Abstract
Cryptococcal infections are primarily caused by two related fungal species: Cryptococcus neoformans and Cryptococcus gattii. It is well known that C. neoformans generally affects immunocompromised hosts; however, C. gattii infection can cause diseases in not only immunocompromised hosts but also immunocompetent individuals. While recent studies suggest that C. gattii infection could dampen pulmonary neutrophil recruitment and inflammatory cytokine production in immunocompetent hosts, the impact of C. gattii infection on the development of their adaptive T helper cell immune response has not been addressed. Here, we report that C. neoformans infection with highly virulent and less virulent strains preferentially induced pulmonary Th1 and Th17 immune responses in the host, respectively. However, fewer pulmonary Th1 and Th17 cells could be detected in mice infected with C. gattii strains. Notably, dendritic cells (DC) in mice infected with C. gattii expressed much lower levels of surface MHC-II and Il12 or Il23 transcripts and failed to induce effective Th1 and Th17 differentiation in vitro. Furthermore, the expression levels of Ip10 and Cxcl9 transcripts, encoding Th1-attracting chemokines, were significantly reduced in the lungs of mice infected with the highly virulent C. gattii strain. Thus, our data suggest that C. gattii infection dampens the DC-mediated effective Th1/Th17 immune responses and downregulates the pulmonary chemokine expression, thus resulting in the inability to mount protective immunity in immunocompetent hosts.
INTRODUCTION
Cryptococcus is an encapsulated basidiomycetous fungus that causes diseases in humans and other animals. The pathogenic sibling species Cryptococcus neoformans and Cryptococcus gattii are different with regard to their natural habitat, geographical distributions, and clinical manifestations (1, 2). While C. neoformans causes meningitis or disseminated diseases in immunocompromised patients, C. gattii predominately involves the lungs, resulting in pneumonia and respiratory failure in healthy individuals (3, 4). However, when invading the central nervous system (CNS), C. gattii is more likely to form cryptococcoma in the brain. A recent study comparing the pathogenesis of C. neoformans H99 and C. gattii R265 strains in murine models demonstrated that C. neoformans grew faster in the brain and caused death by meningoencephalitis, while C. gattii grew faster in the lungs and caused death without producing fulminating meningoencephalitis (4). However, the immunological mechanisms contributing to the differences in pathogenesis between these two species of pathogenic fungi remain unclear.
The host immune response is considered a key factor in determining the development of cryptococcal diseases. While the innate immune system constitutes the first line of defense against cryptococcal infection, adaptive immunity, especially cell-mediated immunity, is required for the control of disease progression. Accumulating evidence suggests that C. gattii may thrive in immunocompetent hosts by suppressing the protective immune response (4–8). Previous studies in humans indicated that C. gattii cell and culture filtrates could not stimulate polymorphonuclear leukocytes migration (6, 8). In the mouse model, C. gattii strains failed to provoke the migration of neutrophils into the lungs of C57BL/6 mice (4, 5). Moreover, lower levels of inflammatory cytokines, including tumor necrosis factor alpha (TNF-α), interleukin 6 (IL-6), and interferon gamma (IFN-γ), were detected in the lungs of mice infected with C. gattii strains (5). Altogether, these studies implicate that the underlying mechanisms by which C. gattii is capable of infecting healthy individuals may be mediated by inhibiting the migration of leukocytes and the induction of the protective immune response.
CD4+ T helper cells play a central role in orchestrating adaptive immune response to various pathogens, including Cryptococcus. Indeed, the incidence of C. neoformans infection increased in patients with deficient numbers of CD4+ T cells. Naive CD4+ T cells can differentiate into T helper cell lineages such as Th1, Th2, and Th17, depending primarily on antigens and the polarizing cytokines present in the microenvironment. Th1 cells primarily produce IFN-γ; Th2 cells produce IL-4, IL-5, and IL-13; and Th17 cells produce IL-17 and IL-17F (9). The observation that an increased susceptibility to cryptococcal infection could occur in mice treated with neutralizing antibodies against IFN-γ, IL-12, and TNF-α strongly suggests the requirement of the Th1 immune response in mounting host protection against C. neoformans (10–13). In contrast, the cytokines secreted by Th2 cells are associated with uncontrolled fungal growth and persistence of C. neoformans infection (11, 14–17). Recently, Th17 cells were shown to be involved in promoting pulmonary clearance of C. neoformans (16, 18, 19). While many studies have investigated the T helper cell immune responses against C. neoformans, little is known about the T helper cell response during pulmonary infection with C. gattii in healthy individuals.
In this study, we characterized T helper cell responses in a mouse model of C. gattii infection. Our results suggest that C. gattii may dampen the capability of an immunocompetent host to mount effective Th1/Th17 immune responses in the lungs by attenuating both the induction of Th1/Th17 cells (through downregulating expression of MHC-II on dendritic cells and the Th1/Th17-inducing cytokines IL-12/IL-23) and their infiltration (through the inhibition of chemokine/chemokine receptor expression), thereby causing fatal pulmonary diseases.
MATERIALS AND METHODS
Animals.
C57BL/6 mice were obtained from The National Laboratory Animal Center, Mahidol University. Female 6- to 8-week-old mice were used for experiments. Mice were housed in enclosed filter-top cages in a pathogen-free animal facility at the Faculty of Allied Health Science, Thammasat University. All animal studies were approved by the Thammasat University Animal Care and Use Committee.
Cryptococcal strains.
We used two sets of reference strains: the highly virulent strains Cryptococcus neoformans H99 (serotype A, VNI) and Cryptococcus gattii R265 (serotype B, VGII) and the less virulent strains Cryptococcus neoformans WM148 (serotype A, VNI) and Cryptococcus gattii WM179 (serotype B, VGI). The reference strains were stored in 25% glycerol at −80°C until use and were maintained on Sabouraud dextrose agar (SDA) at 25°C during the study. For cytokine assays, yeast cells were washed in sterile phosphate-buffered saline (PBS) and heat killed at 56°C for 1 h. The absence of viable organisms was confirmed by plating an aliquot containing 1 × 108 organisms on Sabouraud dextrose agar.
Monoclonal antibodies.
Fluorescein isothiocyanate (FITC)-conjugated anti-CD11b (M1/70), allophycocyanin (APC)-conjugated anti-CD11c (HL3), PerCP-conjugated anti-CD4 (RM4-5), APC-conjugated-anti-Gr.1 (RB6-8C5), phycoerythrin (PE)-conjugated-anti-IL-17 (TC11-18H10), FITC-conjugated anti-IFN-γ (XMG1.2), PE-conjugated-anti-CD80 (16-10A1), PE-conjugated-anti-CD86 (GL1), PE-conjugated-anti-I-A/I-E (major histocompatibility complex class II [MHC-II]) (clone M5/114.15.2), APC-conjugated anti-CD3 (145-2C11), FITC-conjugated anti-CD40 monoclonal antibody (MAb) (clone 3/23), and FITC- and PE-conjugated anti-mouse CD44 (clone IM7) were from BD Pharmingen. APC-conjugated-anti-CXCR3 (220803) and PE-conjugated-anti-CCR6 (140706) were from R&D Systems. PE-conjugated anti-IL-13 (clone eBio13a) and APC-conjugated anti-mouse Foxp3 (clone: FJK-16S) were from eBioscience. Isotype-matched antibodies (from BD Pharmingen, R&D Systems, and eBioscience) were used as controls for nonspecific binding. Cells were analyzed using a FACSCalibur cytometer (BD Biosciences).
Murine model of fungal infections.
For infection, yeast cells were grown for 24 h in Sabouraud dextrose broth in a shaking incubator. The cultures were then washed in phosphate-buffered saline (PBS), counted using a hemocytometer, and resuspended in PBS at a concentration of 1 × 106 yeast cells/ml as previously described (5). Mice were treated with PBS or infected with Cryptococcus spp. by intranasal inoculation (5). After anesthetization with Isoflurane, each mouse received 50 μl of the yeast cell suspension (5.0 × 104 yeast cells/mouse) (5). For the mixed-infection experiments, C. neoformans (5.0 × 104 yeast cells/mouse) was mixed with C. gattii (2.5 × 104, 5 × 104, or 1 × 105 yeast cells/mouse) in a final volume of 50 μl. Infected mice were euthanized using CO2 inhalation at day 7 postinfection. For kinetics studies of effector T cell infiltration and chemokine expression analysis, mice were infected and analyzed at days 1, 7, and 14 postinfection. For lung fungal burden analysis, lungs were homogenized in sterile PBS, diluted, and plated on yeast extract-peptone-dextrose (YPD) agar for colony counts. CFU were enumerated following incubation at 30°C for 48 h. For survival analysis, mice were monitored by inspection twice daily and euthanized if they appeared to be in pain or moribund.
In vitro cocultures of DCs and T cells.
Bone marrow cells were cultured in complete RPMI containing 10% heat-inactivated fetal bovine serum (FBS) in the presence of 20 ng/ml granulocyte-macrophage colony-stimulating factor (GM-CSF) for 3 days. On day 4, fresh medium containing 20 ng/ml GM-CSF was added. On day 6, nonadherent CD11c+ dendritic cells (DCs) were sorted using anti-CD11c-coated magnetic beads, according to the manufacturer's directions (Miltenyi Biotec, Auburn, CA, USA). Enriched CD4+ T cells were isolated from lymph nodes and spleens using magnetic bead positive selection (CD4+ [L3T4] beads; Miltenyi Biotec, Auburn, CA, USA) according to the manufacturer's instructions. Purified bone marrow-derived dendritic cells (BMDCs) (1 × 106 cells/ml) were stimulated with heat-killed Cryptococcus at a ratio of 1:5. After 24 h, activated BMDCs were washed and cocultured with enriched CD4+ T cells at the ratio of 1:2. The activated BMDCs were also analyzed for the expression of costimulatory molecules (via flow cytometry) and cytokines (via real-time PCR analysis). On day 3 of DC-T cell coculture, cultured supernatant was then collected and assessed for cytokine production by enzyme-linked immunosorbent assay (ELISA).
Flow-cytometric analysis.
For the immunofluorescence analysis of lung inflammatory cells, total leukocytes were isolated from murine lungs that had been processed as previously described (5). Single-cell suspensions of lungs or blood were prepared, stained, and analyzed using a FACSCalibur cytometer (BD Biosciences) as described previously (5). As previously described (5), neutrophils were analyzed based on the expression of Gr-1 and CD11b (5); dendritic cells express high levels of CD11b and CD11c, and T helper cells express CD4+ or CD4+ CD3+ for some experiments. The positive populations were created based on the PBS-treated control. For intracellular cytokine staining analysis, lungs or lung draining lymph nodes were prepared for single-cell suspension. Cells in suspension were then restimulated with 500 ng/ml ionomycin and 50 ng/ml phorbol myristate acetate (PMA) in the presence of GolgiStop (BD Biosciences) for 5 h (20). Cells were permeabilized with a Cytofix/Cytoperm kit (BD Biosciences) and analyzed for the expression of cytokines in gated CD4+ or CD4+ CD44+ cells. For intracellular staining of Foxp3, a mouse Foxp3 staining kit was used, according to the manufacturer's protocol (eBioscience).
Antigen-specific cytokine production.
Lung-draining lymph nodes were harvested from mice infected with C. neoformans or C. gattii at various time points after infection or from PBS-treated mice. Single-cell suspensions of lungs or lung-draining lymph nodes were prepared and stimulated with heat-killed C. neoformans or C. gattii (at ratio of 2:1 heat-killed cryptococcus cells to leukocytes). Following a 3-day incubation at 37°C with 5% CO2, culture supernatants were collected and analyzed for the production of cytokines. The antibody pairs for IFN-γ, IL-17, and IL-4 were obtained from BD Pharmingen, the IL-13 ELISA kit was obtained from R&D Systems, and assays were performed according to the manufacturer's instructions.
Real-time RT-PCR analysis.
Lungs were removed from naive or infected mice and homogenized in TRIzol reagent (Invitrogen). Total RNA extracted using TRIzol reagent was used to generate cDNA using oligo(dT), random hexamers, and Moloney murine leukemia virus (MMLV) reverse transcriptase (Invitrogen) (20). To detect cytokine expression, cDNA samples were amplified in IQ SYBR green supermix (Bio-Rad Laboratories). The data were normalized to actin expression (Actb). The primer pairs for the analysis of cytokines, transcription factors, and chemokines were used as previously described (20–22).
Statistical analysis.
Each experiment was conducted two or three times. Data are presented as means and standard deviations (SD). Data were analyzed using one-way analysis of variance (ANOVA) with Tukey's post hoc analysis. Survival data analysis was obtained using Kaplan-Meier survival curves, and P values were obtained from a log-rank test. Fungal burden analysis was evaluated using an unpaired two-tailed t test. All statistical analysis was performed with GraphPad Prism 5 software. A P value of <0.05 was considered significant.
RESULTS
C. gattii-infected mice mount attenuated Th1/Th17 immune responses.
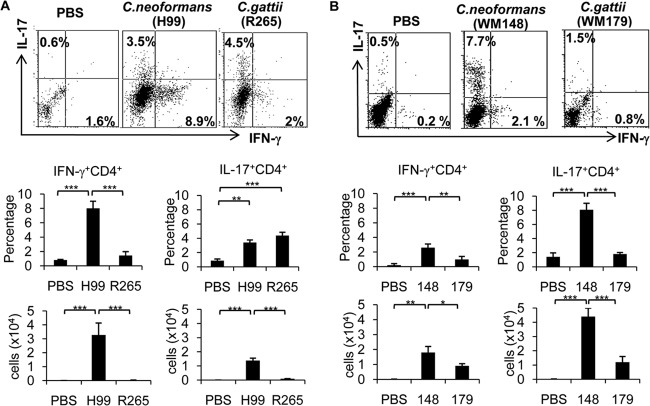
Previous studies suggested that Th1 and Th17 immune responses play critical roles in the clearance of pulmonary C. neoformans infection (19). To understand the T helper cell immune response against C. gattii infection, we analyzed and compared the occurrence of IFN-γ-producing (Th1) and IL-17-producing CD4+ (Th17) cells induced in the lungs of mice following inoculation for 7 days with C. gattii, C. neoformans, or a phosphate-buffered saline (PBS) control by intracellular cytokine staining. The genome-sequenced strains C. neoformans H99 (VNI) and C. gattii R265 (VGII) were selected as representative highly virulent strains (23). Similar to previous findings (4), mice infected with C. gattii strain R265 had higher fungal burdens in the lungs than those infected with C. neoformans strain H99 (see Fig. S1 in the supplemental material). Interestingly, fewer Th1 cells were detected in the lungs of mice infected with the highly virulent C. gattii strain R265 (1.5% ± 0.5%, or 0.05 × 104 ± 0.01 × 104 cells [mean ± SD]) than in mice infected with the highly virulent C. neoformans strain H99 (8% ± 1%, or 3.2 × 104 ± 0.9 × 104 cells) (Fig. 1A). Although we detected comparable percentages of Th17 cells in the lungs of mice infected with the highly virulent strains of C. neoformans and C. gattii (H99-3.4% ± 0.3% versus R265-4.4% ± 0.5%), the absolute numbers of Th17 cells in C. gattii-infected lungs were reduced (H99, 1.3 × 104 ± 0.2 × 104 cells, versus R265, 0.2 × 104 ± 0.05 × 104 cells) (Fig. 1A). Notably, CD44+ CD4+ T cells (effector T cells), but not CD44− CD4+ T cells (naive T cells), primarily secreted the cytokines IFN-γ and IL-17 (see Fig. S2A in the supplemental material). To confirm the effect of C. gattii infection on T helper cell responses, we performed additional analysis of Th1 and Th17 immune responses in mice infected with the other strains of C. neoformans (WM148 [serotype A, VNI]) and C. gattii (WM179 [serotype B, VGI]) (24). These two strains appear to be less virulent, as shown by our survival and fungal burden analysis (see Fig. S1 in the supplemental material). While mice infected with the highly virulent C. neoformans strain H99 had more Th1 cells (8% ± 1%, or 3.2 × 104 ± 0.9 × 104 cells) than Th17 cells (3.4 ± 0.3%, or 1.3 × 104 ± 0.2 × 104 cells) in the lung (Fig. 1A), those infected with the less virulent C. neoformans strain WM148 had more pulmonary Th17 cells (8.1% ± 0.9% or 4.4 × 104 ± 0.6 × 104 cells) than Th1 cells (2.7% ± 0.7%, or 1.5 × 104 ± 0.4 × 104 cells) (Fig. 1B). We observed fewer Th1 cells (1% ± 0.3% or 0.9 × 104 ± 0.2 × 104 cells) and Th17 cells (1.9 ± 0.2% or 1.2 × 104 ± 0.4 × 104 cells) in the lungs of mice infected with C. gattii strain WM179 than those infected with C. neoformans strain WM148 (Fig. 1B).
FIG 1.
C. gattii infection attenuates the Th1/Th17 immune response in the lungs. C57BL/6 mice were treated with PBS (control) or infected with highly virulent strains of C. neoformans (H99) or C. gattii (R265) (A) or with less virulent strains of C. neoformans (WM148) or C. gattii (WM179) (B). Lungs from PBS-treated mice and infected mice were harvested and analyzed for surface CD4 expression and intracellular cytokine expression of IFN-γ and IL-17. The results shown are representative dot plots, average percentage of double-positive cells, and average total cell number (plots were first gated on CD4+ cells, and then cytokine-positive gates were determined by comparison to PBS-treated controls). Data are means ± SD and are representative of three experiments with three to four mice per group. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
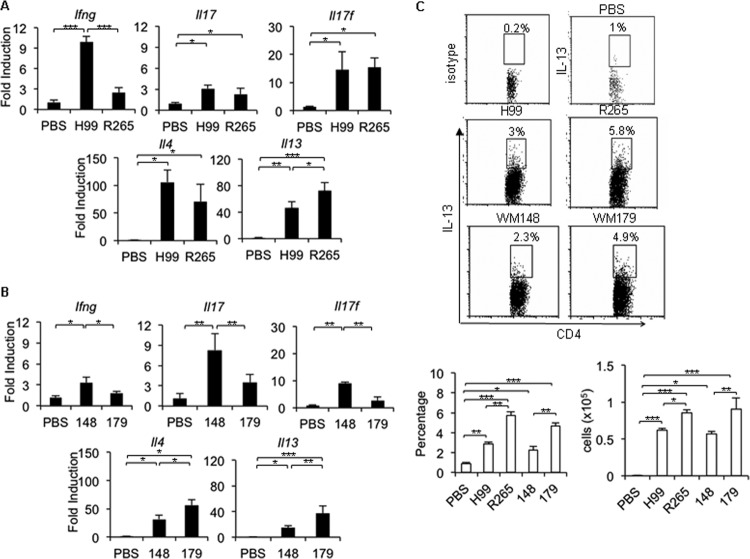
To confirm the above observations, we examined expression of the signature cytokines that specify T helper cell subsets (9). Compared to that in mice treated with PBS, Ifng expression was significantly induced in the lungs of mice infected with C. neoformans (H99 and WM148) but not in those infected with C. gattii (R265 and WM179) (Fig. 2A and B). While Il17 expression was elevated in lungs of mice infected with the highly virulent C. neoformans strain H99 and C. gattii strain R265 (Fig. 2A), infection with C. gattii strain WM179 did not trigger strong induction of Il17 transcript compared to infection with C. neoformans strain WM148 (Fig. 2B). Interestingly, both C. neoformans and C. gattii also triggered a significant increase of Th2 cytokine (Il4 and Il13) expression in the lungs of infected mice (Fig. 2A and B). The expression of Il13 in mice infected with both strains of C. gattii (R265 and WM179) was significantly greater than that in mice infected with C. neoformans (H99 and WM148) (Fig. 2A and B). We confirmed our findings by observing the enhanced expression of intracellular IL-13 in pulmonary CD4+ CD44+ T cells of mice infected with both strains of C. gattii (R265 and WM179) (Fig. 2C). The transcription factors T-bet, RORγt, and GATA3 are known to govern Th1, Th17, and Th2 cell development, respectively (9). We detected upregulation of the Tbet mRNA transcript in lungs of mice infected with C. neoformans (H99 and WM148) but not in those infected with C. gattii (R265 and WM179) (see Fig. S2B and C in the supplemental material). The expression of Rorγt transcript was strongly induced in the lung homogenate of mice infected with C. neoformans strain WM148 but not those infected with C. gattii strain WM179 (see Fig. S2C in the supplemental material). Notably, no significant differences in Gata3, Tgfb, and Foxp3 mRNA expression or FOXP3+ CD4+ T cell infiltration between the lung homogenates of mice infected with C. neoformans and C. gattii was found (Fig. S2B to D in the supplemental material). Collectively, these data suggest that the immunocompetent mice fail to elicit a robust Th1/Th17 immune response against C. gattii infection.
FIG 2.
Reduced Th1/Th17 cytokine expression in C. gattii-infected lungs is associated with an enhanced Th2 immune response. C57BL/6 mice were treated with PBS as a control or infected with a highly virulent strain of C. neoformans (H99) or C. gattii (R265) or with a less virulent strain of C. neoformans (WM148) or C. gattii (WM179). (A and B) Lungs were harvested and analyzed for cytokine expression. Total RNA was isolated from lungs and subjected to cDNA synthesis and subsequent real-time PCR analysis. Data are expressed as fold induction over actin (Actb) expression, with mRNA levels in the uninfected group set as 1. (C) Lungs were analyzed for intracellular cytokine staining of IL-13 and isotype-matched antibody on gated CD4+ CD44+ cells. Data are means ± SD and are representative of three experiments with three to four mice per group. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
C. gattii attenuates Th1/Th17 cell response by downregulating dendritic cell activation and function.
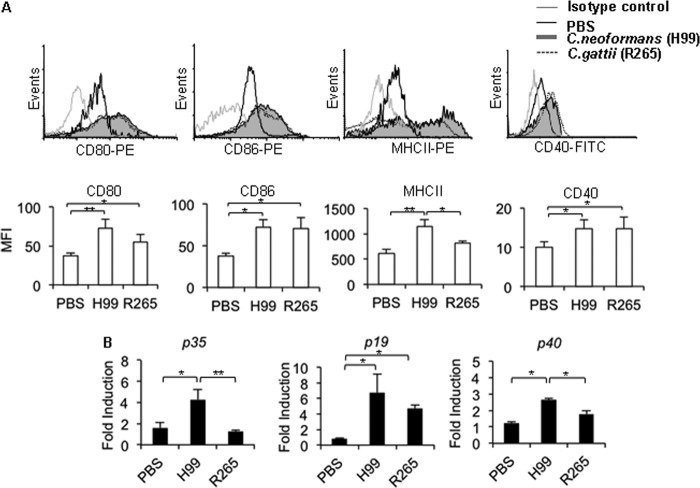
To understand the potential mechanisms involved in the failure to mount an effective Th1/Th17 immune response in C. gattii-infected mice, we examined the characteristics of CD11c+ CD11b+ dendritic cells (DCs) in the lungs of infected mice 7 days after C. neoformans or C. gattii infection. This included examining the expression levels of surface MHC-II and the costimulatory molecules CD80, CD86, and CD40, as well as the genes for lung IL-12 (p35 and p40 subunits) and IL-23 (p19 and p40 subunits), known to drive Th1 and Th17 cell differentiation, respectively (25). Compared to those in PBS-treated mice, pulmonary CD11c+ CD11b+ DCs in mice infected with either C. neoformans (H99 and WM148) or C. gattii (R265 and WM179) upregulated their surface CD80, CD86, and CD40 expression at comparable levels (Fig. 3A; also, see Fig. S3A in the supplemental material). Interestingly, the MHC-II expression level of lung CD11c+ CD11b+ DCs in C. gattii-infected mice (R265 and WM179) was significantly lower than that in C. neoformans-infected mice (H99 and WM148) (Fig. 3A; also, see Fig. S3A in the supplemental material). Moreover, the expression levels of lung Il12 p35 (R265 and WM179), Il23 p19 (WM179), or common subunit p40 (R265) transcripts in mice infected with the C. gattii were significant lower than those in mice infected with C. neoformans (H99 and WM148) (Fig. 3B; also, see Fig. S3B in the supplemental material).
FIG 3.
C. gattii strain R265 infection attenuates the expression of MHC-II on lung DCs and pulmonary IL-12 mRNA. C57BL/6 mice were treated with PBS as an uninfected control or infected with highly virulent C. neoformans (H99) or C. gattii (R265). Lungs were harvested from uninfected or infected mice and analyzed for the expression of CD80, CD86, MHC-II, and CD40 in gated CD11bhiCD11chi cells by flow cytometry (A) or for gene expression of IL-12 (p35/p40) and IL-23 (p19/p40) by quantitative real-time PCR (B). (A) Representative histogram profiles from one of three independent experiments. (B) Total RNA of lungs was isolated and subjected to cDNA synthesis and subsequent real-time PCR analysis of IL-12p35, IL-23p19, and common subunit p40 expression. Data are expressed as fold induction over actin (Actb) expression, with the mRNA levels in the PBS-treated group set as 1. Data are means ± SD and are representative of at least three independent experiments with three to four mice per group. *, P < 0.05; **, P < 0.01.
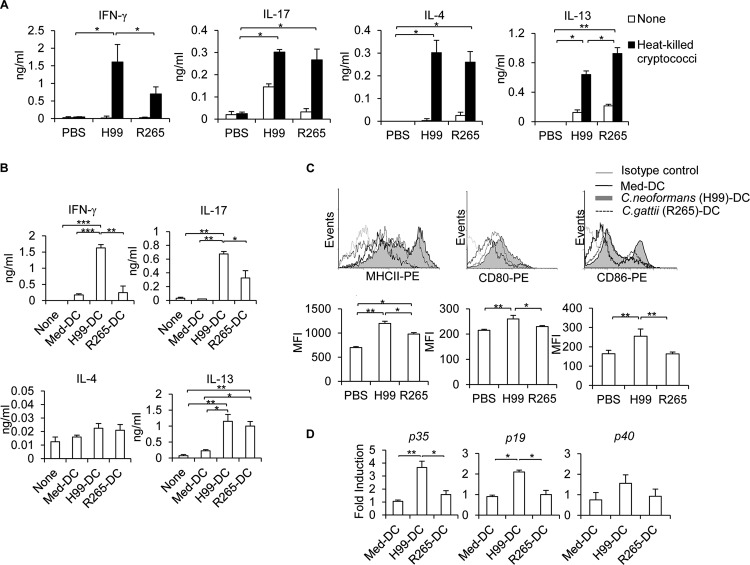
Since IL-12/IL-23 production by DCs and macrophages plays an important role in inducing Th1/Th17 immune responses (9), we analyzed antigen-specific Th1/Th17 cytokine production in lung-draining lymph nodes at 7 days postinfection. Lung-draining lymph nodes harvested from C. neoformans- and C. gattii-infected mice were activated with heat-killed cryptococci for 3 days before the collection of supernatants for the analyses of antigen-specific cytokine production by ELISA. Indeed, we found that draining lymph node cells in mice infected with C. gattii produced significantly lower levels of the Th1 cytokine IFN-γ (R265 and WM179) and the Th17 cytokine IL-17 (WM179) in response to antigen stimulation than those infected with C. neoformans (H99 and WM148) (Fig. 4A; also, see Fig. S4A in the supplemental material). Notably, we also observed much higher antigen-specific IL-13 production in lymph nodes of mice infected with C. gattii than in mice infected with C. neoformans (Fig. 4A; also, see Fig. S4A in the supplemental material). To further investigate whether C. gattii could regulate DC function that leads to inefficient Th1/Th17 cell responses, we stimulated purified bone marrow-derived dendritic cells (BMDCs) with C. neoformans and C. gattii and cocultured them with purified CD4+ T cells from naive mice. Compared to CD4+ T cells cultured with C. gattii (R265 and WM179)-activated DCs, those cultured with C. neoformans (H99 and WM148)-activated DCs produced markedly greater IFN-γ and IL-17 levels (Fig. 4B; also, see Fig. S4B in the supplemental material). However, C. gattii-DC- and C. neoformans-DC-stimulated CD4+ T cells produced comparable amounts of IL-13, but not IL-4, using this DC-T cell coculture experiment in vitro (Fig. 4B). Indeed, C. gattii impaired the activation of bone marrow-derived dendritic cells in vitro, since C. gattii-treated BMDCs failed to upregulate expression of surface markers MHC-II, CD80, and CD86, as well as cytokine transcripts Il12 and Il23, compared to C. neoformans-treated BMDCs (Fig. 4C and D; also, see Fig. S4C and D in the supplemental material). Collectively, these data suggest that C. gattii fails to mount effective antigen-specific Th1/Th17 immune responses by attenuating dendritic cell activation and function.
FIG 4.
C. gattii strain R265 inefficiently elicits antigen-specific Th1/Th17 cytokine production. (A) C57BL/6 mice were treated with PBS as an uninfected control or infected with the highly virulent strain for C. neoformans (H99) or C. gattii (R265). Lung-draining lymph nodes were harvested and cultured with heat-killed Cryptococcus cells. After lymph node cells were restimulated with Cryptococcus for 72 h, supernatants were collected and analyzed for antigen-specific cytokine production by ELISA. (B to D) Purified bone marrow-derived dendritic cells (BMDCs) were stimulated with heat-killed Cryptococcus (H99 and R265). (B) After 24 h, medium-treated DCs (Med-DCs) or activated BMDCs (H99-DC and R265-DC) were washed and cocultured with purified CD4+ T cells from naive mice at a ratio of 1:2. On day 3, culture supernatants were then collected and assessed for cytokine production by ELISA. (C and D) Medium-treated DCs (Med-DCs) or activated BMDCs (H99-DC and R265-DC) were analyzed for the expression of MHC-II, CD80, and CD86 after 24 h of activation by flow cytometry analysis (C) and cytokine gene expression by real-time PCR analysis (D). (C) Representative histogram profile of one of three independent experiments. (D) Total RNA was isolated and subjected to cDNA synthesis and subsequent real-time PCR analysis of IL-12p35, IL-23p19, and common subunit p40 expression. Data are expressed as fold induction over actin (Actb) expression, with the mRNA levels in the medium-treated DCs set as 1. Data are means ± SD and are representative of at least three independent experiments with four mice per group. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Kinetics of the Th1/Th17 cell response in the lungs of mice infected with a highly virulent strain of Cryptococcus gattii.
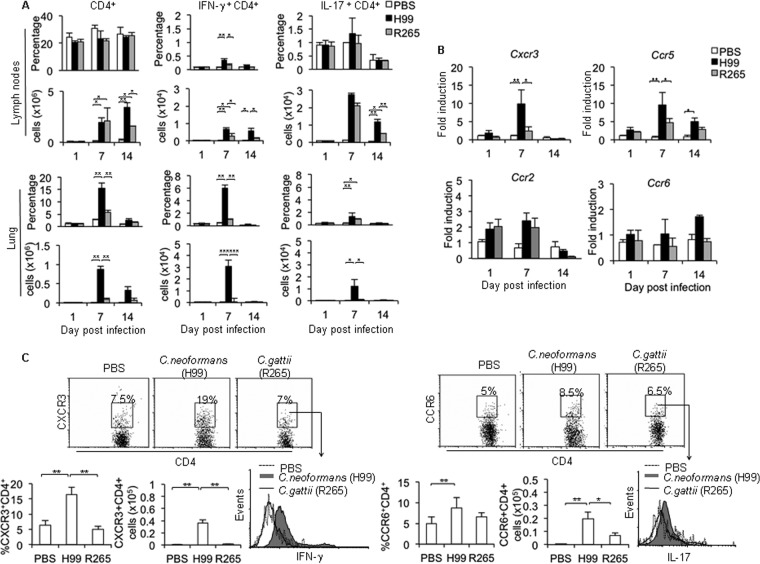
Since we observed the inefficient induction of Th1/Th17 cells in mice infected with C. gattii, we further characterized the kinetics of Th1/Th17 responses in the lungs by using the highly virulent strains of C. neoformans and C. gattii as a representative model for more detailed analysis. Th1 and Th17 cell infiltration in the lungs and draining lymph nodes of mice were assessed on days 1, 7, and 14 postinfection with the highly virulent C. neoformans strain H99 or C. gattii strain R265. While the percentages of CD4+ T cells in lung draining lymph nodes remained constant during infection, pulmonary CD4+ T cells increased, peaked at day 7, and then declined at day 14 following infection with C. neoformans or C. gattii (Fig. 5A). At day 7 postinfection, the numbers of CD4+ T cells in peripheral blood were comparable between C. neoformans and C. gattii-infected mice (see Fig. S5 in the supplemental material); however, mice infected with C. gattii strain R265 had fewer CD4+ T cells in the lungs than C. neoformans H99-infected mice (C. gattii, 5% ± 1%, or 0.15 × 104 ± 0.03 × 104 cells, versus C. neoformans, 13% ± 2.5%, or 0.88 × 104 ± 0.1 × 104 cells) (Fig. 5A; also, see Fig. S6 in the supplemental material). We detected significantly fewer Th1 cells in draining lymph nodes (>2-fold) and a reduced Th1 percentage and absolute number in the lungs (>5-fold) of C. gattii-infected mice than in those of C. neoformans-infected mice (Fig. 5A). Thus, these data indicate that mice infected with the highly virulent C. gattii strain R265 demonstrate impaired Th1 cell recruitment to the infected site.
FIG 5.
Kinetics of Th1/Th17 response and pulmonary chemokine expression in lungs of mice infected with the highly virulent C. gattii infection. C57BL/6 mice were treated with PBS as an uninfected control or infected with highly virulent C. neoformans (H99) or C. gattii (R265). After mice had been infected for 1, 7, or 14 days, their lungs and lung-draining lymph nodes were harvested at the indicated times postinfection. (A) Cells were stained for surface expression of CD4 and intracellular cytokines IFN-γ and IL-17. The results are presented as percentages and absolute numbers of cells. (B) Lungs were homogenized, followed by RNA extraction and cDNA analysis for chemokine receptor gene expression (CXCR3, CCR2, CCR5, and CCR6) by real-time PCR. Data are expressed as fold induction over actin (Actb) expression, with the mRNA levels in the PBS-treated group set as 1. (C) On day 7 postinfection, lungs were prepared as a single-cell suspension and analyzed by flow cytometry for surface expression of CXCR3 and CCR6 (on CD4+ T cells) and the intracellular cytokines IFN-γ (on gated CXCR3+ CD4+) or IL-17 on (CCR6+ CD4+ T cells). The results are presented as dot plots or as percentages or absolute numbers of cells. Values are means ± SD and are representative of three experiments with three to four mice per group. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Next, we further examined and compared the expression patterns of chemokine receptors CXCR3 and CCR5 that were shown to preferentially direct the migration of Th1 cells, as well as CCR6 and CCR2, which are important for Th17 cell and monocyte migration, respectively (26, 27). We found that the expression of CXCR3 and CCR5 transcripts was significantly elevated and peaked on day 7 postinfection with a kinetic pattern similar to that of Th1 cell recruitment in the lungs of C. neoformans-infected mice (Fig. 5B); however, the expression of these chemokine receptors was not induced in lungs of C. gattii-infected mice (Fig. 5B). Moreover, we did not detect significant changes in CCR6 and CCR2 transcripts during the highly virulent C. neoformans and C. gattii infection. In line with gene expression analysis, we showed that surface CXCR3 expression by lung CD4+ T cells was greatly enhanced in mice following C. neoformans infection but remained unchanged in mice after C. gattii infection (Fig. 5C). Interestingly, IFN-γ expression by lung CXCR3+ CD4+ T cells in mice infected with C. neoformans was significantly higher than that in mice infected with C. gattii or treated with PBS (Fig. 5C). While there was no significant difference in percentage of CCR6-expressing CD4+ T cells between mice infected with C. neoformans (8.7% ± 1.5%) or C. gattii (6.7% ± 1%), the absolute number of CCR6+ CD4+ T cells in C. gattii-infected lungs was reduced (C. neoformans, 0.198 × 105 ± 0.05 × 105 cells, versus C. gattii, 0.07 × 105 ± 0.02 × 105 cells) (Fig. 5C). Moreover, the expression of IL-17 by lung CCR6+ CD4+ T cells in C. gattii-infected mice was moderately reduced compared to that in C. neoformans-infected mice (Fig. 5C). Similar chemokine expression patterns were detected in CD44+ CD4+ effector T cells (see Fig. S7 in the supplemental material). Collectively, these data suggest that the highly virulent C. gattii infection may cause impaired Th1 immune responses by affecting not only the Th1 cell induction but also infiltration.
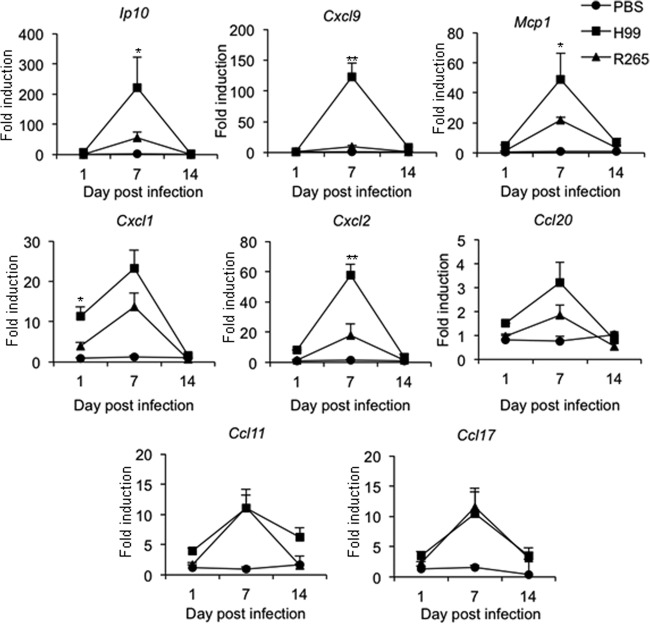
Reduced Th1-promoting chemokine production in mice infected with highly virulent C. gattii R265 is associated with attenuated induction of the Th1 immune response.
To further understand the potential mechanisms that result in the reduced Th1 cell response in the lungs of mice infected with highly virulent C. gattii, R265, we analyzed the expression kinetics of chemokines, known to be involved in the recruitment of T cells (IP-10 and CXCL-9 for Th1, CCL20 for Th17 cells, and CCL17 for Th2 cells) as well as neutrophils (CXCL1 and CXCL2), monocytes/dendritic cells (MCP-1), and eosinophils (CCL11). Lung cells harvested from mice infected with C. neoformans H99 or C. gattii R265 for 1, 7, and 14 days were subjected to RNA extraction, followed by cDNA synthesis and chemokine gene expression analysis by quantitative real-time PCR. Compared to PBS-treated mice, the expression of Ip10, Cxcl9, Mcp1, Cxcl1, Cxcl2, Ccl20, Ccl11, and Ccl17 transcripts in the lung of mice infected with C. neoformans and C. gattii was found to be upregulated, and all reached peak levels at day 7 postinfection (Fig. 6). The expression levels of Cxcl1 and Cxcl2 transcripts (induced at days 1 and 7 postinfection, respectively) and Mcp1 (induced at day 7 postinfection) were significantly higher in C. neoformans-infected mice than in C. gattii-infected mice (Fig. 6). Notably, the expression levels of Ip10 and Cxcl9, the chemokines that selectively attract Th1 lymphocytes, were dramatically elevated in lungs of C. neoformans-infected mice but not in those of C. gattii-infected mice (Fig. 6). In contrast, we found that the expression levels of Ccl20, Ccl11, and Ccl17 were comparable between the lungs of mice infected with C. neoformans and C. gattii.
FIG 6.
Kinetics of pulmonary chemokine expression in response to the highly virulent C. gattii infection. C57BL/6 mice were treated with PBS as an uninfected control or infected with the highly virulent C. neoformans or C. gattii for 1, 7, or 14 days. At the indicated time points, lungs were harvested and analyzed for chemokine gene expression by quantitative real-time PCR. Data are expressed as fold induction over actin (Actb) expression, with the mRNA levels in the PBS-treated group set as 1. Data are means ± SD and are representative of three experiments with three to four mice per group. *, P < 0.05; **, P < 0.01.
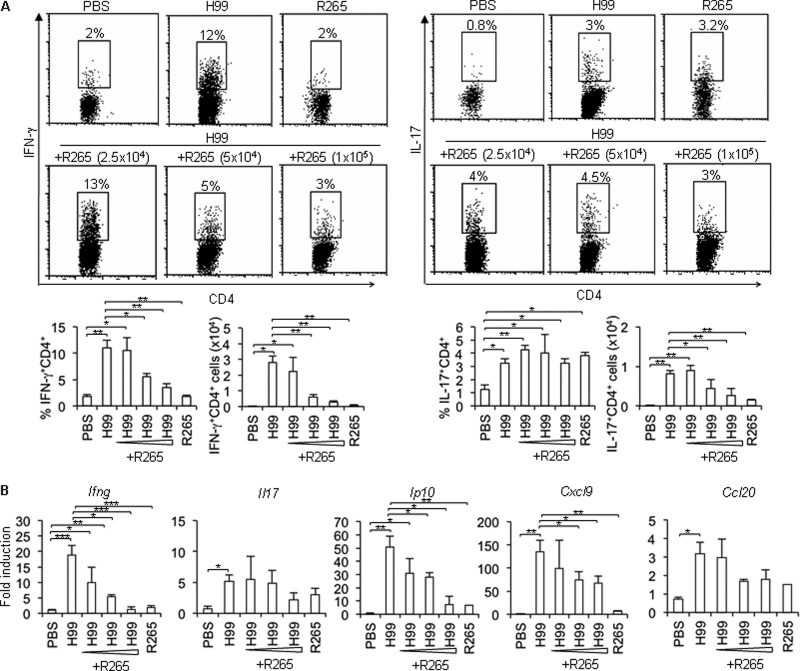
To further confirm the ability of C. gattii to attenuate Th1 cell infiltration, a mixed infection of C. neoformans H99 with increasing doses of C. gattii R265 was given to the mice, and then pulmonary Th1 cell responses and chemokine expression were assessed. As described above, lungs in mice infected with C. neoformans H99 had stronger Th1 immune responses than those in C. gattii R265-infected mice. Interestingly, mice infected with a mixed infection of H99 and increasing doses of R265 had lower Th1 cell infiltration (Fig. 7A) and reduced levels of the Th1-attracting chemokine IP-10 and CXCL-9 in a dose-dependent fashion compared to those infected with only C. neoformans H99 (Fig. 7B). Altogether, these data suggest that the highly virulent C. gattii strain is capable of attenuating adaptive Th1 cell recruitment into the lungs of mice, which are therefore ineffective in controlling pulmonary fungal burdens.
FIG 7.
A mixed infection of the highly virulent C. neoformans and C. gattii infection results in inhibiting Th1 cell infiltration and Th1-attracting chemokine expression. C57BL/6 mice were infected with the highly virulent C. neoformans (H99) or C. gattii (R265) (5 × 104 yeast cells) or mixture of C. neoformans (5 × 104 yeast cells) with different doses of C. gattii (2.5 × 104, 5 × 104, or 1 × 105 yeast cells) for 7 days. Lungs were harvested and subjected to (A) intracellular cytokine staining of IFN-γ and IL-17 in gated CD4+ cells by flow cytometry analysis and (B) cytokine and chemokine gene expression analysis by quantitative real-time PCR. Data are expressed as fold induction over actin (Actb) expression, with the mRNA levels in the PBS-treated group set as 1. Data are means ± SD and are representative of three experiments with three to four mice per group. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
DISCUSSION
An adaptive immune response is crucial for controlling the development of cryptococcal infection and disease persistence (17). For example in HIV patients, T helper cell defects are associated with systemic infection of C. neoformans and life-threatening cryptococcosis (28). C. gattii, recently classified as a separate species, is notable in causing infection in immunocompetent hosts (17). Previous data have suggested that this fungus is capable of suppressing protective immunity by inhibiting neutrophil migration and the inflammatory cytokine response (5). The ability of C. gattii to modulate the T helper cell response, however, has not been clearly addressed. In the present study, we examined the effect of C. gattii on the T helper cell response to better understand the mechanism whereby C. gattii downregulates host immune responses and causes disease in immunocompetent hosts. We found that mice infected with C. gattii, not C. neoformans, fail to mount an efficient Th1/Th17 immune response by attenuating dendritic cell function and chemokine expression.
While protective immunity against C. neoformans was known to be dependent upon the induction of Th1- and Th17-type cytokine responses (11–13), the role of these cells in immunity against C. gattii infection is less clear. Indeed, we observed that mice infected with the highly virulent C. neoformans strain H99 induced a greater Th1 response (compared to Th17) in the lungs, whereas Th1 cells in mice infected with the highly virulent C. gattii strain R265 were markedly reduced. However, the less virulent C. neoformans strain WM148 induced higher Th17 than Th1 responses in the lungs, and the frequencies of Th17 cells were greatly decreased in mice infected with the less virulent C. gattii strain WM179. It is possible that the C. neoformans strain with less virulence may induce a lower strength of T cell stimulation that favors a Th17 response over Th1 (29). Indeed, we could detect IL-17 secretion by cells from lung-draining lymph nodes of C. neoformans- and C. gattii-infected mice in the absence of restimulation with antigen-specific heat-killed cryptococci, suggesting innate cellular sources of IL-17 during fungal infection. Besides Th17 cells, IL-17 can also be produced by non-CD4+ T cells, including γδ T cells, NKT cells, neutrophils, and CD8+ T cells. It is likely that IL-17 derived from innate immune cells may play important roles in enhancing effective pulmonary immune responses in the early phase of infection. How IL-17 functions in response to C. neoformans and C. gattii infection has not been clearly defined and requires further investigation. Interestingly, the higher numbers of IL-13+ CD4+ T cells were observed in both strains of C. gattii-infected mice, although GATA3 was not upregulated. Type 2 cytokines such as IL-4 and IL-13 were known to attenuate host immune responses against C. neoformans and C. gattii by promoting intracellular yeast cell proliferation in vitro (16). It is likely that greater induction of Th2 cytokines such as IL-13 by C. gattii infection may create a lung environment that allows them to grow faster than during C. neoformans infection. It will be interesting to further investigate the role of IL-13 and its regulation during C. gattii infection. Indeed, we did not detect enhanced IL-13 expression in CD4+ T cells that were stimulated with C. gattii-treated DCs compared to C. neoformans-treated DCs in vitro, suggesting that other innate immune cells, such as type 2 innate lymphoid cells (ILC2), may contribute to creation of a microenvironment potentiating a Th2 immune response during C. gattii infection in vivo. These data indicate that C. gattii attenuates Th1 and Th17 immune responses and may enhance a Th2 type bias that favors a more deleterious Th2 immune response.
The activation and determination of T helper cell response depend on several signals, including types of antigens, the expression of MHC-II, costimulatory molecules, and cytokines. IL-12 and IL-23 are derived from antigen-presenting cells and play important roles in promoting the differentiation of Th1 and Th17 cells, respectively (9). IL-12 was known to be important for controlling pulmonary and disseminated infection with C. neoformans (13, 30). IL-23p19 knockout mice infected with C. neoformans serotype D exhibited decreased survival times and higher organ burdens, which correlate with impaired IL-17 production (18, 31). We found that C. gattii dampened the induction of Th1 cell response (R265 and WM179) and Th17 immunity (WM179) in infected mice. Our data showing reduced expression levels of MHC-II in lung DCs and pulmonary cytokines IL-12/IL-23, attenuated production of antigen-specific Th1/Th17 cytokines in lung draining lymph nodes, and failure to generate Th1/Th17 cells upon stimulation with C. gattii-treated DCs in vitro indicate that Th1 and Th17 cells, which are critical for the host against fungal infection, are inefficiently developed in response to C. gattii infection. Similar to our findings, human dendritic cells exposed to C. gattii failed to mature and were incompetent to activate T cells (32).
Kinetic studies of T helper cell responses indicated that the highly virulent C. gattii strain R265 may impair the migration of Th1 cells into the lungs. Analysis of chemokine receptor expression in the lungs revealed that the expression levels of both CXCR3 and CCR5 were highly elevated and their expression kinetics were correlated positively with the pattern of effector Th1 cell migration into the lungs of mice following C. neoformans strain H99 infection but not in mice infected with C. gattii strain R265. Previous studies have shown that CD4+ Th1 cells preferentially express CCR5 and CXCR3, which may regulate migration of these cells into the site of infection (33). Expression of the CXCR3 chemokine receptor was required for optimal generation of IFN-γ-secreting Th1 cells in vivo, and expression of CCR5 is required for host defense and survival after infection with C. neoformans (34, 35). Chemokines play important roles in the recruitment, migration, and trafficking of immune cells to specific sites in inflammatory tissues (27). To characterize the initial events that instruct pulmonary inflammatory cell infiltration preceding the effector T helper cell response, we compared the expression levels of chemokines in the lungs. IP-10 (CXCL10) and CXCL9 are ligands for the chemokine receptor CXCR3, which is categorized functionally as a Th1 chemokine (27). CCL17, interacting with CCR4, contributes to the recruitment of Th2 cells (27). Consistent with our studies on CXCR3 expression, we found that expression levels of the induced Ip10 and Cxcl9, but not Ccl17, transcripts were significantly lower in mice infected with C. gattii than those in mice infected with C. neoformans. These data indicate that C. gattii strain R265 may selectively inhibit the induction of Th1-attracting chemokine expression in the lung, which results in impaired Th1 cell migration. Moreover, infection with C. gattii strain R265, but not C. neoformans strain H99, also dampened the induction of expression of Cxcl1, Cxcl2, and Mcp1, which encode chemokines critical for neutrophils, dendritic cells, and monocytes, but not the induction of Ccl11 expression, which drives eosinophil recruitment. Therefore, mice infected with C. gattii fail to recruit neutrophils and dendritic cells to the lungs, and these cells may be essential for mounting Th1 immune responses. Further studies to understand the underlying mechanisms that are involved in the downregulation of chemokine expression during C. gattii infection may facilitate the design of treatments to prevent the persistence of diseases.
In conclusion, we provide direct evidence that infection with C. gattii attenuates adaptive T helper cell responses by dampening the induction of Th1/Th17 cells. Further analyses of the kinetics of T helper cell responses when infected with highly virulent C. gattii indicate that this fungus is capable of inhibiting chemokine/chemokine receptor expression, which results in selectively reduced Th1 cell migration into the lungs. Although our analysis was limited to a few strains of C. neoformans and C. gattii, the finding that C. gattii attenuates the immune response in infected mice by dampening dendritic cell activation and immune cell migration may provide new insight into the underlying mechanisms C. gattii utilizes to infect immunocompetent hosts and cause fatal lung infection. Previous studies suggested that mice infected with C. gattii strain R265 may die due to overwhelming intrapulmonary growth, whereas the infection of C. neoformans strain H99 primarily causes fulminating brain disease (4). Although the overall survival rates of mice infected with C. neoformans (H99) and those infected with C. gattii (R265) were similar, the fungal burdens in C. gattii (R265 and WM179)-infected lungs were significantly greater than those in C. neoformans (H99 and WM148)-infected lungs, supporting the notion that the suppression of neutrophil migration and adaptive Th1 immunity after C. gattii infection further contributes to the uncontrolled growth of C. gattii in the lung. We could not exclude the possibility that other factors, including fungal virulence factors, host immune components, and genetics, may result in the differences in disease progression and survival. Future studies of the Th1 and Th17 immune response to the C. gattii infection by other strains may provide more evidence supporting our current observations that can be instrumental for understanding the complex immune responses to Cryptococcus infection.
Supplementary Material
ACKNOWLEDGMENTS
We thank the Faculty of Allied Health Sciences, Thammasat University, for their support and Hoainam T. Nguyen-Jackson for her critical reading of the manuscript.
This work was supported by grants from the National Science and Technology Development Agency (Discovery Based Development [DD] grant) and Thammasat University, Thailand.
We declare no conflicting financial interests.
Footnotes
Published ahead of print 30 June 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.01773-14.
REFERENCES
- 1.Lin X, Heitman J. 2006. The biology of the Cryptococcus neoformans species complex. Annu. Rev. Microbiol. 60:69–105. 10.1146/annurev.micro.60.080805.142102 [DOI] [PubMed] [Google Scholar]
- 2.Speed B, Dunt D. 1995. Clinical and host differences between infections with the two varieties of Cryptococcus neoformans. Clin. Infect. Dis. 21:28–34, 35–26. 10.1093/clinids/21.1.28 [DOI] [PubMed] [Google Scholar]
- 3.Chen S, Sorrell T, Nimmo G, Speed B, Currie B, Ellis D, Marriott D, Pfeiffer T, Parr D, Byth K. 2000. Epidemiology and host- and variety-dependent characteristics of infection due to Cryptococcus neoformans in Australia and New Zealand. Australasian Cryptococcal Study Group. Clin. Infect. Dis. 31:499–508 [DOI] [PubMed] [Google Scholar]
- 4.Ngamskulrungroj P, Chang Y, Sionov E, Kwon-Chung KJ. 2012. The primary target organ of Cryptococcus gattii is different from that of Cryptococcus neoformans in a murine model. mBio 3:e00103-12. 10.1128/mBio.00103-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheng PY, Sham A, Kronstad JW. 2009. Cryptococcus gattii isolates from the British Columbia cryptococcosis outbreak induce less protective inflammation in a murine model of infection than Cryptococcus neoformans. Infect. Immun. 77:4284–4294. 10.1128/IAI.00628-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wright L, Bubb W, Davidson J, Santangelo R, Krockenberger M, Himmelreich U, Sorrell T. 2002. Metabolites released by Cryptococcus neoformans var. neoformans and var. gattii differentially affect human neutrophil function. Microbes Infect. 4:1427–1438. 10.1016/S1286-4579(02)00024-2 [DOI] [PubMed] [Google Scholar]
- 7.Brouwer AE, Siddiqui AA, Kester MI, Sigaloff KC, Rajanuwong A, Wannapasni S, Chierakul W, Harrison TS. 2007. Immune dysfunction in HIV-seronegative, Cryptococcus gattii meningitis. J. Infect. 54:e165–168. 10.1016/j.jinf.2006.10.002 [DOI] [PubMed] [Google Scholar]
- 8.Dong ZM, Murphy JW. 1995. Effects of the two varieties of Cryptococcus neoformans cells and culture filtrate antigens on neutrophil locomotion. Infect. Immun. 63:2632–2644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pappu BP, Angkasekwinai P, Dong C. 2008. Regulatory mechanisms of helper T cell differentiation: new lessons learned from interleukin 17 family cytokines. Pharmacol. Ther. 117:374–384. 10.1016/j.pharmthera.2007.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herring AC, Lee J, McDonald RA, Toews GB, Huffnagle GB. 2002. Induction of interleukin-12 and gamma interferon requires tumor necrosis factor alpha for protective T1-cell-mediated immunity to pulmonary Cryptococcus neoformans infection. Infect. Immun. 70:2959–2964. 10.1128/IAI.70.6.2959-2964.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Decken K, Kohler G, Palmer-Lehmann K, Wunderlin A, Mattner F, Magram J, Gately MK, Alber G. 1998. Interleukin-12 is essential for a protective Th1 response in mice infected with Cryptococcus neoformans. Infect. Immun. 66:4994–5000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kawakami K, Tohyama M, Teruya K, Kudeken N, Xie Q, Saito A. 1996. Contribution of interferon-gamma in protecting mice during pulmonary and disseminated infection with Cryptococcus neoformans. FEMS Immunol. Med. Microbiol. 13:123–130. 10.1111/j.1574-695X.1996.tb00225.x [DOI] [PubMed] [Google Scholar]
- 13.Kawakami K, Tohyama M, Xie Q, Saito A. 1996. IL-12 protects mice against pulmonary and disseminated infection caused by Cryptococcus neoformans. Clin. Exp. Immunol. 104:208–214. 10.1046/j.1365-2249.1996.14723.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kawakami K, Hossain Qureshi M, Zhang T, Koguchi Y, Xie Q, Kurimoto M, Saito A. 1999. Interleukin-4 weakens host resistance to pulmonary and disseminated cryptococcal infection caused by combined treatment with interferon-gamma-inducing cytokines. Cell Immunol. 197:55–61. 10.1006/cimm.1999.1557 [DOI] [PubMed] [Google Scholar]
- 15.Muller U, Stenzel W, Kohler G, Werner C, Polte T, Hansen G, Schutze N, Straubinger RK, Blessing M, McKenzie AN, Brombacher F, Alber G. 2007. IL-13 induces disease-promoting type 2 cytokines, alternatively activated macrophages and allergic inflammation during pulmonary infection of mice with Cryptococcus neoformans. J. Immunol. 179:5367–5377. 10.4049/jimmunol.179.8.5367 [DOI] [PubMed] [Google Scholar]
- 16.Voelz K, Lammas DA, May RC. 2009. Cytokine signaling regulates the outcome of intracellular macrophage parasitism by Cryptococcus neoformans. Infect. Immun. 77:3450–3457. 10.1128/IAI.00297-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Voelz K, May RC. 2010. Cryptococcal interactions with the host immune system. Eukaryot. Cell 9:835–846. 10.1128/EC.00039-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kleinschek MA, Muller U, Brodie SJ, Stenzel W, Kohler G, Blumenschein WM, Straubinger RK, McClanahan T, Kastelein RA, Alber G. 2006. IL-23 enhances the inflammatory cell response in Cryptococcus neoformans infection and induces a cytokine pattern distinct from IL-12. J. Immunol. 176:1098–1106. 10.4049/jimmunol.176.2.1098 [DOI] [PubMed] [Google Scholar]
- 19.Zhang Y, Wang F, Tompkins KC, McNamara A, Jain AV, Moore BB, Toews GB, Huffnagle GB, Olszewski MA. 2009. Robust Th1 and Th17 immunity supports pulmonary clearance but cannot prevent systemic dissemination of highly virulent Cryptococcus neoformans H99. Am. J. Pathol. 175:2489–2500. 10.2353/ajpath.2009.090530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Angkasekwinai P, Park H, Wang YH, Chang SH, Corry DB, Liu YJ, Zhu Z, Dong C. 2007. Interleukin 25 promotes the initiation of proallergic type 2 responses. J. Exp. Med. 204:1509–1517. 10.1084/jem.20061675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Filippo K, Henderson RB, Laschinger M, Hogg N. 2008. Neutrophil chemokines KC and macrophage-inflammatory protein-2 are newly synthesized by tissue macrophages using distinct TLR signaling pathways. J. Immunol. 180:4308–4315. 10.4049/jimmunol.180.6.4308 [DOI] [PubMed] [Google Scholar]
- 22.Angkasekwinai P, Srimanote P, Wang YH, Pootong A, Sakolvaree Y, Pattanapanyasat K, Chaicumpa W, Chaiyaroj S, Dong C. 2013. Interleukin-25 (IL-25) promotes efficient protective immunity against Trichinella spiralis infection by enhancing the antigen-specific IL-9 response. Infect. Immun. 81:3731–3741. 10.1128/IAI.00646-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fraser JA, Giles SS, Wenink EC, Geunes-Boyer SG, Wright JR, Diezmann S, Allen A, Stajich JE, Dietrich FS, Perfect JR, Heitman J. 2005. Same-sex mating and the origin of the Vancouver Island Cryptococcus gattii outbreak. Nature 437:1360–1364. 10.1038/nature04220 [DOI] [PubMed] [Google Scholar]
- 24.Meyer W, Castaneda A, Jackson S, Huynh M, Castaneda E. 2003. Molecular typing of IberoAmerican Cryptococcus neoformans isolates. Emerg. Infect. Dis. 9:189–195. 10.3201/eid0902.020246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dong C. 2006. Diversification of T-helper-cell lineages: finding the family root of IL-17-producing cells. Nat. Rev. Immunol. 6:329–334. 10.1038/nri1807 [DOI] [PubMed] [Google Scholar]
- 26.Bromley SK, Mempel TR, Luster AD. 2008. Orchestrating the orchestrators: chemokines in control of T cell traffic. Nat. Immunol. 9:970–980. 10.1038/ni.f.213 [DOI] [PubMed] [Google Scholar]
- 27.D'Ambrosio D, Mariani M, Panina-Bordignon P, Sinigaglia F. 2001. Chemokines and their receptors guiding T lymphocyte recruitment in lung inflammation. Am. J. Respir. Crit. Care Med. 164:1266–1275. 10.1164/ajrccm.164.7.2103011 [DOI] [PubMed] [Google Scholar]
- 28.Perfect JR, Dismukes WE, Dromer F, Goldman DL, Graybill JR, Hamill RJ, Harrison TS, Larsen RA, Lortholary O, Nguyen MH, Pappas PG, Powderly WG, Singh N, Sobel JD, Sorrell TC. 2010. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the infectious diseases society of America. Clin. Infect. Dis. 50:291–322. 10.1086/649858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Purvis HA, Stoop JN, Mann J, Woods S, Kozijn AE, Hambleton S, Robinson JH, Isaacs JD, Anderson AE, Hilkens CM. 2010. Low-strength T-cell activation promotes Th17 responses. Blood 116:4829–4837. 10.1182/blood-2010-03-272153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hoag KA, Lipscomb MF, Izzo AA, Street NE. 1997. IL-12 and IFN-gamma are required for initiating the protective Th1 response to pulmonary cryptococcosis in resistant C.B-17 mice. Am. J. Respir. Cell Mol. Biol. 17:733–739. 10.1165/ajrcmb.17.6.2879 [DOI] [PubMed] [Google Scholar]
- 31.Kleinschek MA, Muller U, Schutze N, Sabat R, Straubinger RK, Blumenschein WM, McClanahan T, Kastelein RA, Alber G. 2010. Administration of IL-23 engages innate and adaptive immune mechanisms during fungal infection. Int. Immunol. 22:81–90. 10.1093/intimm/dxp117 [DOI] [PubMed] [Google Scholar]
- 32.Huston SM, Li SS, Stack D, Timm-McCann M, Jones GJ, Islam A, Berenger BM, Xiang RF, Colarusso P, Mody CH. 2013. Cryptococcus gattii is killed by dendritic cells, but evades adaptive immunity by failing to induce dendritic cell maturation. J. Immunol. 191:249–261. 10.4049/jimmunol.1202707 [DOI] [PubMed] [Google Scholar]
- 33.Qin S, Rottman JB, Myers P, Kassam N, Weinblatt M, Loetscher M, Koch AE, Moser B, Mackay CR. 1998. The chemokine receptors CXCR3 and CCR5 mark subsets of T cells associated with certain inflammatory reactions. J. Clin. Invest. 101:746–754. 10.1172/JCI1422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huffnagle GB, McNeil LK, McDonald RA, Murphy JW, Toews GB, Maeda N, Kuziel WA. 1999. Cutting edge: role of C-C chemokine receptor 5 in organ-specific and innate immunity to Cryptococcus neoformans. J. Immunol. 163:4642–4646 [PubMed] [Google Scholar]
- 35.Groom JR, Richmond J, Murooka TT, Sorensen EW, Sung JH, Bankert K, von Andrian UH, Moon JJ, Mempel TR, Luster AD. 2012. CXCR3 chemokine receptor-ligand interactions in the lymph node optimize CD4+ T helper 1 cell differentiation. Immunity 37:1091–1103. 10.1016/j.immuni.2012.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.