Abstract
The collagen adhesin Acm was the first virulence determinant reported to be important for the pathogenesis of Enterococcus faecium in a rat infective endocarditis model. We had previously reported that there was a slight growth delay associated with acm allelic replacement (cat) mutant strain TX6051 used in that study. Recently, we generated a nonpolar markerless acm deletion mutant and did not observe a delay in growth. We therefore performed comparative genome sequence analysis of wild-type strain TX82 and TX6051 and found a single mutation, a nonsense mutation in the ccpA gene of TX6051. After correcting this mutation, the growth defect of TX6051 was abolished, implicating a role for CcpA in the growth of E. faecium. To confirm this, we created a ccpA deletion mutant of TX82, which also exhibited a slight delay in growth. Furthermore, the ccpA deletion mutant was attenuated (P = 0.0024) in a mixed-inoculum (TX82 plus TX82 ΔccpA) rat endocarditis model and also in an in vitro competitive growth assay; a ccpA-complemented strain showed neither reduced growth nor reduced virulence. We also found attenuation in the endocarditis model with the new acm deletion mutant although not as great as that previously observed with TX6051 carrying the ccpA mutation. Taken together, our data confirm the role of Acm in the pathogenesis of endocarditis. We also show that CcpA affects the growth of E. faecium, that an intact ccpA gene is important for full virulence, and that a ccpA mutation was partly responsible for the highly attenuated phenotype of TX6051.
INTRODUCTION
Enterococcus faecium has become one of the most problematic causes of nosocomial infections in the United States over the last 2 decades and is found as a cause of various infections, including endocarditis, bacteremia in neutropenic patients, urinary tract infections, and transplant- and device-associated infections (1). A recent survey indicated that slightly more than one-third of the hospital-associated infections caused by enterococci can be attributed to E. faecium when the organisms were identified to the species level (2). Infections with multidrug-resistant E. faecium strains are a major clinical threat due to limited treatment options. The emergence of resistance to ampicillin and the subsequent acquisition of resistance to vancomycin have been associated with the progression of this harmless commensal organism into an important nosocomial pathogen in the United State (3–5). In addition, E. faecium isolates recovered from hospital-associated infections (HA clade or subclade A1) (4, 6) are also characterized by the presence of many putative or confirmed virulence-associated traits, including the expression of collagen adherence mediated by Acm (adhesin of collagen from E. faecium) (7), Esp (enterococcal surface protein) (8), a plasmid encoding a hyaluronidase-like protein (Hylfm, now annotated family 84 glycosyltransferase) (9), MSCRAMMs (microbial surface component recognizing adhesive matrix molecules) (10, 11), EmpABC (forming E. faecium pili [previously known as EbpABCfm]) (11), and a putative phosphotransferase system important for gut colonization during antibiotic treatment (12). Moreover, the presence of insertion sequence (IS) elements and a high level of recombination events have also contributed to the evolution and rise of this organism in the nosocomial environment (13, 14).
The ability of E. faecium to adhere to collagen is mediated primarily by Acm (15). Acm is expressed only by HA clade strains, and an inactive gene was found in many commensal isolates via insertion sequences or stop codons; however, acm was found intact in all infective endocarditis isolates (16). Previously, we reported that in a rat endocarditis model, the acm allelic replacement mutant (TX6051 [TX82 Δacm::cat]) was highly attenuated compared to wild-type (WT) strain TX82 (17). We also observed that TX6051 produced small colonies and that the doubling time was long compared to the wild type (7). However, colony counts were equal beyond about 9 h. Since the genetic tools to restore the deleted gene had not yet been developed and the plasmid vectors that we tested were not stable in vivo, we were not able to determine if the growth defect and attenuation were attributed solely to the deletion of the acm gene. With the development of efficient genetic manipulation techniques (18, 19), we recently generated a markerless, nonpolar deletion of acm, which exhibited growth characteristics similar to those of the wild type. This prompted us to look for the actual cause of the growth defect associated with TX6051.
Here, we show that the previously reported, highly attenuated phenotype exhibited by an acm allelic replacement mutant is partly mediated by the presence of a nonsense mutation in the ccpA gene and that CcpA, a global transcriptional regulator of carbon catabolite repression (CR) in Gram-positive bacteria, affects the growth of E. faecium. We also demonstrate the importance of CcpA in infective endocarditis in a rat model, likely due to its effect on growth, and confirm the contribution of Acm to virulence.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Strains and plasmids used in this study are listed in Table 1. Escherichia coli strains were routinely grown at 37°C in Luria-Bertani (LB) broth or agar, and enterococci were grown in brain heart infusion (BHI) or M17 broth or agar (Difco Laboratories). For enterococci, the following antibiotic concentrations were used: 10 μg/ml chloramphenicol, 10 μg/ml erythromycin, and 200 μg/ml gentamicin. For E. coli, the concentrations used were 30 μg/ml kanamycin and 25 μg/ml gentamicin.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Relevant characteristic(s)a | Reference(s) or source |
|---|---|---|
| Strains | ||
| E. faecium | ||
| TX82 | Endocarditis isolate; Vanr Ampr | 7 |
| TX6051 | TX82 Δacm::cat; acm allelic replacement mutant with an incidental ccpA premature stop codon | 7; this study |
| TX6086 | TX82 Δacm; nonpolar markerless acm deletion mutant | This study |
| TX6130 | TX6051::ccpA nucleotide mutation corrected | This study |
| TX6127 | TX82 ΔccpA; ccpA deletion mutant | This study |
| TX6140 | TX6127 complemented with ccpA (in situ in the chromosome) | This study |
| TX6145 | TX6127 complemented with 300 bp of the ccpA gene | This study |
| E. coli | ||
| DH5α | E. coli cloning host | Invitrogen |
| EC1000 | E. coli host strain, provides RepA | 45 |
| E. faecalis CK111 | Conjugative donor for genetic manipulations | 18 |
| Plasmids | ||
| pHOU1 | Plasmid for mutagenesis; Genr | 19 |
| TX6143 | Plasmid for ccpA gene deletion; 820 bp upstream and 912 bp downstream of the ccpA gene cloned into pHOU1 | This study |
| TX6144 | Plasmid for initial 300-bp complementation of the ccpA gene; 452 bp upstream of the start codon of ccpA along with the initial 300 bp of ccpA and 507 bp downstream of the stop codon of the ccpA gene cloned into pHOU1 | This study |
| TX6146 | Plasmid for correcting the ccpA gene mutation of TX6051; fragment containing 863 bp upstream and 572 bp downstream of the ccpA stop codon cloned into pHOU1 | This study |
| TX6147 | Plasmid for acm gene deletion; flanking regions of the acm gene cloned into pHOU1 | This study |
| TX6148 | Plasmid for second-step complementation (restoration) of the ccpA gene; fragment containing bp 21–1020 of the ccpA gene along with 507 bp of downstream sequence cloned into pHOU1 | This study |
Ampr, ampicillin resistance; Genr, gentamicin resistance; Vanr, vancomycin resistance.
Genomic sequencing.
Sequence reads of the E. faecium strains (TX82 and TX6051) were generated with the Illumina IIx genome analyzer and assembled at the Washington University Genome Institute. Genome-wide variant detection (read mapping) and comparisons of contigs were performed by using standard methods as described previously (20).
Construction of a markerless nonpolar deletion mutant of acm.
The acm gene along with the upstream region (1,036 bp) and the downstream region (473 bp) were PCR amplified by using specific primers (data not shown), cloned into pBluescript, and subcloned into pHOU1 between BamHI and EcoRI sites; pHOU1 contains a pheS* allele, encoding a phenylalanine tRNA synthetase with altered substrate specificity, which confers susceptibility to p-chloro-phenylalanine (p-Cl-Phe) (17). The acm coding region, except for the 239 bp at the 5′ end, was deleted by digestion with MscI, followed by self-ligation and transformation into competent E. coli EC1000 cells, as described previously (19). This recombinant plasmid, our deletion construct (TX6147), was then electroporated into CK111 and transferred to TX82 by filter mating. Single-crossover integrants were selected on BHI plates containing gentamicin and erythromycin and then replated onto MM9YEG medium (M9-based medium supplemented with yeast extract, salts, and glucose) containing p-Cl-Phe (7 mM) to select for excision of pHOU1. Excision of pHOU1 was confirmed by an absence of growth on BHI-gentamicin agar; colonies lacking acm were detected by PCR, and one of these was designated TX6086. The correct deletion was confirmed by sequencing of the PCR product obtained by using outside primers specific for the flanking regions of the cloned gene and by pulsed-field gel electrophoresis (PFGE).
Correction of the mutation in the ccpA gene of TX6051.
To correct the mutation in the ccpA gene of TX6051, part of the ccpA coding region (863 bp at the C-terminal end) together with the downstream sequence (572 bp) was PCR amplified and cloned into plasmid pHOU1 between PstI and SphI sites, and this construct (TX6146) was introduced into TX6051 and processed as described above. Replacement of the mutated ccpA gene with the wild-type ccpA gene (generating TX6130) was confirmed by the absence of growth on BHI-gentamicin agar, by sequencing of the PCR product obtained using primers specific for the flanking regions of the cloned gene, and by PFGE.
Construction of a ccpA deletion mutant and its complementation.
To construct a ccpA deletion mutant of TX82, genomic DNA regions flanking the ccpA gene (820 bp upstream and 912 bp downstream) were amplified by overlap extension PCR using primers with BamHI and PstI restriction sites and cloned into plasmid pHOU1 (TX6143), and the deletion mutant (TX82 ΔccpA, designated TX6127) was prepared as described above.
To construct a ccpA chromosomally complemented strain (i.e., restoration of the wild-type gene back into its native location), the ccpA gene along with the upstream and downstream sequences were amplified by using overlapping primers, which ensured the removal of the BamHI site in the ccpA gene (silent mutation), in order to make a distinction between the wild type and strains with ccpA complemented in the native chromosomal site. Because of the difficulty in the cloning of this ccpA complementation construct (the entire ccpA gene along with the upstream and downstream sequences) in E. coli, a two-step complementation strategy was employed. Initially, a construct (TX6144) was made, including the upstream genomic sequence (452 bp) of the ccpA gene along with the initial 300 bp of ccpA and 507 bp from the stop codon of the ccpA gene, and was used to insert the first 300 bp of the ccpA gene back into the ccpA deletion mutant (TX6127), resulting in TX6145 (TX82 ΔccpA::300 bp ccpA [ccpA deletion mutant complemented with the initial 300 bp of the ccpA gene]). This was followed by the introduction of a construct (TX6148) containing the ccpA gene (21 bp from the start codon to bp 1020) and the downstream sequence (507 bp) into strain TX6145 (TX82 ΔccpA::300 bp ccpA) to create TX6140 (TX82 ΔccpA::ccpA).
Growth curves.
Cultures grown overnight were inoculated into BHI broth at a dilution of 1:100. The cultures were then grown at 37°C, and aliquots were removed at 0, 2, 4, 6, 9, and 24 h for determination of their CFU on BHI agar.
Experimental endocarditis.
Aortic valve endocarditis was induced in white Sprague-Dawley rats according to our previously reported method (21). The wild type and mutants were tested in a mixed-infection competition assay by inoculating a mixture of bacteria (approximately 1:1 by the optical density [OD]) intravenously via the tail vein, 24 h after catheterization; the cultures were then serially diluted and plated to determine the actual CFU and percentage of each strain that went into the inoculum. Animals were sacrificed 48 h after infection; hearts were aseptically removed; the aortic valves were excised, weighed, and homogenized in 1 ml of saline; and dilutions were plated onto BHI medium. After 24 h, all colonies that grew from an aortic valve (up to 47/rat, thus accommodating two rats' colonies and controls in a 96-well microtiter plate) were picked into wells of microtiter plates containing BHI medium, grown overnight, and then replica plated onto a filter overlaid onto BHI agar. DNA lysates from colonies were hybridized under high-stringency conditions (22), using intragenic DNA probes of acm, ccpA, and ddl (23) to generate the percentages of wild-type and mutant colonies of the bacteria recovered from aortic valves. Data were expressed as percentages of WT and mutant colonies per aortic valve and analyzed by the paired t test using GraphPad Prism version 4.00 for Windows (GraphPad Software, San Diego, CA). The rat experimental procedures were carried out in accordance with the institutional policies and the guidelines stipulated by the Animal Welfare Committee, University of Texas Health Science Center at Houston.
Growth competition assay.
For the growth competition assay, 0.1 ml each of cultures (∼2 × 109 bacteria/ml) of TX82 and TX6127 grown overnight were added to 4.8 ml of BHI broth and grown at 37°C with gentle shaking, aliquots were removed at 4 and 24 h, and dilutions were plated onto BHI medium. Even though the strains were distinguishable by colony size differences, for specificity, we employed DNA hybridization using intragenic DNA probes of acm and ccpA to determine the percentages of wild-type and mutant colonies. The experiment was performed three times, and statistical analyses were performed by two-way analysis of variance (ANOVA).
Biofilm assay.
Cultures of TX82 or TX6127 grown overnight were diluted 1:100 in tryptic soy broth (TSB) containing 0.25% glucose, and 200 μl of this mixture was inoculated into the wells of 96-well polystyrene microtiter plates. After 24 h of incubation at 37°C, biofilm formation was quantified by measuring the OD at 570 nm (OD570) of crystal violet-stained wells, as described previously (24, 25). The assay was repeated twice with a minimum of 8 wells each time. Statistical analyses were performed by the Mann-Whitney test.
RESULTS
The growth defect of TX6051 is due to a nonsense mutation in its ccpA gene.
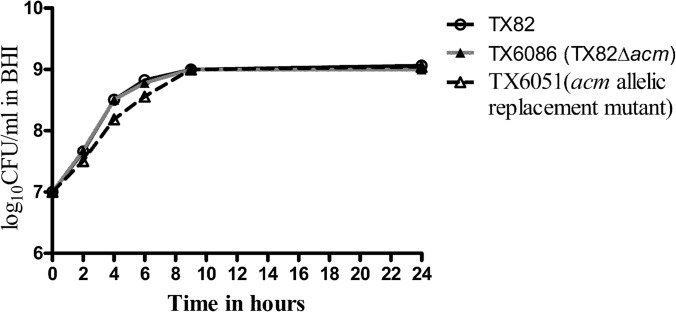
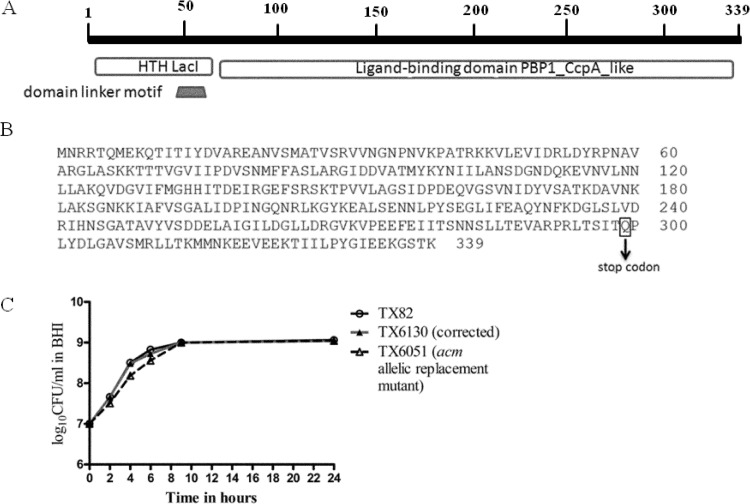
In order to determine whether the growth delay observed with TX6051 is indeed due to the effect of the deletion of acm, we constructed a markerless, nonpolar acm gene deletion mutant. Surprisingly, this mutant did not exhibit any growth defect, unlike TX6051 (Fig. 1), while both strains showed a loss of collagen binding (data not shown). This suggested that there may be one or more extraneous mutations in the genome of TX6051 which caused the growth defect. This prompted us to perform genomic sequencing of TX6051, which revealed the presence of a nonsense mutation in the ccpA gene. We did not find any mutations other than the ccpA single nucleotide nonsense mutation. The domain organization of the CcpA protein is shown in Fig. 2A. The ccpA gene encodes a protein of 339 amino acids (aa) that contains a helix-turn-helix (HTH) DNA-binding domain of the LacI family of transcriptional regulators at the N terminus (aa 15 to 66); a C-terminal ligand-binding domain (aa 71 to 337), which exhibits the type 1 periplasmic protein-binding fold; and a linker (aa 55 to 64) connecting these two domains (26).
FIG 1.
Effect of acm deletion on growth of TX82. Shown are growth curves of wild-type E. faecium strain TX82, its nonpolar acm deletion mutant (TX6086), and the previously described acm allelic replacement mutant (TX6051). Cells were grown in BHI broth at 37°C, and aliquots were removed at 0, 2, 4, 6, 9, and 24 h for determination of their CFU counts on BHI agar. Data are representative of two independent experiments.
FIG 2.
Diagrammatic representation of the CcpA protein and effect of the ccpA nonsense mutation on growth of TX82. (A) Schematic representation of the CcpA protein indicating conserved domains. (B) Amino acid sequence of CcpA of TX82 indicating the position of the nonsense mutation. (C) Growth curve of wild-type E. faecium strain TX82, the previously described acm allelic replacement mutant (TX6051), and TX6051 with a corrected ccpA gene (TX6130). Cells were grown in BHI broth at 37°C, and aliquots were removed at 0, 2, 4, 6, 9, and 24 h for determination of their CFU counts on BHI agar. Data are representative of two independent experiments.
The nonsense mutation in the ccpA gene of TX6051 occurred at the 299th amino acid, converting glutamine to a stop codon and thereby causing premature termination (Fig. 2B). To confirm that the observed growth defect is due solely to the mutation in the ccpA gene, we corrected the ccpA mutation in TX6051 and examined the growth curve; correction of the ccpA nonsense mutation completely abrogated the growth defect associated with TX6051 (Fig. 2C), suggesting that the C-terminal (aa 71 to 337) ligand-binding domain is responsible for the regulation of growth.
Deletion of ccpA affects growth of TX82.
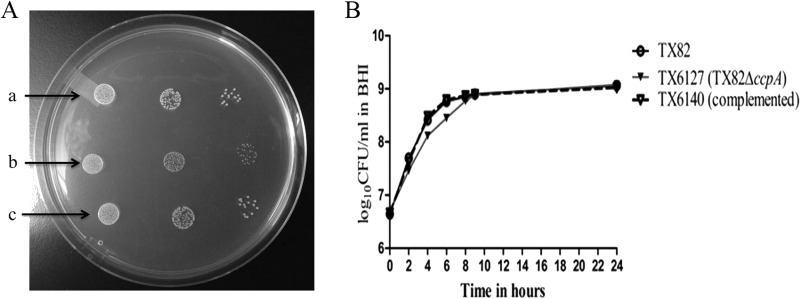
Since our data showed an effect of CcpA on growth, we next sought to determine the consequence of the deletion of the complete ccpA gene on growth and virulence. For this, we constructed a markerless nonpolar ccpA gene mutant (TX6127) in E. faecium TX82. As with TX6051, ccpA deletion mutant colonies were small compared to those of TX82 and TX6140, the ccpA chromosomally complemented strain (Fig. 3A). In addition, it showed a growth defect in broth almost identical to that observed for TX6051 carrying the ccpA single nucleotide mutation, in the lag phase and early log phase, compared to TX82 (Fig. 3B).
FIG 3.
Effect of ccpA deletion on growth of TX82. (A) Colony characteristics of wild-type E. faecium strain TX82 (a), the ccpA deletion mutant (TX6127) (b), and TX6127 complemented with ccpA (TX6140) (c). A serially diluted suspension of bacteria grown overnight was spotted onto BHI agar plates. (B) Growth curve of wild-type E. faecium strain TX82, its ccpA deletion mutant (TX6127), and TX6127 complemented with ccpA (TX6140). Data are representative of two independent experiments.
CcpA is important for virulence of E. faecium in experimental endocarditis.
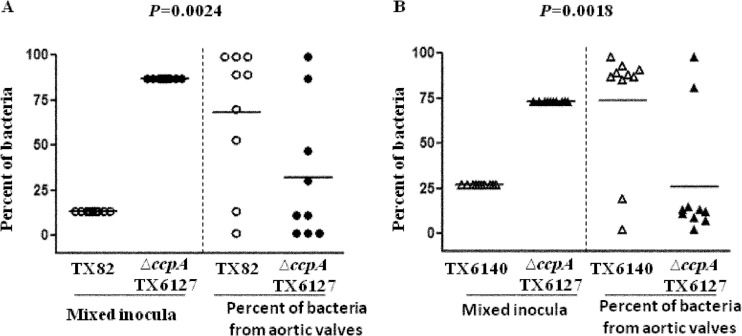
Next, we wanted to determine whether CcpA is important for the infection process during endocarditis. For this, 9 catheterized rats were inoculated intravenously through the tail vein with a mixture of wild-type strain TX82 and the ccpA deletion mutant (TX6127). Although we had aimed for approximately equal CFU/ml estimated by equal ODs, the actual bacterial mean CFU determined for TX82 and TX6127 from the inocula were 2.1 × 108 CFU/rat (representing 13% of the inoculum) and 1.4 × 109 CFU/rat (representing 87% of the inoculum), respectively. Even though the inoculum mixture contained a higher percentage of the ccpA deletion mutant than of the wild type, plating of the bacteria recovered from aortic valves showed a (geometric) mean of 6.3 × 104 CFU/g of aortic valve, ranging from 1.3 × 101 to 7.9 × 106 CFU/g. This was followed by Southern hybridization of individual colonies, which revealed that 68% of the total bacteria were wild-type strain TX82, compared to only 32% for the ccpA deletion mutant (TX6127) (P = 0.0024) (Fig. 4A). This demonstrates a clear dominance of TX82 over the ccpA deletion mutant and indicates that CcpA is important in infective endocarditis.
FIG 4.
Attenuation of ccpA deletion mutant strain TX6127 in a rat endocarditis model. (A) Percentages of wild-type (TX82) and ccpA deletion mutant (TX6127) bacteria recovered from the initial inoculum (left) and from aortic valves of 9 rats 48 h after infection (right). Horizontal lines indicate means (P = 0.0024 by paired t test) for the percentage of total bacteria in the aortic valve versus that in the inoculum. (B) Percentages of ccpA chromosomally complemented (TX6140) and ccpA deletion mutant (TX6127) bacteria recovered from the initial inoculum (left) and from aortic valves 48 h after infection of 10 rats (right). Horizontal lines indicate means (P = 0.0018 by paired t test) for the percentage of total bacteria in the aortic valve versus that in the inoculum for each rat.
In order to conclusively show the importance of CcpA in endocarditis, we infected 10 rats with an inoculum mixture of ccpA deletion mutant strain TX6127 (1.4 × 109 bacteria/rat, representing 73% of the inoculum) and ccpA chromosomally complemented strain TX6140 (5.2 × 108 bacteria/rat, representing 27% of the inoculum). The geometric mean CFU recovered from aortic valves was 4.5 × 104 CFU/g of aortic valve, ranging from 1.3 × 101 to 2.8 × 107 CFU/g. The ccpA chromosomally complemented strain was recovered at a significantly higher percentage (81.5%) than the ccpA deletion mutant (18.5%) (P = 0.0018). Taken together, these findings demonstrate the importance of CcpA for virulence in experimental endocarditis (Fig. 4B).
Deletion of ccpA also causes reduced competitiveness in mixed in vitro growth and reduced biofilm formation capacity.
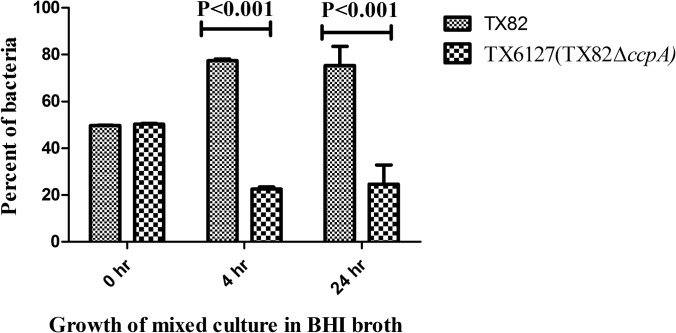
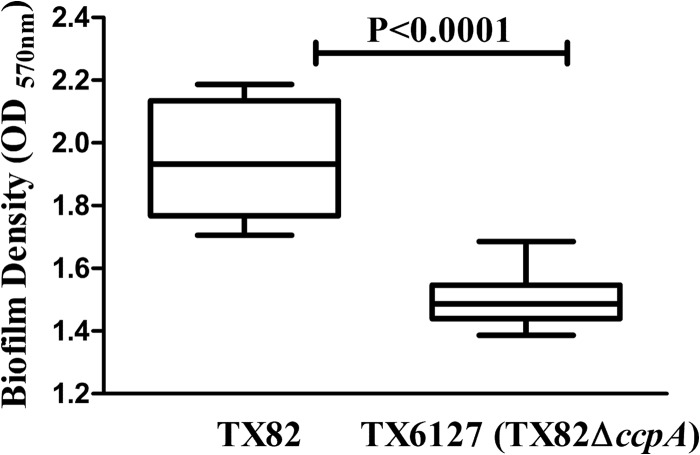
Since the ccpA deletion mutant (TX6127) showed attenuation in the endocarditis model, we next sought to determine whether this effect was correlated with an in vitro mixed-growth defect or reduced biofilm formation. For this, we performed a growth competition assay. The percentage of colonies recovered was higher for wild-type strain TX82 than for ccpA deletion mutant strain TX6127 (77.3% versus 23.7% and 75.3% versus 24.7% for the wild type and the ccpA deletion mutant at 4 h and 24 h, respectively; P < 0.001 by two-way ANOVA), suggesting that the growth defect plays a major role in the attenuated phenotype exhibited by the ccpA deletion mutant in the endocarditis model (Fig. 5). Next, we measured biofilm formation of these strains, grown independently in microtiter plates, and observed that the ccpA deletion mutant exhibited reduced biofilm formation compared to that of the wild type (median OD570 values of 1.486 and 1.933 for TX6127 and TX82, respectively; P < 0.0001) (Fig. 6). This raises the possibility that the capacity to form biofilm may also contribute to the attenuated phenotype of the ccpA deletion mutant in the infective endocarditis model.
FIG 5.
Effect of ccpA deletion on competitive growth in a mixed culture. Equal quantities of cultures (2 × 109 bacteria/ml) of the wild type (TX82) and the ccpA deletion mutant (TX6127) grown overnight were added to BHI broth and grown at 37°C with gentle shaking. Aliquots were removed at 4 and 24 h, and dilutions were plated onto BHI agar. The bars represent the means ± standard errors of percentages of each strain in the mixed culture in BHI broth at 4 and 24 h after inoculation (P < 0.001by two-way ANOVA).
FIG 6.
Effect of ccpA deletion on biofilm formation by TX82. Biofilm formation by the wild type (TX82) and the ccpA deletion mutant (TX6127) was quantitated by measuring the OD570 of crystal violet-stained wells. Horizontal lines indicate median OD570 values, and interquartile ranges, with the minimum and maximum values marked by whiskers, represent combined data from two independent assays with 8 wells per experiment. Statistical analyses were performed by a Mann-Whitney test.
Acm also contributes to the infectivity of TX82 in experimental endocarditis.
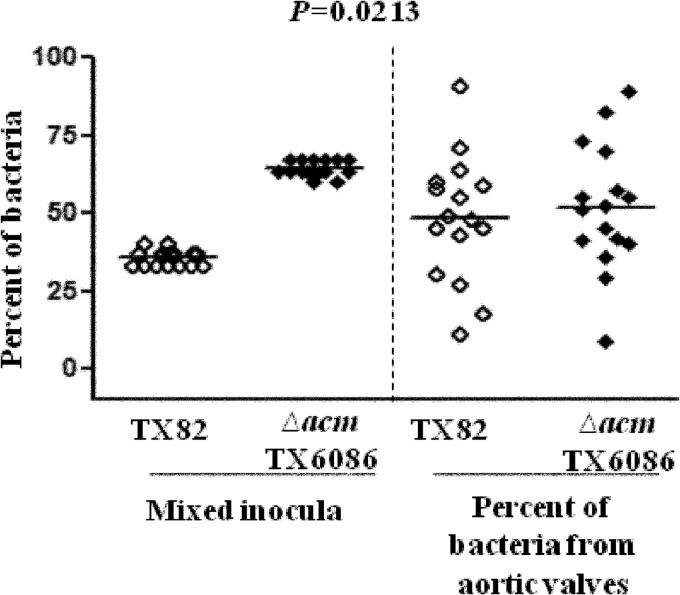
Next, we wanted to determine whether Acm actually has a role in infective endocarditis in the absence of a ccpA mutation. Toward this goal, we tested wild-type strain TX82 and its markerless nonpolar acm deletion mutant (TX6086) in the endocarditis model. For this, 16 rats were used, as described above, with a mixture of the wild type and the acm deletion mutant. The CFU of the wild type and mutant strain TX6086 in the inoculum were 2.5 ×109 CFU (38%) and 4 × 109 CFU (62%) for TX82 and TX6086, respectively. The mean percentages of CFU recovered from aortic valves (geometric mean of 3.9 × 105 CFU/g of aortic valve, ranging from 4.4 × 101 to 5.1 × 107 CFU/g) were 48% and 52% for TX82 and TX6086, respectively (P = 0.0213), indicating an advantage of TX82 over the acm mutant (TX6086), even in the absence of the ccpA mutation (Fig. 7).
FIG 7.
Attenuation of a nonpolar acm deletion mutant (TX6086) in a rat endocarditis model. Percentages of wild-type and acm deletion mutant (TX6086) bacteria recovered from the initial inoculum (left) and from aortic valves 48 h after infection of 16 rats (right) are shown. Horizontal lines indicate means (P = 0.0213 by paired t test) for the percentage of total bacteria in the aortic valve versus that in the inoculum for each rat.
DISCUSSION
We have previously shown that the expression of a functional acm gene confers a collagen adherence phenotype to clinical E. faecium strains and found evidence that Acm expression occurred in vivo in all patients with infective endocarditis, even when not expressed in vitro (16). In addition, acm allelic replacement mutant strain TX6051 was found to be highly attenuated in a rat endocarditis model and exhibited a slight growth delay compared to wild-type TX82 (7, 17). To follow up on this finding, we recently created a nonpolar markerless acm deletion mutant and did not observe any growth delay or defect, as was seen with acm allelic replacement mutant strain TX6051, which suggested that the presence of one or more extraneous mutations in the genome of TX6051 might have caused the defect in growth. By comparative genomic sequence analysis of the wild type and the acm allelic replacement mutant, we then found that there is a nonsense mutation in the ccpA gene of TX6051, leading to premature termination of CcpA.
CcpA is a global transcriptional regulator belonging to the LacI-GalR family of transcriptional regulators and is the major regulator of carbon catabolite repression (CR) in low-G+C Gram-positive bacteria. CR refers to the repression of metabolism of nonpreferred carbon sources when preferred sugar sources are available. When glucose or other easily metabolized sugars are present, Hpr kinase is activated and causes the phosphorylation of the phosphocarrier protein HPr, which is a component of the phosphoenol pyruvate-carbohydrate phosphotransferase system (PTS) (27). This initiates complex formation between CcpA and Hpr (Ser46-P), which binds to CREs (catabolite-responsive elements) of catabolite-responsive genes and either represses or activates gene transcription. The role of CcpA in growth and sugar utilization was previously demonstrated for many low-G+C Gram-positive bacteria, including Enterococcus faecalis, Staphylococcus aureus, Streptococcus pneumoniae, Streptococcus mutans, and Lysinibacillus sphaericus (28–31). However, its role in E. faecium is not yet known. Thus, we sought to determine whether the ccpA mutation was responsible for the transient growth delay observed for TX6051 by correcting the mutation in the ccpA gene of TX6051 as well as by creating a ccpA gene deletion mutant. After the nonsense mutation in ccpA was corrected, the transient growth defect observed for TX6051 reverted to normal, and after deletion of the ccpA gene, a reduced initial growth rate and smaller colony size were observed. Furthermore, the reduced growth and the smaller colonies caused by the deletion of the ccpA gene were completely reversed by chromosomal complementation of the ccpA deletion mutant with an intact ccpA gene. Thus, the present study demonstrates that CcpA is important for the regulation of growth and colony size in E. faecium.
Despite the fact that most of the transcriptional regulatory functions of CcpA are associated with the uptake and utilization of carbohydrates, CcpA is also involved in the regulation of amino acid biosynthesis, sporulation, and toxin production (32–35). CcpA has been linked to biofilm formation in many bacteria, including S. aureus, Staphylococcus epidermidis, Bacillus subtilis, and Clostridium perfringens (36–39). Interestingly, CcpA-dependent regulation has also been demonstrated for the expression of many virulence factors in Gram-positive bacterial pathogens, including S. aureus, Bacillus anthracis, group A streptococci, and E. faecalis (30, 40–43). Moreover, deletion of ccpA led to decreased virulence of S. pneumoniae in a pneumonia model and reduced colonization of the nasopharynx (41). Similarly, a ccpA deletion mutant strain of B. anthracis was highly attenuated in a murine model of infection (42). Attenuation of virulence was also observed when the ccpA gene of Streptococcus suis was deleted (44). Nevertheless, until now, it had not been shown that CcpA played any part in the pathogenesis of enterococcal infections or endocarditis. In the present study, when rats were inoculated intravenously with a mixture of TX82 and the ccpA deletion mutant, TX82 was found to have a statistically significant advantage over the ccpA deletion mutant with respect to the capacity to infect aortic valves, implicating CcpA as being important for full infectivity in the pathogenesis of endocarditis. This was further confirmed by the increased recovery of a ccpA-complemented strain from aortic valves compared to the recovery of ccpA deletion mutant strain when a mixture of both strains was used to infect rats. The increase in the percentage of recovered colonies observed in the presence of ccpA may be due to the upregulation of expression of certain virulence-associated genes, which in turn might provide enhanced adherence, colonization, and persistence properties to E. faecium, as observed for other pathogens (41, 42, 44). Alternatively, CcpA might also favor the increased uptake and utilization of carbohydrates from the surroundings, thereby increasing the metabolic fitness and success of E. faecium during the infection process. Thus, in order to determine the effect of the growth defect on the attenuated virulence phenotype exhibited by the ccpA deletion mutant, we performed a growth competition assay, and the results suggested that the wild-type strain outcompetes the ccpA mutant strain when they are mixed and grown competitively, attributing a main role for the growth defect in the reduced-virulence property. However, biofilm formation, a key factor in the pathogenicity and persistence properties of many microbes, was also found to be reduced in the ccpA deletion mutant strain compared to the wild type when these strains were independently grown into a biofilm in microtiter plates. Thus, our results suggest that the attenuation observed with the ccpA deletion mutant may be attributable to the pleiotropic regulatory effects of this transcriptional regulator, possibly affecting genes involved in carbon utilization which may affect fitness as well as possibly having an effect on virulence genes, a subject which we are currently pursuing (S. R. Somarajan and B. E. Murray, unpublished data).
As it became evident that CcpA is important in the pathogenesis of infective endocarditis and also contributed to the attenuation of TX6051, we sought to clarify whether Acm actually has a role in endocarditis, in the absence of a ccpA mutation. After infecting rats with a mixture of the wild type and the nonpolar markerless acm deletion mutant, we found that the acm deletion mutant infected valves at a significantly lower level than did wild-type strain TX82, although the level of colonization was not as drastically reduced as the level that we observed in our previous study with the acm allelic replacement mutant harboring the ccpA mutation (17); nonetheless, the results showed that Acm is important in the pathogenesis of infective endocarditis, which may also account for the presence of anti-Acm antibodies in all patients with E. faecium endocarditis (16).
In conclusion, our study indicates that Acm and CcpA contribute independently to the pathogenesis of infective endocarditis. It is not yet clear how CcpA affects the pathogenesis of E. faecium infections, and this subject warrants future study.
ACKNOWLEDGMENTS
We thank Karen Jacquez-Palaz for her technical assistance and Hongyu Gao for help with the analysis of sequence data.
This work was supported in part by NIH grant R01 AI067861 from the NIAID to Barbara E. Murray and NIH grant U54 HG004968 to George M. Weinstock.
Footnotes
Published ahead of print 9 June 2014
REFERENCES
- 1.Arias CA, Murray BE. 2012. The rise of the Enterococcus: beyond vancomycin resistance. Nat. Rev. Microbiol. 10:266–278. 10.1038/nrmicro2761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hidron AI, Edwards JR, Patel J, Horan TC, Sievert DM, Pollock DA, Fridkin SK. 2008. NHSN annual update: antimicrobial-resistant pathogens associated with healthcare-associated infections. Annual summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2006-2007. Infect. Control Hosp. Epidemiol. 29:996–1011. 10.1086/591861 (Erratum, 30:107, 2009, ) [DOI] [PubMed] [Google Scholar]
- 3.Murray BE. 2000. Vancomycin-resistant enterococcal infections. N. Engl. J. Med. 342:710–721. 10.1056/NEJM200003093421007 [DOI] [PubMed] [Google Scholar]
- 4.Galloway-Pena JR, Nallapareddy SR, Arias CA, Eliopoulos GM, Murray BE. 2009. Analysis of clonality and antibiotic resistance among early clinical isolates of Enterococcus faecium in the United States. J. Infect. Dis. 200:1566–1573. 10.1086/644790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Somarajan SR, Murray BE. 2013. Could a phosphotransferase system provide the means to control outbreaks of Enterococcus faecium infection? J. Infect. Dis. 207:1633–1636. 10.1093/infdis/jit080 [DOI] [PubMed] [Google Scholar]
- 6.Lebreton F, van Schaik W, McGuire AM, Godfrey P, Griggs A, Mazumdar V, Corander J, Cheng L, Saif S, Young S, Zeng Q, Wortman J, Birren B, Willems RJ, Earl AM, Gilmore MS. 2013. Emergence of epidemic multidrug-resistant Enterococcus faecium from animal and commensal strains. mBio 4(4):e00534-13. 10.1128/mBio.00534-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nallapareddy SR, Singh KV, Murray BE. 2006. Construction of improved temperature-sensitive and mobilizable vectors and their use for constructing mutations in the adhesin-encoding acm gene of poorly transformable clinical Enterococcus faecium strains. Appl. Environ. Microbiol. 72:334–345. 10.1128/AEM.72.1.334-345.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Willems RJ, Homan W, Top J, van Santen-Verheuvel M, Tribe D, Manzioros X, Gaillard C, Vandenbroucke-Grauls CM, Mascini EM, van Kregten E, van Embden JD, Bonten MJ. 2001. Variant esp gene as a marker of a distinct genetic lineage of vancomycin-resistant Enterococcus faecium spreading in hospitals. Lancet 357:853–855. 10.1016/S0140-6736(00)04205-7 [DOI] [PubMed] [Google Scholar]
- 9.Rice LB, Carias L, Rudin S, Vael C, Goossens H, Konstabel C, Klare I, Nallapareddy SR, Huang W, Murray BE. 2003. A potential virulence gene, hylEfm, predominates in Enterococcus faecium of clinical origin. J. Infect. Dis. 187:508–512. 10.1086/367711 [DOI] [PubMed] [Google Scholar]
- 10.Sillanpaa J, Nallapareddy SR, Prakash VP, Qin X, Hook M, Weinstock GM, Murray BE. 2008. Identification and phenotypic characterization of a second collagen adhesin, Scm, and genome-based identification and analysis of 13 other predicted MSCRAMMs, including four distinct pilus loci, in Enterococcus faecium. Microbiology 154:3199–3211. 10.1099/mic.0.2008/017319-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sillanpaa J, Prakash VP, Nallapareddy SR, Murray BE. 2009. Distribution of genes encoding MSCRAMMs and pili in clinical and natural populations of Enterococcus faecium. J. Clin. Microbiol. 47:896–901. 10.1128/JCM.02283-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang X, Top J, de Been M, Bierschenk D, Rogers M, Leendertse M, Bonten MJ, van der Poll T, Willems RJ, van Schaik W. 2013. Identification of a genetic determinant in clinical Enterococcus faecium strains that contributes to intestinal colonization during antibiotic treatment. J. Infect. Dis. 207:1780–1786. 10.1093/infdis/jit076 [DOI] [PubMed] [Google Scholar]
- 13.Leavis HL, Willems RJ, van Wamel WJ, Schuren FH, Caspers MP, Bonten MJ. 2007. Insertion sequence-driven diversification creates a globally dispersed emerging multiresistant subspecies of E. faecium. PLoS Pathog. 3:e7. 10.1371/journal.ppat.0030007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Been M, van Schaik W, Cheng L, Corander J, Willems RJ. 2013. Recent recombination events in the core genome are associated with adaptive evolution in Enterococcus faecium. Genome Biol. Evol. 5:1524–1535. 10.1093/gbe/evt111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nallapareddy SR, Weinstock GM, Murray BE. 2003. Clinical isolates of Enterococcus faecium exhibit strain-specific collagen binding mediated by Acm, a new member of the MSCRAMM family. Mol. Microbiol. 47:1733–1747. 10.1046/j.1365-2958.2003.03417.x [DOI] [PubMed] [Google Scholar]
- 16.Nallapareddy SR, Singh KV, Okhuysen PC, Murray BE. 2008. A functional collagen adhesin gene, acm, in clinical isolates of Enterococcus faecium correlates with the recent success of this emerging nosocomial pathogen. Infect. Immun. 76:4110–4119. 10.1128/IAI.00375-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nallapareddy SR, Singh KV, Murray BE. 2008. Contribution of the collagen adhesin Acm to pathogenesis of Enterococcus faecium in experimental endocarditis. Infect. Immun. 76:4120–4128. 10.1128/IAI.00376-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kristich CJ, Chandler JR, Dunny GM. 2007. Development of a host-genotype-independent counterselectable marker and a high-frequency conjugative delivery system and their use in genetic analysis of Enterococcus faecalis. Plasmid 57:131–144. 10.1016/j.plasmid.2006.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Panesso D, Montealegre MC, Rincon S, Mojica MF, Rice LB, Singh KV, Murray BE, Arias CA. 2011. The hylEfm gene in pHylEfm of Enterococcus faecium is not required in pathogenesis of murine peritonitis. BMC Microbiol. 11:20. 10.1186/1471-2180-11-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arias CA, Panesso D, McGrath DM, Qin X, Mojica MF, Miller C, Diaz L, Tran TT, Rincon S, Barbu EM, Reyes J, Roh JH, Lobos E, Sodergren E, Pasqualini R, Arap W, Quinn JP, Shamoo Y, Murray BE, Weinstock GM. 2011. Genetic basis for in vivo daptomycin resistance in enterococci. N. Engl. J. Med. 365:892–900. 10.1056/NEJMoa1011138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singh KV, Nallapareddy SR, Sillanpaa J, Murray BE. 2010. Importance of the collagen adhesin ace in pathogenesis and protection against Enterococcus faecalis experimental endocarditis. PLoS Pathog. 6:e1000716. 10.1371/journal.ppat.1000716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singh KV, Coque TM, Weinstock GM, Murray BE. 1998. In vivo testing of an Enterococcus faecalis efaA mutant and use of efaA homologs for species identification. FEMS Immunol. Med. Microbiol. 21:323–331. 10.1111/j.1574-695X.1998.tb01180.x [DOI] [PubMed] [Google Scholar]
- 23.Dutka-Malen S, Evers S, Courvalin P. 1995. Detection of glycopeptide resistance genotypes and identification to the species level of clinically relevant enterococci by PCR. J. Clin. Microbiol. 33:24–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mohamed JA, Huang W, Nallapareddy SR, Teng F, Murray BE. 2004. Influence of origin of isolates, especially endocarditis isolates, and various genes on biofilm formation by Enterococcus faecalis. Infect. Immun. 72:3658–3663. 10.1128/IAI.72.6.3658-3663.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Almohamad S, Somarajan SR, Singh KV, Nallapareddy SR, Murray BE. 2014. Influence of isolate origin and presence of various genes on biofilm formation by Enterococcus faecium. FEMS Microbiol. Lett. 353:151–156. 10.1111/1574-6968.12418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marchler-Bauer A, Lu S, Anderson JB, Chitsaz F, Derbyshire MK, DeWeese-Scott C, Fong JH, Geer LY, Geer RC, Gonzales NR, Gwadz M, Hurwitz DI, Jackson JD, Ke Z, Lanczycki CJ, Lu F, Marchler GH, Mullokandov M, Omelchenko MV, Robertson CL, Song JS, Thanki N, Yamashita RA, Zhang D, Zhang N, Zheng C, Bryant SH. 2011. CDD: a conserved domain database for the functional annotation of proteins. Nucleic Acids Res. 39:D225–D229. 10.1093/nar/gkq1189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Warner JB, Lolkema JS. 2003. CcpA-dependent carbon catabolite repression in bacteria. Microbiol. Mol. Biol. Rev. 67:475–490. 10.1128/MMBR.67.4.475-490.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li J, Huang C, Zheng D, Wang Y, Yuan Z. 2012. CcpA-mediated enhancement of sugar and amino acid metabolism in Lysinibacillus sphaericus by NMR-based metabolomics. J. Proteome Res. 11:4654–4661. 10.1021/pr300469v [DOI] [PubMed] [Google Scholar]
- 29.Leboeuf C, Leblanc L, Auffray Y, Hartke A. 2000. Characterization of the ccpA gene of Enterococcus faecalis: identification of starvation-inducible proteins regulated by ccpA. J. Bacteriol. 182:5799–5806. 10.1128/JB.182.20.5799-5806.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seidl K, Stucki M, Ruegg M, Goerke C, Wolz C, Harris L, Berger-Bachi B, Bischoff M. 2006. Staphylococcus aureus CcpA affects virulence determinant production and antibiotic resistance. Antimicrob. Agents Chemother. 50:1183–1194. 10.1128/AAC.50.4.1183-1194.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abranches J, Nascimento MM, Zeng L, Browngardt CM, Wen ZT, Rivera MF, Burne RA. 2008. CcpA regulates central metabolism and virulence gene expression in Streptococcus mutans. J. Bacteriol. 190:2340–2349. 10.1128/JB.01237-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Antunes A, Camiade E, Monot M, Courtois E, Barbut F, Sernova NV, Rodionov DA, Martin-Verstraete I, Dupuy B. 2012. Global transcriptional control by glucose and carbon regulator CcpA in Clostridium difficile. Nucleic Acids Res. 40:10701–10718. 10.1093/nar/gks864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Varga J, Stirewalt VL, Melville SB. 2004. The CcpA protein is necessary for efficient sporulation and enterotoxin gene (cpe) regulation in Clostridium perfringens. J. Bacteriol. 186:5221–5229. 10.1128/JB.186.16.5221-5229.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nuxoll AS, Halouska SM, Sadykov MR, Hanke ML, Bayles KW, Kielian T, Powers R, Fey PD. 2012. CcpA regulates arginine biosynthesis in Staphylococcus aureus through repression of proline catabolism. PLoS Pathog. 8:e1003033. 10.1371/journal.ppat.1003033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Antunes A, Martin-Verstraete I, Dupuy B. 2011. CcpA-mediated repression of Clostridium difficile toxin gene expression. Mol. Microbiol. 79:882–899. 10.1111/j.1365-2958.2010.07495.x [DOI] [PubMed] [Google Scholar]
- 36.Varga JJ, Therit B, Melville SB. 2008. Type IV pili and the CcpA protein are needed for maximal biofilm formation by the Gram-positive anaerobic pathogen Clostridium perfringens. Infect. Immun. 76:4944–4951. 10.1128/IAI.00692-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stanley NR, Britton RA, Grossman AD, Lazazzera BA. 2003. Identification of catabolite repression as a physiological regulator of biofilm formation by Bacillus subtilis by use of DNA microarrays. J. Bacteriol. 185:1951–1957. 10.1128/JB.185.6.1951-1957.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seidl K, Goerke C, Wolz C, Mack D, Berger-Bachi B, Bischoff M. 2008. Staphylococcus aureus CcpA affects biofilm formation. Infect. Immun. 76:2044–2050. 10.1128/IAI.00035-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sadykov MR, Hartmann T, Mattes TA, Hiatt M, Jann NJ, Zhu Y, Ledala N, Landmann R, Herrmann M, Rohde H, Bischoff M, Somerville GA. 2011. CcpA coordinates central metabolism and biofilm formation in Staphylococcus epidermidis. Microbiology 157:3458–3468. 10.1099/mic.0.051243-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shelburne SA, III, Keith D, Horstmann N, Sumby P, Davenport MT, Graviss EA, Brennan RG, Musser JM. 2008. A direct link between carbohydrate utilization and virulence in the major human pathogen group A Streptococcus. Proc. Natl. Acad. Sci. U. S. A. 105:1698–1703. 10.1073/pnas.0711767105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Iyer R, Baliga NS, Camilli A. 2005. Catabolite control protein A (CcpA) contributes to virulence and regulation of sugar metabolism in Streptococcus pneumoniae. J. Bacteriol. 187:8340–8349. 10.1128/JB.187.24.8340-8349.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chiang C, Bongiorni C, Perego M. 2011. Glucose-dependent activation of Bacillus anthracis toxin gene expression and virulence requires the carbon catabolite protein CcpA. J. Bacteriol. 193:52–62. 10.1128/JB.01656-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gao P, Pinkston KL, Bourgogne A, Cruz MR, Garsin DA, Murray BE, Harvey BR. 2013. Library screen identifies Enterococcus faecalis CcpA, the catabolite control protein A, as an effector of Ace, a collagen adhesion protein linked to virulence. J. Bacteriol. 195:4761–4768. 10.1128/JB.00706-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tang Y, Wu W, Zhang X, Lu Z, Chen J, Fang W. 2012. Catabolite control protein A of Streptococcus suis type 2 contributes to sugar metabolism and virulence. J. Microbiol. 50:994–1002. 10.1007/s12275-012-2035-3 [DOI] [PubMed] [Google Scholar]
- 45.Leenhouts K, Buist G, Bolhuis A, ten Berge A, Kiel J, Mierau I, Dabrowska M, Venema G, Kok J. 1996. A general system for generating unlabelled gene replacements in bacterial chromosomes. Mol. Gen. Genet. 253:217–224. 10.1007/s004380050315 [DOI] [PubMed] [Google Scholar]