Abstract
Serratia marcescens is a Gram-negative bacterium that thrives in a wide variety of ambient niches and interacts with an ample range of hosts. As an opportunistic human pathogen, it has increased its clinical incidence in recent years, being responsible for life-threatening nosocomial infections. S. marcescens produces numerous exoproteins with toxic effects, including the ShlA pore-forming toxin, which has been catalogued as its most potent cytotoxin. However, the regulatory mechanisms that govern ShlA expression, as well as its action toward the host, have remained unclear. We have shown that S. marcescens elicits an autophagic response in host nonphagocytic cells. In this work, we determine that the expression of ShlA is responsible for the autophagic response that is promoted prior to bacterial internalization in epithelial cells. We show that a strain unable to express ShlA is no longer able to induce this autophagic mechanism, while heterologous expression of ShlA/ShlB suffices to confer on noninvasive Escherichia coli the capacity to trigger autophagy. We also demonstrate that shlBA harbors a binding motif for the RcsB regulator in its promoter region. RcsB-dependent control of shlBA constitutes a feed-forward regulatory mechanism that allows interplay with flagellar-biogenesis regulation. At the top of the circuit, activated RcsB downregulates expression of flagella by binding to the flhDC promoter region, preventing FliA-activated transcription of shlBA. Simultaneously, RcsB interaction within the shlBA promoter represses ShlA expression. This circuit offers multiple access points to fine-tune ShlA production. These findings also strengthen the case for an RcsB role in orchestrating the expression of Serratia virulence factors.
INTRODUCTION
Serratia marcescens is a highly ubiquitous Gram-negative enteric bacterium that can be isolated from most abiotic environmental sources (air, soil, and water), as well as from plants, insects, and nematodes. In the clinical setting, together with the Serratia liquefaciens complex (Serratia liquefaciens, Serratia proteamaculans, and Serratia grimesii), S. marcescens causes the majority of human Serratia infections and is the cause of urinary tract, respiratory, wound, ocular, cardiac, bloodstream, and surgical infections, mostly affecting intensive care unit patients (1). Its capacity to adhere and persist attached to hospital instrumentation and prostheses (2); its resistance to disinfection procedures; and the increasingly reported acquisition of resistance to lactams, cephalosporins, and aminoglycosides (1, 3) make S. marcescens a current health threat worldwide. However, no clear picture of the mechanisms that allow Serratia to succeed in the infected host has yet emerged.
In our previous work, we have demonstrated that S. marcescens is able to be internalized by nonphagocytic cells. We showed that, once inside the cell, Serratia is able to inhabit and proliferate inside large membrane-bound compartments. These vesicles exhibit autophagic-like features, as they acquire markers typically recruited throughout the progression of autophagosome biogenesis in the antibacterial process (4). However, the majority of the autophagic Serratia-containing vacuole population is nonacidic and has no degradative properties, indicating alteration of the normal delivery to lysosomal compartments. These results reveal that Serratia maneuvers the normal progression of host cell traffic, and they contribute to explaining the potential for Serratia to establish infection and persist in the host (4).
We demonstrated that flagellar expression was essential for the bacteria to adhere to epithelial cells and that this contact was required for subsequent internalization in nonphagocytic cells, suggesting that Serratia flagellar apparatus components can act as adhesins (4). Interestingly, when a mutant strain impeded in flagellum expression (flhDC) was coincubated with epithelial cells, early onset of the autophagic process was induced in the whole cultured epithelial cell layer, identical to the one induced by the wild-type (wt) strain. Moreover, heat-killed bacteria were unable to induce autophagy, and treatment of Serratia with chloramphenicol strongly diminished its ability to promote autophagy from the outside of the eukaryotic cells (4). These results indicated that an actively synthesized protein factor, most likely an exoprotein, was engaged in triggering autophagy prior to invasion.
In this work, we demonstrate that the expression of ShlA is required to provoke the extracellular induction of autophagy that takes place before S. marcescens is internalized in epithelial cells. ShlA is a pore-forming toxin that belongs to the two-partner secretion (also known as type Vb secretion [5]) family and is encoded by the shlBA operon (6, 7). Two-partner secretion (Tps) systems are composed of two cognate components: a β-barrel protein (in this system, ShlB) that is inserted in the outer bacterial membrane and serves as the translocator of the active component, in this case ShlA. ShlB not only secretes, but also mediates the activation of ShlA (8, 9). Several works have supported the notion that ShlA is a dominant factor in the virulence program of S. marcescens: ShlA was shown to exert cytotoxic effects on epithelial cells and fibroblasts (10), and shlBA mutant strains were highly attenuated in mice, Caenorhabditis elegans, and Drosophila melanogaster invasion models (11–13). Our findings reveal that ShlA induces an autophagic process that would aid in shaping the features of the vacuole, where the bacterium survives and proliferates. Therefore, they shed light on the relevance of this toxin in the development of Serratia infection in diverse host organisms.
Earlier work from our group pointed to the S. marcescens Rcs system as a key player in the regulation of the expression of virulence determinants of the bacterium. The Rcs system constitutes a signal transduction phosphorelay that was first identified in Escherichia coli as the regulator of capsule synthesis (14). The system is composed of two inner membrane sensor proteins of the two-component family RcsC and RcsD and an associated transcriptional response regulator, RcsB. Depending on its phoshorylation status, RcsB binds to the promoter regions of its target genes, activating or repressing their transcription. Three additional components, RcsF, a membrane-anchored lipoprotein that can channel stimuli to the RcsC sensor; IgaA, which is able to repress RcsC activity; and RcsA, which coregulates a set of genes, together with RcsB, can be part of the Rcs signaling cascade (14, 15). We have previously demonstrated that the Rcs system is able to modulate swimming and swarming, the two motile capacities of Serratia that depend on a functional flagellum. This regulation is due to RcsB transcriptional control of the expression of flhDC, which encodes the master regulator of the flagellar biogenesis transcriptional cascade, FlhDC (16). The S. marcescens RcsF/RcsCDB signaling cascade is activated by the deficiency of the enterobacterial common antigen (ECA), an exopolysaccharidic structure assembled by the wec cluster-encoded enzymes, provoking RcsB-mediated inhibition of flhDC expression (16, 17). Concomitantly, we found that, in S. marcescens, RcsB is also involved in the thermoregulated production of outer membrane vesicles (OMVs), which pack and deliver a specific cargo of potential virulence determinants, including lipolytic, proteolytic, and chitinolytic enzymes (18).
In this work, we demonstrate that RcsB is able to exert control of the expression of the shlBA operon in a coherent feed-forward regulatory circuit that involves the flagellar regulatory cascade. These results not only highlight the implication of RcsB in the control of the expression of Serratia virulence traits, but also reveal a finely tuned regulatory network required to control the production of ShlA, an S. marcescens dominant pathogenic factor.
MATERIALS AND METHODS
Materials.
α-Minimal essential medium (α-MEM) was obtained from Invitrogen (Argentina). Fetal calf serum (FCS) was obtained from Internegocios S.A. The antibiotics kanamycin (50 μg/ml), ampicillin (100 μg/ml), chloramphenicol (20 μg/ml), spectinomycin (100 μg/ml), and gentamicin (30 μg/ml) were provided by Sigma (Argentina).
Rabbit anti-S. marcescens polyclonal antibodies were prepared in our laboratory, and the secondary goat anti-rabbit IgG(H+L) antibodies conjugated with Cy3 were provided by Zymed Laboratories.
Bacterial strains and plasmids.
The strains and plasmids used in this study are listed in Table 1.
TABLE 1.
Strains and plasmids used in this study
| Strains and plasmids | Genotype and/or comments | Source or reference |
|---|---|---|
| S. marcescens | ||
| Wild type | S. marcescens RM66262; clinical isolate | 17 |
| rcsB | rcsB::pKNOCK-Gmr | 16 |
| rcsA | rcsA::pKNOCK- Gmr | 16 |
| rcsC | rcsC::pKNOCK-Gmr | 16 |
| rcsF | rcsF::pKNOCK-Gmr | 16 |
| fliA | fliA::pKNOCK-Cmr | This work |
| flhD | flhD::pKNOCK-Cmr | 4 |
| shlB | shlB::pKNOCK-Spr | This work |
| wecG | wecG::pKNOCK-Cmr | 16 |
| phlA | ΔphlAB::Cmr | 17 |
| wecG rcsB | wecG::pKNOCK-Cm; mini-Tn5::rcsB; Kmr | 16 |
| phlA rcsB | ΔphlAB::Cm; rcsB::pKNOCK-Gmr | This work |
| phlA shlB | ΔphlAB::Cm; shlB::pKNOCK-Spr | This work |
| fliA/pBB2 | fliA::pKNOCK-Cmr/pBB2; Kmr | This work |
| flhD/pBB2 | flhD::pKNOCK-Cmr/pBB2; Kmr | This work |
| fliA/pBB2::fliA | fliA::pKNOCK-Cmr/pBB2::fliA; Kmr | This work |
| flhD/pBB2::flhDC | flhD::pKNOCK-Cmr/pBB2::flhDC; Kmr | 4 |
| fliA/pBB2::flhDC | fliA::pKNOCK-Cmr/pBB2::flhDC; Kmr | This work |
| flhD/pBB2::fliA | flhD::pKNOCK-Cmr/pBB2::fliA; Kmr | This work |
| wt/pBB2 | Wild-type strain/pBB2; Kmr | This work |
| wt/pBB2::fliA | Wild-type strain/pBB2::fliA; Kmr | This work |
| wt/pBB2::flhDC | Wild-type strain/pBB2::flhDC; Kmr | This work |
| shlB/pBB2 | shlB::pKNOCK-Spr/pBB2; Kmr | This work |
| shlB/pBB2::fliA | shlB::pKNOCK-Spr/pBB2::fliA; Kmr | This work |
| shlB/pBB2::flhDC | shlB::pKNOCK-Spr/pBB2::flhDC; Kmr | This work |
| rcsB/pBB1 | rcsB::pKNOCK-Gmr/pBB1 lacI; Cmr | This work |
| rcsB/pBB1::rcsB | rcsB::pKNOCK-Gmr/pBB1 lacI::rcsB; Cmr | This work |
| fliA/pBB5 | fliA::pKNOCK-Cmr/pBB5; Gmr | This work |
| flhD/pBB5 | flhD::pKNOCK-Cmr/pBB5; Gmr | This work |
| fliA/pBB5::rcsB | fliA::pKNOCK-Cmr/pBB5::rcsB; Gmr | This work |
| flhD/pBB5::rcsB | flhD::pKNOCK-Cmr/pBB5::rcsB; Gmr | This work |
| wecG/pBB2 | wecG::pKNOCK-Cmr/pBB2; Kmr | This work |
| wecG/pBB2::fliA | wecG::pKNOCK-Cmr/pBB2::fliA; Kmr | This work |
| wecG/pBB2::flhDC | wecG::pKNOCK-Cmr/pBB2::flhDC; Kmr | This work |
| wt/ppromshlBA-gfp | Wild-type/pPROBE(NT)::promshlBA; Kmr | This work |
| fliA/ppromshlBA-gfp | fliA::pKNOCK−Cmr/pPROBE(NT)::promshlBA; Kmr | This work |
| flhD/ppromshlBA-gfp | flhD::pKNOCK-Cmr/pPROBE(NT)::promshlBA; Kmr | This work |
| wecG/ppromshlBA-gfp | wecG::pKNOCK-Cmr/pPROBE(NT)::promshlBA; Kmr | This work |
| rcsB/ppromshlBA-gfp | rcsB::pKNOCK-Gmr/pPROBE(NT)::promshlBA; Kmr | This work |
| E. coli | ||
| XL1-Blue | recA1 endA1 gyrA96 thi-1 hsdR17 supE44 relA1 lacTcR | Stratagene |
| W3110 | λ− IN(rrnD-rrnE)1 rph-1 | 50 |
| W3110/pT7 | T7 overexpression vector; Ampr | This work |
| W3110/pES14 | pT7–5::shlBA; Ampr | This work |
| W3110/pBB2 | pBB2; Kmr | This work |
| W3110/pphlAB | pBB2::phlAB; Kmr | This work |
| BL21(DE3), pLysS | F− ompT hsdSB(rB− mB−) gal dcm (DE3) pLysS(Cmr) | Stratagene |
| Plasmids | ||
| pBB1 | pBBR1-MCS1 Cmr; broad host range | 51 |
| pBB2 | pBBR1-MCS2 Kmr; broad host range | 51 |
| pBB5 | pBBR1-MCS5 Gmr; broad host range | 51 |
| pflhDC | pBBR1-MCS2::flhDC; Kmr | 4 |
| pT7 | T7 overexpression vector; Ampr | 52 |
| pES14 | pT7-5:shlBA; Ampr | 7 |
| pphlAB | pBBR1-MCS2::phlAB; Kmr | 17 |
| pBB2::shlBA | pBBR1-MCS2::shlBA; Kmr | This work |
| pBB1::rcsB | pBBR1-MCS1::rcsB; Cmr | This work |
| pBB5::rcsB | pBBR1-MCS5::rcsB; Gmr | This work |
| pBB2::fliA | pBBR1-MCS2::fliA; Kmr | This work |
| pBB2::flhDC | pBBR1-MCS2::flhDC; Kmr | 4 |
| ppromshlBA-gfp | pPROBE(NT)::promshlBA; Kmr | This work |
Plasmid pES14 was kindly provided by Silke I. Patzer (Max Planck Institute for Developmental Biology, Tuebingen, Germany) and was introduced into competent E. coli W3110 and E. coli BL21(DE3) pLysS cells by electroporation.
S. marcescens shlB was disrupted by integration of the suicide plasmid pKNOCK-Spr, derived from plasmid pKNOCK-Gm (19). Briefly, an internal fragment of 460 bp from the shlB gene was amplified by PCR using primers FwmutShlB (BamHI) and RvmutShlB (XhoI) (Table 2). The purified PCR product was digested with the BamHI and XhoI restriction enzymes, and the fragment was ligated into the BamHI and XhoI sites of pKNOCK-GmR. Then, the gentamicin cassette was interrupted by ligation to a spectinomycin cassette (20) in the BglII site. The resulting plasmid was introduced into competent E. coli SM10 (λpir) cells by electroporation and then mobilized into the S. marcescens wild-type strain by conjugation. Insertional mutants were confirmed by PCR analysis. For complementing S. marcescens shlB, pES14 was digested with EcoRI and HindIII restriction enzymes, and the operon shlBA was ligated into the EcoRI and HindIII sites of pBBR1-MCS2 Kmr. The resulting plasmid was mobilized into S. marcescens shlB by conjugation.
TABLE 2.
Primers used in this study
| Primer | Sequence (5′–3′)a |
|---|---|
| PromShlBFwEcoRI | CGGAATTCTATTTCTCGCCGCTGG |
| PromShlBRvBamHI | CGGGATCCGTGCTGACCAACAGC |
| FwmutShlB (BamHI) | CGCGGATCCCGCGTGCAAATTGTTC |
| RevmutShlB (XhoI) | CCGCTCGAGCCGTACGGCAGCGAAT |
| PromflhDFw | AAATGGCGGTCAATAAGTGAG |
| PromflhDRv | GTACCCATATTCCCCATC |
| FliAserratiaNter | TCGCCCGGGGTGAGCGATCTGTATAC |
| FliActerrevserratia | TCCAAGCTTCAGGTATCGTTCGCC |
| FlhDserratiaNter | TCGCCCGGGATGGGGAATATGGGTAC |
| FlhDcterrevserratia | TCCAAGCTTCAGGCTCTTTTCTTCG |
| RcsBFwXhoI | CCGCTCGAGATGAATAACCTGAACG |
| RcsBRvBamHI | CGGGATCCTTAGTCTTTGTCCAACGG |
| rcsBHis-ParallelNcoIFw | CATGCCATGGGAATGAATAACCTGAACG |
| rcsBHis-Parallel XhoIRv | CCGCTCGAGTTAGTCTTTGTCCAACGG |
Restriction sites are underlined.
S. marcescens fliA was disrupted by integration of the suicide plasmid pKNOCK-Cmr (19). Briefly, an internal fragment of 692 bp from the gene fliA was amplified by PCR using primers FliAserratiaNter and FliActerrevserratia (Table 2). The purified PCR product was digested with the NarI restriction enzyme, and a fragment of 350 bp was ligated into the ClaI site of pKNOCK-Cmr. The resulting plasmid was introduced into competent E. coli SM10 (λpir) cells by electroporation and then mobilized into the S. marcescens wild-type strain by conjugation. Insertional mutants were confirmed by PCR analysis. For complementing S. marcescens fliA, pBBR1-MCS2 Kmr was digested with XhoI, and the 5′ overhanging end was filled by treatment with Klenow fragment. Finally, it was digested with the HindIII restriction enzyme. fliA was amplified by PCR using primers FliA-serratia-Nter and FliA-cter-rev-serratia, and the purified PCR product was digested with the SmaI and HindIII restriction enzymes. The fragment was ligated into pBBR1-MCS2 Kmr, and the resulting plasmid was mobilized into S. marcescens by conjugation.
The S. marcescens phlA rcsB and phlA shlB strains were constructed through the mobilization of plasmids rcsB::pKNOCK-GmR and shlB::pKNOCK-Spr, respectively, into the S. marcescens phlA strain by conjugation.
To analyze the transcriptional level of shlBA, the promoter region of the operon was amplified by PCR using the primers Prom ShlBFwEcoRI and PromShlBRvBamHi (Table 2). The purified PCR product was digested with the EcoRI and BamHI restriction enzymes and was ligated into the same sites of pBBR1-MCS2 Kmr. The resulting plasmid was introduced into competent E. coli XL1-Blue cells by electroporation, and then it was digested with HindIII and BamHI restriction enzymes. The purified fragment of 539 bp was ligated into the same sites of pPROBE(NT) (21) and introduced into competent E. coli XL1-Blue cells by electroporation. The plasmid ppromshlBA-gfp was mobilized by conjugation into the S. marcescens wild-type strain and the fliA, flhD, wecG, and rcsB mutant strains.
To construct the pBB1 lacI::rcsB Cmr and pBB5::rcsB GmR plasmids, the rcsB gene was amplified from the S. marcescens wild-type strain chromosome by PCR, using primers RcsBFwXhoI and RcsBRvBamHI (Table 2). The PCR product was cloned into the XhoI-BamHI-digested pBBR1-MCS1 Cmr and pBBR1-MCS5 GmR plasmids. The resulting plasmids were mobilized into bacterial strains by conjugation.
Bacterial and cell culture.
Bacteria were routinely grown in Miller′s Luria-Bertani (LB) medium supplemented with antibiotics at 30°C or 37°C, as indicated.
Stably transfected Chinese hamster ovary (CHO) cells overexpressing enhanced green fluorescent protein (EGFP)-LC3 (CHO-EGFP-LC3) were grown in α-MEM supplemented with 10% FCS at 37°C and 5% CO2.
Hemolysin activity assay.
S. marcescens and E. coli strains were grown overnight (ON) in LB medium, without shaking, at 30°C or 37°C, respectively, as indicated. Two hundred microliters of the bacterial suspension was incubated with 200 μl of a suspension of human red blood cells in phosphate-buffered saline (PBS) for 1 h at 30°C. In another set of experiments, overnight cultures of S. marcescens were diluted 1:100 in fresh LB medium and cultivated for 3 h without agitation at 30°C. Dilutions (1:4) of the exponential cultures were prepared with PBS, and a 200-μl aliquot of each hemolytic sample was incubated with 200 μl of erythrocyte suspension for 1 h at 30°C. After centrifugation, the amount of hemoglobin released into the supernatant was determined by measuring absorbance at 562 nm. The absorbance of each sample was normalized to the optical density at 600 nm (OD600) of the corresponding culture. The 100% activity level was determined by incubating 200 μl of human red blood cells with 200 μl of water. The relative activity was plotted, using the wild-type strain hemolytic activity value as the unit.
Determination of ShlA protein levels.
Ten milliliters of saturated cultures grown in LB medium at 30°C was centrifuged for 5 min at 5,000 × g, and the supernatant was separated. The spent medium was filtered with 0.2-μm acetate-cellulose filters, precipitated with 12% trichloroacetic acid for 30 min at 4°C, and centrifuged for 30 min at 30,000 × g. The precipitated proteins were resuspended in protein sample buffer. One milliliter of trichloroacetic acid-precipitated spent medium was loaded onto 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels. Hemolysin levels were determined by Western blotting using S. marcescens anti-ShlA rabbit polyclonal antibodies.
ShlA rabbit polyclonal antibodies.
Hemolysin was purified as described previously (22). Briefly, E. coli BL21(DE3), pLysS harboring the pES14 plasmid was grown in LB medium at 37°C, and gene expression was induced at an OD600 of 0.5 by addition of isopropyl thio-β-d-galactoside (final concentration, 0.5 mM). After further incubation for 2 h, the cells were sedimented (10,000 × g for 15 min), and the supernatant containing the ShlA protein was precipitated by addition of solid ammonium sulfate (to 45% saturation) on ice. After 1 h of incubation at 0°C, the protein was sedimented by centrifugation at 15,000 × g (30 min; 4°C). The protein was suspended in 1 ml buffer (50 mM HEPES, pH 8), and desalted on a PD30 Sepharose column (GE Healthcare). The eluent was applied to a Mono-S (HR 5/5) cation-exchange column (GE Healthcare) equilibrated with HEPES buffer. The column was washed with 15 ml HEPES buffer, and the bound proteins were eluted by a linear NaCl gradient (0 to 500 mM) in 25 ml HEPES buffer. Fractions of 1 ml were collected. The purified hemolysin was used to immunize New Zealand White rabbits, as previously described (17).
Construction, expression, and purification of RcsB-His×6.
A His×6-tagged RcsB protein (RcsB-His×6)-tagged fusion gene was constructed by PCR using primers rcsBHis pParalellNcoIFw (Table 2) and rcsBHis-Parallel XhoIRv (Table 2) and then cloned into the NcoI and XhoI sites of the pHisParallel vector (23) to obtain the plasmid pHisParallel::orfrcsB-HisNter. RcsB-His×6 was expressed in E. coli BL21(DE3) pLysS. Cells were grown in 500 ml of LB medium at 37°C to an OD600 of 0.5. Following induction with 0.25 μM isopropyl-β-d-thiogalactopyranoside (IPTG) and incubation for 4 h at 25°C, cells were harvested and disrupted by sonication. The RcsB-His×6 protein was purified using an Ni2+-nitrilotriacetic acid-agarose affinity chromatography column according to the QIA expression purification protocol (Qiagen) and exhaustively dialyzed against 20 mM Tris-HCl (pH 7.4), 500 mM NaCl. The protein concentration was determined by a bicinchoninic acid assay (Bio-Rad), and the protein profile of the purified RcsB-His×6 protein was analyzed by SDS-PAGE (24).
DNase I footprinting assay.
DNase I protection assays were done with DNA fragments corresponding to the shlB and flhD promoter regions. The DNA fragments were amplified by PCR using the appropriately 32-P-labeled primers (PromShlBFwEcoRI, PromShlBRvBamHI, and PromflhDRv [Table 2]). Approximately 6.0 fmol of each DNA fragment was incubated with purified RcsB-His×6 protein (see the legend to Fig. 6 for the amounts) at room temperature for 30 min in a 20-μl volume of binding reaction mixture. The RcsB-His×6 protein was previously phosphorylated by incubation with acetyl phosphate (0.9 ng/μl) at room temperature for 30 min. The binding buffer used for protein-DNA incubation contained 25 mM Tris-HCl (pH 8), 50 mM NaCl, 5 mM MgCl2, 5 mM dithiothreitol, 10% glycerol, 2.0 ng/μl salmon sperm DNA, and 0.025 mg/ml bovine serum albumin. DNase I (0.05 U; Life Technologies, Inc.) was added, and the mixture was incubated for 90 s at room temperature in a final volume of 100 μl. The reaction was stopped by adding 90 μl of 20 mM EDTA (pH 8.0) solution. DNA fragments were purified by phenol-chloroform extraction and resuspended in 6.0 μl of sequencing stop solution buffer (10 mM NaOH, 95% formamide, 0.05% bromophenol blue, 0.05% xylene cyanol). Samples were analyzed by denaturing polyacrylamide (6%) gel electrophoresis by comparison with a DNA sequence ladder generated with the appropriate primer. After electrophoresis, the gel was dried and autoradiographed (25).
FIG 6.
Influence of the flagellar regulatory cascade and of RcsB over autophagy induction. CHO-EGFP-LC3 cells were infected with the S. marcescens wild-type, shlB, flhD, fliA, wecG, rcsB, or wecG rcsB strain and fixed at the indicated time points. Intracellular bacteria were detected with anti-Serratia antibodies coupled with a secondary antibody labeled with Cy3. (A) The percentages of cells containing LC3 puncta were determined by confocal microscopy. At least 300 cells were counted for each condition. The averages and SD are shown for three independent experiments performed in duplicate in each case. Statistical analysis was performed using one-way ANOVA and the Tukey-Kramer multiple-comparison test. **, P < 0.01; ***, P < 0.001; statistically significantly different from wild-type S. marcescens. (B) Cells were visualized by confocal laser microscopy. (a and b) Representative images of control noninvaded cells. (c to p) Cells infected with the S. marcescens wild-type (c and d), shlB (e and f), flhD (g and h), fliA (i and j), wecG (k and l), rcsB (m and n), or wecG rcsB (panels o and p) strain. Representative DIC and merged images are shown. Bars, 10 μm.
shlBA gene expression assays.
Ten microliters of overnight LB medium cultures of S. marcescens strains carrying ppromshlBA-gfp was mixed with 1 ml of fresh LB medium with kanamycin, and 100 μl of the mixture was incubated in a 96-well black microtiter plate at 30°C without agitation for 18 h. Fluorescence (excitation wavelength [λexc] = 485 nm; emission wavelength [λem] = 528 nm) and OD600 readings were determined every 60 min with a 96-microwell plate reader (Synergy2 or BioTek ELx808 microplate reader). The means and standard deviations for triplicate analysis were calculated.
Autophagy assay.
The autophagy assay was performed as a gentamicin protection assay (26) with modifications as follows. CHO-GFP-LC3 cells were cultured in 24-well plates until they reached 50% confluence. S. marcescens or E. coli cultures were grown without shaking overnight at 30°C. The bacterial cultures were washed once with PBS, and an appropriate volume was added to each well to reach a multiplicity of infection (MOI) of 10. The plates were centrifuged for 10 min at 1,000 rpm and incubated for 1 h at 37°C and 5% CO2. Then, the cells were washed repeatedly with PBS and medium supplemented with 10% FCS, and 30 μg/ml gentamicin was added, or in other set of experiments, the cells were left without treatment. At the indicated time points, the cells were washed four times with PBS and fixed with 0.5 ml 3% paraformaldehyde solution in PBS.
Indirect immunofluorescence and confocal microscopy.
Infected cells overexpressing EGFP-LC3 were fixed with 0.5 ml of 3% paraformaldehyde solution in PBS for 10 min and permeabilized with 0.1% Triton X-100 solution in PBS for 5 min. Subsequently, the cells were incubated with primary polyclonal antibodies against S. marcescens (1:500) and detected by incubation with anti-rabbit Cy3-conjugated secondary antibodies (1:150). The cells were mounted with SlowFadeAntifade reagent in glycerol-PBS (Molecular Probes). Finally, the cells were analyzed by confocal microscopy with a Nikon Eclipse TE-2000-E2 and the EZ-C1 3.20 Free Viewer program (Nikon, Japan). At least 200 cells from three independent experiments were scored using confocal microscopy. The percentage of colocalization was calculated as the ratio between the number of internalized bacteria that colocalized with EGFP-LC3 and the total number of infected cells.
Statistical analysis.
Statistical analysis was performed using one-way analysis of variance (ANOVA) and the Tukey-Kramer multiple-comparison test with an overall significance level of 0.05. The asterisks in the plots denote the values among the treatment groups for which a statistically significant difference was determined.
RESULTS
ShlA is responsible for inducing autophagy in nonphagocytic epithelial cells.
We previously determined that upon contact with Serratia, an autophagic response was induced in epithelial cells prior to bacterial internalization. Our results showed that, although the flagellum is involved in early bacterium-host cell adhesion, neither the flagellum nor the phospholipase PhlA, which is dependent on the integrity of the flagellar structure for its secretion, was the factor implicated in autophagy induction (4).
Hertle et al. (10) had previously shown that the recombinant ShlA pore-forming toxin of S. marcescens produced in E. coli was able to exert a cytotoxic action on human epithelial cells. They attributed this effect to the capacity of ShlA to induce ATP depletion and K+ efflux from epithelial cells, which was also observed when fibroblasts were assayed. It was later demonstrated that K+ efflux can promote the onset of the autophagic response (27–30). In light of these results, we conjectured that ShlA could be the virulence factor responsible for the induction of autophagy.
To test if the expression of the ShlA exoprotein was able to induce autophagy, the shlBA operon was expressed from the pES14 plasmid in the nonpathogenic E. coli strain W3110. The expression and activity of ShlA in E. coli were verified by immunodetection using anti-ShlA polyclonal antibodies and by the determination of hemolytic activity using human red blood cells, respectively, as described in Materials and Methods (Fig. 1A). The CHO cells expressing EGFP-LC3 were challenged by coincubation with either wild-type Serratia or the E. coli strain carrying the empty vector or the shlBA-containing plasmid, followed by a previously set up gentamicin protection protocol (4). As expected, E. coli W3110 was not able to invade CHO cells, and this behavior was not altered when the strain carried the pES14 plasmid (data not shown). The autophagy phenotype (revealed by the EGFP-LC3 punctate pattern as opposed to the homogeneous distribution of green fluorescence in nontreated cells) was induced in CHO cells expressing EGFP-LC3 when either wild-type Serratia or the E. coli W3110/pES14 strain was used in the assay (Fig. 1B, compare EGFP-LC3 fluorescence patterns in cells shown in images d and h to controls in images b and f). In agreement with our previous results (4), no induction of autophagy was detected with E. coli cells carrying either the empty vector or the pphlAB plasmid, used as controls for the assay (Fig. 1B, j and l). pphlBA harbors the phlAB operon that encodes phospholipase PhlA together with PhlB, a protein which, in complex with PhlA, protects the bacterial cell from lipolytic action before secretion (31). At 300 min postinfection (p.i.), in the absence of extracellular bacteria, which were eliminated by gentamicin action, homogeneous cytoplasmic LC3 labeling was observed only in noninvaded cells. Conversely, cells evidencing the presence of intracellular Serratia showed the characteristic LC3 dotted pattern (compare matching panels in Fig. 1B and C). Figure 1D shows the percentage of cells showing LC3 puncta calculated from each assay. These results indicate that, when extracellular bacteria are no longer present, autophagy occurs only in infected cells. We reason that in noninvaded cells, and in the absence of an external stimulus, autophagosome turnover provokes the clearance of the membrane-recruited LC3 autophagic marker, which would be replaced by the nonlipidated cytoplasmic form.
FIG 1.
S. marcescens ShlA induces autophagy in CHO cells. (A) S. marcescens or E. coli strains were grown ON, without shaking, at 30°C or 37°C, respectively. The saturated cultures were centrifuged, and the supernatant was filtered with 0.2 μm acetate-cellulose filters, precipitated with 12% trichloroacetic acid, and centrifuged for 30 min at 30,000 × g. The precipitated proteins were resuspended in protein sample buffer and loaded onto SDS-PAGE gels. Hemolysin levels were determined by Western blotting using S. marcescens anti-ShlA rabbit polyclonal antibodies. To determine the hemolytic activity, 200 μl of the bacterial suspension was incubated with 200 μl of a human red blood cell suspension for 1 h at 30°C. The amount of released hemoglobin in the supernatant was determined by measuring absorbance at 562 nm. The absorbance of each sample was normalized to the OD600 of the corresponding culture. Relative activity was plotted by considering the wild-type strain hemolytic activity value as the unit. The averages and standard deviations (SD) are shown for four independent experiments performed in duplicate in each case. Statistical analysis was performed using one-way ANOVA and the Tukey-Kramer multiple-comparison test. **, P < 0.01; ***, P < 0.001; statistically significantly different from wild-type S. marcescens. At the top are shown representative images of the immunodetection assay; each lane corresponds to the sample analyzed for hemolytic activity in the plot below. (B and C) CHO-EGFP-LC3 cells were infected with wild-type S. marcescens (c and d) or with noninvasive E. coli W3110/pT7 (e and f), E. coli W3110/pES14 (g and h), E. coli W3110/pBB2 (i and j), or E. coli W3110/pphlAB (k and l) and fixed at 120 min p.i. (B) or treated with gentamicin and fixed at 300 min p.i. (C). Noninfected cells are shown as a control (a and b). The cells were visualized by confocal microscopy. Representative differential interference contrast (DIC) and green fluorescence from EGFP-LC3 images are shown. Bars, 10 μm. (D) Quantitative measurement of the autophagy assay. CHO-EGFP-LC3 cells subjected to the assay depicted in panels B and C were visualized by confocal microscopy. At least 300 infected cells were counted for each condition. The averages and SD are shown for three independent experiments performed in duplicate in each case. Statistical analysis was performed using one-way ANOVA and the Tukey-Kramer multiple-comparison test. ***, P < 0.001.
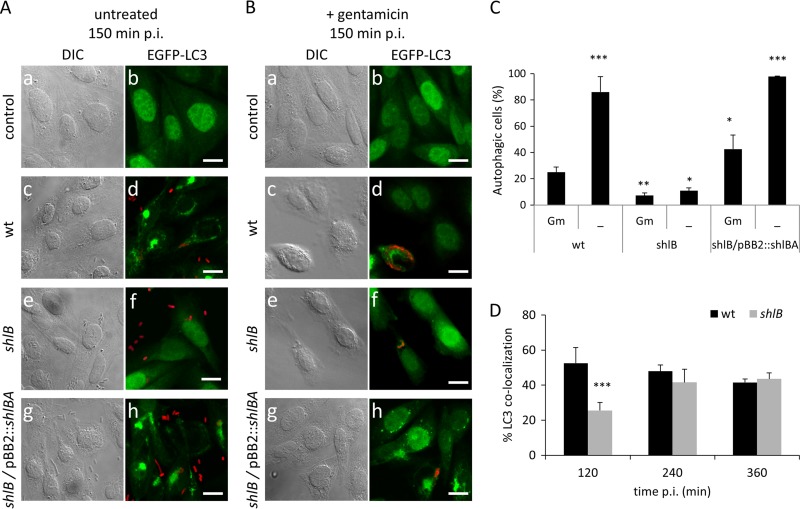
To confirm that ShlA was involved in the external activation of autophagy, we constructed a Serratia mutant strain in which shlB was disrupted by insertion of a spectinomycin resistance cassette (shlB::Spr). Because shlBA is an operon (7) and ShlB is required for ShlA activation and secretion, the shlB mutant strain was unable to both activate and secrete the ShlA hemolysin. The lack of ShlA expression and the hemolytic capacity of the shlB mutant strain were verified by immunodetection and by measuring hemolytic activity (Fig. 1A, compare wt and shlB). Challenged CHO EGFP-LC3 cells were split in two, and extracellular bacteria were either eliminated by gentamicin 60 min p.i. or left untreated. The cells were then fixed, stained, and microscopically inspected 150 min p.i.
When the EGFP-LC3 CHO cell invasion assay was performed with the shlB mutant strain, no LC3 puncta were detected in noninvaded cells (Fig. 2A and B, compare shlB images). The capacity of the shlB mutant strain to induce the autophagic phenotype, as in the wild-type strains, was recovered when it was complemented with the shlBA operon in trans (Fig. 2, h). In fact, the complemented strain provoked a stronger punctate LC3-EGFP fluorescent pattern than the wild-type strain (Fig. 2A and B, compare images d to h). This can be attributed to the high levels of ShlA expressed from the plasmid that would accordingly lengthen the stimulus effect and the time for LC3II to LC3I turnover (Fig. 2B, h). Indeed, ShlA expression and hemolytic activity in the complemented shlB/pBB2::shlBA strain was higher than that determined for the wild-type strain (Fig. 1A). After gentamicin treatment, either with the wild type or with the shlB mutant strain, LC3 puncta were only observed clustered in a restricted area tightly surrounding the invading bacteria, while homogeneous LC3 distribution was observed in the rest of the CHO cell cytoplasm (Fig. 2B, d and f).
FIG 2.
A Serratia mutant strain impeded in ShlA expression is unable to trigger autophagy from the extracellular space. CHO-EGFP-LC3 cells were infected with wild-type S. marcescens (c and d), the S. marcescens shlB strain (e and f), or the S. marcescens shlB/pBB2::shlBA strain (g and h) and fixed at 150 min p.i. (A) or treated with gentamicin and fixed at 150 min p.i. (B). Noninfected cells are shown as a control (a and b). The cells were visualized by confocal microscopy. Representative DIC and merged fluorescence images, green for EGFP-LC3 and red for bacteria, are shown. Bars, 10 μm. (C) CHO-EGFP-LC3 cells subjected to the assay depicted in panels A and B were visualized by confocal microscopy. At least 300 infected cells were counted for each condition. The averages and SD are shown for three independent experiments performed in duplicate in each case. Statistical analysis was performed using one-way ANOVA and the Tukey-Kramer multiple-comparison test. *, P < 0.05; **, P < 0.01; ***, P < 0.001; statistically significantly different from wild-type S. marcescens. (D) CHO-EGFP-LC3 cells were infected with wild-type S. marcescens or the S. marcescens shlB strain and treated with gentamicin at 60 min p.i. and fixed at the indicated times. The percentages of colocalization of bacteria with EGFP-LC3 were determined by confocal microscopy. At least 300 infected cells were counted for each condition. The averages and SD are shown for three independent experiments performed in triplicate in each case. Statistical analysis was performed using one-way ANOVA and the Tukey-Kramer multiple-comparison test. ***, P < 0.001; statistically significantly different from wild-type S. marcescens.
To further analyze whether externally induced autophagy precedes the intracellular formation of the autophagosome-like Serratia-containing vacuole (SeCV), we also scored the total number of cells that showed LC3 puncta (Fig. 2A to C). When extracellular bacteria were not eliminated by gentamicin, the cells that displayed the LC3 dotted pattern exceeded by 3.5-fold the scores determined for gentamicin-treated cells, provided the bacteria expressed ShlA (Fig. 2A and C). As observed in the representative captured images, even when gentamicin has eliminated extracellular bacteria, the autophagic phenotype (evidenced by cells containing LC3 puncta) was more persistent in noninvaded cells in the case of the shlB/pBB2::shlBA strain compared to the wild-type strain, due to high ShlA expression (Fig. 2B and C). We also monitored colocalization of Serratia with LC3 at 120, 240, and 360 min p.i., following the gentamicin protection protocol (Fig. 2D). Once internalization had taken place, the autophagic phenotype was only observed associated with cells containing SeCVs. Although a delay was observed for the shlB mutant strain levels at 120 min p.i., at 240 or at 360 min, shlB reached numbers of cells containing LC3 puncta equivalent to those in the wild-type strain (Fig. 2D). Together, these results indicate that when host cells are challenged with Serratia, the vast majority of exposed cells undergo autophagy induction, and that this trigger depends on ShlA expression. Once internalization has occurred, ShlA is no longer essential to sustain LC3 recruitment to the SeCV within the invaded cell, suggesting that bacterial effectors other that ShlA could be involved in the autophagy process intracellularly.
ShlA is transcriptionally modulated by RcsB.
In light of these results, we sought to gain insight into the regulatory mechanisms that govern ShlA expression. We had shown that the flagellar biogenesis was transcriptionally modulated by the RcsCDB system, with flhDC under negative regulation by RcsB (17). While analyzing this mechanism, we concomitantly observed that the rcsB mutant showed exacerbated hemolytic activity. Therefore, we hypothesized that this increase could be due to a transcriptional cascade involving the Rcs flagellar circuit under the control of shlBA expression.
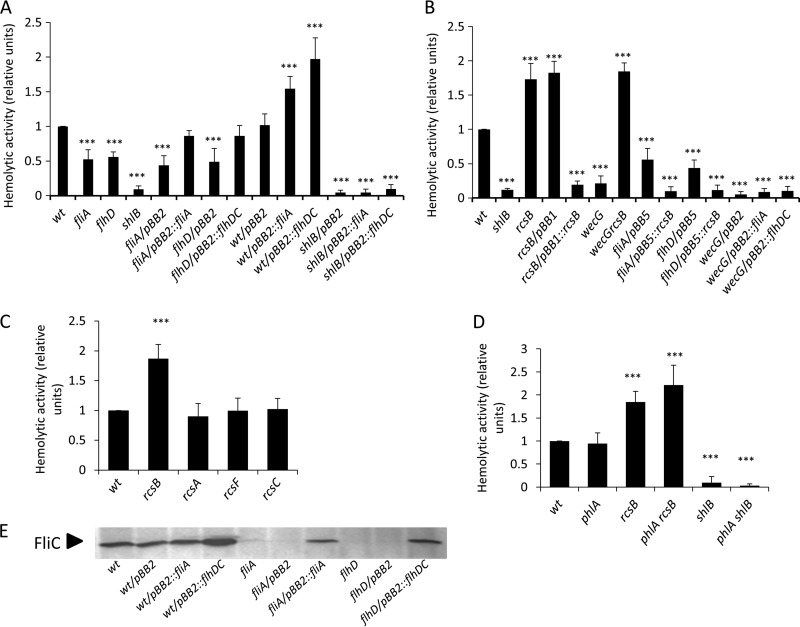
To examine this possibility, we assayed the hemolytic capacities of the S. marcescens flhD and fliA mutant strains compared to the wild-type strain. In both mutant strains, we observed a partial decrease (ca. 50% reduction) in hemolytic activity levels that was restored to the wild-type level when FlhDC or FliA was expressed in trans from the pBB2::flhDC or pBB2::fliA plasmid, respectively (Fig. 3A). Overexpression of FliA or FlhDC in the wild-type background rendered up to 2-fold-increased hemolytic activity levels compared to those attained in the wild-type strain (Fig. 3A). We also overexpressed FliA or FlhDC in an shlB mutant strain. As shown in Fig. 3A, no induction of a second hemolytic activity was detected in these strains. Together, these results indicated an inducing action of the flagellar regulatory cascade over shlA expression. On the other hand, the rcsB mutant strain showed a 2-fold increase in hemolytic capacity relative to the wild-type strain (Fig. 3B).
FIG 3.
Modulation of hemolytic activity by the flagellar regulatory cascade and the Rcs system. The hemolytic activity of each S. marcescens strain was determined by hemolysis assay in liquid culture. Log-phase cells (A600 = 0.25), grown at 30°C in liquid LB medium without agitation, were harvested, resuspended in PBS buffer, and incubated at 30°C with an equal volume of erythrocytes, as described in Materials and Methods. Hemolytic activity values (relative units) were calculated relative to the hemolytic activity of the wild-type strain, considered the unit. The averages and SD are shown for four independent experiments performed in duplicate in each case. Statistical analysis was performed using one-way ANOVA and the Tukey-Kramer multiple-comparison test. ***, P < 0.001; statistically significantly different from wild-type S. marcescens. (A) Hemolytic activity of wild-type, fliA, flhD, and shlB mutant strains and the strains harboring either the empty vector or pBB2::fliA and pBB2::flhDC plasmids was analyzed. (B) Hemolytic activity of wild-type, shlB, rcsB, wecG, wecGrcsB, and flagellar-mutant strains and the same strains carrying the empty vectors or pBB5::rcsB, pBB1::rcsB; pBB2::fliA, or pBB2::flhDC was tested. (C) Hemolytic activity of mutant strains in Rcs system components, rcsB, rcsA, rcsF, and rcsC, was assayed. (D) Hemolytic activity from the phlA strain or the phlA rcsB or phlA shlB double-mutant strain was assayed. The shlB strain was used as a control in panels A, B, and D. The values shown are the means of at least three independent experiments, and the error bars indicate standard deviations. (E) Immunodetection of the flagellin FliC. Whole-cell extracts from S. marcescens grown ON at 30°C were analyzed by Western blotting with anti-flagellin polyclonal antibodies. The image is representative of at least three independent immunodetection assays.
A strong (5-fold) reduction in hemolytic activity was detected when an Rcs-inducing condition, i.e., a wecG mutant background in which ECA assembly is impaired (16, 17), was used in the assay. Under this condition, we obtained hemolytic levels similar to those measured for the shlB strain (Fig. 3B). As expected, the hemolytic capacity of the wecG mutant was restored to rcsB levels in a wecG rcsB double-mutant strain (Fig. 3B). Moreover, the overexpression of RcsB inhibited hemolytic activity in either an flhD or an fliA mutant background (Fig. 3B). In accordance with this result, neither pBB2::flhDC nor pBB2::fliA could complement a wecG mutant strain (Fig. 3B, wecG/pBB2::fliA and wecG/pBB2::flhDC). In addition, no effect was detected when an rcsA, rcsF, or rcsC genetic background was compared to the wild type (Fig. 3C, compare wt with rcsA, rcsF, and rcsC hemolytic levels). Therefore, our results indicate that RcsB downregulates hemolysin expression. They also provide evidence that RcsB inhibitory action can be exerted, bypassing the flagellar regulatory cascade, and that under the assayed conditions, RcsF/RcsC/RcsA signaling components have no role in the control of ShlA expression levels.
Previous work showed that the expression of the PhlA phospholipase is activated by FliA and that PhlA secretion depends on the integrity of the type III flagellar secretory apparatus (32). Furthermore, it was shown that PhlA was able to cause cell lysis in the presence of phospholipids (33). Therefore, to rule out the possibility that PhlA expression could be partially responsible for the observed induction of the cytolytic activity mediated by the activation of the flagellar cascade, a phlA mutant strain was also tested. As shown in Fig. 3D, hemolytic activity levels measured in a wild-type, rcsB, or shlB background were not affected by the deletion of phlA. The functionality of pBB2::flhDC and pBB2::fliA in the complementation of flhD or fliA mutant strains, as well as the effects of FlhDC and FliA overexpression in the wild-type strain background, was also examined by immunodetection of flagellin expression (Fig. 3E). This assay confirmed the proper functional expression of both flagellar transcriptional regulators from the pBB2 plasmids.
To analyze the observed regulatory effects at the transcriptional level, we monitored shlBA gene expression profiles by detecting fluorescence over time from a ppromshlBA-gfp transcriptional reporter in the aforementioned mutant genetic backgrounds. ppromshlBA harbors 539 bp that comprise 500 bp upstream of the shlB ATG initiation codon and 39 bp of the shlB coding region fused to the green fluorescent protein (GFP)-encoding gene (see plasmid construction details in Materials and Methods). As shown in Fig. 4, and in good correlation with the hemolytic activity results (Fig. 3A and B), shlBA expression was repressed in the fliA or flhD strains and was further inhibited in the wecG mutant background, while strong activation was detected in an rcsB strain compared to the wild-type strain. The fact that at 16 h fliA or flhD mutants reached wild-type transcriptional activity levels shows that, as has also been demonstrated by the results from hemolytic activity measurements (Fig. 3A), FliA control over shlBA expression is not an on-off regulation. Therefore, we can infer that shlBA expression can also be directed by other transcriptional factors, such as Fur (34), depending on the growth phase or environmental conditions of the bacterial culture. Together, our results provide evidence that, in addition to the repression channeled by the FlhDC/FliA-dependent circuit, RcsB is also able to exert direct inhibition of shlBA expression.
FIG 4.
Influence of the flagellar regulatory cascade and the Rcs system over shlBA transcriptional expression. Overnight cultures of the S. marcescens wt/ppromshlBA-gfp, fliA/ppromshlBA-gfp, flhD/ppromshlBA-gfp, wecG/ppromshlBA-gfp, and rcsB/ppromshlBA-gfp strains grown in LB medium were diluted in fresh LB medium and incubated in a 96-well black microtiter plate at 30°C without agitation for 18 h. Fluorescence (λexc = 485 nm; λem = 528 nm) and OD600 readings were determined every 60 min, with a 96-microwell Synergy2 or BioTek ELx808 microplate reader, respectively. The means and standard deviations for three independent assays performed in duplicate in each case were calculated. Statistical analysis was performed using one-way ANOVA and the Tukey-Kramer multiple-comparison test. ***, P < 0.001; statistically significantly different from wild-type S. marcescens.
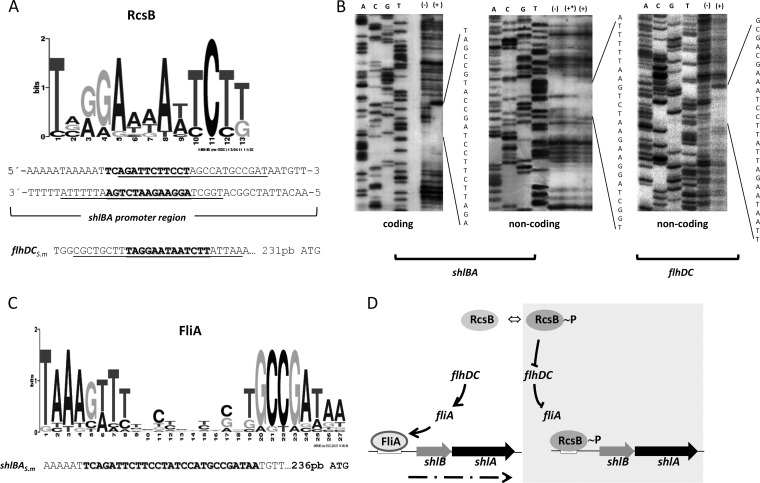
A bioinformatics search for a putative RcsB-binding site in the shlBA promoter region by use of the MEME/MAST motif detection programs (35, 36) showed the presence of a putative RcsB-binding motif 254 bp upstream of the shlB ATG initiation codon (Fig. 5A). To assess RcsB direct binding to the promoter region of shlBA, a DNase I footprinting assay was performed using purified RcsB-His×6, as described in Materials and Methods. As shown in Fig. 5B, RcsB-His×6 protected an overlapping region from nucleotide (nt) −249 to −265 relative to the shlB translational ATG start site. This protected region overlaps the in silico-predicted sequence that displays 12 matches to the RcsB consensus binding motif (Fig. 5A). The RcsB protection within the predicted RcsB motif in the promoter region of flhDC corroborated our previous results (17) and indicated that the His×6 tag did not alter RcsB functionality (Fig. 5A and B). We also explored the effect of the phosphorylation status of RcsB on the ability of the regulator to interact with DNA. For this purpose, when using the shlBA noncoding strand, we performed the footprinting assay using RcsB, either not treated or previously incubated with acetyl-phosphate, as the phosphoryl donor (Fig. 5B, middle). Acetyl-phosphate-treated RcsB exerted stronger protection than an equivalent concentration of untreated RcsB, indicating that the phosphorylated response regulator displays higher affinity for its target site in the shlBA promoter.
FIG 5.
RcsB binds to the promoter region of the S. marcescens shlBA and flhDC operons. (A) Logo showing the consensus motif for the RcsB-binding site and the predicted RcsB-binding site sequences in the promoter regions of S. marcescens (S.m.) flhDC and shlBA. (B) DNA footprinting analysis was performed on end-labeled coding and noncoding strands of the shlB promoter region and on the noncoding strand for the flhDC promoter region. The DNA fragments were incubated with purified RcsB-His×6 at a final concentration of 0 (−) or 400 pmol (+*) or with acetyl-phosphate preincubated with RcsB-His×6 at a final concentration of 400 pmol (+). The positions of the protected areas were determined by comparison with sequence ladders obtained by using the same labeled primer that was used as a probe. The RcsB-protected regions are indicated, with the respective nucleotide sequences. (C) Logo showing the consensus motif for the FliA-binding site and the predicted FliA-binding site sequences in the promoter regions of S. marcescens shlBA. DNA-protected regions obtained by the DNA footprinting assays are underlined (A), while the binding motifs that match the RcsB or FliA consensus motifs are indicated in boldface (C). (D) Schematic overview of the RcsB-dependent regulatory circuit that governs shlBA expression. (Left) When RcsB is inactive (RcsB, nonphosphorylated RcsB protein), FliA protein expression drives shlBA operon transcription by interaction with the FliA-binding motif within the shlBA operon promoter region (dashed arrow, shlBA transcript). (Right) Activated RcsB (RcsB∼P, phosphorylated RcsB protein) represses the transcriptional activity of the flagellar flhDC-dependent transcriptional cascade, inhibiting FliA expression, while it simultaneously downregulates shlBA transcription by direct interaction with the RcsB-binding motif within the shlBA operon promoter region.
These results establish that RcsB is able to control the transcriptional level of shlBA expression by direct interaction with a specific recognition motif within the shlBA promoter region. Interestingly, a predictive in silico analysis of the shlBA promoter region in search of a putative FliA DNA-binding motif by use of the MEME/MAST motif detection programs (35, 36) showed a consensus FliA box that partially overlaps the RcsB-binding motif (Fig. 5C). Therefore, we propose an RcsB-dependent regulatory circuit that is summarized in the scheme shown in Fig. 5D.
In light of these results, we also examined the impact of the RcsB-dependent regulatory mechanism on the control of ShlA-mediated induction of the autophagic process in epithelial cells. With this aim, we coincubated S. marcescens strains (wild type and mutants) with EGFP-LC3-CHO cells and scored the total number of cells that showed LC3 puncta at 60 and 120 min p.i. As shown in Fig. 6A, at 120 min p.i., the induction of autophagy was increased 114% ± 10.5% in the rcsB background while it was 65.9% ± 1.2% diminished in the wecG mutant strain (RcsB-inducing conditions), in good agreement with shlBA expression being transcriptionally repressed by RcsB. The levels achieved by the double wecG rcsB strain were identical to the values obtained for the rcsB strain, corroborating the role of RcsB in the transduction of the inducing signal. Also in accordance with our previous results, a 26% ± 7.5% reduction in the autophagic cell score compared to the wild-type level was observed when the fliA or flhD strain was assayed. Discrepancies between the magnitude of the inhibitory or inducing effects determined for each strain when hemolytic activity levels and autophagy scores were compared (Fig. 6A and 3) can be attributed to differences in each assay's conditions, to distinct membrane peculiarities of the target cells, and/or to toxin dose thresholds required to achieve each biological effect. Figure 6B shows representative microscopic analysis of images captured from the assay obtained at 60 and 120 min p.i. The exacerbated EGFP-LC3 punctate pattern became evident when the rcsB or the wecG rcsB strain was used (Fig. 6B, m to p), while the cells challenged with the fliA and flhD strains had an intermediate phenotype (Fig. 6B, e to h). shlB strain-exposed cells show no detectable difference from untreated cells (Fig. 6B, compare c and d to e and f). At 60 and 120 min p.i., the wecG strain (Fig. 6B, k and l) shows a subtle autophagic phenotype (fine, scattered EGFP-LC3 puncta), which is consistent with the reduced shlBA transcriptional and hemolytic activities measured for the strain (Fig. 3 and 4).
DISCUSSION
We previously established that S. marcescens could trigger a noncanonical autophagic cascade from outside host epithelial cells prior to the internalization process (4). In this work, we identify the bacterial factor required for this induction as ShlA. To our knowledge, this is the first report that shows a factor exported by a bacterial two-partner secretion system that is responsible for the onset of autophagy in eukaryotic cells. To confirm that ShlA is the required factor, we showed that the capacity to promote autophagy in epithelial cells could be conferred on a noninvasive E. coli strain by the recombinant expression of the Serratia ShlB/ShlA Tps system. However, taking together the facts that (i) bacterium-free, filtered supernatants from Serratia growth culture are unable to induce autophagy in CHO cells (data not shown) and (ii) the purified-ShlA hemolytic-activity half-life is estimated to be 3 min at 37°C (37), our results cannot rule out the possibility that another bacterial product or structural component, shared by E. coli and Serratia, might be involved in ShlA stabilization or in allowing the pore-forming toxin to exert its action.
Recent reports support the hypothesis that pathogenic bacteria that reside and/or proliferate inside intracellular vacuoles, such as Shigella, Listeria, or Salmonella, induce the autophagic response by affecting cellular metabolism (38, 39). This would be accomplished by promoting rapid depletion of the amino acid intracellular pools that provoke mTOR kinase inhibition, which in turn results in derepression of the autophagic process. Interestingly, Tattoli et al. (38) showed that aseptic damage to the host plasma membrane or lysosomes was sufficient to trigger amino acid starvation pathways, but only lysosomal damage resulted in mTOR inhibition, arguing that the pathway is triggered only by bacteria from within intracellular compartments. Indeed, the effectors responsible for autophagy induction identified in Listeria and Salmonella, LLO- (38) and SPI-1-dependent factors (40), respectively, were shown to damage the bacterium-containing vacuole membrane.
Kloft et al. (28, 29) found that epithelial cell lines exposed to diverse purified pore-forming toxins (Staphylococcus aureus alpha-toxin, Vibrio cholerae cytolysin, streptolysin O, or E. coli hemolysin) activated pathways leading to autophagy. Later, Gonzalez et al. (41) added to this finding, showing analogous results with LLO from Listeria monocytogenes and aerolysin from Aeromonas hydrophila. Additionally, Byrne et al. (30) have shown that, in macrophages, treatment with micromolar concentrations of the inonophore valynomycin was sufficient to stimulate accumulation of LC3+ vacuoles. In sum, their assays showed that a drop in the cellular ATP/AMP ratio, an increase in K+ efflux, and the concomitant amino acid depletion caused by cell plasma membrane disruption are the signals that are able to induce the autophagic process by the activation of different transduction pathways. This induction promotes a quiescent state that favors homeostasis recovery and repair of membrane damage to the injured cell.
We previously established that, after internalization in nonphagocytic cells, S. marcescens is able to reside and multiply intracellularly inside autophagic-like vacuoles (4). The results shown in this work indicate that when Serratia encounters the host cell, autophagy induction takes place in two stages. The early interaction with bacteria exposes epithelial cells to the action of the ShlA pore-forming toxin. We can conjecture that the metabolic imbalance, probably prompted by K+ efflux, generated by ShlA-mediated damage would promote the observed autophagic response in the vast majority of cells. Then, a comparatively small percentage of cells are effectively invaded by Serratia. We previously demonstrated that early after internalization, Serratia establishes itself within a vacuole that recruits prototypical autophagic markers, i.e., LC3 and Rab7 (4). Our results indicate that ShlA would not be required either for internalization into cells or for recruitment of LC3 to the SeCV. Conceivably, Serratia effectors, such as proteolytic or lipolitic exoenzymes, could be responsible for damage to the endocytic vacuole, engaging the autophagic machinery that contributes to shape SeCV features. Nevertheless, whether wild-type SeCV vacuoles are identical to their hemolysin mutant strain-containing counterparts should be further examined. In this context, our ongoing experiments focus on finding out whether externally ShlA-triggered autophagy paves the way for subsequent steps of successful Serratia internalization, establishment, and proliferation inside the SeCV.
We have previously shown that mutations in genes coding for the components of the Rcs signaling cascade counteract the defects in Serratia flagellum-dependent motility inflicted by alterations in the enterobacterial common antigen assembly. This finding led us to demonstrate that in S. marcescens, RcsB inhibits the expression of flhDC (16, 17). We have now confirmed direct binding of S. marcescens RcsB to a conserved RcsB recognition motif within the flhDC promoter region. This regulatory feature adds new evidence about the conservation of RcsB as a repressor of the FlhDC-dependent pathway among Enterobacteriaceae (14, 42). Additionally, we show that the abrogation of either flhDC or fliA expression downregulates shlBA expression, indicating that downstream of FlhDC in the hierarchical flagellar cascade, FliA induces shlBA expression. In keeping with this result, overexpression of either FlhDC or FliA in a wild-type genetic background induces shlBA expression. Together, these results and the fact that a predicted conserved FliA-binding motif is found by bioinformatics analysis upstream of the shlBA coding region (Fig. 5C) strongly suggest that FliA is able to activate ShlA expression by direct induction of shlBA transcription. FliA-mediated regulation of ShlA expression also helps to explain the results obtained by Soo et al. in an S. marcescens CH-1 pigmented strain (43) showing that the RssAB system could partially repress shlBA in an FlhDC-dependent manner.
The hierarchical control of the expression of a Tps-dependent cytotoxin by the flagellar regulatory cascade has also been shown for Proteus mirabilis hpmBA (44) and Xenorhabdus nematophila xaxAB (45). We are tempted to speculate that this could be a common regulatory feature for those opportunistic bacteria that alternate between environmental and host-associated lifestyles, such as Edwardsiella (46, 47), Erwinia, Photorhabdus, Pseudomonas, and Xylella, which were found to carry ShlBA orthologues encoded in their genomes (6, 48, 49). In S. marcescens, the fact that ShlA is coupled with flagellum expression also argues in favor of the conjecture that both bacterial determinants are required prior to the internalization of the bacterium into host epithelial cells: the flagellum operates as an attachment factor (4), while ShlA provokes transient membrane destabilization, a low energy consumption status, and the onset of signaling pathways that might favor later invasion steps.
Here, we show that shlBA expression is highly induced in an rcsB mutant strain while it is severely repressed in a wecG mutant in which the Rcs system is activated and demonstrate that RcsB is able to recognize a conserved binding motif in the promoter region of the shlBA operon. These findings, together with the fact that RcsB overexpression inhibits shlBA expression in either an flhD or an fliA mutant background, reveal that RcsB is able to downregulate shlBA expression in an FlhDC/FliA-independent manner. In sum, we show that the expression of shlBA is subjected to a transcriptional feed-forward regulatory circuit dependent on RcsB that can involve or bypass the flagellar regulatory pathway. No participation of canonical Rcs phosphorelay components (RcsC, RcsA, and RcsF) was detected, suggesting either that nonphosphorylated RcsB is proficient in downregulating shlBA expression and this action depends on RcsB intracellular levels or that signaling molecules other that RcsC/RcsD/RcsF can channel the message onto RcsB, defining its phosphorylation status. Interestingly, the identified RcsB box partially overlaps the predicted FliA-binding motif (Fig. 5) within the shlBA regulatory region. Additionally, we have previously demonstrated that under RcsB-activating conditions, fliA expression is repressed (17). Our results show that inactivation of FliA expression in the fliA mutant strain reduces the hemolytic activity only by ca. 50% and that this correlates with the reduced values of shlBA transcriptional activity (the results are shown in Fig. 3 and 4). Further repression levels are achieved by direct binding of RcsB to the DNA recognition motif. This repression was observed when RcsB was overexpressed from a plasmid or when the Rcs system was activated by the abolition of ECA expression. Together, these results anticipate a scenario in which, in response to environmental cues, the FliA-mediated induction of shlBA expression can switch to RcsB-dependent repression, or vice versa, by occupancy replacement of one regulator by the other in the DNA-binding region.
A phenotypical outcome from different settings of the regulatory circuit that controls ShlA expression was demonstrated by autophagic induction in epithelial cells (Fig. 6). In light of these results, we can also infer that this feed-forward regulatory path has evolved to provide multiple access points for the fine-tuning of ShlA expression, highlighting the functional importance of this cytotoxin throughout the Serratia life cycle. In addition to the regulation of flagellum and ShlA expression, we have previously shown that S. marcescens RcsB is involved in controlling the production of OMVs, which are vehicles for the delivery of virulence factors (18). These findings define RcsB as one master regulator that commands the expression of multiple phenotypes that endow S. marcescens with its pathogenic potential. Together, this knowledge provides new tools to design strategies to fight Serratia infections.
ACKNOWLEDGMENTS
We are grateful to Rodrigo Vena (confocal laser microscopy) and to Dolores Campos (tissue culture) for excellent technical assistance. We thank María Isabel Colombo and Emanuel Campoy for advice in the early setting of autophagy assays; Griselda V. Fedrigo for performing preliminary assays; M. F. Alexeyev for providing the pKNOCK plasmid series; Volkmar Braun and Silke I. Patzer for providing pES14; Carlos Cotorruelo for providing the red blood cells from the Immuno-Hematology Service–Rosario School of Biochemistry; Fernando C. Soncini, Javier F. Mariscotti, and the anonymous reviewers for critically reading the manuscript; and Javier Palatnik for help with digital art.
This work was supported by grants from the Agencia Nacional de Promoción Científica y Tecnológica and from the Consejo Nacional de Investigaciones Científicas y Tecnológicas (CONICET), Argentina, to E.G.V. E.G.V. is a career investigator of CONICET, Argentina. T.M.S. and G.D.V. have fellowships from CONICET, Argentina. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Published ahead of print 9 June 2014
REFERENCES
- 1.Mahlen SD. 2011. Serratia infections: from military experiments to current practice. Clin. Microbiol. Rev. 24:755–791. 10.1128/CMR.00017-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kramer A, Schwebke I, Kampf G. 2006. How long do nosocomial pathogens persist on inanimate surfaces? A systematic review. BMC Infect. Dis. 6:130. 10.1186/1471-2334-6-130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stock I, Grueger T, Wiedemann B. 2003. Natural antibiotic susceptibility of strains of Serratia marcescens and the S. liquefaciens complex: S. liquefaciens sensu stricto, S. proteamaculans and S. grimesii. Int. J. Antimicrob. Agents 22:35–47. 10.1016/S0924-8579(02)00163-2 [DOI] [PubMed] [Google Scholar]
- 4.Fedrigo GV, Campoy EM, Di Venanzio G, Colombo MI, Garcia Vescovi E. 2011. Serratia marcescens is able to survive and proliferate in autophagic-like vacuoles inside non-phagocytic cells. PLoS One 6:e24054. 10.1371/journal.pone.0024054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Desvaux M, Parham NJ, Henderson IR. 2004. Type V protein secretion: simplicity gone awry? Curr. Issues Mol. Biol. 6:111–124 [PubMed] [Google Scholar]
- 6.Hertle R. 2005. The family of Serratia type pore forming toxins. Curr. Protein Pept. Sci. 6:313–325. 10.2174/1389203054546370 [DOI] [PubMed] [Google Scholar]
- 7.Poole K, Schiebel E, Braun V. 1988. Molecular characterization of the hemolysin determinant of Serratia marcescens. J. Bacteriol. 170:3177–3188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pramanik AK, Konninger U, Selvam A, Braun V. 2014. Secretion and activation of the Serratia marcescens hemolysin by structurally defined ShlB mutants. Int. J. Med. Microbiol. 304:351–359. 10.1016/j.ijmm.2013.11.021 [DOI] [PubMed] [Google Scholar]
- 9.Walker G, Hertle R, Braun V. 2004. Activation of Serratia marcescens hemolysin through a conformational change. Infect. Immun. 72:611–614. 10.1128/IAI.72.1.611-614.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hertle R, Hilger M, Weingardt-Kocher S, Walev I. 1999. Cytotoxic action of Serratia marcescens hemolysin on human epithelial cells. Infect. Immun. 67:817–825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kurz CL, Chauvet S, Andres E, Aurouze M, Vallet I, Michel GP, Uh M, Celli J, Filloux A, De Bentzmann S, Steinmetz I, Hoffmann JA, Finlay BB, Gorvel JP, Ferrandon D, Ewbank JJ. 2003. Virulence factors of the human opportunistic pathogen Serratia marcescens identified by in vivo screening. EMBO J. 22:1451–1460. 10.1093/emboj/cdg159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin CS, Horng JT, Yang CH, Tsai YH, Su LH, Wei CF, Chen CC, Hsieh SC, Lu CC, Lai HC. 2010. RssAB-FlhDC-ShlBA as a major pathogenesis pathway in Serratia marcescens. Infect. Immun. 78:4870–4881. 10.1128/IAI.00661-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nehme NT, Liegeois S, Kele B, Giammarinaro P, Pradel E, Hoffmann JA, Ewbank JJ, Ferrandon D. 2007. A model of bacterial intestinal infections in Drosophila melanogaster. PLoS Pathog. 3:e173. 10.1371/journal.ppat.0030173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Majdalani N, Gottesman S. 2005. The Rcs phosphorelay: a complex signal transduction system. Annu. Rev. Microbiol. 59:379–405. 10.1146/annurev.micro.59.050405.101230 [DOI] [PubMed] [Google Scholar]
- 15.Mariscotti JF, Garcia-del Portillo F. 2009. Genome expression analyses revealing the modulation of the Salmonella Rcs regulon by the attenuator IgaA. J. Bacteriol. 191:1855–1867. 10.1128/JB.01604-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Castelli ME, Vescovi EG. 2011. The Rcs signal transduction pathway is triggered by enterobacterial common antigen structure alterations in Serratia marcescens. J. Bacteriol. 193:63–74. 10.1128/JB.00839-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Castelli ME, Fedrigo GV, Clementin AL, Ielmini MV, Feldman MF, Garcia Vescovi E. 2008. Enterobacterial common antigen integrity is a checkpoint for flagellar biogenesis in Serratia marcescens. J. Bacteriol. 190:213–220. 10.1128/JB.01348-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McMahon KJ, Castelli ME, Garcia Vescovi E, Feldman MF. 2012. Biogenesis of outer membrane vesicles in Serratia marcescens is thermoregulated and can be induced by activation of the Rcs phosphorelay system. J. Bacteriol. 194:3241–3249. 10.1128/JB.00016-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alexeyev MF. 1999. The pKNOCK series of broad-host-range mobilizable suicide vectors for gene knockout and targeted DNA insertion into the chromosome of gram-negative bacteria. Biotechniques 26:824–826, 828 [DOI] [PubMed] [Google Scholar]
- 20.Reece KS, Phillips GJ. 1995. New plasmids carrying antibiotic-resistance cassettes. Gene 165:141–142. 10.1016/0378-1119(95)00529-F [DOI] [PubMed] [Google Scholar]
- 21.Miller WG, Leveau JH, Lindow SE. 2000. Improved gfp and inaZ broad-host-range promoter-probe vectors. Mol. Plant Microbe Interact. 13:1243–1250. 10.1094/MPMI.2000.13.11.1243 [DOI] [PubMed] [Google Scholar]
- 22.Schonherr R, Hilger M, Broer S, Benz R, Braun V. 1994. Interaction of Serratia marcescens hemolysin (ShlA) with artificial and erythrocyte membranes. Demonstration of the formation of aqueous multistate channels. Eur. J. Biochem. 223:655–663 [DOI] [PubMed] [Google Scholar]
- 23.Sheffield P, Garrard S, Derewenda Z. 1999. Overcoming expression and purification problems of RhoGDI using a family of “parallel” expression vectors. Protein Expr. Purif. 15:34–39. 10.1006/prep.1998.1003 [DOI] [PubMed] [Google Scholar]
- 24.Aguirre A, Lejona S, Vescovi EG, Soncini FC. 2000. Phosphorylated PmrA interacts with the promoter region of ugd in Salmonella enterica serovar Typhimurium. J. Bacteriol. 182:3874–3876. 10.1128/JB.182.13.3874-3876.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aguirre A, Cabeza ML, Spinelli SV, McClelland M, Garcia Vescovi E, Soncini FC. 2006. PhoP-induced genes within Salmonella pathogenicity island 1. J. Bacteriol. 188:6889–6898. 10.1128/JB.00804-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sciara MI, Spagnuolo C, Jares-Erijman E, Garcia Vescovi E. 2008. Cytolocalization of the PhoP response regulator in Salmonella enterica: modulation by extracellular Mg2+ and by the SCV environment. Mol. Microbiol. 70:479–493. 10.1111/j.1365-2958.2008.06427.x [DOI] [PubMed] [Google Scholar]
- 27.von Hoven G, Kloft N, Neukirch C, Ebinger S, Bobkiewicz W, Weis S, Boller K, Janda KD, Husmann M. 2012. Modulation of translation and induction of autophagy by bacterial exoproducts. Med. Microbiol. Immunol. 201:409–418. 10.1007/s00430-012-0271-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kloft N, Neukirch C, Bobkiewicz W, Veerachato G, Busch T, von Hoven G, Boller K, Husmann M. 2010. Pro-autophagic signal induction by bacterial pore-forming toxins. Med. Microbiol. Immunol. 199:299–309. 10.1007/s00430-010-0163-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kloft N, Busch T, Neukirch C, Weis S, Boukhallouk F, Bobkiewicz W, Cibis I, Bhakdi S, Husmann M. 2009. Pore-forming toxins activate MAPK p38 by causing loss of cellular potassium. Biochem. Biophys. Res. Commun. 385:503–506. 10.1016/j.bbrc.2009.05.121 [DOI] [PubMed] [Google Scholar]
- 30.Byrne BG, Dubuisson JF, Joshi AD, Persson JJ, Swanson MS. 2013. Inflammasome components coordinate autophagy and pyroptosis as macrophage responses to infection. mBio 4:e00620-12. 10.1128/mBio.00620-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Givskov M, Molin S. 1993. Secretion of Serratia liquefaciens phospholipase from Escherichia coli. Mol. Microbiol. 8:229–242. 10.1111/j.1365-2958.1993.tb01567.x [DOI] [PubMed] [Google Scholar]
- 32.Givskov M, Eberl L, Christiansen G, Benedik MJ, Molin S. 1995. Induction of phospholipase- and flagellar synthesis in Serratia liquefaciens is controlled by expression of the flagellar master operon flhD. Mol. Microbiol. 15:445–454. 10.1111/j.1365-2958.1995.tb02258.x [DOI] [PubMed] [Google Scholar]
- 33.Shimuta K, Ohnishi M, Iyoda S, Gotoh N, Koizumi N, Watanabe H. 2009. The hemolytic and cytolytic activities of Serratia marcescens phospholipase A (PhlA) depend on lysophospholipid production by PhlA. BMC Microbiol. 9:261. 10.1186/1471-2180-9-261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Poole K, Braun V. 1988. Iron regulation of Serratia marcescens hemolysin gene expression. Infect. Immun. 56:2967–2971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bailey TL. 2002. Discovering novel sequence motifs with MEME. Curr. Protoc. Bioinformatics Chapter 2:Unit 2.4. 10.1002/0471250953.bi0204s00 [DOI] [PubMed] [Google Scholar]
- 36.Bailey TL, Boden M, Buske FA, Frith M, Grant CE, Clementi L, Ren J, Li WW, Noble WS. 2009. MEME SUITE: tools for motif discovery and searching. Nucleic Acids Res. 37:W202–W208. 10.1093/nar/gkp335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schiebel E, Schwarz H, Braun V. 1989. Subcellular location and unique secretion of the hemolysin of Serratia marcescens. J. Biol. Chem. 264:16311–16320 [PubMed] [Google Scholar]
- 38.Tattoli I, Sorbara MT, Vuckovic D, Ling A, Soares F, Carneiro LA, Yang C, Emili A, Philpott DJ, Girardin SE. 2012. Amino acid starvation induced by invasive bacterial pathogens triggers an innate host defense program. Cell Host Microbe 11:563–575. 10.1016/j.chom.2012.04.012 [DOI] [PubMed] [Google Scholar]
- 39.Tattoli I, Sorbara MT, Yang C, Tooze SA, Philpott DJ, Girardin SE. 2013. Listeria phospholipases subvert host autophagic defenses by stalling pre-autophagosomal structures. EMBO J. 32:3066–3078. 10.1038/emboj.2013.234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tattoli I, Philpott DJ, Girardin SE. 2012. The bacterial and cellular determinants controlling the recruitment of mTOR to the Salmonella-containing vacuole. Biol. Open. 1:1215–1225. 10.1242/bio.20122840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gonzalez MR, Bischofberger M, Freche B, Ho S, Parton RG, van der Goot FG. 2011. Pore-forming toxins induce multiple cellular responses promoting survival. Cell Microbiol. 13:1026–1043. 10.1111/j.1462-5822.2011.01600.x [DOI] [PubMed] [Google Scholar]
- 42.Huang YH, Ferrieres L, Clarke DJ. 2006. The role of the Rcs phosphorelay in Enterobacteriaceae. Res. Microbiol. 157:206–212. 10.1016/j.resmic.2005.11.005 [DOI] [PubMed] [Google Scholar]
- 43.Soo PC, Horng YT, Wei JR, Shu JC, Lu CC, Lai HC. 2008. Regulation of swarming motility and flhDC(Sm) expression by RssAB signaling in Serratia marcescens. J. Bacteriol. 190:2496–2504. 10.1128/JB.01670-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fraser GM, Claret L, Furness R, Gupta S, Hughes C. 2002. Swarming-coupled expression of the Proteus mirabilis hpmBA haemolysin operon. Microbiology 148:2191–2201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lanois A, Jubelin G, Givaudan A. 2008. FliZ, a flagellar regulator, is at the crossroads between motility, haemolysin expression and virulence in the insect pathogenic bacterium Xenorhabdus. Mol. Microbiol. 68:516–533. 10.1111/j.1365-2958.2008.06168.x [DOI] [PubMed] [Google Scholar]
- 46.Strauss EJ, Ghori N, Falkow S. 1997. An Edwardsiella tarda strain containing a mutation in a gene with homology to shlB and hpmB is defective for entry into epithelial cells in culture. Infect. Immun. 65:3924–3932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Williams ML, Lawrence ML. 2005. Identification and characterization of a two-component hemolysin from Edwardsiella ictaluri. Vet. Microbiol. 108:281–289. 10.1016/j.vetmic.2005.04.017 [DOI] [PubMed] [Google Scholar]
- 48.Brillard J, Duchaud E, Boemare N, Kunst F, Givaudan A. 2002. The PhlA hemolysin from the entomopathogenic bacterium Photorhabdus luminescens belongs to the two-partner secretion family of hemolysins. J. Bacteriol. 184:3871–3878. 10.1128/JB.184.14.3871-3878.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mazar J, Cotter PA. 2007. New insight into the molecular mechanisms of two-partner secretion. Trends Microbiol. 15:508–515. 10.1016/j.tim.2007.10.005 [DOI] [PubMed] [Google Scholar]
- 50.Yura T, Mori H, Nagai H, Nagata T, Ishihama A, Fujita N, Isono K, Mizobuchi K, Nakata A. 1992. Systematic sequencing of the Escherichia coli genome: analysis of the 0–2.4 min region. Nucleic Acids Res. 20:3305–3308. 10.1093/nar/20.13.3305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kovach ME, Elzer PH, Hill DS, Robertson GT, Farris MA, Roop RM, II, Peterson KM. 1995. Four new derivatives of the broad-host-range cloning vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene 166:175–176. 10.1016/0378-1119(95)00584-1 [DOI] [PubMed] [Google Scholar]
- 52.Tabor S, Richardson CC. 1985. A bacteriophage T7 RNA polymerase/promoter system for controlled exclusive expression of specific genes. Proc. Natl. Acad. Sci. U. S. A. 82:1074–1078. 10.1073/pnas.82.4.1074 [DOI] [PMC free article] [PubMed] [Google Scholar]