Abstract
Brucella spp. are facultative intracellular Gram-negative coccobacilli responsible for brucellosis, a worldwide zoonosis. We observed that Brucella melitensis is able to persist for several weeks in the blood of intraperitoneally infected mice and that transferred blood at any time point tested is able to induce infection in naive recipient mice. Bacterial persistence in the blood is dramatically impaired by specific antibodies induced following Brucella vaccination. In contrast to Bartonella, the type IV secretion system and flagellar expression are not critically required for the persistence of Brucella in blood. ImageStream analysis of blood cells showed that following a brief extracellular phase, Brucella is associated mainly with the erythrocytes. Examination by confocal microscopy and transmission electron microscopy formally demonstrated that B. melitensis is able to invade erythrocytes in vivo. The bacteria do not seem to multiply in erythrocytes and are found free in the cytoplasm. Our results open up new areas for investigation and should serve in the development of novel strategies for the treatment or prophylaxis of brucellosis. Invasion of erythrocytes could potentially protect the bacterial cells from the host's immune response and hamper antibiotic treatment and suggests possible Brucella transmission by bloodsucking insects in nature.
INTRODUCTION
Brucella is a facultative intracellular alphaproteobacterium that causes abortion and infertility in mammals and leads to a debilitating febrile illness in humans. Although it is rarely fatal, human brucellosis can progress into a severe chronic disease if left untreated (1, 2). Despite significant progress, the incidence of human brucellosis remains very high in areas of endemicity (3). Complete eradication of Brucella would be impractical due to its presence in a large range of wild mammals (4, 5). Moreover, antibiotic treatment is costly, and patients frequently suffer from relapse of the illness (6, 7). Vaccination actually remains the only rational strategy to confer protection on populations living in countries of endemicity. Unfortunately, there is currently no available vaccine against human brucellosis, since all commercial animal vaccines are based on live attenuated strains of Brucella (8) that are still virulent in humans. Efforts to develop an effective vaccine are impaired by our poor knowledge of the Brucella life cycle in vivo and of the protective immune response induced by infection. In particular, the mechanisms of early Brucella dissemination in vivo remain largely unknown. A prominent characteristic of brucellosis is its long incubation period. The current hypothesis is that this period, corresponding to reduced activation of innate immunity, opens an “immunological window” and gives Brucella the chance to spread throughout the organism to establish a replication niche within phagocytic cells (9).
Bacteremia is one major characteristic of human brucellosis (10) and is often associated with an increased risk of relapse (6, 11). Moreover, positive blood cultures are synonymous with secondary seeding and development of focal complications of the disease. Bacteremia in mice infected by intraperitoneal injection, the most frequent route of experimental infection, has been reported in some studies (12, 13). However, to our knowledge, the mechanism of Brucella persistence in the blood has never been investigated. In this study, we monitored bacteremia in mice for several weeks after the intraperitoneal inoculation of Brucella melitensis strain 16M constitutively expressing the mCherry fluorescent tracer. The location of B. melitensis in blood cells was analyzed by ImageStream analysis and confocal microscopy. We also investigated the impact of type IV secretion system and flagellum deficiencies and of specific antibodies on the persistence of the bacteria in blood.
Our results show that B. melitensis is able to persist for at least 2 weeks in the blood of infected mice after intraperitoneal inoculation. The bacteria were first located extracellularly but were then found mainly inside erythrocytes. To our knowledge, this particular niche of infection has never been described in brucellosis, although it is relatively common in other bacteria of the Alphaproteobacteria phylum, such as Anaplasma (14) and Bartonella (15). Circulating antibodies induced by vaccination drastically reduced blood persistence of the bacteria following challenge.
MATERIALS AND METHODS
Ethics statement.
The animal handling and procedures used in this study complied with current European legislation (directive 86/609/EEC) and the corresponding Belgian law, “Arrêté royal relatif à la protection des animaux d'expérience du 6 Avril 2010 publié le 14 Mai 2010“ (Royal Decree on the protection of experimental animals of 6 April 2010, published on 14 May 2010). The complete protocol was reviewed and approved by the Animal Welfare Committee of the University of Namur (permit number 05-558).
Mice and reagents.
MuMT−/− C57BL/6 mice (16) and C57BL/6 mice transgenic for green fluorescent protein (GFP) under the control of the ubiquitin C promoter (Ubi-GFP) (17) were purchased from The Jackson Laboratory (Bar Harbor, ME). Wild-type C57BL/6 mice were purchased from Harlan (Bicester, United Kingdom) and were used as the control. All wild-type and deficient mice used in this study were bred in the Gosselies animal facility of the Free University of Brussels (ULB) (Belgium).
B. melitensis strain 16M (biotype 1, ATCC 23456) was grown in a biosafety level III laboratory facility. Cultures grown overnight under shaking at 37°C in 2YT medium (Luria-Bertani broth with a double quantity of yeast extract) to stationary phase were washed twice in phosphate-buffered saline (PBS) (3,500 × g, 10 min) as described previously (18). When appropriate, we used B. melitensis strain 16M which stably expresses the mCherry protein (mCherry-Br), a previously described rapidly maturing variant of the red fluorescent protein DsRed (19), under the control of a strong Brucella species promoter named PsojA (20). The construction of the mCherry-Br strain has been described previously in detail (21). We also used the B. melitensis strain 16M producing GFP (constructed by transferring the plasmid pBBR1-GFP-SOG [22] by mating), the virB mutant B. melitensis strain 16M (deficient for the type IV secretion system [23]), and the fliC mutant B. melitensis strain 16M (deficient for flagellin expression [24]).
Mouse infection.
Mice were injected intraperitoneally (i.p.) with the appropriate dose of B. melitensis in 500 μl of PBS. Control animals were injected with the same volume of PBS. The infectious doses were validated by plating serial dilutions of inoculum. Mice were bled at selected time points and sacrificed at the end of the experiment.
Blood transfer experiment.
Mice were injected i.p. (500 μl) with fresh blood from infected donor mice. Blood was diluted 20-fold in PBS before immediate administration. Two weeks later, spleens were recovered in PBS–0.1% Triton X-100. Serial dilutions in PBS were performed, followed by plating on 2YT medium plates. The CFU counts were recorded after 4 days of culture at 37°C.
Antibiotic treatment.
Mice were injected i.p. with 4 × 104 CFU of B. melitensis or with PBS. After resting for 3 weeks, both the immunized and control mice were treated with antibiotics for 3 weeks. The treatment was a combination of rifampin (12 mg/kg of body weight) and streptomycin (450 mg/kg) (adapted from the method in reference 25) prepared fresh daily and given in the drinking water. An additional treatment was given i.p. and consisted of 5 injections of streptomycin (300 mg/kg) throughout the 3 weeks of oral treatment.
RAW cell infection.
RAW cells grown in 6-well tissue culture plates were inoculated with mCherry-Br at a multiplicity of infection (MOI) of 300 in 2 ml of cell culture medium (Dulbecco's modified Eagle medium [DMEM]–10% fetal bovine serum [FBS]). Cells were then centrifuged at 1,200 × g for 10 min at 4°C and placed under 5% CO2 at 37°C. After 1 h, the cells were washed two times in PBS and incubated further with cell culture medium supplemented with 50 μg/ml gentamicin until the end of the infection time. After the indicated incubation time, the cells were washed three times in PBS, collected, and centrifuged at 1,200 × g for 10 min at 4°C. The cells were fixed in 300 μl of PBS–4% paraformaldehyde (PFA) for further ImageStream analysis.
Bacterial count.
For bacterial counts in the blood, 70 μl of blood was collected from the tail into heparinized capillary tubes at the selected time points and diluted in PBS–0.1% Triton X-100 (Sigma). Serial dilutions were performed in PBS, followed by plating on 2YT medium plates. CFU were counted after 4 days of culture at 37°C.
To separate the blood cells from the plasma, 140 μl of blood was collected and diluted 4 times to allow better separation. Samples were then centrifuged at 250 × g for 10 min at room temperature (RT), and each blood fraction was recovered. An additional centrifugation (8,000 × g, 2 min, RT) was performed with the supernatant of samples from mice infected with a low dose of bacteria (4 × 104 CFU). This was necessary to concentrate the bacteria. The supernatant fraction was diluted in PBS when necessary, while the pellet fraction was diluted in PBS–0.1% Triton X-100. Each fraction was then plated on 2YT, and CFU were counted after 4 days of culture at 37°C.
Erythrocyte purification.
Blood was collected from the tail into heparinized capillary tubes at the selected time points, diluted in PBS, and immediately filtered using Plasmodipur filters (EuroProxima), following the recommendation of the manufacturer. Plasmodipur filters remove leukocytes from blood (26, 27).
Cytofluorometric analysis.
Blood was collected from the tail into heparinized capillary tubes at the selected time points and diluted in PBS. After washing, blood cells were first incubated in saturating doses of purified 2.4G2 (anti-mouse Fc receptor; ATCC) in 200 μl PBS–0.2% bovine serum albumin (BSA)–0.02% NaN3 (fluorescence-activated cell sorter [FACS] buffer) for 20 min on ice to prevent antibody binding to Fc receptor. Blood cells were stained on ice with various fluorescent monoclonal antibody (MAb) combinations in FACS buffer and further collected on a FACScalibur cytofluorimeter (Becton, Dickinson [BD]). We purchased the following MAbs from BD Biosciences: fluorescein isothiocyanate (FITC)-coupled 145-2C11 (anti-CD3ε), phycoerythrin (PE)-coupled 1A8 (anti-Ly-6G), and Alexa 647-coupled M5/114.15.2 (anti-major histocompatibility complex class II [anti-MHC-II]).
Immunofluorescence microscopy on fixed bacteria.
Blood from ubiquitin-GFP C57BL/6 mice infected with 5 × 107 CFU of mCherry-Br was diluted 1/1 (without preliminary wash or centrifugation) for 15 min in PBS containing 4% paraformaldehyde (pH 7.4) before being smeared on slides. The slides were dried overnight at room temperature and then mounted in Fluoro-Gel medium containing 4′,6-diamidino-2-phenylindole (DAPI) nucleic acid stain (Electron Microscopy Sciences, Hatfield, PA). The labeled cells were visualized with an Axiovert M200 inverted microscope (Zeiss, Iena, Germany) equipped with a high-resolution monochrome camera (AxioCam HR; Zeiss). Images (1,384 by 1,036 pixels [0.16 μm/pixel]) were acquired sequentially for each fluorochrome using an LD-Plan-NeoFluar 63×/0.75 numerical-aperture (NA) dry objective and recorded as 8-bit gray-level zvi files. Images were exported as TIFF files, and figures were created using Canvas 7 software.
Immunofluorescence microscopy of live bacteria.
Wild-type C57BL/6 mice were infected with 5 × 107 CFU of GFP- or mCherry-expressing Brucella melitensis. Blood samples were harvested at 3 h and 24 h postinfection and immediately diluted 10× in PBS. For the observation, the samples were directly dropped on an agarose pad (solution of 1% agarose in PBS), covered with a coverslip, and sealed with Valap (1/3 Vaseline, 1/3 lanolin, and 1/3 paraffin wax). The sealed pad was placed at 4°C for 10 min before imaging with a Nikon 80i (objective phase-contrast, 100×, plan Apo) connected to a Hamamatsu Orca-Er camera in a biosafety level III laboratory facility.
Multispectral imaging flow cytometry (ImageStream).
Events were imaged using multispectral imaging flow cytometry (ImageStream 100; Amnis Corp., Washington). At least 60,000 events were collected from each sample (100,000 for blood samples), and the data were analyzed using image analysis software (IDEAS; Amnis Corp., Washington). Bright-field images of the cells and bacteria were recorded in channel 2, and mCherry bacteria were recorded in channel 4. The acquisition gate included all cells and bacteria and excluded fragments of cells and cell aggregates. Gate R1 was based on the mCherry signal and allowed for the identification of bacteria inside and outside blood cells. The distinction between overlapping bacteria and extracellular (free or adherent) bacteria was made by a combination of the erosion mask applied on the bright field image and the intensity mask at 50% in the specific mCherry channel and using the intensity feature of IDEAS software. Briefly, a bacterium was tagged as intracellular when its fluorescent image overlapped with the bright-field image of blood cells. The intracellular or extracellular location of the bacteria was always confirmed by examination of the images.
Confocal microcopy.
Confocal analyses were performed using the LSM780 confocal system fitted on an Observer Z1 inverted microscope equipped with an alpha Plan Apochromat 63×/1.46 NA oil immersion objective (Zeiss, Iena, Germany). The 488-nm excitation wavelength of the argon/2 laser, a main dichroic HFT 488, and a band-pass (BP) emission filter (BP 500 to 530 nm) were used for selective detection of the enhanced GFP (eGFP) fluorochrome. The 543-nm excitation wavelength of the HeNe1 laser, a main dichroic HFT 488/543/633, and a long-pass emission filter (BP 580 to 650 nm) were used for selective detection of the mCherry fluorochrome. Stacks of 1-μm-thick (i.e., 1 Airy Unit for the red fluorophore) optical sections (volume sampled, 27 by 27 by 5.2 μm, 512 by 512 pixels; scaling, x and y, 0.053 μm; z, 0.370 μm) were collected sequentially for each fluorochrome throughout the thickness of the object and stored as 16-bit *.lsm files. File stacks were processed for deconvolution using the Huyghens software program (Scientific Volume Imaging BV, Hilversum, The Netherlands), and three-dimensional (3D) rendering was performed using Imaris software (Bitplane AG, Zurich, Switzerland). Images were exported as 8-bit uncompressed *.TIF image files.
Scanning electron microcopy.
Samples were fixed overnight at 4°C in 4% PFA–0.1 M cacodylate buffer (pH 7.2) and postfixed in OsO4 (2%) in the same buffer. After serial dehydration, samples were dried to the critical point and coated with platinum by standard procedures. Observations were made using a Tecnai field emission gun (FEG) ESEM Quanta 200 (FEI) instrument, and images were processed by using the SIS iTEM (Olympus) software program.
Transmission electron microcopy.
Infected red blood cells were fixed overnight in 2.5% glutaraldehyde in cacodylate buffer, postfixed in 2% osmium tetroxide, serially dehydrated in ethanol, and embedded in epoxy resin (Agar 100 resin; Agar Scientific, United Kingdom). Ultrathin 70-nm sections were obtained, mounted on copper-Formvar-carbon grids (EMS, United Kingdom), and stained with uranyl acetate and lead citrate by standard procedures. Observations were made on a Tecnai 10 electron microscope (FEI, Eindhoven, The Netherlands), and images were captured with a Veleta charge-coupled-device (CCD) camera and processed using the AnalySIS and Adobe Photoshop software programs.
ELISA.
Specific murine IgM, IgG1, IgG2a, and IgG3 isotypes were determined by enzyme-linked immunosorbent assay (ELISA). Polystyrene plates (Nunc 269620) were coated with heat-killed B. melitensis (107 CFU/ml). After incubation overnight at 4°C, plates were blocked for 2 h at RT with 200 μl of PBS-3.65% casein. The plates were then incubated for 1 h at RT with 50 μl of serial dilutions of the serum in PBS-3.5% casein. Sera from naive mice were used as the negative control. After 4 washes with PBS, isotype-specific goat anti-mouse horseradish peroxidase conjugates were added (50 μl/well) at appropriate dilutions (anti-IgM from Sigma; LO-MG1-13 HRPO, LO-MG2a-9 HRPO, and LO-MG3-13 HRPO from Lo-Imex). After 1 h of incubation at room temperature, the plates were washed 4 times in PBS, and 100 μl/well of substrate solution (BD OptEiA) was added to each well. After 10 min of incubation at room temperature in the dark, the enzyme reaction was stopped by adding 25 μl/well of 2 N H2SO4, and the absorbance was measured at 450 nm.
Statistical analysis.
We used a (Wilcoxon-)Mann-Whitney test provided by the GraphPad Prism software program to statistically analyze our results. Each group of deficient mice was compared to wild-type mice. P values of <0.05 were considered to represent a significant difference.
RESULTS
Long-term persistence of B. melitensis in the bloodstream.
Bacteremia is common in human brucellosis (10), but reports of the presence of Brucella in the blood of infected mice are very rare and old (13). In order to quantify the persistence of bacteria in the blood of infected mice, we collected samples from wild-type C57BL/6 mice infected with different doses (4 × 104, 1 × 106, or 5 × 107 CFU) of mCherry-expressing Brucella melitensis (mCherry-Br) and determined the bacterial titers (Fig. 1A) at different time points postinfection (p.i.). Surprisingly, we observed that bacteria persist for at least 2 weeks in the blood of infected mice (Fig. 1A). When a high dose of bacteria (5 × 107 CFU) was inoculated, 100% of the mice (Fig. 1B) displayed a mean of almost 5 × 103 CFU/ml of blood at 13 days p.i. Clearance of the bacteria appeared to be dose dependent, since at a more classical dose (4 × 104 CFU), only 28% of the mice showed detectable blood bacteremia, and these displayed a mean of 102 CFU/ml of blood at 13 days p.i. (Fig. 1A and B).
FIG 1.
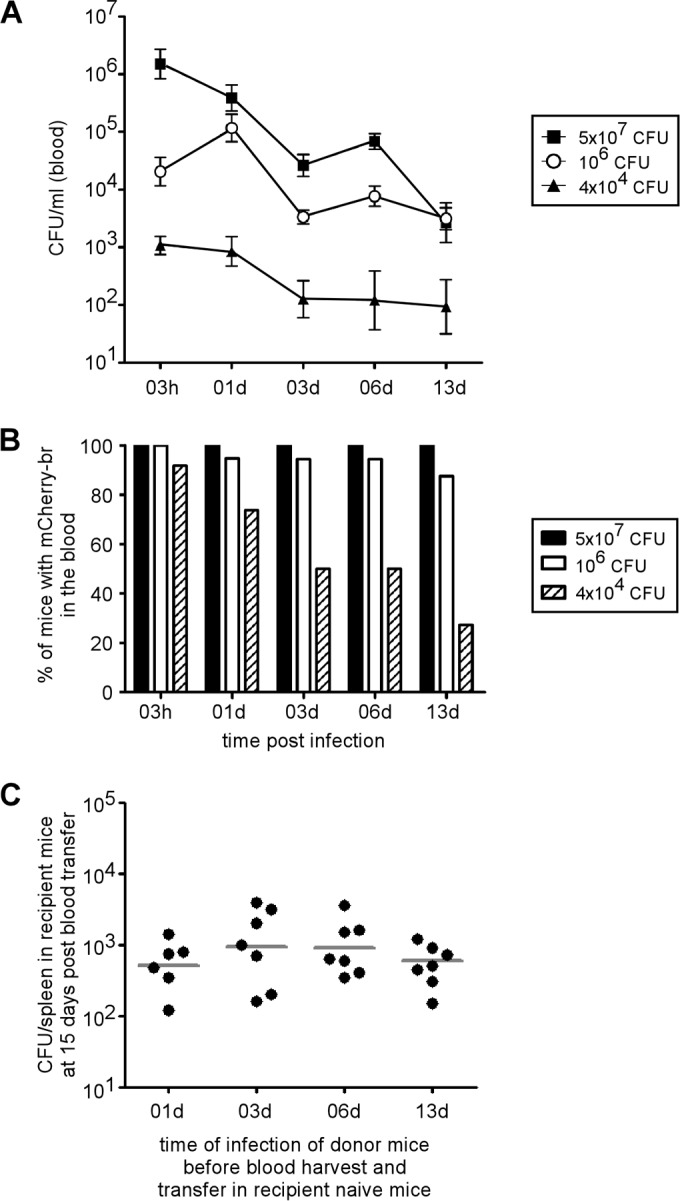
Persistence of infectious B. melitensis in the blood of mice after i.p. infection. (A and B) Wild-type C57BL/6 mice were injected i.p. with 4 × 104, 106, or 5 × 107 CFU of mCherry-expressing B. melitensis (mCherry-Br). and blood was collected at selected time points. The data represent either the means ± SEM of mCherry-Br CFU per ml of blood (A) or the percentage of mice that still had bacteria in the blood (B). The results are representative of two independent experiments. (C) Wild-type C57BL/6 mice were injected i.p. with 106 CFU of mCherry-Br, and blood was collected at 1 (01d), 3, 6, and 13 days p.i. Blood was immediately diluted 20× in PBS and transferred i.p. (500 μl) to recipient wild-type C57BL/6 mice. Two weeks after blood transfer, the mice were sacrificed and the spleens were harvested. The data represent the CFU per spleen and are representative of two independent experiments. The gray bars represent the medians.
In order to confirm the presence of infectious Brucella in the blood of infected mice, naive (recipient) C57BL/6 mice were injected intraperitoneally (i.p.) with fresh blood from mice infected with 106 CFU for 1, 3, 6, and 13 days (Fig. 1C). Two weeks later, all recipient mice displayed CFU counts in the spleen, showing that the blood transferred from infected mice contained living and virulent Brucella melitensis.
Type IV secretion system and flagella are not critically implicated in blood persistence of Brucella.
Several bacterial pathogens, such as Bartonella spp. (28), are well known to persist in the bloodstream during infection. In a Bartonella experimental mouse model, it has been demonstrated that both the type IV secretion system (29, 30) and flagella (31, 32) are necessary for persistence in blood. In order to test their role in Brucella persistence in the blood, naive C57BL/6 mice were injected i.p. with 106 CFU of the wild type, virB mutant (deficient for the type IV secretion system [23]), or fliC mutant (deficient for flagellum expression [24]) of B. melitensis strain 16M. We observed that wild-type and mutant strains displayed similar blood persistence until 3 days p.i. (Fig. 2). We observed a lower CFU count of the VirB mutant in the blood only at 6 and 13 days p.i. No significant differences between wild-type and FliC mutant strains were observed at all time points tested.
FIG 2.
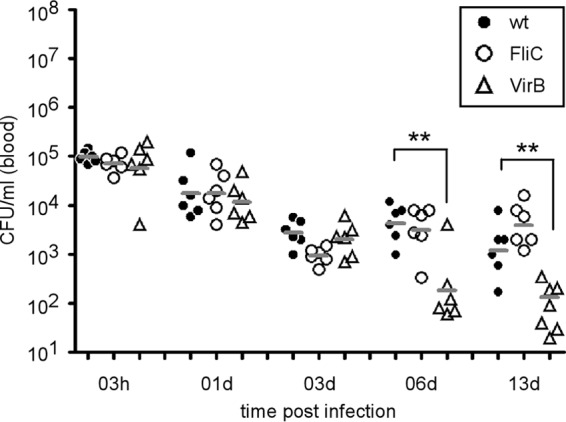
Type IV secretion system (VirB)-deficient and flagellum (FliC)-deficient strains of B. melitensis are able to persist in the blood of infected mice until day 3. Wild-type C57BL/6 mice were injected i.p. with 106 CFU of the wild-type (wt), FliC, or VirB strain of B. melitensis, and blood was collected at the indicated time points. The data represent the CFU per ml of blood of individual mice and are representative of three independent experiments. The gray bars represent the medians. **, P < 0.01.
Vaccination-induced specific antibodies are able to reduce Brucella persistence in the bloodstream.
Some studies have reported that natural IgM antibodies play a crucial role in the early trapping of viral and bacterial particles. This capture reduces pathogen dissemination and infection of organs (33, 34). In order to determine the impact of natural antibodies on the persistence of Brucella in blood, we compared the CFU levels of Brucella in the blood of wild-type and MuMT−/− mice (B cell deficient) infected i.p. with 5 × 107 CFU (Fig. 3A). Our results showed that the complete absence of B cells and antibodies in MuMT−/− mice did not affect the bacterial count in the blood between 1 and 3 days p.i., suggesting that natural antibodies are not able to impair Brucella persistence in the blood in our experimental model.
FIG 3.
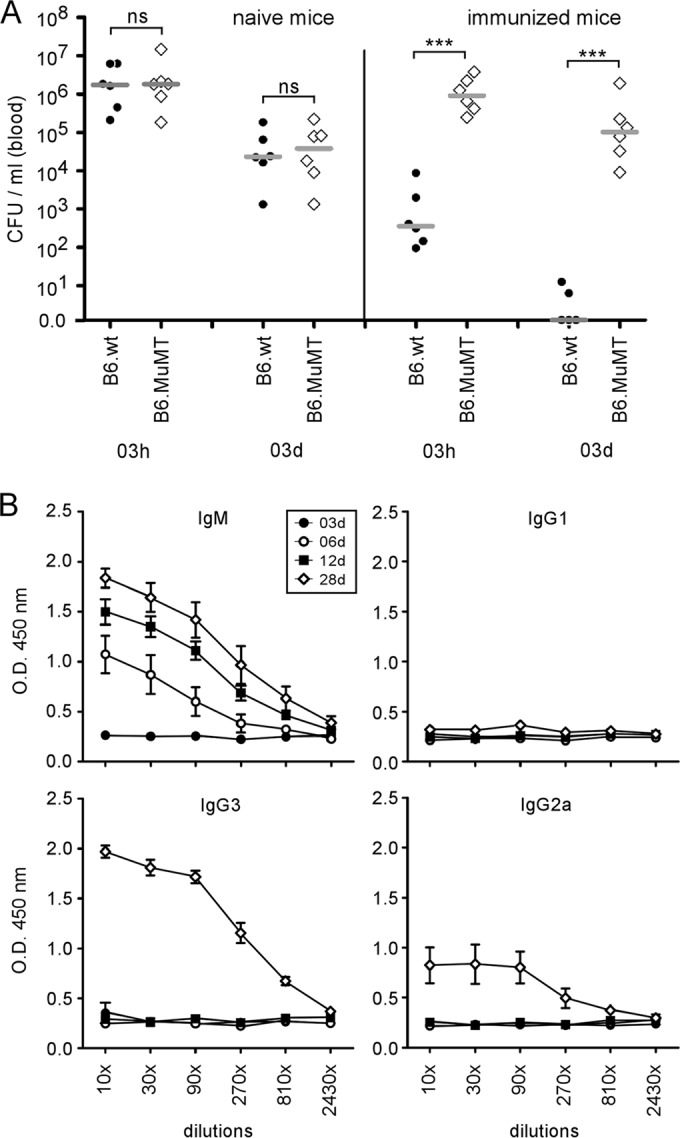
Natural antibodies do not prevent dissemination of B. melitensis in the blood, whereas vaccine-induced specific antibodies reduce the bacteremia following a secondary infection. (A) Wild-type and MuMT−/− C57BL/6 mice were immunized i.p. with 4 × 104 CFU of B. melitensis (vaccinated group) or injected i.p. with PBS (naive group) and then treated with antibiotics for 3 weeks, as described in Materials and Methods. Sixty days after immunization, all mice were injected i.p. with 5 × 107 CFU of mCherry-Br, and blood was collected at the indicated time points. The data represent the CFU per ml of blood and are representative of three independent experiments. The gray bars represent the medians. ***, P < 0.001; ns, not significant. (B) Wild-type C57BL/6 mice were infected with 4 × 104 CFU of B. melitensis. Serum was collected at the indicated time points, and ELISA was performed to determine the isotype distribution of the Brucella-specific antibodies. The data represent the means ± SEM of results for 6 mice. O.D., optical density.
Kinetic analyses of Brucella-specific antibodies in the serum of Brucella-infected mice showed specific IgM antibodies appearing at 6 days, whereas IgG2 and IgG3 antibodies were detectable by 28 days p.i. (Fig. 3B). In order to test the impact of infection-induced specific antibodies on the persistence of Brucella in the blood, mice immunized previously (60 days before) with live virulent bacteria and cured of infection with antibiotics were challenged i.p. with 5 × 107 CFU of mCherry-Br. In agreement with findings of a previous study (35), we observed that the persistence of Brucella in the blood was strongly reduced in wild-type immunized mice but not in MuMT−/− immunized mice, demonstrating that specific antibodies are able to impair the persistence of Brucella in the blood.
Brucella is mostly cell associated in blood.
Given the strong impact of specific antibodies on persistence of Brucella in the blood, its long-term persistence in blood must mean that the bacteria are harbored in an intracellular location in order to escape from circulating antibodies.
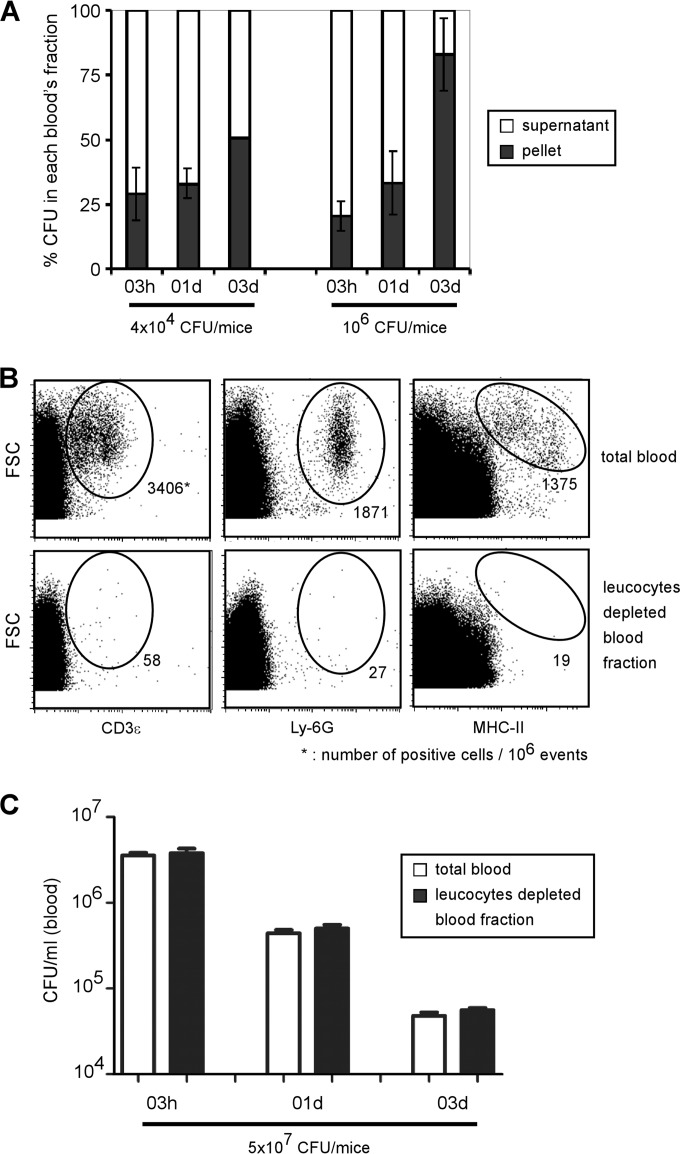
In order to determine the location of Brucella in the blood during infection, we compared the CFU levels in the cellular fraction (pellet) and plasma fraction (supernatant) obtained following centrifugation of blood samples from mice infected with 4 × 104 or 106 CFU (Fig. 4A). At 3 h p.i., the bacteria were found mainly in the plasma (70 to 75%), and only 20 to 30% of the bacteria were found in the pellet. However, 3 days p.i., the percentage in the pellet increased, to reach about 50 to 80%, suggesting that Brucella progressively associates with blood cells.
FIG 4.
Blood location of B. melitensis after i.p. infection. (A) Wild-type C57BL/6 mice were injected i.p. with 4 × 104 or 106 CFU of mCherry-Br, and blood was collected at the indicated time points. Samples were diluted and centrifuged to separate blood cells from plasma. The data represent the mean ± SD of the percentage of CFU in each blood fraction and are representative of two independent experiments. (B and C) Wild-type C57BL/6 mice were injected i.p. with 5 × 107 CFU of mCherry-Br, and blood was collected at the indicated time points. Samples were diluted and immediately filtered with Plasmodipur filters. (B) Total blood cells and purified erythrocytes were analyzed by flow cytometry using anti-CD3ε, anti-Ly-6G, and anti-MHC-II antibodies in order to confirm the elimination of all leukocytes. (C) The data represent the CFU per ml of total blood or purified erythrocytes and are representative of two independent experiments with 3 mice per group.
Brucella has been mainly found to infect myeloid cells in vivo in mice (21, 36, 37). We therefore postulated that elimination of leukocytes should result in a reduction in the level of CFU. Using Plasmodipur filters (26, 27), we purified erythrocytes from mice infected with 5 × 107 CFU at different times after infection. Flow cytometry analysis confirmed the drastic elimination of all CD3+ (T cells), Ly-6G+ (neutrophils), and MHC-II+ (monocytes and B cells) leukocytes after filtration (Fig. 4B). Surprisingly, total blood cells and purified erythrocytes displayed similar CFU levels at all times tested (Fig. 4C), suggesting that bacteria are associated with the erythrocyte-enriched fraction rather than with leukocytes. In order to test the ability of erythrocytes to transfer brucellosis, naive C57BL/6 mice were injected i.p. with purified erythrocytes from 3-day 5 × 107 CFU-infected mice. Two weeks later, all i.p. injected mice displayed CFU counts in the spleen, confirming that purified erythrocytes are able to transfer infectious B. melitensis in mice (data not shown).
Brucella associates preferentially with erythrocytes in blood.
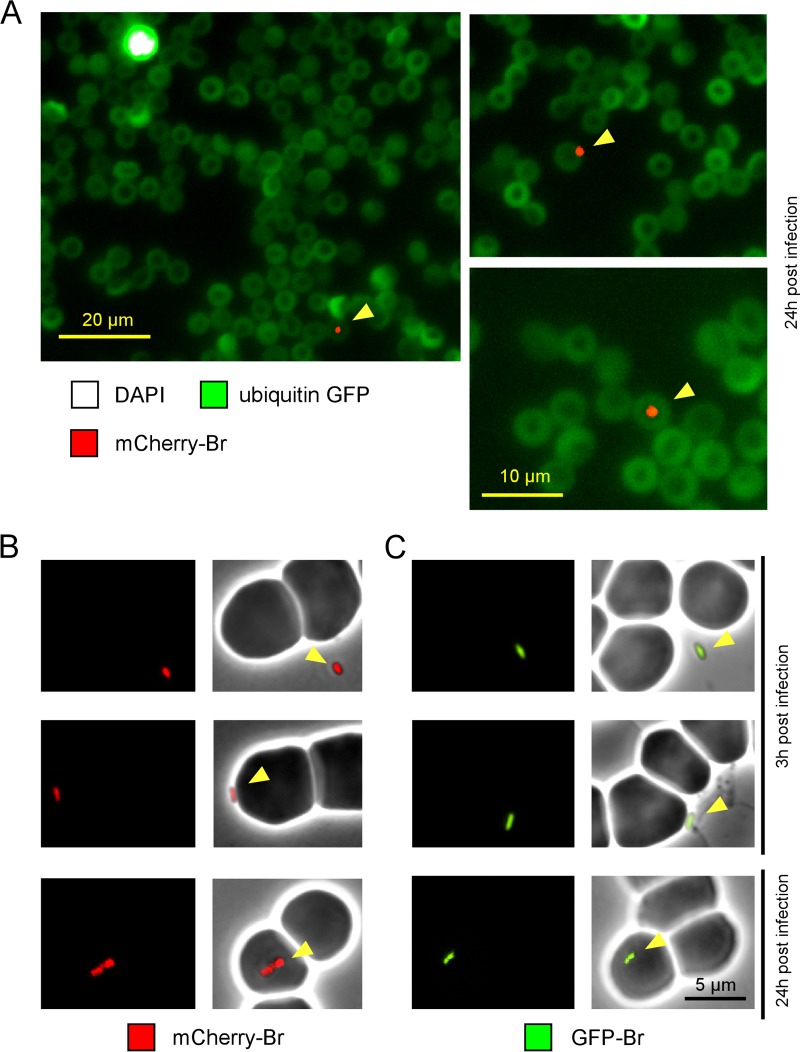
In order to clearly establish the location of Brucella in blood during infection, we performed fluorescence microscopic analyses of blood samples from infected mice. mCherry-expressing Brucella (mCherry-Br) (5 × 107 CFU) was injected i.p. into transgenic C57BL/6 mice expressing GFP under the control of the ubiquitin promoter (17), and blood was collected at 3 and 24 h p.i. Note that the collected blood was immediately diluted in paraformaldehyde (PFA) for fixation without centrifugation or staining in order to avoid artifacts. Thirty minutes after fixation, blood was smeared on slides and air dried overnight, and DAPI staining was performed with mounting medium.
Interestingly, whatever the time point tested, microscopic observations never showed the binding of Brucella to nucleated (DAPI+) cells. In agreement with results shown in Fig. 4C, Brucella was found either free or in close association with the erythrocytes at 3 h p.i. (35 free and 45 associated out of 80 bacteria observed). At 24 h, Brucella was mainly found associated with the erythrocytes (8 free and 52 associated out of 60 bacteria observed; several examples of association are shown in Fig. 5A). These observations were confirmed in the absence of fixation and mounting medium with mCherry-Br (Fig. 5B) and GFP-expressing Brucella (GFP-Br) (Fig. 5C).
FIG 5.
Immunofluorescence microscopy of fixed or live bacteria. (A) Ubiquitin-GFP C57BL/6 mice were infected i.p. with 5 × 107 CFU of mCherry-Br and bled at 3 h and 24 h p.i. The blood was fixed and then smeared on slides. The slides were dried overnight and then mounted in Fluoro-Gel medium containing DAPI nucleic acid stain (Electron Microscopy Sciences, Hatfield, PA) and examined under a fluorescence microscope. Brucella was found associated with erythrocytes mainly at 24 h, and thus we present only images starting at 24 h p.i. The figure shows examples of mCherry bacteria associated with DAPI- erythrocytes. The panels are color coded according to the antigen examined. Scale bar = 20 or 10 μm, as indicated. The data are representative of at least 3 independent experiments with a total of more 200 bacteria observed. (B and C) Wild-type C57BL/6 mice were infected with 5 × 107 CFU of mCherry-Br (B) or GFP-Br (C). Blood samples were harvested at 3 h and 24 h p.i. and immediately diluted 10× in PBS. Freshly harvested blood samples were directly dropped on an agarose pad and sealed with VALAP before observation under a fluorescence microscope in a biosafety level III laboratory facility. The data are representative of at least 3 independent experiments, with a total of more than 30 bacteria observed under each condition.
In order to more rigorously and more easily determine the location of Brucella in the blood, we performed a multispectral imaging flow cytometry (ImageStream) analysis (Fig. 6) of blood samples from wild-type-infected mice. This technique allowed us to analyze 100,000 cellular events per blood sample. Note that, as previously, blood was immediately diluted in PFA for fixation without centrifugation to avoid cell lysis or infection.
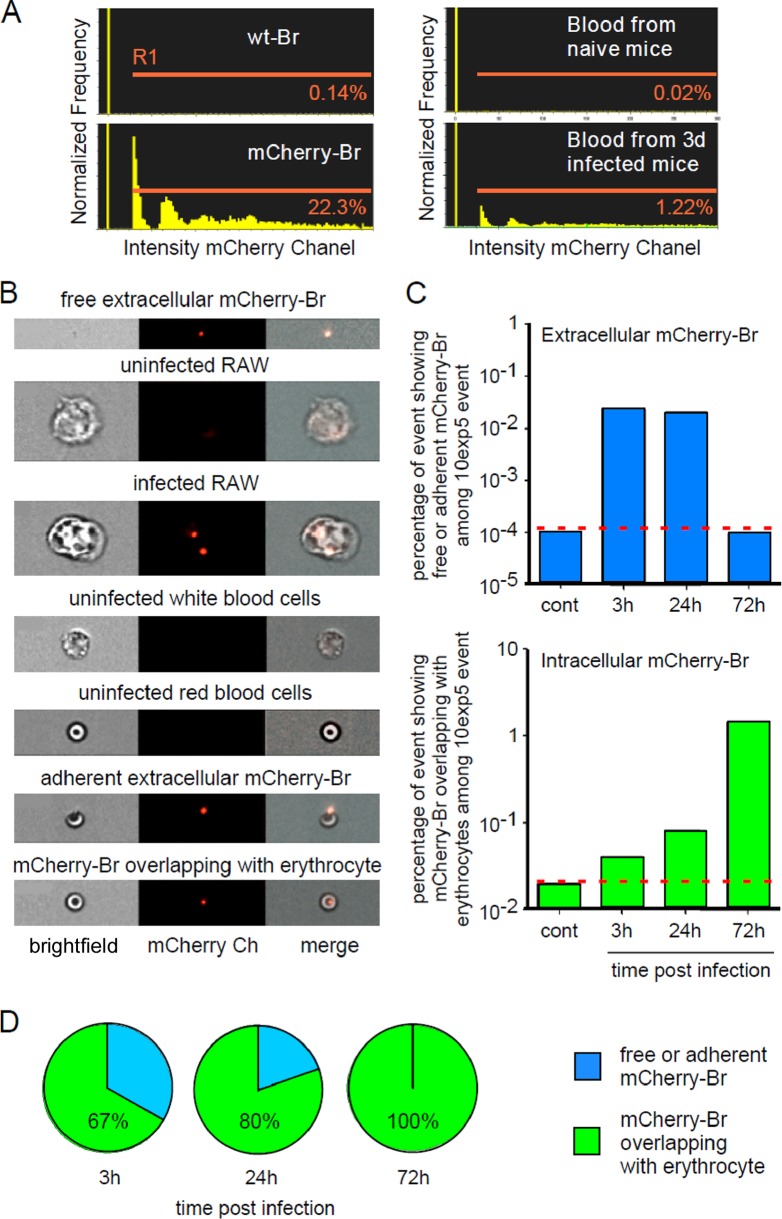
FIG 6.
ImageStream analysis of blood from infected mice. Wild-type C57BL/6 mice were injected i.p. with 5 × 107 CFU of mCherry-Br, and blood was collected at 3 h, 24 h, and 72 h p.i. The blood samples were immediately fixed by 3-fold dilution in 4% paraformaldehyde and then analyzed by multispectral imaging flow cytometry (ImageStream 100). As controls, we used a fresh culture of wild-type Brucella, mCherry-Br, and a macrophage cell line (RAW cells) at 3 h post-in vitro infection with mCherry-Br. (A) Comparison of the percentage of events displaying a positive mCherry (channel 4) fluorescent signal (gate R1) in blood from control and infected mice. (B) Representative images of free bacteria and uninfected and infected cells in channels 2 and 4. (C and D) Kinetic analysis of the percentages of free or adherent bacteria (in blue) and bacteria overlapping with cells (in green) found in blood samples from control and infected mice. The location of bacteria was defined by erosion mask. These data are representative of three independent experiments.
In a preliminary experiment, we confirmed that ImageStream analysis was able to detect mCherry-Br fluorescence in blood samples from infected mice (Fig. 6A). We also tested whether ImageStream is able to discriminate, based on morphological and fluorescent parameters, between free and adherent extracellular mCherry-Br blood cells and between uninfected and infected RAW cells (Fig. 6B). In these experiments, uninfected RAW cells and RAW cells infected in vitro with mCherry-Br were used as internal controls to demonstrate that we are able to visualize Brucella inside phagocytic nucleated cells.
Unfortunately, we observed that the low fluorescence of mCherry-Br does not allow us to perform cell surface staining (data not shown). Consequently, cells were identified based only on morphological aspects, but, as illustrated in Fig. 6B, white and red blood cells are easily distinguishable. We classified Brucella as “free,” “adherent,” or “overlapping with a cell” using a combination of the erosion mask applied on the bright-field image and the intensity mask in the specific mCherry channel (see Materials and Methods). Examples of “free,” “adherent,” and “overlapping with a cell” Brucella are showed in Fig. 6B.
Kinetic ImageStream analysis of infected blood (Fig. 6C) showed that some bacteria were found extracellularly (free or adhering to cells) at 3 and 24 h p.i. (±0.05% of the total acquired events) but not at 72 h p.i. In contrast, the percentage of bacteria found that overlapped with erythrocytes increased exponentially until 72 h p.i. (up to 1.5% of the total acquired events). Independently of the time point tested, we never observed binding or overlapping of Brucella with white blood cells or several bacteria inside erythrocytes.
In summary, ImageStream analysis showed that following a brief free extracellular stage, Brucella rapidly associated with erythrocytes. At 3 h p.i., 67% of the Brucella events were already identified as “Brucella overlapping with erythrocytes” (Fig. 6D). At 24 h and 72 h p.i., this percentage peaked at 80 and 100%, respectively (Fig. 6D).
We have carefully examined the possibility that phagocytosis causes alteration of the mCherry signal and therefore hampers the detection of mCherry-Br in phagocytic cells, such as neutrophils and monocytes. In our experimental model, ImageStream was able to detect mCherry-Br inside infected RAW macrophages. A previous study by our group demonstrated that mCherry-Br was also detectable inside inducible nitric oxide synthase (iNOS)-expressing inflammatory dendritic cells in situ and that the mCherry signal is well correlated with the detection of Brucella antigen by antibodies (21). Together, these data strongly suggest that our experimental procedures allow us to detect mCherry-Br-infected phagocytic cells in the bloodstream. However, we cannot formally exclude the possibility that phagocytic cells represent a minor fraction of infected cells in the bloodstream.
Fluorescent and electron microscopic analysis demonstrated the in vivo invasion of erythrocytes by Brucella.
ImageStream analysis allowed us to visualize a great number of blood cells, but the image resolution was poor and the images of bacteria overlapping with erythrocytes did not formally prove the intracellular location of Brucella inside erythrocytes.
In order to investigate this point, we performed scanning electron microscopic and confocal microscopic analyses of blood samples from infected mice.
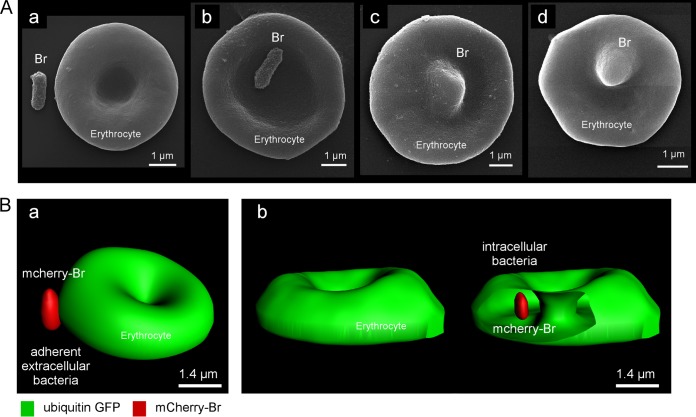
Scanning electron microscopy of blood from 24 h infected mice (Fig. 7A) showed that Brucella was either found free in the blood (a) or adherent to erythrocytes (b). We also observed deformed erythrocytes (Fig. 7A, c and d), suggesting the presence of Brucella inside the erythrocytes. Using confocal microscopy, we analyzed blood samples from 24-h-mCherry-Br-infected ubiquitin-GFP mice. A Z-stack analysis was performed on erythrocytes presenting adherent mCherry-Br or a clear overlap with mCherry-Br. These images were processed for 3D reconstruction using the Imaris program (Fig. 7B). We confirmed by this technique that in all cases (n = 10) of clear overlap selected, bacteria were found inside the erythrocytes.
FIG 7.
Brucella is found associated with or inside erythrocytes by scanning electron and confocal microscopy. (A) Scanning electron microscopy images from blood collected in mice 3 h p.i. by i.p. injection of 5 × 107 CFU of Brucella. Extracellular bacteria (a), attachment of Brucella to erythrocytes (b), and deformed erythrocytes, suggesting Brucella infection (c and d), are shown. (B) Confocal microscopy images of blood collected in mice 24 h p.i. by i.p. injection of 5 × 107 CFU of Brucella. Attachment of Brucella to erythrocytes (a) and Brucella inside erythrocytes (b) are shown.
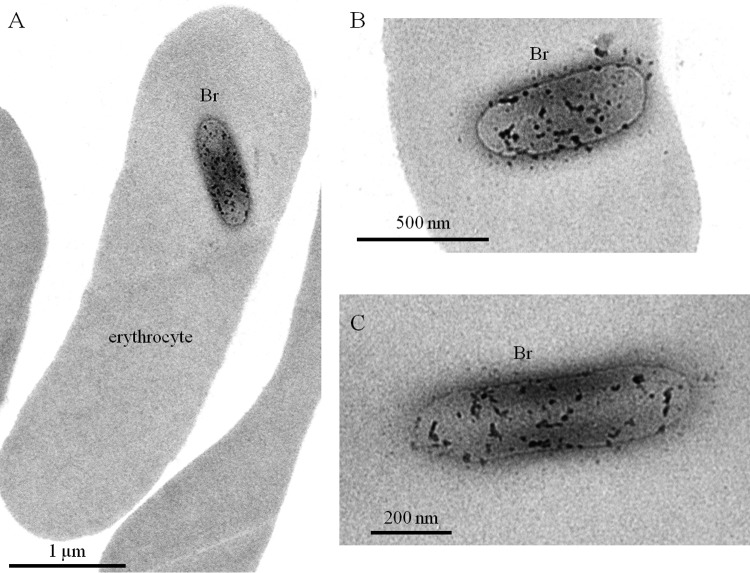
Brucella is known to cause extramedullary hematopoiesis (38), resulting in the appearance of large numbers of immature blood cells in the spleen. In order to exclude all doubts regarding the capacity of Brucella to infect mature erythrocytes in vivo and to gain more information on the mechanism(s) of invasion, we performed transmission electron microscopic (TEM) analyses of blood cells from 24-h-infected mice. The images (Fig. 8A) confirmed that the infected blood cells were mature erythrocytes, since all infected cells observed (n = 16) never displayed nuclei and organelles. Intraerythrocytic Brucella did not appear surrounded by a vacuole (Fig. 8B and C), suggesting that these bacteria were free in the cytoplasm of the erythrocytes.
FIG 8.
Brucella is not surrounded by a vacuole in erythrocytes. Transmission electron microscopy analysis of erythrocytes from mice 24 h post-infection by i.p. injection of 5 × 107 CFU of Brucella.
DISCUSSION
We used ImageStream analysis, confocal microscopy, and electron microscopy to provide the first demonstration of erythrocyte invasion during the persistence of B. melitensis in the bloodstream following intraperitoneal infection.
In vitro studies have shown that Brucella can invade and replicate in a range of nonphagocytic cells (trophoblasts, epithelial cells, and fibroblasts) (39–42). However, until now, the virulence of Brucella in vivo has been associated only with its ability to invade and replicate in phagocytic cells, such as macrophages and dendritic cells (21, 36, 37). To our knowledge, our results establish for the first time the persistence of Brucella within erythrocytes of infected mice. Surprisingly, during our study, we never observed the association of Brucella with phagocytic white blood cells, and depletion of leukocytes using Plasmodipur filters did not reduce the ability of blood from infected mice to transfer infection in naive mice. Our internal control, RAW infected cells, suggested that phagocytosis did not impair the detection of mCherry under our experimental conditions. In addition, a previous study showed that mCherry-Br can be detected in vivo in activated iNOS+ macrophages and inflammatory dendritic cells (21). A study by Braude et al. dating from 1951 (13) reported the phagocytosis of Brucella abortus by polymorphonuclear leukocytes in the blood of mice 3 h after the intraperitoneal inoculation of 1012 CFU. However, this observation may have been an artifact due to the injection of a very high dose of bacteria, 1,000-fold superior to the 109-CFU lethal dose in mice. In conclusion, our study does not exclude that white blood cells could be infected during the course of infection by Brucella melitensis but strongly suggests that in the model used, erythrocytes are the cells most frequently infected in the blood.
Gentamicin treatment is frequently used to discriminate between intra- and extracellular bacterium location. We have performed a brief (1 h, 4°C) gentamicin treatment at different concentrations with free bacteria and blood samples from mice infected for 3 or 72 h. Note that the samples were immediately plated after treatment without centrifugation to avoid erythrocyte lysis. Our data showed that bacteria in the “72 h blood” group are more resistant than either free bacteria or bacteria from “3 h blood” groups for all gentamicin concentrations tested. We observed that >50% of the bacteria in the “72 h blood” group survived to the treatment with 200 μg/ml of gentamicin. In contrast, only around 10% resisted in other groups (data not shown). Gentamicin is generally presented as an antibiotic that acts only on extracellular bacteria, but this is an oversimplification. Several studies have demonstrated that gentamicin can kill intracellular bacteria inside macrophages (43). In addition, pharmacokinetic studies have shown that gentamicin slowly penetrates human erythrocytes (44). We suspect that infected erythrocytes may be more fragile and susceptible to being lysed during in vitro incubation. This can explain why bacteria, even when located inside erythrocytes, remain partially sensitive to gentamicin in our model.
In the model of erythrocyte infection by Bartonella spp., persistence in blood and erythrocyte invasion have been reported to be drastically reduced in the absence of a functional type IV secretion system (29, 30) or flagella (31, 32). In our Brucella model of infection, a bacterial strain deficient for flagella (FliC mutant) was able to persist in the blood at a level that was similar to that of the wild-type strain, thus demonstrating that flagella are not implicated in blood persistence and presumably in erythrocyte invasion. Deficiency for the type IV secretion system (VirB mutant) did not affect early persistence of bacteria in the blood. Only blood CFU levels appeared to be reduced by 1 log at a later time point. Since persistence of the VirB mutant in the spleen is known to be reduced at a later time point (23; data not shown), we can only conclude that the type IV secretion system is not critically implicated in blood persistence, since this strain remains able to persist until 13 days p.i., in contrast to the complete inability of Bartonella type IV secretion system mutants to cause bacteremia (29).
We also investigated whether the control of B. melitensis persistence in the blood involves circulating antibodies and B cells. Some studies have reported that natural antibodies play an important protective function in the control of early dissemination of viruses (vesicular stomatitis virus, vaccinia virus, and lymphocytic choriomeningitis virus) and facultative intracellular bacteria (Listeria monocytogenes) (33, 34). Natural antibodies could increase the early trapping of pathogens in secondary lymphoid organs by enhancing phagocytosis. Previously, several groups have analyzed the role of natural antibodies in an experimental model of Brucella, with contradictory results. Natural antibodies have been reported to promote the control of an attenuated Brucella mutant by facilitating its elimination by phagocytosis (45). Many serum transfer experiments have demonstrated that antibodies play a positive role in protecting mice against Brucella infection (46–48). In this study, we were unable to detect Brucella-specific antibodies in naive mice or during the 3 first days p.i. Specific IgM antibodies were first detected at 6 days and IgG antibodies at 28 days, with a predominance of the IgG2a and IgG3 isotypes. This observation and the fact that the numbers of bacteria found in the bloodstream during infection appear to be similar in wild-type and MuMT−/− mice demonstrated that natural antibodies had no or only a minor impact on Brucella trapping in naive mice under our experimental conditions. In contrast, we observed that specific circulating antibodies present in vaccinated wild-type mice dramatically reduced the bacterial count persisting in the blood after a secondary infection. This suggests that the blockage of Brucella erythrocyte colonization could constitute an important step in the protective immune response against Brucella infection.
Although erythrocytes do not appear to constitute a replication niche for Brucella, since each infected cell contained only a single bacterium, they could serve as an important part of the pathogen reservoir. Given the long life-span of erythrocytes (49), Brucella could potentially persist hidden in this environment for several months. The undulating fever characteristic of human brucellosis and the high risk of relapse also argue for regularly occurring new bacteremia waves. In this context, the failure to replicate within erythrocytes could be advantageous for Brucella.
Of course, our data were obtained using the murine experimental model of infection with Brucella melitensis, and their relevance to other Brucella species and in the natural host remains to be demonstrated. However, bacteremia is common in human brucellosis (11), and an in vitro study has reported that Brucella is able to bind to sialic acid residues on human erythrocytes (50), suggesting that erythrocyte invasion could also occur in patients.
From an evolutionary point of view, the unexpected ability of Brucella to infect erythrocytes is not so surprising. Indeed, several bacterial pathogens belonging to the Alphaproteobacteria (Bartonella spp. [28] and Anaplasma marginale [51]) and Gammaproteobacteria (Francisella tularensis [52, 53]) phyla persist inside erythrocytes in the bloodstream during infection and are transmitted to the host by ticks or fleas. Numerous old studies (5, 54–57) and a study published in 2013 (58) have documented the presence of Brucella in bloodsucking arthropods (ticks and fleas) under natural conditions. These observations, combined with our demonstration of the ability of B. melitensis to infect erythrocytes in vivo, render plausible the hypothesis that under natural conditions, erythrocytes could constitute an alternative reservoir for Brucella and that Brucella could be transmitted to the host by bloodsucking arthropods. We hope that this hypothesis will be investigated in the future.
In conclusion, invasion of erythrocytes could constitute a crucial unexpected step that promotes the evasion of host immune defenses. This new trait of Brucella pathogenesis opens up new areas of research. Since erythrocytes are not capable of endocytosis (59), we will now need to define the precise mechanism of Brucella uptake by the cell and identify the virulence factors necessary for this process. We have tested several in vitro protocols to infect erythrocytes from naive mice. Unfortunately, under conditions where RAW cells were fully infected, we never observed erythrocyte infection (data not shown). In addition, it would be interesting to investigate the homing of infected erythrocytes in tissues and whether they trigger a different immune response than other infected cells. We are thus left with the exciting challenge of determining the importance of this mechanism to pathogenesis and transmission of the infection.
ACKNOWLEDGMENTS
This work was supported by grants from the Fonds National de la Recherche Scientifique (FNRS) (FRSM FNRS convention 3.4.600.06.F, Belgium), from the Communauté Française de Belgique (Action de Recherches Concertées 08/13-015), and from the Interuniversity Attraction Poles Programme initiated by the Belgian Science Policy Office (http://www.belspo.be/belspo/iap/index_en.stm). E.M. is a research associate from the FRS-FNRS (Belgium). M.-A.V. and D.H.M. hold Ph.D. grants from the FRS-FNRS (Belgium). A.M. and M.D. hold Ph.D. grants from the FRIA (Belgium).
The funding agencies played no role in the design of the study, collection and analysis of the data, decision to publish, or preparation of the manuscript.
Footnotes
Published ahead of print 7 July 2014
J.-J.L. and E.M. are joint senior authors of this work.
REFERENCES
- 1.Godfroid J, Cloeckaert A, Liautard JP, Kohler S, Fretin D, Walravens K, Garin-Bastuji B, Letesson JJ. 2005. From the discovery of the Malta fever's agent to the discovery of a marine mammal reservoir, brucellosis has continuously been a re-emerging zoonosis. Vet. Res. 36:313–326. 10.1051/vetres:2005003 [DOI] [PubMed] [Google Scholar]
- 2.Cutler SJ, Whatmore AM, Commander NJ. 2005. Brucellosis—new aspects of an old disease. J. Appl. Microbiol. 98:1270–1281. 10.1111/j.1365-2672.2005.02622.x [DOI] [PubMed] [Google Scholar]
- 3.Pappas G, Papadimitriou P, Akritidis N, Christou L, Tsianos EV. 2006. The new global map of human brucellosis. Lancet Infect. Dis. 6:91–99. 10.1016/S1473-3099(06)70382-6 [DOI] [PubMed] [Google Scholar]
- 4.Gregoire F, Mousset B, Hanrez D, Michaux C, Walravens K, Linden A. 2012. A serological and bacteriological survey of brucellosis in wild boar (Sus scrofa) in Belgium. BMC Vet. Res. 8:80. 10.1186/1746-6148-8-80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zheludkov MM, Tsirel'son LE. 2010. Reservoirs of Brucella infection in nature. Biol. Bull. 37:709–715. 10.1134/S106235901007006X [DOI] [Google Scholar]
- 6.Solera J, Martinez-Alfaro E, Espinosa A, Castillejos ML, Geijo P, Rodriguez-Zapata M. 1998. Multivariate model for predicting relapse in human brucellosis. J. Infect. 36:85–92. 10.1016/S0163-4453(98)93342-4 [DOI] [PubMed] [Google Scholar]
- 7.Ariza J, Corredoira J, Pallares R, Viladrich PF, Rufi G, Pujol M, Gudiol F. 1995. Characteristics of and risk factors for relapse of brucellosis in humans. Clin. Infect. Dis. 20:1241-1249. 10.1093/clinids/20.5.1241 [DOI] [PubMed] [Google Scholar]
- 8.Schurig GG, Sriranganathan N, Corbel MJ. 2002. Brucellosis vaccines: past, present and future. Vet. Microbiol. 90:479–496. 10.1016/S0378-1135(02)00255-9 [DOI] [PubMed] [Google Scholar]
- 9.Martirosyan A, Moreno E, Gorvel JP. 2011. An evolutionary strategy for a stealthy intracellular Brucella pathogen. Immunol. Rev. 240:211–234. 10.1111/j.1600-065X.2010.00982.x [DOI] [PubMed] [Google Scholar]
- 10.Yagupsky P. 1999. Detection of Brucellae in blood cultures. J. Clin. Microbiol. 37:3437–3442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pappas G, Papadimitriou P. 2007. Challenges in Brucella bacteraemia. Int. J. Antimicrob. Agents 30(Suppl 1):S29–S31. 10.1016/j.ijantimicag.2007.06.011 [DOI] [PubMed] [Google Scholar]
- 12.Rajashekara G, Glover DA, Krepps M, Splitter GA. 2005. Temporal analysis of pathogenic events in virulent and avirulent Brucella melitensis infections. Cell Microbiol. 7:1459–1473. 10.1111/j.1462-5822.2005.00570.x [DOI] [PubMed] [Google Scholar]
- 13.Braude AI. 1951. Studies in the pathology and pathogenesis of experimental brucellosis. II. The formation of the hepatic granuloma and its evolution. J. Infect. Dis. 89:87–94 [DOI] [PubMed] [Google Scholar]
- 14.Kocan KM, de la Fuente J, Guglielmone AA, Melendez RD. 2003. Antigens and alternatives for control of Anaplasma marginale infection in cattle. Clin. Microbiol. Rev. 16:698–712. 10.1128/CMR.16.4.698-712.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harms A, Dehio C. 2012. Intruders below the radar: molecular pathogenesis of Bartonella spp. Clin. Microbiol. Rev. 25:42–78. 10.1128/CMR.05009-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kitamura D, Roes J, Kuhn R, Rajewsky K. 1991. A B cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin mu chain gene. Nature 350:423–426. 10.1038/350423a0 [DOI] [PubMed] [Google Scholar]
- 17.Schaefer BC, Schaefer ML, Kappler JW, Marrack P, Kedl RM. 2001. Observation of antigen-dependent CD8+ T-cell/dendritic cell interactions in vivo. Cell Immunol. 214:110–122. 10.1006/cimm.2001.1895 [DOI] [PubMed] [Google Scholar]
- 18.Copin R, De Baetselier P, Carlier Y, Letesson JJ, Muraille E. 2007. MyD88-dependent activation of B220-CD11b+LY-6C+ dendritic cells during Brucella melitensis Infection. J. Immunol. 178:5182–5191. 10.4049/jimmunol.178.8.5182 [DOI] [PubMed] [Google Scholar]
- 19.Shaner NC, Campbell RE, Steinbach PA, Giepmans BN, Palmer AE, Tsien RY. 2004. Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein. Nat. Biotechnol. 22:1567–1572. 10.1038/nbt1037 [DOI] [PubMed] [Google Scholar]
- 20.Kohler S, Ouahrani-Bettache S, Layssac M, Teyssier J, Liautard JP. 1999. Constitutive and inducible expression of green fluorescent protein in Brucella suis. Infect. Immun. 67:6695–6697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Copin R, Vitry MA, Hanot Mambres D, Machelart A, De Trez C, Vanderwinden JM, Magez S, Akira S, Ryffel B, Carlier Y, Letesson JJ, Muraille E. 2012. In situ microscopy analysis reveals local innate immune response developed around Brucella infected cells in resistant and susceptible mice. PLoS Pathog. 8:e1002575. 10.1371/journal.ppat.1002575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ouahrani-Bettache S, Porte F, Teyssier J, Liautard JP, Kohler S. 1999. pBBR1-GFP: a broad-host-range vector for prokaryotic promoter studies. Biotechniques 26:620–622 [DOI] [PubMed] [Google Scholar]
- 23.Delrue RM, Martinez-Lorenzo M, Lestrate P, Danese I, Bielarz V, Mertens P, De Bolle X, Tibor A, Gorvel JP, Letesson JJ. 2001. Identification of Brucella spp. genes involved in intracellular trafficking. Cell Microbiol. 3:487–497. 10.1046/j.1462-5822.2001.00131.x [DOI] [PubMed] [Google Scholar]
- 24.Leonard S, Ferooz J, Haine V, Danese I, Fretin D, Tibor A, de Walque S, De Bolle X, Letesson JJ. 2007. FtcR is a new master regulator of the flagellar system of Brucella melitensis 16M with homologs in Rhizobiaceae. J. Bacteriol. 189:131–141. 10.1128/JB.00712-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sathiyaseelan J, Goenka R, Parent M, Benson RM, Murphy EA, Fernandes DM, Foulkes AS, Baldwin CL. 2006. Treatment of Brucella-susceptible mice with IL-12 increases primary and secondary immunity. Cell Immunol. 243:1–9. 10.1016/j.cellimm.2006.10.003 [DOI] [PubMed] [Google Scholar]
- 26.Auburn S, Campino S, Clark TG, Djimde AA, Zongo I, Pinches R, Manske M, Mangano V, Alcock D, Anastasi E, Maslen G, Macinnis B, Rockett K, Modiano D, Newbold CI, Doumbo OK, Ouedraogo JB, Kwiatkowski DP. 2011. An effective method to purify Plasmodium falciparum DNA directly from clinical blood samples for whole genome high-throughput sequencing. PLoS One 6:e22213. 10.1371/journal.pone.0022213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sriprawat K, Kaewpongsri S, Suwanarusk R, Leimanis ML, Lek-Uthai U, Phyo AP, Snounou G, Russell B, Renia L, Nosten F. 2009. Effective and cheap removal of leukocytes and platelets from Plasmodium vivax infected blood. Malar. J. 8:115. 10.1186/1475-2875-8-115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dehio C. 2001. Bartonella interactions with endothelial cells and erythrocytes. Trends Microbiol. 9:279–285. 10.1016/S0966-842X(01)02047-9 [DOI] [PubMed] [Google Scholar]
- 29.Schulein R, Dehio C. 2002. The VirB/VirD4 type IV secretion system of Bartonella is essential for establishing intraerythrocytic infection. Mol. Microbiol. 46:1053–1067. 10.1046/j.1365-2958.2002.03208.x [DOI] [PubMed] [Google Scholar]
- 30.Vayssier-Taussat M, Le Rhun D, Deng HK, Biville F, Cescau S, Danchin A, Marignac G, Lenaour E, Boulouis HJ, Mavris M, Arnaud L, Yang H, Wang J, Quebatte M, Engel P, Saenz H, Dehio C. 2010. The Trw type IV secretion system of Bartonella mediates host-specific adhesion to erythrocytes. PLoS Pathog. 6:e1000946. 10.1371/journal.ppat.1000946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Benson LA, Kar S, McLaughlin G, Ihler GM. 1986. Entry of Bartonella bacilliformis into erythrocytes. Infect. Immun. 54:347–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scherer DC, DeBuron-Connors I, Minnick MF. 1993. Characterization of Bartonella bacilliformis flagella and effect of antiflagellin antibodies on invasion of human erythrocytes. Infect. Immun. 61:4962–4971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ochsenbein AF, Fehr T, Lutz C, Suter M, Brombacher F, Hengartner H, Zinkernagel RM. 1999. Control of early viral and bacterial distribution and disease by natural antibodies. Science 286:2156–2159. 10.1126/science.286.5447.2156 [DOI] [PubMed] [Google Scholar]
- 34.Boes M. 2000. Role of natural and immune IgM antibodies in immune responses. Mol. Immunol. 37:1141–1149. 10.1016/S0161-5890(01)00025-6 [DOI] [PubMed] [Google Scholar]
- 35.Vitry MA, Hanot Mambres D, De Trez C, Akira S, Ryffel B, Letesson JJ, Muraille E. 2014. Humoral immunity and CD4+ Th1 cells are both necessary for a fully protective immune response upon secondary infection with Brucella melitensis. J. Immunol. 192:3740–3752. 10.4049/jimmunol.1302561 [DOI] [PubMed] [Google Scholar]
- 36.Billard E, Cazevieille C, Dornand J, Gross A. 2005. High susceptibility of human dendritic cells to invasion by the intracellular pathogens Brucella suis, B. abortus, and B. melitensis. Infect. Immun. 73:8418–8424. 10.1128/IAI.73.12.8418-8424.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Archambaud C, Salcedo SP, Lelouard H, Devilard E, de Bovis B, Van Rooijen N, Gorvel JP, Malissen B. 2010. Contrasting roles of macrophages and dendritic cells in controlling initial pulmonary Brucella infection. Eur. J. Immunol. 40:3458–3471. 10.1002/eji.201040497 [DOI] [PubMed] [Google Scholar]
- 38.Enright FM, Araya LN, Elzer PH, Rowe GE, Winter AJ. 1990. Comparative histopathology in BALB/c mice infected with virulent and attenuated strains of Brucella abortus. Vet. Immunol. Immunopathol. 26:171–182. 10.1016/0165-2427(90)90065-Z [DOI] [PubMed] [Google Scholar]
- 39.Anderson TD, Meador VP, Cheville NF. 1986. Pathogenesis of placentitis in the goat inoculated with Brucella abortus. I. Gross and histologic lesions. Vet. Pathol. 23:219–226 [DOI] [PubMed] [Google Scholar]
- 40.Detilleux PG, Deyoe BL, Cheville NF. 1990. Penetration and intracellular growth of Brucella abortus in nonphagocytic cells in vitro. Infect. Immun. 58:2320–2328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pizarro-Cerda J, Meresse S, Parton RG, van der Goot G, Sola-Landa A, Lopez-Goni I, Moreno E, Gorvel JP. 1998. Brucella abortus transits through the autophagic pathway and replicates in the endoplasmic reticulum of nonprofessional phagocytes. Infect. Immun. 66:5711–5724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Holland JJ, Pickett MJ. 1956. Intracellular behavior of Brucella variants in chick embryo cells in tissue culture. Proc. Soc. Exp. Biol. Med. 93:476–479. 10.3181/00379727-93-22792 [DOI] [PubMed] [Google Scholar]
- 43.Drevets DA, Canono BP, Leenen PJ, Campbell PA. 1994. Gentamicin kills intracellular Listeria monocytogenes. Infect. Immun. 62:2222–2228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rolain JM, Maurin M, Mallet MN, Parzy D, Raoult D. 2003. Culture and antibiotic susceptibility of Bartonella quintana in human erythrocytes. Antimicrob. Agents Chemother. 47:614–619. 10.1128/AAC.47.2.614-619.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rolan HG, Xavier MN, Santos RL, Tsolis RM. 2009. Natural antibody contributes to host defense against an attenuated Brucella abortus virB mutant. Infect. Immun. 77:3004–3013. 10.1128/IAI.01114-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Winter AJ, Duncan JR, Santisteban CG, Douglas JT, Adams LG. 1989. Capacity of passively administered antibody to prevent establishment of Brucella abortus infection in mice. Infect. Immun. 57:3438–3444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Araya LN, Elzer PH, Rowe GE, Enright FM, Winter AJ. 1989. Temporal development of protective cell-mediated and humoral immunity in BALB/c mice infected with Brucella abortus. J. Immunol. 143:3330–3337 [PubMed] [Google Scholar]
- 48.Montaraz JA, Winter AJ. 1986. Comparison of living and nonliving vaccines for Brucella abortus in BALB/c mice. Infect. Immun. 53:245–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shemin D, Rittenberg D. 1946. The life span of the human red blood cell. J. Biol. Chem. 166:627–636 [PubMed] [Google Scholar]
- 50.Rocha-Gracia RDC, Castaneda-Roldan EI, Giono-Cerezo S, Giron JA. 2002. Brucella sp. bind to sialic acid residues on human and animal red blood cells. FEMS Microbiol. Lett. 213:219–224. 10.1111/j.1574-6968.2002.tb11309.x [DOI] [PubMed] [Google Scholar]
- 51.de la Fuente J, Garcia-Garcia JC, Blouin EF, Saliki JT, Kocan KM. 2002. Infection of tick cells and bovine erythrocytes with one genotype of the intracellular ehrlichia Anaplasma marginale excludes infection with other genotypes. Clin. Diagn. Lab. Immunol. 9:658–668. 10.1128/CDLI.9.3.658-668.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Forestal CA, Malik M, Catlett SV, Savitt AG, Benach JL, Sellati TJ, Furie MB. 2007. Francisella tularensis has a significant extracellular phase in infected mice. J. Infect. Dis. 196:134–137. 10.1086/518611 [DOI] [PubMed] [Google Scholar]
- 53.Horzempa J, O'Dee DM, Stolz DB, Franks JM, Clay D, Nau GJ. 2011. Invasion of erythrocytes by Francisella tularensis. J. Infect. Dis. 204:51–59. 10.1093/infdis/jir221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wellmann G. 1951. Blood sucking insects as mechanical vectors of Brucella. Zentralbl. Bakteriol. Orig. 156:414–426 [PubMed] [Google Scholar]
- 55.Wellmann G. 1952. Experimental studies on the possibility of Brucella transmission by insects. Zentralbl. Bakteriol. Parasitenkd. Infektionskr. Hyg. 159:71–86 [PubMed] [Google Scholar]
- 56.Ozsan K. 1962. Isolation of Brucella melitensis from the fleas Xenopsylla conformis gathered from the burrows of wild rodents. Ann. Inst. Pasteur (Paris) 103:90–92 (In French.) [PubMed] [Google Scholar]
- 57.Gudoshnik AN. 1958. Role of pasture ticks and rodents in dissemination of Brucella. Zh. Mikrobiol. Epidemiol. Immunobiol. 29:113–117 (In Russian.) [PubMed] [Google Scholar]
- 58.Neglia G, Veneziano V, De Carlo E, Galiero G, Borriello G, Francillo M, Campanile G, Zicarelli L, Manna L. 2013. Detection of Brucella abortus DNA and RNA in different stages of development of the sucking louse Haematopinus tuberculatus. BMC Vet. Res. 9:236. 10.1186/1746-6148-9-236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schekman R, Singer SJ. 1976. Clustering and endocytosis of membrane receptors can be induced in mature erythrocytes of neonatal but not adult humans. Proc. Natl. Acad. Sci. U. S. A. 73:4075–4079. 10.1073/pnas.73.11.4075 [DOI] [PMC free article] [PubMed] [Google Scholar]