putative polymorphisms identified in the hasA and hasB genes were confirmed using Sanger sequencing.
Hyaluronic acid assays.
A colorimetric assay using known concentrations of a hyaluronic acid standard were used to quantify hyaluronic acid in a 10-ml mid-exponential-phase broth culture (THY) as previously described (25).
Statistics.
Log rank was used to test for statistical significance of mortality data. A two-tailed t test (unequal variance) was used to compare multiplication factors between strains grown in human blood. A two-tailed Mann-Whitney test was used to compare pathology scores for nonhuman primate virulence studies. Repeated-measure analysis of variance (ANOVA) was used to compare rates of nasopharyngeal colonization between strains. A P value of less than 0.05 was considered significant for all statistical tests.
RESULTS
Carriage strains are less virulent in mouse and nonhuman primate models of necrotizing fasciitis.
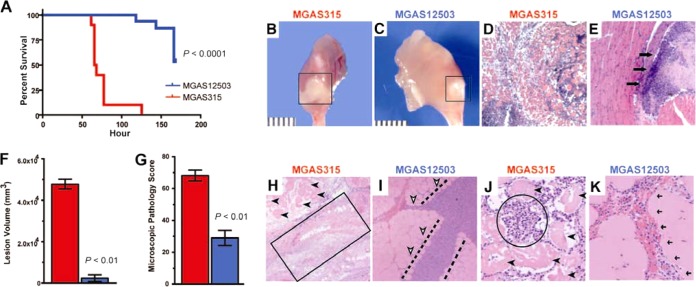
We previously reported that serotype M3 carriage strain MGAS12503, which was isolated from an asymptomatic human carrier, had a significantly higher 90% near-lethal dose (LD90) (that is, was less virulent) for mice than invasive serotype M3 strains after intraperitoneal (i.p.) inoculation (13). To study virulence attributes of the MGAS12503 carriage strain in more detail, we tested the hypothesis that this carriage strain is less virulent in a mouse model of necrotizing fasciitis (NF) (19) than invasive serotype M3 strain MGAS315. Consistent with the hypothesis and the results obtained from the mouse i.p. infection model, strain MGAS12503 was significantly less virulent in the NF model (Fig. 1A). The carriage strain produced significantly less tissue destruction and abscess formation compared to the extensive necrosis observed in mice inoculated with invasive strain MGAS315 (Fig. 1B and C). Similarly, MGAS12503 caused significantly less tissue pathology than invasive strain MGAS315 (Fig. 1D and E).
FIG 1.
The MGAS12503 carriage strain is significantly less virulent than invasive GAS in necrotizing fasciitis models of GAS infection. (A) Survival curve of mice infected with carriage strain MGAS12503 and invasive strain MGAS315. Ten mice in each group were infected intramuscularly with 1 × 107 CFU, as described in Materials and Methods. P value is relative to MGAS315 as determined by log rank test. Gross pathology (B and C) and histopathology (D and E) (×2 original magnification) of mouse hind limb lesions at 48 h postinfection. Mice infected with strain MGAS315 had extensive spreading myonecrosis and tissue destruction (B, D). In contrast, the lesions in mice challenged with the carrier strain (C, E) had small abscesses confined to the inoculation site. The boxed area and arrows demarcate a circumscribed, walled-off lesion. (F) Cynomolgus macaques were inoculated intramuscularly in the anterior thigh (n = 3 monkeys per strain treatment group), and nonviable lesion volume was measured. (G) Microscopic pathology was scored using objective criteria as described in Materials and Methods. (H) Specimens taken from the inoculation site of monkeys infected with invasive strain MGAS315 show extensive necrosis (boxed region) with complete obliteration of the muscle fascicles and intervening fascial planes (original magnification, ×4). Many nonviable cells (black arrowheads) are seen in the background of necrotic debris. (I) In contrast, monkeys infected with carriage strain MGAS12503 have necrosis that is limited to the fascial planes (area between dashed lines), sparing the adjacent muscle tissue, which retains viability (white arrowheads). (J) At higher magnification (×10 original magnification), the tissue from animals infected with strain MGAS315 is completely nonviable (black arrowheads) and extensively infiltrated by PMN leukocytes (circled region). (K) In comparison, muscle tissue from animals infected with strain MGAS12503 was relatively preserved, with a very limited pattern of infiltration that was restricted to the major facial plane (black arrows).
GAS is a human-specific pathogen, and several key virulence factors lack or have poor activity against mouse molecules. For example, the critical GAS virulence factor streptokinase binds plasminogen of humans and nonhuman primates but not that of mice (26). Furthermore, recent research highlights deficiencies in murine models used in the investigation of human inflammatory diseases (27). Thus, we next tested the hypothesis that strain MGAS12503 is less virulent in a nonhuman primate model of NF (19). Consistent with the mouse data, the carriage strain was significantly less virulent in the nonhuman primate than invasive strain MGAS315, as assessed by lesion volume (Fig. 1F), pathology score (Fig. 1G), and magnitude and character of tissue destruction (Fig. 1H to K). Together, the mouse and nonhuman primate virulence studies clearly demonstrated that carriage strain MGAS12503 is less virulent than invasive strain MGAS315.
Carriage strain MGAS12503 contains a frameshift mutation in the hasA gene that eliminates capsule production and decreases virulence.
In our DNA-DNA microarray comparative genome analysis, no polymorphisms relative to invasive strains were identified in MGAS12503 that would account for its significantly decreased virulence or carriage (13). Because of the unambiguous significantly decreased virulence phenotype, we reinvestigated the genome sequence of this strain. We hypothesized that additional polymorphisms may be identified with newer next-generation whole-genome sequencing technology. Using an Illumina instrument and bioinformatic analyses (12), we discovered a previously unidentified frameshift mutation in the hasA gene, which encodes an enzyme required for hyaluronic acid capsule biosynthesis (28). The GAS capsule has been studied extensively since its initial description by Bordet in 1907 (29). Very early studies demonstrated that the GAS capsule is composed of hyaluronic acid, is nonimmunogenic, and is a major virulence factor contributing to resistance to phagocytosis by human polymorphonuclear leukocytes (PMNs) (29–31). Subsequently, the hasA and hasB genes were shown to encode enzymes required for capsule biosynthesis (32). The frameshift mutation in strain MGAS12503 results in a premature stop codon that truncates the HasA protein by 104 amino acids (Fig. 2A).
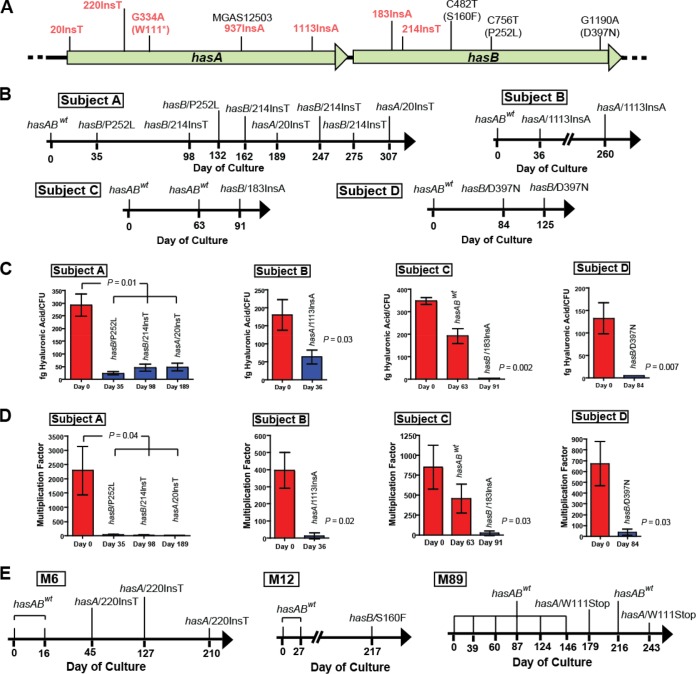
FIG 2.
Location of hasA and hasB mutations identified in serially cultured GAS from humans. (A) The hasA and hasB genes are adjacent to each other in the hasABC operon. Strain MGAS12503 contains a nucleotide insertion at position 937 in hasA. Locations of nucleotide insertions (Ins) identified in other human carriage strains are shown. Mutations resulting in truncation of the predicted protein are in red text. (B) Strains of GAS serotype M3 containing mutations in either hasA or hasB are plotted according to subject and day cultured after initial isolate (day 0). In all subjects, day 0 represents first acquisition of GAS. (C) Hyaluronic acid assays of the initial isolates and representative subsequent isolates of serotype M3. Assays were performed in triplicate as described in Materials and Methods. Error bars represent standard deviations. P values were determined by t test (unequal variance). fg, femtograms. (D) Multiplication of initial isolate and representative subsequent isolates of serotype M3 GAS in human blood. Human blood was inoculated with the indicated strains as described in Materials and Methods. Shown is multiplication after growth in blood from a single donor in triplicate. P value determined by t test (unequal variance). (E) Strains of GAS serotype M6, M12, and M89 containing mutations in either hasA or hasB are plotted according to subject and day cultured after initial isolate, as described for panel B.
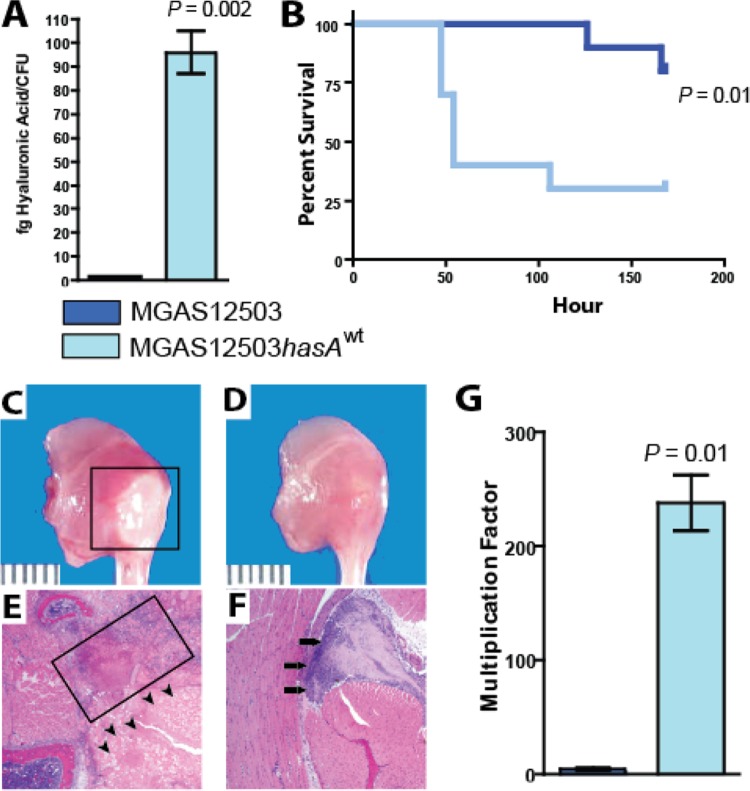
Thus, we hypothesized that this mutation results in loss of capsule biosynthesis, thereby contributing to decreased virulence and the carriage phenotype. Further, we hypothesized that repair of the mutation in hasA would restore capsule production and increase virulence. To test these hypotheses, we generated an isoallelic mutant strain differing from parental strain MGAS12503 only by the presence of a functional hasA gene. The isoallelic mutant strain (MGAS12503hasAwt) produced significantly more hyaluronic acid than wild-type carriage strain MGAS12503, an organism that produced no detectable hyaluronic acid (Fig. 3A).
FIG 3.
Repair of the hasA mutation in strain MGAS12503 restores capsule production and virulence. (A) Hyaluronic acid assays of MGAS12503 and repaired strain MGAS12503hasAwt. Error bars represent standard deviations after performing assays on three biologic replicate samples. Strain MGAS12503 did not produce detectable hyaluronic acid. fg, femtograms. (B) Survival curve of mice infected with MGAS12503 or repaired strain MGAS12503hasAwt. Ten mice in each group were infected intramuscularly with 1 × 107 CFU, as described in Materials and Methods. P values determined by log rank test. (C and D) Mice infected with MGAS12503hasAwt had significantly greater myonecrosis at the site of infection than mice infected with MGAS12503 containing the mutant hasA allele. (E and F) Extensive tissue destruction (boxed area) and nonviable cells were observed in the mouse infected with strain MGAS12503hasAwt, whereas animals infected with MGAS12503 had only small abscesses (black arrows). (G) Multiplication of MGAS12503 and MGAS12503hasAwt in human blood. Whole blood was inoculated with the indicated strains as described in Materials and Methods. Shown is multiplication after growth in blood from a single donor. Similar results were obtained with additional donors. Error bars indicate standard deviations of growth performed in triplicate. P values were determined by t test (unequal variance).
To test the altered-virulence hypothesis, we used a mouse model of NF (19). As anticipated, “repaired” strain MGAS123503hasAwt was significantly more virulent than carriage strain MGAS12503 (Fig. 3B). Consistent with increased virulence, animals injected with strain MGAS12503hasAwt had significantly more extensive necrosis and tissue pathology (Fig. 3C and E) than acapsular carriage strain MGAS12503 (Fig. 3D and F). As noted previously, GAS is a human-specific pathogen, and the possibility exists that the virulence differences observed in mice are not seen in a human system. Inasmuch as the hyaluronic acid capsule is known to impart resistance to phagocytosis by PMNs (29–31), we used a bactericidal assay to test the hypothesis that restoration of capsule production increased survival of the isoallelic mutant (that is, the strain with a wild-type “repaired” hasA allele) in human blood. We observed significantly increased (>100-fold, P = 0.01) survival of repaired strain MGAS12503hasAwt compared to that of isoallelic carriage strain MGAS12503 containing the mutant hasA allele (Fig. 3G). The identification of the frameshift inactivating mutation in the hasA gene in carriage strain MGAS12503 provides a mechanism for the early observations linking decreased capsule production and carriage (33, 34).
Restoration of capsule production by carriage strain MGAS12503 increases bacterial burden in the mouse oropharynx but decreases epithelial cell internalization.
The studies thus far have compared the virulence (i.e., invasive potential) of the carriage strain relative to an invasive strain and an isoallelic-derived strain. We next tested the hypothesis that the carriage strain and repaired isoallelic mutant strain (MGAS12503hasAwt) differed significantly in ability to colonize the mouse oropharynx. Consistent with our hypothesis, isoallelic strain MGAS12503hasAwt colonized the mouse oropharynx with a significantly greater bacterial burden (P < 0.0001; Fig. 4A) than the MGAS12503 parental strain cultured from the human carrier. In fact, the bacterial burden of mice colonized with the repaired MGAS12503hasAwt strain was closely similar to that observed for the invasive MGAS315 strain (Fig. 4).
FIG 4.
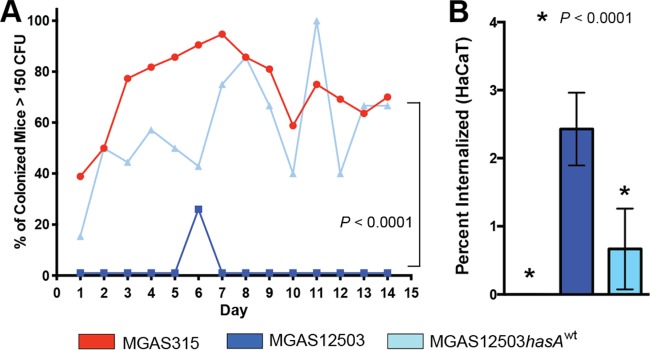
Capsule contributes to bacterial burden in mice but decreases ability to internalize epithelial cells. (A) Nasopharyngeal colonization of mice with GAS strains. Percent of colonized mice with >150 CFU isolated after daily swabbing for each strain: MGAS315 (invasive), MGAS12503 (carrier), and MGAS12503hasAwt (repaired carrier). Mice were inoculated intranasally and swabbed daily for 14 days as described in Materials and Methods. P values were determined by repeated-measures ANOVA. (B) Internalization of MGAS315, MGAS12503, and MGAS12503hasAwt into cultured human epithelial cells (HaCaT) as described in Materials and Methods. Less than 0.1% of strain MGAS315 internalized epithelial cells. P values are relative to the carrier strain MGAS12503 and determined by t test (unequal variance).
Pharyngeal epithelial cell internalization has been suggested as a mechanism for failure to eradicate GAS from the throat following antibiotic therapy (35). Further, it has been shown that GAS isolated from patients with eradication failure have greater ability to internalize epithelial cells than GAS from patients with successful eradication (36). However, the mechanism by which GAS persist in such cases is unknown. We hypothesize that the loss of capsule in MGAS12503 enhances the ability to internalize into human epithelial cells. To test this hypothesis, we performed internalization assays in cultured human epithelial cells (HaCaT) using invasive strain MGAS315, carrier strain MGAS12503, and repaired carrier strain MGAS12503hasAwt. Consistent with our hypothesis, we observed significantly greater ability of the capsule-negative carrier MGAS12503 strain to internalize epithelial cells than either MGAS315 or the repaired isoallelic mutant (Fig. 4B).
Mutations that ablate capsule biosynthesis are present in vivo in humans.
The mutation in hasA was identified in a carriage strain isolated from a healthy human with no recent history of pharyngitis (14). Our data suggest that the GAS capsule contributes to increased bacterial burden during acute infection, and subsequent loss of capsule production contributes to persistence during asymptomatic carriage. These data are consistent with the studies by Hamburger et al. (37) in that individuals with high GAS burdens are most likely to transmit to others and that the GAS bacterial burdens decreased over the course of infection and usually were the same M protein serotype (38). Thus, it is possible, and we believe likely, that the carriage strains typically represent lineal descendants of the strain causing acute pharyngitis in the same individual, rather than new acquisition via transmission from another carrier.
To directly test the hypothesis that mutations in the hyaluronic acid synthesis genes arise naturally in vivo in humans, we compared the genome sequences of M3 strains cultured serially from nine unrelated individuals over time (24). Whole-genome sequence data were obtained from a total of 37 GAS strains. We discovered that strains from 4 of the 9 individuals studied (44%) developed mutations over time in the hasAB genes, essential for capsule biosynthesis (Fig. 2B). GAS isolates cultured from a single individual were very closely related, differing on average by only 4 core genome polymorphisms, which means they are highly unlikely to represent reinfection with a new (unrelated) strain. Relative to the initial strain, strains subsequently isolated from subject A contained either one nonsynonymous nucleotide substitution in hasB (resulting in a P252L replacement in a highly conserved amino acid) or a nucleotide insertion in either hasA or hasB (Fig. 2A and B). Similarly, organisms from subject B contained a nucleotide insertion in hasA, and bacteria from subject C contained a nucleotide insertion in hasB (Fig. 2A and B). Each nucleotide insertion resulted in a shifted reading frame and is predicted to truncate the resulting protein and ablate capsule biosynthesis in these strains, as observed in strain MGAS12503. Two isolates from subject D have a nonsynonymous nucleotide substitution in hasB (D397N; also conserved among sequenced GAS strains) (Fig. 2A and B). Consistent with the identified mutations negatively affecting GAS capsule expression, we observed significantly reduced hyaluronic acid production and survival in human blood in each representative isolate from each subject (Fig. 2C and D). These findings are consistent with a recent study identifying mutations that negatively affect capsule production in GAS carrier strains isolated during a longitudinal nonhuman primate study (12). Also of note, different strains cultured longitudinally from subject A had three mutually exclusive, independent mutations in the hyaluronic acid capsule biosynthesis genes (Fig. 2A and B), further emphasizing the link between capsule downregulation and GAS carriage.
Mutations in capsule biosynthesis genes arise in vivo in multiple GAS M protein serotype human isolates.
It is possible that the mutations in the capsule biosynthesis genes observed thus far are unique to serotype M3 GAS. Thus, we next sought to test the hypothesis that capsule-ablating mutations commonly arise in other GAS serotypes during carriage. Whole-genome sequence data were obtained from an additional 44 GAS strains representing 10 epidemiologic-independent individuals (in some instances cultured over 8 months) and 3 GAS M types (Fig. 2E). In total, the GAS serotypes examined (M3, M6, M12, and M89) represent approximately 35% of all serotypes reported to cause pharyngitis in the United States and Canada (39, 40). Similar to serotype M3 GAS, isolates cultured from a single individual were very closely related, with an average core genome single-nucleotide polymorphism (SNP) difference ranging from a low of 1.6 (M6) to a high of 53.6 (M89). We discovered that, relative to the initially cultured strain, subsequent isolates from 3 of the 10 subjects developed mutations in hasA or hasB that are predicted to negatively affect the GAS hyaluronic acid capsule (Fig. 2E). Consistent with the identified carriage-associated mutations negatively affecting GAS capsule expression, we identified significantly reduced hyaluronic acid production in representative isolates harboring mutations in the hasAB genes relative to the initially infecting strain from the corresponding subject (data not shown). In summary, GAS strains from 7 of 19 (37%) epidemiologically independent subjects studied developed mutations during the course of carriage that reduced or eliminated capsule biosynthesis. Thus, it is clear that mutations reducing capsule biosynthesis are the dominant genetic change in this cohort of multiple GAS serotypes that are common causes of pharyngitis and other infections. In combination with the virulence and colonization data, our data definitively demonstrate that mutations negatively affecting capsule biosynthesis arise over time in the posterior pharynx of humans.
DISCUSSION
The past several decades have seen dramatic advances in understanding how bacterial pathogens cause a diverse array of disease in humans. In contrast, little attention has been given to the study of molecular factors contributing to carriage, despite the fact that for many bacterial pathogens it is a far more common lifestyle than clinically significant infection. We have discovered that mutations resulting in loss of capsule biosynthesis arise during adaptation of GAS to the human pharynx. Our findings echo recent reports that Pseudomonas aeruginosa (41) and Burkholderia dolosa (42) strains in chronically colonized cystic fibrosis patients have mutations in genes encoding known and potentially novel virulence factors or virulence factor regulators.
Our findings are consistent with early studies that reported GAS throat isolates cultured from asymptomatic humans lack observable capsule (33, 34) and were subsequently shown to produce less hyaluronic acid capsule than infecting strains of GAS (7). The identification of frameshift-inactivating mutations in the hasA or hasB genes in carrier strains provides a molecular mechanism for the early observations linking decreased capsule production and carriage. Unencapsulated strains of other bacterial pathogens, such as N. meningitidis (43), S. pneumoniae (44), Streptococcus agalactiae (group B streptococcus) (45), and Haemophilus influenzae (46), have been frequently isolated from carriers. More recently, it has been shown that S. pneumoniae differentially regulates capsule production in a niche-specific manner such that decreased capsule production promotes colonization (47). Thus, decreasing or eliminating capsule production in multiple bacterial pathogens appears to offer an advantage to persistence on mucosal surfaces.
The data begin to unravel the molecular genetic basis underlying the decades-old observation that asymptomatically carried GAS strains are often unencapsulated. It is possible that immune evasion by masking of surface proteins through increased capsule production (48) and active participation of capsule in adherence to epithelial cells (49, 50) contribute to the observed differences in colonization of the oropharynx between the encapsulated and unencapsulated strains. However, other studies have shown that compared to poorly encapsulated GAS, encapsulated strains have reduced adherence to and internalization by epithelial cells (25). This phenomenon has also been observed in H. influenzae (51) and S. pneumoniae (52) strains lacking capsule. Some investigators have postulated that adherence and subsequent internalization of GAS into epithelial cells contributes to persistent colonization (53) and failure to eradicate GAS after treatment of acute pharyngitis (35). Very recent studies provide evidence that GAS may not only survive epithelial internalization (54) but, under some circumstances, escape autophagy and replicate within host epithelial cells (55). Survival within epithelial cells may provide GAS carrier strains with a “safe haven” by limiting antibiotic and immune system exposure. It could be that increased capsule production may contribute to acute symptomatic infection, but as symptoms abate, loss of capsule allows for greater adherence and internalization, signifying adaptation to the human host and persistence.
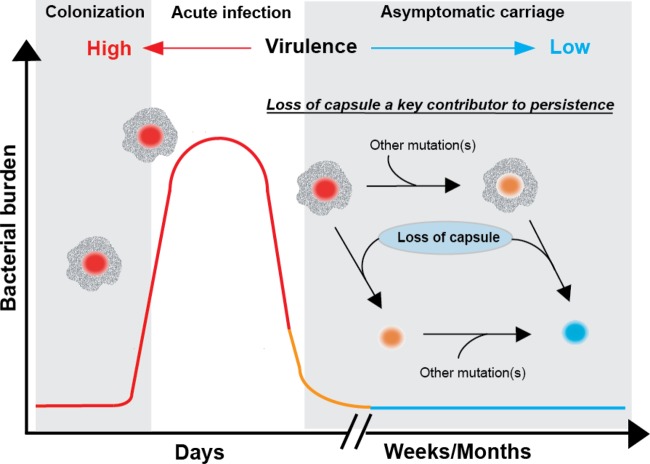
These findings should not be interpreted to mean that mutations that reduce or ablate capsule synthesis are the sole molecular events responsible for carriage in all strains. Clearly it is possible, and expected, that mutations in other genes that detrimentally alter virulence could in principle contribute to the carrier phenotype in GAS (Fig. 5) and other bacterial pathogens. For instance, early studies reported a decrease in M protein in GAS strains isolated from carrier strains compared to that in organisms recovered from acute infection (56, 57). Consistent with this, we previously reported that a serotype M3 GAS carrier isolate had a 195-bp deletion in emm, the gene encoding M protein, that removed the hypervariable N terminus (13). The strains examined in this study did not contain polymorphisms in emm predicted to alter M protein production. Thus, the data indicate that mutations eliminating capsule production are the single predominant category of polymorphisms in organisms serially cultured from the pharynx. Our findings suggest a common mechanism contributing to the numerically frequent carriage phenotype of organisms generally considered to be frank pathogens.
FIG 5.
Loss of hyaluronic acid capsule is a key contributor to GAS asymptomatic carriage. Three stylized phases of GAS pharyngeal infection are shown, based on Virtaneva et al. (58). Bacterial numbers are highest during the acute infection phase (37, 58). In acute infection, encapsulated GAS strains with higher virulence (red circles) predominate. During asymptomatic carriage, selection of GAS strains with mutations negatively affecting capsule takes place along with additional undefined mutations. Loss of capsule can occur early or after other mutations have already occurred, ultimately resulting in loss of virulence (blue circles) and adaptation to the human host.
ACKNOWLEDGMENTS
A.R.F. was supported by the Pediatric Infectious Disease Society–St. Jude Children's Research Hospital Fellowship Award in Basic Research and the Robert Wood Johnson–Harold Amos Medical Faculty Development Program Award.
We thank D. L. Kasper for supplying pJL1055. A. A. Ayeras, C. Cantu, M. E. Watkins, T. Humbird, J. Greaver, L. Jenkins, and T. L. Blasdel assisted with the nonhuman primate studies. Some strains were supplied by D. R. Johnson and E. L. Kaplan for a fee. We thank K. Stockbauer, D. R. Johnson, and E. L. Kaplan for suggestions to improve the manuscript.
Footnotes
Published ahead of print 14 July 2014
REFERENCES
- 1.Bogaert D, van Belkum A, Sluijter M, Luijendijk A, de Groot R, Rumke HC, Verbrugh HA, Hermans PW. 2004. Colonisation by Streptococcus pneumoniae and Staphylococcus aureus in healthy children. Lancet 363:1871–1872. 10.1016/S0140-6736(04)16357-5 [DOI] [PubMed] [Google Scholar]
- 2.Graham PL, III, Lin SX, Larson EL. 2006. A U.S. population-based survey of Staphylococcus aureus colonization. Ann. Intern. Med. 144:318–325. 10.7326/0003-4819-144-5-200603070-00006 [DOI] [PubMed] [Google Scholar]
- 3.Shaikh N, Leonard E, Martin JM. 2010. Prevalence of streptococcal pharyngitis and streptococcal carriage in children: a meta-analysis. Pediatrics 126:e557–e564. 10.1542/peds.2009-2648 [DOI] [PubMed] [Google Scholar]
- 4.Yazdankhah SP, Caugant DA. 2004. Neisseria meningitidis: an overview of the carriage state. J. Med. Microbiol. 53:821–832. 10.1099/jmm.0.45529-0 [DOI] [PubMed] [Google Scholar]
- 5.Martin JM, Green M, Barbadora KA, Wald ER. 2004. Group A streptococci among school-aged children: clinical characteristics and the carrier state. Pediatrics 114:1212–1219. 10.1542/peds.2004-0133 [DOI] [PubMed] [Google Scholar]
- 6.O'Loughlin RE, Roberson A, Cieslak PR, Lynfield R, Gershman K, Craig A, Albanese BA, Farley MM, Barrett NL, Spina NL, Beall B, Harrison LH, Reingold A, Van Beneden C. 2007. The epidemiology of invasive group A streptococcal infection and potential vaccine implications: United States, 2000–2004. Clin. Infect. Dis. 45:853–862. 10.1086/521264 [DOI] [PubMed] [Google Scholar]
- 7.Seastone CV. 1943. The occurrence of mucoid polysaccharide in hemolytic streptococci of human origin. J. Exp. Med. 77:21–28. 10.1084/jem.77.1.21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuttner AG, Krumwiede E. 1944. Observations on the Epidemiology of streptococcal pharyngitis and the relation of streptococcal carriers to the occurrence of outbreaks. J. Clin. Invest. 23:139–150. 10.1172/JCI101477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gordon JE, Badger GF. 1934. The isolation time of scarlet fever. Am. J. Public Health Nations Health 24:438–448. 10.2105/AJPH.24.5.438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wannamaker LW. 1954. The epidemiology of streptococcal infection, p 157–175 In McCarty M. (ed), Streptococcal infections. Columbia University Press, New York, NY [Google Scholar]
- 11.Craven DE, Peppler MS, Frasch CE, Mocca LF, McGrath PP, Washington G. 1980. Adherence of isolates of Neisseria meningitidis from patients and carriers to human buccal epithelial cells. J. Infect. Dis. 142:556–568. 10.1093/infdis/142.4.556 [DOI] [PubMed] [Google Scholar]
- 12.Shea PR, Beres SB, Flores AR, Ewbank AL, Gonzalez-Lugo JH, Martagon-Rosado AJ, Martinez-Gutierrez JC, Rehman HA, Serrano-Gonzalez M, Fittipaldi N, Ayers SD, Webb P, Willey BM, Low DE, Musser JM. 2011. Distinct signatures of diversifying selection revealed by genome analysis of respiratory tract and invasive bacterial populations. Proc. Natl. Acad. Sci. U. S. A. 108:5039–5044. 10.1073/pnas.1016282108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beres SB, Richter EW, Nagiec MJ, Sumby P, Porcella SF, DeLeo FR, Musser JM. 2006. Molecular genetic anatomy of inter- and intraserotype variation in the human bacterial pathogen group A streptococcus. Proc. Natl. Acad. Sci. U. S. A. 103:7059–7064. 10.1073/pnas.0510279103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoe NP, Fullerton KE, Liu M, Peters JE, Gackstetter GD, Adams GJ, Musser JM. 2003. Molecular genetic analysis of 675 group A streptococcus isolates collected in a carrier study at Lackland Air Force Base, San Antonio, Texas. J. Infect. Dis. 188:818–827. 10.1086/377644 [DOI] [PubMed] [Google Scholar]
- 15.Musser JM, Hauser AR, Kim MH, Schlievert PM, Nelson K, Selander RK. 1991. Streptococcus pyogenes causing toxic-shock-like syndrome and other invasive diseases: clonal diversity and pyrogenic exotoxin expression. Proc. Natl. Acad. Sci. U. S. A. 88:2668–2672. 10.1073/pnas.88.7.2668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gryllos I, Grifantini R, Colaprico A, Cary ME, Hakansson A, Carey DW, Suarez-Chavez M, Kalish LA, Mitchell PD, White GL, Wessels MR. 2008. PerR confers phagocytic killing resistance and allows pharyngeal colonization by group A streptococcus. PLoS Pathog. 4:e1000145. 10.1371/journal.ppat.1000145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li J, Kasper DL, Ausubel FM, Rosner B, Michel JL. 1997. Inactivation of the alpha C protein antigen gene, bca, by a novel shuttle/suicide vector results in attenuation of virulence and immunity in group B streptococcus. Proc. Natl. Acad. Sci. U. S. A. 94:13251–13256. 10.1073/pnas.94.24.13251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carroll RK, Shelburne SA, III, Olsen RJ, Suber B, Sahasrabhojane P, Kumaraswami M, Beres SB, Shea PR, Flores AR, Musser JM. 2011. Naturally occurring single amino acid replacements in a regulatory protein alter streptococcal gene expression and virulence in mice. J. Clin. Invest. 121:1956–1968. 10.1172/JCI45169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Olsen RJ, Sitkiewicz I, Ayeras AA, Gonulal VE, Cantu C, Beres SB, Green NM, Lei B, Humbird T, Greaver J, Chang E, Ragasa WP, Montgomery CA, Cartwright J, Jr, McGeer A, Low DE, Whitney AR, Cagle PT, Blasdel TL, DeLeo FR, Musser JM. 2010. Decreased necrotizing fasciitis capacity caused by a single nucleotide mutation that alters a multiple gene virulence axis. Proc. Natl. Acad. Sci. U. S. A. 107:888–893. 10.1073/pnas.0911811107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lukomski S, Montgomery CA, Rurangirwa J, Geske RS, Barrish JP, Adams GJ, Musser JM. 1999. Extracellular cysteine protease produced by Streptococcus pyogenes participates in the pathogenesis of invasive skin infection and dissemination in mice. Infect. Immun. 67:1779–1788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shelburne SA, III, Sumby P, Sitkiewicz I, Okorafor N, Granville C, Patel P, Voyich J, Hull R, DeLeo FR, Musser JM. 2006. Maltodextrin utilization plays a key role in the ability of group A streptococcus to colonize the oropharynx. Infect. Immun. 74:4605–4614. 10.1128/IAI.00477-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.LaPenta D, Rubens C, Chi E, Cleary PP. 1994. Group A streptococci efficiently invade human respiratory epithelial cells. Proc. Natl. Acad. Sci. U. S. A. 91:12115–12119. 10.1073/pnas.91.25.12115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lancefield RC. 1957. Differentiation of group A streptococci with a common R antigen into three serological types, with special reference to the bactericidal test. J. Exp. Med. 106:525–544. 10.1084/jem.106.4.525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnson DR, Kurlan R, Leckman J, Kaplan EL. 2010. The human immune response to streptococcal extracellular antigens: clinical, diagnostic, and potential pathogenetic implications. Clin. Infect. Dis. 50:481–490. 10.1086/650167 [DOI] [PubMed] [Google Scholar]
- 25.Schrager HM, Rheinwald JG, Wessels MR. 1996. Hyaluronic acid capsule and the role of streptococcal entry into keratinocytes in invasive skin infection. J. Clin. Invest. 98:1954–1958. 10.1172/JCI118998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schroeder B, Boyle MD, Sheerin BR, Asbury AC, Lottenberg R. 1999. Species specificity of plasminogen activation and acquisition of surface-associated proteolytic activity by group C streptococci grown in plasma. Infect. Immun. 67:6487–6495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seok J, Warren HS, Cuenca AG, Mindrinos MN, Baker HV, Xu W, Richards DR, McDonald-Smith GP, Gao H, Hennessy L, Finnerty CC, Lopez CM, Honari S, Moore EE, Minei JP, Cuschieri J, Bankey PE, Johnson JL, Sperry J, Nathens AB, Billiar TR, West MA, Jeschke MG, Klein MB, Gamelli RL, Gibran NS, Brownstein BH, Miller-Graziano C, Calvano SE, Mason PH, Cobb JP, Rahme LG, Lowry SF, Maier RV, Moldawer LL, Herndon DN, Davis RW, Xiao W, Tompkins RG, the Inflammation and Host Response to Injury, Large Scale Collaborative Research Program 2013. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc. Natl. Acad. Sci. U. S. A. 110:3507–3512. 10.1073/pnas.1222878110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.DeAngelis PL, Papaconstantinou J, Weigel PH. 1993. Isolation of a Streptococcus pyogenes gene locus that directs hyaluronan biosynthesis in acapsular mutants and in heterologous bacteria. J. Biol. Chem. 268:14568–14571 [PubMed] [Google Scholar]
- 29.Bordet J. 1909. A contribution to the study of antistreptococcal serum, p 104–133 Studies in immunity, 1st ed. John Wiley and Sons, New York, NY [Google Scholar]
- 30.Foley MJ, Wood WB., Jr 1959. Studies on the pathogenicity of group A streptococci. II. The antiphagocytic effects of the M protein and the capsular gel. J. Exp. Med. 110:617–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kass EH, Seastone CV. 1944. The role of the mucoid polysaccharide (hyaluronic acid) in the virulence of group A hemolytic streptococci. J. Exp. Med. 79:319–330. 10.1084/jem.79.3.319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ashbaugh CD, Alberti S, Wessels MR. 1998. Molecular analysis of the capsule gene region of group A streptococcus: the hasAB genes are sufficient for capsule expression. J. Bacteriol. 180:4955–4959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Todd EW, Lancefield RC. 1928. Variants of hemolytic streptococci; their relation to type-specific substance, virulence, and toxin. J. Exp. Med. 48:751–767. 10.1084/jem.48.6.751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ward HK, Lyons C. 1935. Studies on the hemolytic streptococcus of human origin: I. Observations on the virulent, attenuated, and avirulent variants. J. Exp. Med. 61:515–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaplan EL, Chhatwal GS, Rohde M. 2006. Reduced ability of penicillin to eradicate ingested group A streptococci from epithelial cells: clinical and pathogenetic implications. Clin. Infect. Dis. 43:1398–1406. 10.1086/508773 [DOI] [PubMed] [Google Scholar]
- 36.Sela S, Neeman R, Keller N, Barzilai A. 2000. Relationship between asymptomatic carriage of Streptococcus pyogenes and the ability of the strains to adhere to and be internalised by cultured epithelial cells. J. Med. Microbiol. 49:499–502 [DOI] [PubMed] [Google Scholar]
- 37.Hamburger M, Green MJ, Hamburger VG. 1945. The problem of the dangerous carrier of hemolytic streptococci. II. Spread of infection by individuals with strongly positive nose cultures who expelled large numbers of hemolytic streptococci. J. Infect. Dis. 79:68–81 [DOI] [PubMed] [Google Scholar]
- 38.Hamburger M, Jr, Green MJ, Hamburger VG. 1945. The problem of the “dangerous carrier” of hemolytic streptococci. I. Number of hemolytic streptococci expelled by carriers with positive and negative nose cultures. J. Infect. Dis. 77:68–81 [DOI] [PubMed] [Google Scholar]
- 39.Shea PR, Ewbank AL, Gonzalez-Lugo JH, Martagon-Rosado AJ, Martinez-Gutierrez JC, Rehman HA, Serrano-Gonzalez M, Fittipaldi N, Beres SB, Flores AR, Low DE, Willey BM, Musser JM. 2011. Group A streptococcus emm gene types in pharyngeal isolates, Ontario, Canada, 2002–2010. Emerg. Infect. Dis. 17:2010–2017. 10.3201/eid1711.110159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shulman ST, Tanz RR, Dale JB, Beall B, Kabat W, Kabat K, Cederlund E, Patel D, Rippe J, Li Z, Sakota V. 2009. Seven-year surveillance of North American pediatric group a streptococcal pharyngitis isolates. Clin. Infect. Dis. 49:78–84. 10.1086/599344 [DOI] [PubMed] [Google Scholar]
- 41.Smith EE, Buckley DG, Wu Z, Saenphimmachak C, Hoffman LR, D'Argenio DA, Miller SI, Ramsey BW, Speert DP, Moskowitz SM, Burns JL, Kaul R, Olson MV. 2006. Genetic adaptation by Pseudomonas aeruginosa to the airways of cystic fibrosis patients. Proc. Natl. Acad. Sci. U. S. A. 103:8487–8492. 10.1073/pnas.0602138103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lieberman TD, Michel JB, Aingaran M, Potter-Bynoe G, Roux D, Davis MR, Jr, Skurnik D, Leiby N, LiPuma JJ, Goldberg JB, McAdam AJ, Priebe GP, Kishony R. 2011. Parallel bacterial evolution within multiple patients identifies candidate pathogenicity genes. Nat. Genet. 43:1275–1280. 10.1038/ng.997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Caugant DA, Kristiansen BE, Froholm LO, Bovre K, Selander RK. 1988. Clonal diversity of Neisseria meningitidis from a population of asymptomatic carriers. Infect. Immun. 56:2060–2068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hanage WP, Kaijalainen T, Saukkoriipi A, Rickcord JL, Spratt BG. 2006. A successful, diverse disease-associated lineage of nontypeable pneumococci that has lost the capsular biosynthesis locus. J. Clin. Microbiol. 44:743–749. 10.1128/JCM.44.3.743-749.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Davies HD, Adair C, McGeer A, Ma D, Robertson S, Mucenski M, Kowalsky L, Tyrell G, Baker CJ. 2001. Antibodies to capsular polysaccharides of group B streptococcus in pregnant Canadian women: relationship to colonization status and infection in the neonate. J. Infect. Dis. 184:285–291. 10.1086/322029 [DOI] [PubMed] [Google Scholar]
- 46.Howard AJ, Dunkin KT, Millar GW. 1988. Nasopharyngeal carriage and antibiotic resistance of Haemophilus influenzae in healthy children. Epidemiol. Infect. 100:193–203. 10.1017/S0950268800067327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shainheit MG, Mule M, Camilli A. 2014. The core promoter of the capsule operon of Streptococcus pneumoniae is necessary for colonization and invasive disease. Infect. Immun. 82:694–705. 10.1128/IAI.01289-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hyams C, Camberlein E, Cohen JM, Bax K, Brown JS. 2010. The Streptococcus pneumoniae capsule inhibits complement activity and neutrophil phagocytosis by multiple mechanisms. Infect. Immun. 78:704–715. 10.1128/IAI.00881-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ashbaugh CD, Moser TJ, Shearer MH, White GL, Kennedy RC, Wessels MR. 2000. Bacterial determinants of persistent throat colonization and the associated immune response in a primate model of human group A streptococcal pharyngeal infection. Cell. Microbiol. 2:283–292. 10.1046/j.1462-5822.2000.00050.x [DOI] [PubMed] [Google Scholar]
- 50.Cywes C, Stamenkovic I, Wessels MR. 2000. CD44 as a receptor for colonization of the pharynx by group A streptococcus. J. Clin. Invest. 106:995–1002. 10.1172/JCI10195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.St. Geme JW, III, Cutter D. 1996. Influence of pili, fibrils, and capsule on in vitro adherence by Haemophilus influenzae type b. Mol. Microbiol. 21:21–31. 10.1046/j.1365-2958.1996.6241331.x [DOI] [PubMed] [Google Scholar]
- 52.Munoz-Elias EJ, Marcano J, Camilli A. 2008. Isolation of Streptococcus pneumoniae biofilm mutants and their characterization during nasopharyngeal colonization. Infect. Immun. 76:5049–5061. 10.1128/IAI.00425-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Molinari G, Chhatwal GS. 1998. Invasion and survival of Streptococcus pyogenes in eukaryotic cells correlates with the source of the clinical isolates. J. Infect. Dis. 177:1600–1607. 10.1086/515310 [DOI] [PubMed] [Google Scholar]
- 54.O'Seaghdha M, Wessels MR. 2013. Streptolysin O and its cotoxin NAD-glycohydrolase protect group A streptococcus from xenophagic killing. PLoS Pathog. 9:e1003394. 10.1371/journal.ppat.1003394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Barnett TC, Liebl D, Seymour LM, Gillen CM, Lim JY, Larock CN, Davies MR, Schulz BL, Nizet V, Teasdale RD, Walker MJ. 2013. The globally disseminated M1T1 clone of group A streptococcus evades autophagy for intracellular replication. Cell Host Microbe 14:675–682. 10.1016/j.chom.2013.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Krause RM, Rammelkamp CH., Jr 1962. Studies of the carrier state following infection with group A streptococci. II. Infectivity of streptococci isolated during acute pharyngitis and during the carrier state. J. Clin. Invest. 41:575–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rothbard S, Watson RF. 1948. Variation occurring in group A streptococci during human infection. Progressive loss of M substance correlated with increasing susceptibility to bacteriostasis. J. Exp. Med. 87:521–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Virtaneva K, Porcella SF, Graham MR, Ireland RM, Johnson CA, Ricklefs SM, Babar I, Parkins LD, Romero RA, Corn GJ, Gardner DJ, Bailey JR, Parnell MJ, Musser JM. 2005. Longitudinal analysis of the group A streptococcus transcriptome in experimental pharyngitis in cynomolgus macaques. Proc. Natl. Acad. Sci. U. S. A. 102:9014–9019. 10.1073/pnas.0503671102 [DOI] [PMC free article] [PubMed] [Google Scholar]