Abstract
Toxoplasma gondii is an obligate intracellular parasite that can cause severe neurological disease in infected humans. CD40 is a receptor on macrophages that plays a critical role in controlling T. gondii infection. We examined the regulation of CD40 on the surface of T. gondii-infected bone marrow-derived macrophages (BMdMs). T. gondii induced CD40 expression both at the transcript level and on the cell surface, and interestingly, the effect was parasite strain specific: CD40 levels were dramatically increased in type II T. gondii-infected BMdMs compared to type I- or type III-infected cells. Type II induction of CD40 was specific to cells harboring intracellular parasites and detectable as early as 6 h postinfection (hpi) at the transcript level. CD40 protein expression peaked at 18 hpi. Using forward genetics with progeny from a type II × type III cross, we found that CD40 induction mapped to a region of chromosome X that included the gene encoding the dense granule protein 15 (GRA15). Using type I parasites stably expressing the type II allele of GRA15 (GRA15II), we found that type I GRA15II parasites induced the expression of CD40 on infected cells in an NF-κB-dependent manner. In addition, stable expression of hemagglutinin-tagged GRA15II in THP-1 cells resulted in CD40 upregulation in the absence of infection. Since CD40 signaling contributes to interleukin-12 (IL-12) production, we examined IL-12 from infected macrophages and found that CD40L engagement of CD40 amplified the IL-12 response in type II-infected cells. These data indicate that GRA15II induction of CD40 promotes parasite immunity through the production of IL-12.
INTRODUCTION
Toxoplasma gondii is an obligate intracellular parasite and the causative agent of toxoplasmosis. An estimated 30% of the world population is infected with this protozoan parasite (1). Acute infection with T. gondii is characterized by proliferation and dissemination of the fast-growing tachyzoite form of the parasite, followed by encystment of the parasite as slow-growing bradyzoites that establish a persistent chronic infection for the duration of the host's lifetime.
A robust innate immune response is initiated rapidly following T. gondii infection and is responsible for establishing a first line of defense. Myeloid cells, such as monocytes, dendritic cells, and macrophages, are among the first immune cells to migrate to the site of infection and subsequently become activated (2). CD40 is a cell surface receptor that plays a pivotal role in macrophage activation and parasite immunity. The engagement between CD40 and CD40L (CD154), expressed by antigen-presenting cells (APCs) and activated CD4+ T cells, respectively, results in the establishment of antimicrobial programs that contribute to enhanced in vivo control against a number of pathogens (3, 4), including T. gondii (5, 6). CD40 engagement on macrophages leads to the production of nitric oxide (NO) (7) and the induction of autophagy (8), both of which limit the survival of intracellular pathogens. In the case of T. gondii infection, CD40 establishes an antimicrobial program that contributes to parasite control independently of other established gamma interferon (IFN-γ)- and p47 GTPase-dependent mechanisms of defense (6, 9).
CD40 engagement has long been known to result in interleukin-12 (IL-12) production (10–12), which is critical for parasite control. IL-12 is produced by activated APCs shortly after T. gondii infection (13, 14) and is necessary for priming adaptive immunity (15). CD40 induction of IL-12 leads to the release of IFN-γ (16), a key mediator of host resistance during T. gondii infection (17). In addition, since T cells are crucial for controlling T. gondii infection (18, 19), disruption of sustained IL-12 production diminishes Th1 immune responses and promotes parasite growth and dissemination.
Although CD40 plays a critical role in host defense against T. gondii and is induced during infection (5, 20), how this receptor is regulated during parasite infection remains unknown. Lipopolysaccharide (LPS) stimulation of macrophages and microglia has been shown to induce CD40 transcription via STAT-1 and NF-κB p65 and p50 nuclear translocation and binding to the CD40 promoter (21). In addition to NF-κB, binding of the transcription factor specificity protein 1 (Sp1) to the CD40 promoter was necessary for optimal CD40 induction in response to LPS (22). STAT-1 signaling was found to be critical for CD40 expression following IFN-γ stimulation (23), whereas IL-4 inhibits CD40 expression through STAT6 signaling (24). We examined how CD40 is regulated during T. gondii infection. We found that CD40 is induced in a strain-specific manner and that the type II parasite dense granule protein 15 (GRA15) is sufficient for CD40 induction in macrophages through NF-κB signaling. Moreover, engagement of CD40 with CD40L resulted in enhanced IL-12 responses in macrophages infected with a type II strain of T. gondii.
MATERIALS AND METHODS
Mammalian and parasite cell culture.
Human foreskin fibroblasts (HFFs) and human embryonic kidney 293T (293T) Phoenix-E cells were cultured in Dulbecco's modified Eagle's medium (DMEM; Thermo Fisher Scientific, Logan, UT) supplemented with 10% heat-inactivated fetal calf serum (FCS; HyClone, Logan, UT), 2 mM l-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin (D-10% medium). Bone marrow-derived macrophages (BMdMs) from C57BL/6 mice were generated as previously described (25) and cultured in D-10% medium supplemented with 10% macrophage colony-stimulating factor for 5 days. Cells were then seeded overnight before T. gondii infection assays. Splenocytes were isolated from C57BL/6 mice as described previously (26). THP-1 cells were cultured in R-10% medium, consisting of RPMI 1640 (Thermo Fisher Scientific, Logan, UT) supplemented with 10% heat-inactivated FBS, 2 mM l-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin. Where indicated, THP-1 cells were stimulated with 5 mM phorbol myristate acetate (PMA) for 24 h, washed, and then cultured in fresh R-10% medium for an additional 24 h.
T. gondii tachyzoites of type I (RHgfpluc [27]), type II (ME49fLuc; generously provided by John Boothroyd), and type III (CΔLuc123 [28]) and transgenic type I tachyzoites stably expressing the type II allele of GRA15 (type I GRA15II) (29) were used for infection of BMdMs. All parasite strains constitutively expressed green fluorescent protein (GFP) and were maintained by serial passage in confluent HFF monolayers as previously described (30).
All mammalian and parasite cell cultures were maintained in incubators set at 37°C with 5% CO2. Cell lines and parasite strains were tested monthly for Mycoplasma contamination and confirmed to be negative. All research involving mice was carried out in compliance with the Institutional Animal Care and Use Committee at the University of California, Irvine (UCI).
Antibodies and flow cytometry.
BMdMs, PMA-stimulated THP-1 cells, and THP-1 cells transduced with hemagglutinin (HA)-tagged GRA15 (THP-1+GRA15II-HA) were resuspended in fluorescence-activated cell sorting (FACS) wash (phosphate-buffered saline [PBS] with 2% FCS) containing anti-Fc receptor antibody (for mouse, clone 2.4G2 [BD Biosciences, San Jose, CA]; for human, human Fc receptor binding inhibitor [eBioscience, San Diego, CA]) and incubated on ice for 10 min. For cell surface marker staining, all antibodies were resuspended in FACS wash. BMdMs were pelleted by centrifugation, stained with biotinylated control Ig or biotinylated anti-mouse CD40 (clone 3/23; BioLegend, San Diego, CA) on ice for 30 min, and then stained with streptavidin-phycoerythrin (PE) on ice for 15 min. Splenocytes were stained with anti-mouse CD19-PE-cyan 7 (Cy7) (clone 6D5; BioLegend), anti-mouse CD11c-PE-Cy7 (clone N418; BioLegend), or anti-mouse CD40-allophycocyanin (APC) (clone 3/23; BioLegend). Human THP-1 cells were stained with anti-human CD40-PE (clone 5C3; BioLegend) or a mouse IgG1-PE control antibody. After the final wash, the cells were fixed with 4% paraformaldehyde (PFA) prior to analysis by flow cytometry.
All flow cytometry samples were examined using a FACSCalibur cytometer with CellQuest software (BD Biosciences, San Jose, CA) for acquisition and FlowJo software (Tree Star, Ashland, OR) for analysis. For the mock-treated cells, the histograms depict the total cell population. Since GFP-expressing T. gondii parasites were used for the infection and flow cytometry experiments, the histograms for the infected cells depict those cells that fell within the GFP-positive (GFP+) gate.
Gene expression analysis by qPCR.
BMdMs from C57BL/6 mice were infected as described above. At 6 h postinfection (hpi), RNA was harvested using an RNeasy kit (Qiagen, Germantown, MD) and treated with DNase I (Invitrogen, Carlsbad, CA). cDNA was generated and used as the template in real-time quantitative PCR (qPCR) with primers specific for mouse CD40: forward primer 5′-GCTATGGGGCTGCTTGTTGA-3′ and reverse primer 5′-ATGGGTGGCATTGGGTCTTC-3′. Mouse glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used for normalization using the following primers: forward primer 5′-GCATGGCCTTCCGTGTTC-3′ and reverse primer (5′-GATGTCATCATACTTGGCAGGTTT-3′. qPCR was performed in triplicate using a Bio-Rad iCycler apparatus and iTaq Universal SYBR green Supermix (Bio-Rad, Hercules, CA).
The data from the qPCR were analyzed using the threshold cycle (2−ΔΔCT) method (31). The values obtained for CD40 expression were normalized to those for GAPDH expression, and the data are expressed as the ratio of mRNA levels. Error bars reflect the standard deviation from triplicate samples. In all qPCR assays, cDNA generated in the absence of reverse transcriptase was used as a negative control to detect contaminating genomic DNA. No amplification was observed in the samples tested in the absence of reverse transcriptase or in samples containing water in the place of DNA template.
QTL mapping.
CD40 mean fluorescence intensity values generated from BMdMs infected with 19 F1 progeny derived from crosses between a type II strain and a type III strain (28, 32) were analyzed using R/qtl software (33) and the existing T. gondii genetic map (34). Quantitative trait locus (QTL) peaks were identified using the marker regression method (mr). The overall significance level was determined using 1,000 permutations of the genotype data.
NF-κB inhibitor assay.
BMdMs were cultured for 24 h in medium treated with dimethyl sulfoxide (DMSO) as a vehicle control or with the IκB kinase (IKK) inhibitor PS1145 (Sigma-Aldrich, St. Louis, MO) (35). The concentration used was selected based on the findings of dose-response experiments that we performed to select the lowest concentration that showed specific effects on the target (data not shown). After 24 h, the medium was replaced with untreated macrophage medium and the cells were mock infected or infected with type I or type I GRA15II T. gondii parasites. At 1 hpi, the cells were washed and the medium was replaced with fresh macrophage medium or macrophage medium supplemented with DMSO or PS1145. The cells were harvested at 18 hpi for antibody staining and flow cytometry analysis for CD40 expression.
Retroviral transduction.
The open reading frame (ORF) for T. gondii type II GRA15 (GRA15II) with a C-terminal HA epitope tag was cloned into the BamHI and EcoRI sites of the pMX-puro retroviral vector to generate the vector IP9. This construct was generated by performing nested primer PCR using the following primers: forward primer GRA15-BamHI (5′-GCTTCAGGATCCATGATAATTCGGTGGCTTGGGTATCTTACGG-3′), reverse primer 1 (primer GRA15-HA; 5′-TCTGGGACGTCGTATGGGTATGGAGTTACCGCTGATTGTGTGTCCC-3′), and reverse primer 2 (primer GRA15-HA-EcoRI; 5′-GTAATGGAATTCTCAAGCGTAGTCTGGGACGTCGTATGGGTATGGAGTTA-3′). Phoenix-E packaging cells were transfected with IP9 (GRA15II-HA-pMX-puro) or the parental pMX-puro plasmid as a negative control using the Lipofectamine 2000 reagent (Life Technologies, Carlsbad, CA) according to the manufacturer's protocol. Retroviral supernatants were harvested on days 2 and 3 posttransfection and centrifuged at 1,200 rpm for 10 min to clarify the supernatant and remove cell debris. THP-1 cells were infected with the retrovirus by centrifugation at 2,500 rpm for 2 h at 25°C in the presence of 8 μg/ml hexadimethrine bromide (Sigma-Aldrich, St. Louis, MO). At 40 h postinfection, the transduced cells were placed in selection medium containing 2 μg/ml puromycin (Thermo Fisher Scientific, Logan, UT). Western blotting was performed after 5 days in puromycin selection medium to confirm the expression of GRA15II-HA.
Western blotting.
Transduced THP-1 cells were washed with ice-cold 1× PBS, and cell lysates were generated by the addition of 2× Laemmli sample buffer containing 10% β-mercaptoethanol. The lysates were separated by SDS-PAGE and transferred to polyvinylidene difluoride (PVDF) membranes for immunoblotting. The membranes were blotted with a horseradish peroxidase (HRP)-conjugated antibody against the HA epitope tag (Cell Signaling, Danvers, MA). The membranes were developed with the Amersham ECL Prime reagent (GE Healthcare, Waukesha, WI) and detected using a Nikon camera, as previously described (36).
Transient transfection.
293T cells were used to generate CD40L-expressing transfectants (293T-CD40L). 293T cells were transfected with the CD40L-MIEG-hCD4 plasmid containing the complete coding sequence for the murine CD40L (plasmid 40355; Addgene, Cambridge, MA) using Lipofectamine 2000 (Life Technologies, Carlsbad, CA) according to the manufacturer's protocol. To generate control cells, the parental 293T cells were treated with Lipofectamine 2000 in the absence of plasmid. The control 293T cells and CD40L-expressing 293T cells were cultured for 48 h at 37°C before addition to the BMdMs for the coculture experiments.
CD40L stimulation assays.
At day 6 of culture, the BMdMs were mock infected or infected with type I, type II, or type I GRA15II parasites, as described above. At 18 hpi, 293T or CD40L-expressing 293T cells were added to BMdMs at a ratio of 1:2 (293T cells/BMdMs) without changing the medium. At 26 hpi, the cell culture supernatants were collected to measure IL-12p70 by enzyme-linked immunosorbent assay (ELISA) (BioLegend, San Diego, CA).
Statistical analysis.
Unless otherwise indicated, an unpaired, two-tailed Student's t test was used to determine statistical significance. Differences were considered significant when P was <0.05.
RESULTS
Type II T. gondii induces high levels of CD40 on infected macrophages.
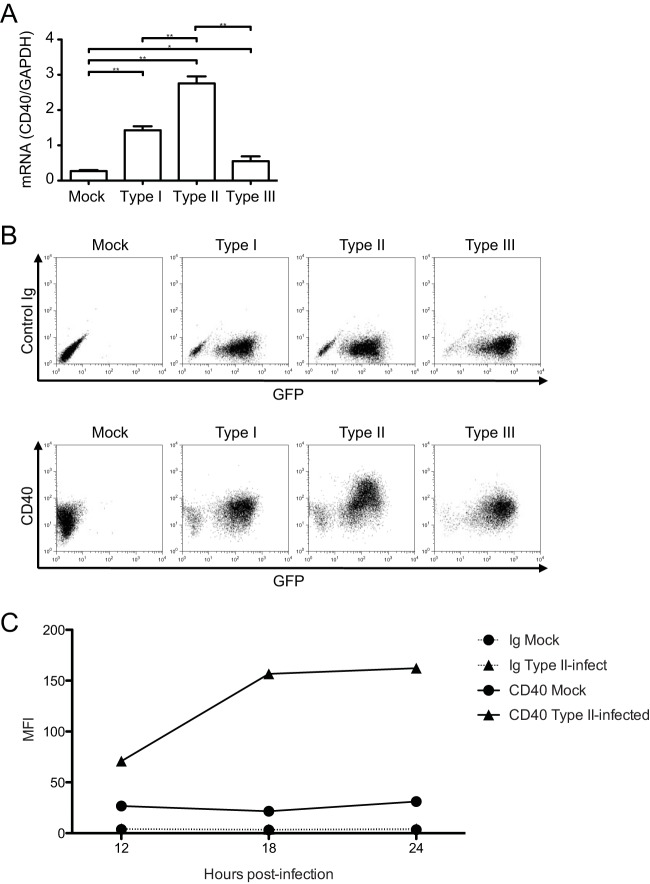
We evaluated the expression of the costimulatory molecule CD40 on macrophages following T. gondii infection. After infecting primary mouse BMdMs with types I, II, or III parasites, we observed that CD40 transcript levels were increased in all infected samples at 6 hpi. However, the increase in CD40 transcript levels was significantly higher in BMdMs infected with type II parasites than in mock-infected cells (10-fold change, P < 0.05) or cells infected with either type I or III parasites (Fig. 1A). We then investigated whether there was a strain-specific induction of CD40 on the surface of infected cells. Flow cytometry analysis of infected BMdMs at 18 hpi revealed that the highest induction of CD40 was observed in cells infected with the type II strain (Fig. 1B). Moreover, CD40 was specifically upregulated in the infected, GFP+ population, and the cells with the highest level of GFP had the strongest CD40 upregulation. To examine whether infection induces CD40 in other types of immune cells, cultures of splenocytes harvested from C57BL/6 mice were infected with type II T. gondii parasites and CD40 was modestly induced on the infected CD19+ (B cell) and CD11c+ (dendritic cell) populations (see Fig. S1 in the supplemental material). We next investigated the kinetics of CD40 induction following infection with the type II strain of T. gondii. CD40 cell surface protein levels were increased by 12 hpi and peaked at 18 hpi in T. gondii-infected BMdMs compared to their levels in mock-infected cells (Fig. 1C). These data indicate that T. gondii induced CD40 expression on infected macrophages and that a high level of CD40 induction occurred in a parasite strain-specific manner.
FIG 1.
Expression of CD40 during T. gondii infection. (A) BMdMs were mock infected or infected with type I, II, or III T. gondii tachyzoites. RNA was harvested at 6 hpi for cDNA synthesis and analysis by qPCR for CD40 transcript levels. The level of expression of CD40 relative to that of GAPDH is shown for each condition. Error bars represent the standard deviations of technical triplicates. (B) BMdMs were mock infected or infected with GFP-expressing T. gondii, as described in the legend to panel A, harvested at 18 hpi, and stained with a control Ig or monoclonal antibodies against CD40. (C) BMdMs were mock infected or infected with type II T. gondii, harvested at 12, 18, or 24 hpi, and stained with control Ig or anti-CD40 monoclonal antibodies, and the mean fluorescence intensity (MFI) values for each sample were plotted. *, P < 0.05: **, P < 0.01 (Student's t test). For all three panels, one representative set from at least three independent experiments is shown.
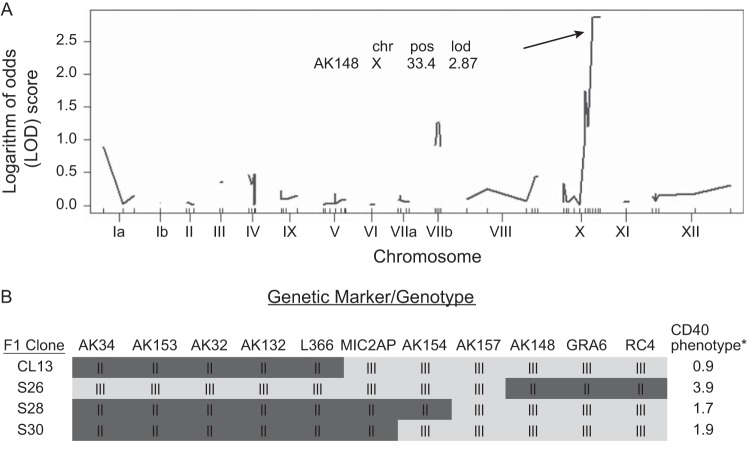
The observation that the high level of CD40 induction was strain specific suggested the possibility of a genetic basis for the phenotype. In this case, the phenotype should segregate among recombinant progeny generated by a cross between the type II and either the type I or type III parental strains. Indeed, by comparing CD40 levels on macrophages infected with type II × type III recombinant progeny lines, we found that some strains induced high CD40 levels, whereas other strains induced CD40 at a low level (see Table S1 in the supplemental material). We analyzed CD40 levels in BMdMs infected with 19 F1 recombinant progeny and used QTL mapping to identify the genetic locus responsible for this effect. QTL mapping identified three peaks on chromosomes Ia, VIIb, and X (Fig. 2A). The presence of more than one peak suggests that the CD40-inducing phenotype is a multilocus trait. Although the peak observed on the right arm of chromosome X (logarithm of odds score = 2.87; P = 0.145) was not statistically significant, we chose to further investigate this genetic region since it showed the strongest association with the phenotype of high CD40 induction. To do this, we chose two strains from the original 19 F1 recombinant progeny as well as two additional strains (S26 and S28 [28]) that had recombinations in the right arm of chromosome X for more detailed analysis. Using the annotated genotype maps for these four strains (28), we found that strains with the type II genotype between marker AK157 and the end of chromosome X induced high levels of CD40 expression, whereas those with the type III genotype had low levels of CD40 induction (Fig. 2B). Specifically, CL13 and S30 did not induce high levels of CD40 (Fig. 2B) and strain S28 also only weakly upregulated CD40 expression; however, strain S26 induced the high CD40 expression phenotype (Fig. 2B). The responsible 0.46-megabase region of chromosome X contains 91 genes, 31 of which encode putative secretory proteins, including GRA15 (29).
FIG 2.
QTL mapping of the CD40 induction phenotype. (A) QTL scan using raw data from BMdMs infected with 19 F1 progeny from a type II × type III cross. A nonsignificant logarithm of odds (LOD) peak was identified near marker AK148 (P = 0.145). The LOD score cutoff for genome-wide significance (P ≤ 0.05) was 4.50. chr X, chromosome X; pos, position (centiMorgan). (B) Genotypes and phenotypes of four F1 progeny used to further map the locus responsible for the CD40 induction phenotype. *, fold change in expression of T. gondii-infected cells compared with mock-infected cells.
GRA15II induces CD40 on infected macrophages.
To test if GRA15 mediates CD40 induction in macrophages infected with the type II strain, we infected BMdMs with type I or type II parasites or type I parasites expressing the type II allele of GRA15 (called type I GRA15II). As expected, type I parasites did not induce robust CD40 cell surface expression on infected BMdMs. In contrast, the expression of CD40 was increased on cells infected with type I GRA15II and type II parasites (Fig. 3A). To evaluate if human macrophage-like cells upregulate CD40 following infection with type I, type II, or type I GRA15II parasites, THP-1 cells differentiated with phorbol myristate acetate (PMA) were infected with each of these three parasite strains. Similar to murine macrophages, human THP-1 differentiated macrophages were found to increase CD40 expression after infection with either type II or type I GRA15II parasites but not with type I parasites (Fig. 3B).
FIG 3.
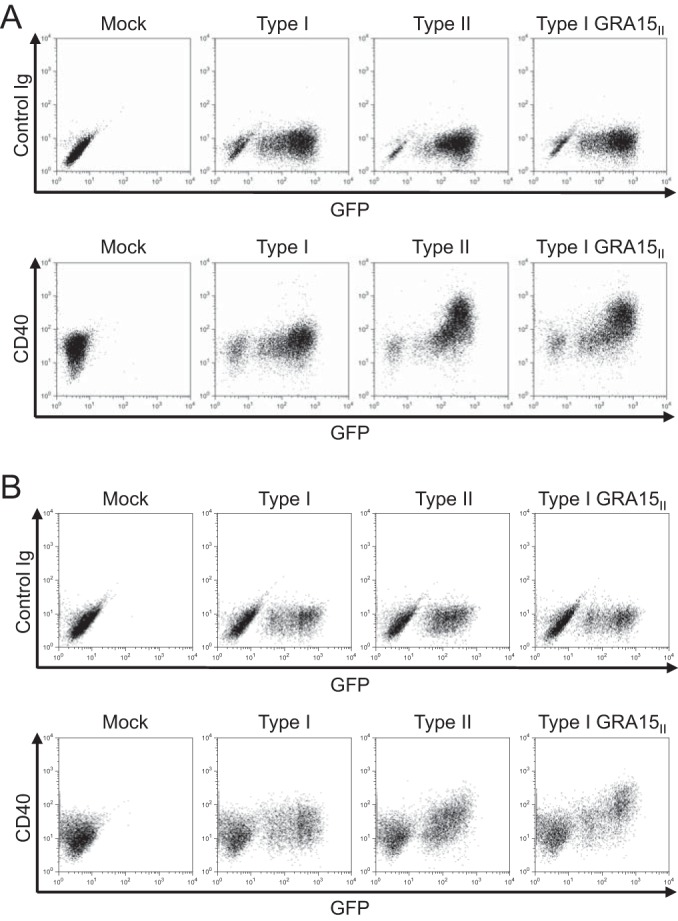
Role of GRA15 in CD40 induction during T. gondii infection. BMdMs (A) or THP-1 cells (B) differentiated with PMA were mock infected or infected with type I, type II, or type I GRA15II parasites. All of these parasite lines express GFP. Cells were harvested at 18 hpi and stained with control Ig or anti-CD40 monoclonal antibodies. One representative set of results from at least three independent experiments is shown for both panels.
Recent work demonstrated that the protein encoded by the type II allele of GRA15 (GRA15II) mediates sustained NF-κB nuclear translocation in T. gondii-infected cells (29) and induces the production of IL-12 in mouse macrophages (29) and IL-1β in human monocytes (30). Additionally, NF-κB signaling has been found to play a role in LPS-induced CD40 expression in macrophages (21). To evaluate if GRA15II-induced CD40 expression was NF-κB dependent, we analyzed CD40 levels on type I GRA15II parasite-infected BMdMs treated with the IκB kinase (IKK) inhibitor PS1145 (35). Infection with type I GRA15II parasites resulted in CD40 upregulation in untreated macrophages or DMSO-treated macrophages, as expected. However, CD40 expression on BMdMs treated with the NF-κB inhibitor was markedly reduced compared to that on untreated or vehicle control-treated cells (Fig. 4). These data indicate that GRA15II mediates the induction of CD40 in both infected human THP-1 cells and mouse macrophages and that NF-κB signaling contributes to this induction.
FIG 4.
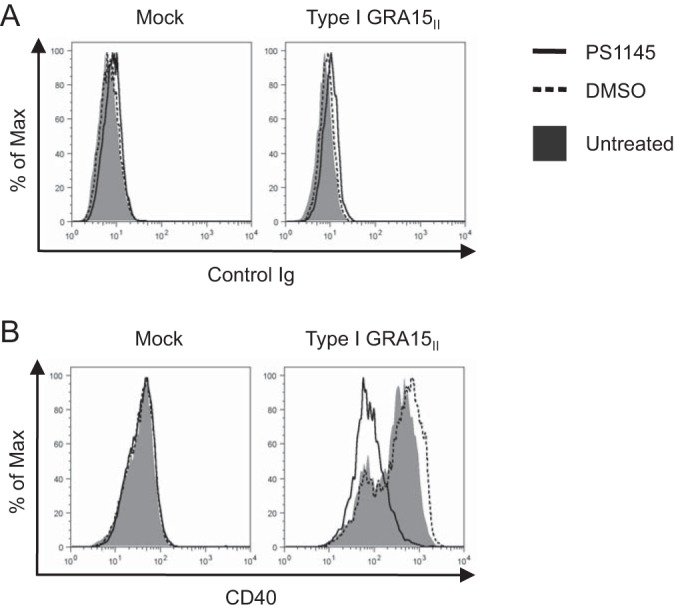
GRA15II-induced CD40 expression following NF-κB inhibition. BMdMs were cultured in macrophage medium (untreated) or medium supplemented with DMSO or the IKK inhibitor PS1145 (10 μM). After 24 h, the cells were mock infected or infected with type I GRA15II T. gondii in untreated macrophage medium. At 1 hpi, the cells were washed and the medium was replaced with fresh macrophage medium or macrophage medium supplemented with DMSO or PS1145. The cells were harvested at 18 hpi and stained with control Ig (A) or anti-CD40 monoclonal antibodies (B). The histograms shown are representative of those from at least two independent experiments. Max, maximum.
GRA15II is sufficient for T. gondii-induced CD40 expression.
To evaluate if GRA15II alone is sufficient to induce CD40 expression in the absence of parasite infection, we generated a retroviral vector containing the cDNA of the GRA15II allele fused to the sequence for the HA epitope tag. THP-1 cells were stably transduced with this vector (GRA15II-HA) or the parental pMX-puro vector (pMX-puro) as a control. We confirmed the expression of the HA-tagged GRA15II by Western blotting (Fig. 5A). Expression of GRA15II-HA in THP-1 cells was sufficient to induce the expression of CD40, even in the absence of parasite infection (Fig. 5B).
FIG 5.
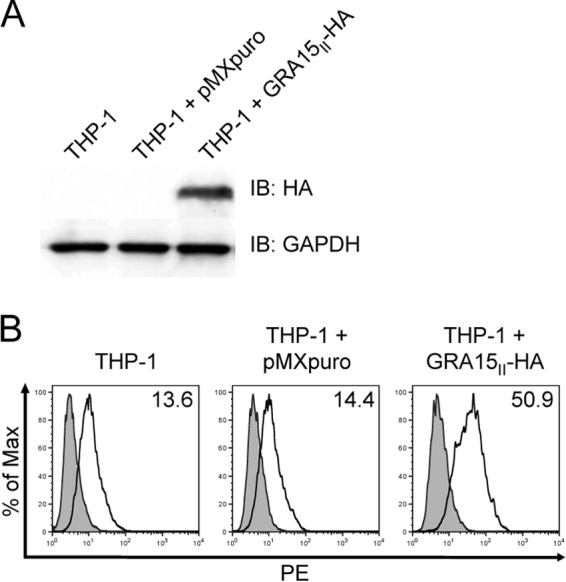
Characterizing GRA15II-expressing THP-1 cells. A retroviral vector containing the cDNA of the GRA15II allele fused to the sequence for the HA epitope tag was generated (GRA15II-HA-pMX-puro). THP-1 cells were stably transduced with this vector (GRA15II-HA) or the parental pMX-puro vector (pMXpuro) as a control. (A) Lysates were generated from stably transduced cells, and the expression of GRA15II-HA was examined by immunoblotting (IB) with an anti-HA monoclonal antibody or anti-GAPDH as a loading control. (B) Stably transduced cells were collected and stained with control Ig (gray) or anti-CD40 (white) monoclonal antibodies. In each histogram plot, the numbers in the upper right corner represent the mean fluorescence intensity of CD40 expression. One representative set of results from four independent experiments from two independent transductions is shown for both panels.
CD40 expression on infected macrophages enhances IL-12 production following CD40L stimulation.
In addition to their role as professional APCs, macrophages are capable of eliciting cell-intrinsic effector functions through CD40 signaling. For example, CD40 and CD40L engagement has been shown to induce the transcription and secretion of IL-12p40 (37). Since CD40 levels are upregulated on macrophages infected with type II parasites, we hypothesized that type II-infected cells may have enhanced CD40-mediated effector functions. To investigate this possibility, we measured the production of the biologically active IL-12p70 heterodimer by BMdMs that were mock infected or infected with type I, type II, or type I GRA15II parasites for 18 h and then cultured with 293T cells or with 293T cells expressing murine CD40L for an additional 8 h. The level of production of IL-12p70 by mock-infected and type I-infected BMdMs was below the level of detection, regardless of whether the cells were cultured with control 293T cells or CD40L-expressing 293T cells (Fig. 6). In contrast, infection of BMdMs with the type II or type I GRA15II parasites resulted in the production and secretion of IL-12p70 when cultured alone or with 293T cells. Notably, culturing BMdMs infected with the type II or type I GRA15II parasites with CD40L-expressing 293T cells resulted in a significant increase in the production of IL-12p70. These data suggest that the engagement of CD40 on macrophages infected with type II T. gondii contributes to the elevated production of IL-12 in response to interaction with CD40L.
FIG 6.
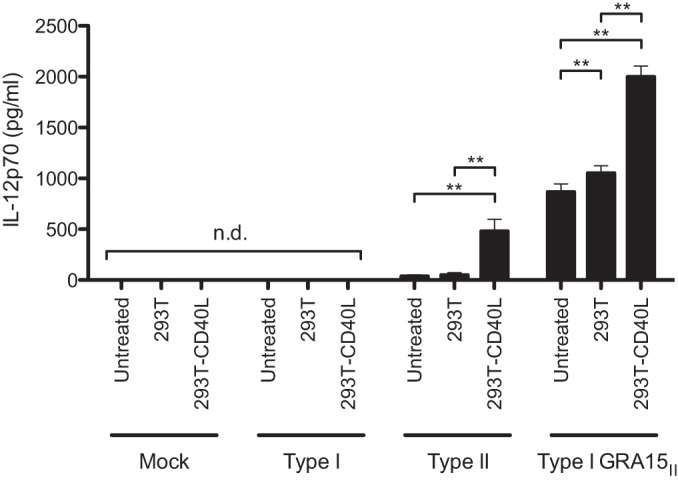
CD40 stimulation of T. gondii-infected macrophages. BMdMs were mock infected or infected with type I, type II, or type I GRA15II parasites. At 18 hpi, 293T cells or CD40L-expressing 293T cells were added to BMdMs at a ratio of 1:2 (293T cells/BMdMs). At 26 hpi, the IL-12p70 released into the culture medium was analyzed by ELISA. The error bars represent the standard deviations of biological triplicates. n.d., not detected. **, P < 0.01 (Student's t test).
DISCUSSION
The establishment of host defense against infection with T. gondii is initiated by cells of the innate immune response, which help to shape the course of the infection. Effective parasite control requires a robust immune response without the induction of immunopathology. This is achieved through coordinated communication between innate immune cells and the regulation of signaling cascades triggered by cell surface immune receptors.
The modulation of immune receptors on macrophages during the acute stage of infection can have profound implications for the development of the ensuing immune response. This led us to investigate the mechanisms behind the observed induction of CD40 on infected macrophages following infection with T. gondii. It has previously been shown that CD40 is upregulated on mouse macrophages (5) and human monocytes (20) after T. gondii infection. We found that there is a parasite strain specificity to this induction: the type II strain of T. gondii induced high levels of expression of CD40, whereas the types I and III strains induced only low levels of CD40 expression. The protein encoded by the GRA15II allele drove CD40 expression in a NF-κB-dependent manner. Furthermore, we showed that the induction of CD40 in cells infected with parasites expressing GRA15II contributed to the establishment of a Th1-conducive microenvironment through the production of IL-12p70 after stimulation with CD40L. By investigating the mechanism of CD40 induction by the type II strain of T. gondii, we have identified a parasite factor that contributes to CD40 induction and found a role for infected cells in the establishment of protective immunity during parasite infection.
CD40 and CD40L engagement contributes to enhanced parasite clearance through the activation of macrophages and other APCs (38). CD40/CD40L interaction leads to the induction of an antimicrobial program in macrophages (8, 39) and is also critical for controlling T. gondii infection in mice (5). This results in a reduction in the T. gondii burden in infected cells (9, 40). Despite the elevated levels of CD40 on type II-infected macrophages, we did not observe a strain-specific enhancement in parasite clearance in these cells due to CD40 signaling (data not shown). CD40 engagement also leads to the production of IL-12, tumor necrosis factor alpha, and nitric oxide, which have been shown to play key roles in host defense (11, 12, 41, 42). These cytokines and effectors produced in response to CD40 engagement are important for establishing an immune response capable of controlling intracellular pathogens through Th1-mediated immunity (3, 4).
CD40 signal strength can profoundly affect p38 and extracellular signal-regulated kinase (ERK) mitogen-activated protein kinase (MAPK) signaling and IL-12 production (12, 43, 44), as well as T cell skewing through reciprocal regulation of IL-12 and IL-4 (15, 45). We demonstrate that macrophages infected with CD40-inducing T. gondii parasite strains dramatically increase IL-12 production following CD40-CD40L stimulation: type II-infected macrophages cultured with CD40L-expressing cells exhibited a more than 12-fold increase in IL-12p70 production compared to type II-infected cells that were cultured with the control cells. The enhanced CD40 expression may amplify the strength of the signal mediated by CD40L stimulation. Consistent with our findings, it is known that strong CD40 engagement, achieved by increasing the concentration of recombinant soluble CD40L, results in robust Th1-skewing conditions due to preferential p38 signaling and subsequently more IL-12p40 and p70 production (44). In contrast, weak CD40 engagement of T cells was found to enhance Th2 immunity due to elevated ERK activation and subsequently enhanced IL-10 expression (44). Our observation that type II T. gondii-infected macrophages produce higher levels of IL-12p40 following CD40 engagement suggests that these infected cells contribute to the promotion of Th1 responses.
T. gondii proteins secreted into infected host cells have been shown to alter host cell signaling and gene transcription (46–48) as well as antimicrobial processes (49–51). Interestingly, however, there are parasite strain-specific effects on the modulation of host cell signaling pathways, including the JAK/STAT, NF-κB, and MAPK pathways. A growing body of work has demonstrated that the differences in the modulation of these host cell signaling pathways are a result of strain-specific polymorphisms in the secreted factors released into host cells during infection (48, 52–54). Consequently, polymorphisms in secreted parasite proteins can differentially influence macrophage polarization and effector functions: type I parasites induce an alternative activation state, whereas type II strains induce a classical activation state (55). Type I T. gondii parasites actively dampen the responsiveness of infected host cells by directly activating STAT3 signaling, rendering cells refractory to IFN-γ stimulation (51). Lang et al. demonstrated that the suppressed responsiveness to IFN-γ is the result of chromatin remodeling following infection with the type I RH strain of T. gondii (47). Our data showing CD40 upregulation by the type II strain of T. gondii are consistent with this paradigm, and CD40 upregulation primes macrophages for a proinflammatory program following receptor engagement (45, 55).
The pathways that mediate CD40 induction after T. gondii infection were unknown. The fact that CD40 was induced in a strain-specific manner enabled us to use QTL analysis to identify a genomic region that associates with CD40 induction. QTL analysis indicated that the genomic locus responsible for CD40 induction contained the gene GRA15 (TGME49_275470), which encodes the dense granule protein GRA15. GRA15 was the first T. gondii dense granule protein shown to modulate host cell NF-κB signaling due to sustained nuclear translocation of p65 (29). The GRA15 allele of type II T. gondii encodes a protein consisting of 635 amino acids, while the GRA15 allele of type I parasites contains a frameshift mutation that leads to a premature stop codon and a final protein product of 312 amino acids. Additionally, the type I and type III alleles of GRA15 contain an insertion/deletion mutation near the C terminus of the protein, along with 5 other amino acids that are polymorphic (29). We demonstrate that GRA15II is sufficient to induce CD40 expression even in the absence of parasite infection.
Surprisingly, we observed that not all type II strains induce CD40 expression. Whereas infections with independently obtained ME49 strains highly upregulated CD40, the Prugniaud strain (56) did not (see Fig. S2 in the supplemental material). It has previously been reported that there are phenotypic differences between different type I lines (57) and between different type II lines (58). Yang et al. demonstrated that phenotypic differences in NF-κB activation and IL-12 production between type I parasites were partially explained by polymorphisms in GRA15 (57). Sequence analysis of the GRA15 coding sequence for the ME49 and Prugniaud strains revealed that they were 100% identical (data not shown), whereas analysis of the genomic region upstream of the GRA15 transcription start site revealed the presence of a 2-nucleotide insertion in the Prugniaud strain. The significance of these additional nucleotides has yet to be determined. One possible explanation for the observed differences in CD40 induction between type II parasite isolates is that CD40 expression is regulated by additional parasite factors that differ in function or expression level between the type II strains that we have evaluated. CD40 expression induced by the various recombinant progeny was not strictly binary, suggesting that while CD40 induction is primarily dependent on GRA15, it is likely to be influenced by additional parasite factors. Indeed, QTL analysis revealed the presence of an additional peak on chromosome Ia and on chromosome VIIb (Fig. 2A). There is a precedent for the dual regulation of host pathways by secreted proteins, such as ROP5 and ROP18. Strain differences in the parasites' susceptibility to killing by IFN-γ-stimulated mouse embryonic fibroblasts were dependent on the expression of both virulent polymorphic genes encoding ROP5 E. L. and ROP18 J. A. (48). In addition, the protein encoded by the virulent allele of ROP16 in type I parasites has been shown to antagonize the activity of GRA15 (29). Further work characterizing additional parasite factors that enhance or inhibit GRA15 activity may help to explain the differences in CD40 induction observed in distinct type II isolates.
Here we report a novel function for GRA15 in CD40 induction and demonstrate that CD40 signaling amplifies the IL-12 response in type II-infected macrophages and may contribute to the Th1 environment established during infection with type II strains of T. gondii.
Supplementary Material
ACKNOWLEDGMENTS
We thank all members of the A. L. Edinger, D. A. Fruman, N. S. Morrissette, E. L. Nelson, J. A. Prescher, and A. J. Tenner labs for helpful discussion on this project. We also thank John Boothroyd and Jeroen Saeij for generously providing parasites.
This work was supported by NIH NIGMS grant R25GM055246 (to P.C.), a UCI faculty mentor program fellowship (to P.M.), a President's Dissertation Year fellowship (to P.M.), NIH immunology training grant T32AI60573 (to K.S.H.), ACS IRG-98-279-07 (to M.B.L.), AHA scientist development grant 10SDG3140025 (to M.B.L.), and setup funds from the UCI School of Biological Sciences (to M.B.L.).
We have no conflict of interest to declare.
Footnotes
Published ahead of print 14 July 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.01615-14.
REFERENCES
- 1. Montoya JG, Liesenfeld O. 2004. Toxoplasmosis. Lancet 363:1965–1976. 10.1016/S0140-6736(04)16412-X [DOI] [PubMed] [Google Scholar]
- 2. Miller CM, Boulter NR, Ikin RJ, Smith NC. 2009. The immunobiology of the innate response to Toxoplasma gondii. Int. J. Parasitol. 39:23–39. 10.1016/j.ijpara.2008.08.002 [DOI] [PubMed] [Google Scholar]
- 3. Soong L, Xu JC, Grewal IS, Kima P, Sun J, Longley BJ, Ruddle NH, McMahon-Pratt D, Flavell RA. 1996. Disruption of CD40-CD40 ligand interactions results in an enhanced susceptibility to Leishmania amazonensis infection. Immunity 4:263–273. 10.1016/S1074-7613(00)80434-3 [DOI] [PubMed] [Google Scholar]
- 4. Kamanaka M, Yu P, Yasui T, Yoshida K, Kawabe T, Horii T, Kishimoto T, Kikutani H. 1996. Protective role of CD40 in Leishmania major infection at two distinct phases of cell-mediated immunity. Immunity 4:275–281. 10.1016/S1074-7613(00)80435-5 [DOI] [PubMed] [Google Scholar]
- 5. Reichmann G, Walker W, Villegas EN, Craig L, Cai G, Alexander J, Hunter CA. 2000. The CD40/CD40 ligand interaction is required for resistance to toxoplasmic encephalitis. Infect. Immun. 68:1312–1318. 10.1128/IAI.68.3.1312-1318.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Subauste CS, Wessendarp M. 2006. CD40 restrains in vivo growth of Toxoplasma gondii independently of gamma interferon. Infect. Immun. 74:1573–1579. 10.1128/IAI.74.3.1573-1579.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Awasthi A, Mathur R, Khan A, Joshi BN, Jain N, Sawant S, Boppana R, Mitra D, Saha B. 2003. CD40 signaling is impaired in L. major-infected macrophages and is rescued by a p38MAPK activator establishing a host-protective memory T cell response. J. Exp. Med. 197:1037–1043. 10.1084/jem.20022033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Andrade RM, Wessendarp M, Gubbels M-J, Striepen B, Subauste CS. 2006. CD40 induces macrophage anti-Toxoplasma gondii activity by triggering autophagy-dependent fusion of pathogen-containing vacuoles and lysosomes. J. Clin. Invest. 116:2366–2377. 10.1172/JCI28796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Andrade RM, Portillo J-AC, Wessendarp M, Subauste CS. 2005. CD40 signaling in macrophages induces activity against an intracellular pathogen independently of gamma interferon and reactive nitrogen intermediates. Infect. Immun. 73:3115–3123. 10.1128/IAI.73.5.3115-3123.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mason N, Aliberti J, Caamano JC, Liou H-C, Hunter CA. 2002. Cutting edge: identification of c-Rel-dependent and -independent pathways of IL-12 production during infectious and inflammatory stimuli. J. Immunol. 168:2590–2594. 10.4049/jimmunol.168.6.2590 [DOI] [PubMed] [Google Scholar]
- 11. Kato T, Hakamada R, Yamane H, Nariuchi H. 1996. Induction of IL-12 p40 messenger RNA expression and IL-12 production of macrophages via CD40-CD40 ligand interaction. J. Immunol. 156:3932–3938 [PubMed] [Google Scholar]
- 12. Kennedy MK, Picha KS, Fanslow WC, Grabstein KH, Alderson MR, Clifford KN, Chin WA, Mohler KM. 1996. CD40/CD40 ligand interactions are required for T cell-dependent production of interleukin-12 by mouse macrophages. Eur. J. Immunol. 26:370–378. 10.1002/eji.1830260216 [DOI] [PubMed] [Google Scholar]
- 13. Khan IA, Matsuura T, Kasper LH. 1994. Interleukin-12 enhances murine survival against acute toxoplasmosis. Infect. Immun. 62:1639–1642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hunter CA, Subauste CS, Van Cleave VH, Remington JS. 1994. Production of gamma interferon by natural killer cells from Toxoplasma gondii-infected SCID mice: regulation by interleukin-10, interleukin-12, and tumor necrosis factor alpha. Infect. Immun. 62:2818–2824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stuber E, Strober W, Neurath M. 1996. Blocking the CD40L-CD40 interaction in vivo specifically prevents the priming of T helper 1 cells through the inhibition of interleukin 12 secretion. J. Exp. Med. 183:693–698. 10.1084/jem.183.2.693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yap G, Pesin M, Sher A. 2000. Cutting edge: IL-12 is required for the maintenance of IFN-gamma production in T cells mediating chronic resistance to the intracellular pathogen, Toxoplasma gondii. J. Immunol. 165:628–631. 10.4049/jimmunol.165.2.628 [DOI] [PubMed] [Google Scholar]
- 17. Suzuki Y, Orellana MA, Schreiber RD, Remington JS. 1988. Interferon-gamma: the major mediator of resistance against Toxoplasma gondii. Science 240:516–518. 10.1126/science.3128869 [DOI] [PubMed] [Google Scholar]
- 18. Suzuki Y, Remington JS. 1988. Dual regulation of resistance against Toxoplasma gondii infection by Lyt-2+ and Lyt-1+, L3T4+ T cells in mice. J. Immunol. 140:3943–3946 [PubMed] [Google Scholar]
- 19. Gazzinelli R, Xu Y, Hieny S, Cheever A, Sher A. 1992. Simultaneous depletion of CD4+ and CD8+ T lymphocytes is required to reactivate chronic infection with Toxoplasma gondii. J. Immunol. 149:175–180 [PubMed] [Google Scholar]
- 20. Subauste CS, Wessendarp M, Sorensen RU, Leiva LE. 1999. CD40-CD40 ligand interaction is central to cell-mediated immunity against Toxoplasma gondii: patients with hyper IgM syndrome have a defective type 1 immune response that can be restored by soluble CD40 ligand trimer. J. Immunol. 162:6690–6700 [PubMed] [Google Scholar]
- 21. Qin H, Wilson CA, Lee SJ, Zhao X, Benveniste EN. 2005. LPS induces CD40 gene expression through the activation of NF-kappaB and STAT-1alpha in macrophages and microglia. Blood 106:3114–3122. 10.1182/blood-2005-02-0759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tone M, Tone Y, Babik JM, Lin C-Y, Waldmann H. 2002. The role of Sp1 and NF-kappa B in regulating CD40 gene expression. J. Biol. Chem. 277:8890–8897. 10.1074/jbc.M109889200 [DOI] [PubMed] [Google Scholar]
- 23. Nguyen VT, Benveniste EN. 2000. Involvement of STAT-1 and Ets family members in interferon-gamma induction of CD40 transcription in microglia/macrophages. J. Biol. Chem. 275:23674–23684. 10.1074/jbc.M002482200 [DOI] [PubMed] [Google Scholar]
- 24. Nguyen VT, Benveniste EN. 2000. IL-4-activated STAT-6 inhibits IFN-gamma-induced CD40 gene expression in macrophages/microglia. J. Immunol. 165:6235–6243. 10.4049/jimmunol.165.11.6235 [DOI] [PubMed] [Google Scholar]
- 25. Hamerman JA, Tchao NK, Lowell CA, Lanier LL. 2005. Enhanced Toll-like receptor responses in the absence of signaling adaptor DAP12. Nat. Immunol. 6:579–586. 10.1038/ni1204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Morgado P, Ong Y-C, Boothroyd JC, Lodoen MB. 2011. Toxoplasma gondii induces B7-2 expression through activation of JNK signal transduction. Infect. Immun. 79:4401–4412. 10.1128/IAI.05562-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pernas L, Boothroyd JC. 2010. Association of host mitochondria with the parasitophorous vacuole during Toxoplasma infection is not dependent on rhoptry proteins ROP2/8. Int. J. Parasitol. 40:1367–1371. 10.1016/j.ijpara.2010.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Saeij JPJ, Boyle JP, Coller S, Taylor S, Sibley LD, Brooke-Powell ET, Ajioka JW, Boothroyd JC. 2006. Polymorphic secreted kinases are key virulence factors in toxoplasmosis. Science 314:1780–1783. 10.1126/science.1133690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rosowski EE, Lu D, Julien L, Rodda L, Gaiser RA, Jensen KDC, Saeij JPJ. 2011. Strain-specific activation of the NF-kappa B pathway by GRA15, a novel Toxoplasma gondii dense granule protein. J. Exp. Med. 208:195–212. 10.1084/jem.20100717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gov L, Karimzadeh A, Ueno N, Lodoen MB. 2013. Human innate immunity to Toxoplasma gondii is mediated by host caspase-1 and ASC and parasite GRA15. mBio 4(4):e00255-13. 10.1128/mBio.00255-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408. 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- 32. Sibley LD, Boothroyd JC. 1992. Virulent strains of Toxoplasma gondii comprise a single clonal lineage. Nature 359:82–85. 10.1038/359082a0 [DOI] [PubMed] [Google Scholar]
- 33. Arends D, Prins P, Jansen RC, Broman KW. 2010. R/qtl: high-throughput multiple QTL mapping. Bioinformatics 26:2990–2992. 10.1093/bioinformatics/btq565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Khan A, Taylor S, Su C, Mackey AJ, Boyle J, Cole R, Glover D, Tang K, Paulsen IT, Berriman M, Boothroyd JC, Pfefferkorn ER, Dubey JP, Ajioka JW, Roos DS, Wootton JC, Sibley LD. 2005. Composite genome map and recombination parameters derived from three archetypal lineages of Toxoplasma gondii. Nucleic Acids Res. 33:2980–2992. 10.1093/nar/gki604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hideshima T, Chauhan D, Richardson P, Mitsiades C, Mitsiades N, Hayashi T, Munshi N, Dang L, Castro A, Palombella V, Adams J, Anderson KC. 2002. NF-kappa B as a therapeutic target in multiple myeloma. J. Biol. Chem. 277:16639–16647. 10.1074/jbc.M200360200 [DOI] [PubMed] [Google Scholar]
- 36. Khoury MK, Parker I, Aswad DW. 2010. Acquisition of chemiluminescent signals from immunoblots with a digital single-lens reflex camera. Anal. Biochem. 397:129–131. 10.1016/j.ab.2009.09.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yoshimoto T, Nagase H, Ishida T, Inoue JI, Nariuchi H. 1997. Induction of interleukin-12 p40 transcript by CD40 ligation via activation of nuclear factor-κB. Eur. J. Immunol. 27:3461–3470. 10.1002/eji.1830271247 [DOI] [PubMed] [Google Scholar]
- 38. Stout RD, Suttles J, Xu J, Grewal IS, Flavell RA. 1996. Impaired T cell-mediated macrophage activation in CD40 ligand-deficient mice. J. Immunol. 156:8–11 [PubMed] [Google Scholar]
- 39. Portillo J-AC, Okenka G, Reed E, Subauste A, Van Grol J, Gentil K, Komatsu M, Tanaka K, Landreth G, Levine B, Subauste CS. 2010. The CD40-autophagy pathway is needed for host protection despite IFN-γ-dependent immunity and CD40 induces autophagy via control of P21 levels. PLoS One 5:e14472. 10.1371/journal.pone.0014472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Andrade RM, Wessendarp M, Portillo J-AC, Yang J-Q, Gomez FJ, Durbin JE, Bishop GA, Subauste CS. 2005. TNF receptor-associated factor 6-dependent CD40 signaling primes macrophages to acquire antimicrobial activity in response to TNF-alpha. J. Immunol. 175:6014–6021. 10.4049/jimmunol.175.9.6014 [DOI] [PubMed] [Google Scholar]
- 41. Schulz O, Edwards AD, Schito M, Aliberti J, Manickasingham S, Sher A, Reis e Sousa C. 2000. CD40 triggering of heterodimeric IL-12 p70 production by dendritic cells in vivo requires a microbial priming signal. Immunity 13:453–462. 10.1016/S1074-7613(00)00045-5 [DOI] [PubMed] [Google Scholar]
- 42. Straw AD, MacDonald AS, Denkers EY, Pearce EJ. 2003. CD154 plays a central role in regulating dendritic cell activation during infections that induce Th1 or Th2 responses. J. Immunol. 170:727–734. 10.4049/jimmunol.170.2.727 [DOI] [PubMed] [Google Scholar]
- 43. Yanagawa Y, Onoe K. 2006. Distinct regulation of CD40-mediated interleukin-6 and interleukin-12 productions via mitogen-activated protein kinase and nuclear factor kappaB-inducing kinase in mature dendritic cells. Immunology 117:526–535. 10.1111/j.1365-2567.2006.02329.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mathur RK, Awasthi A, Wadhone P, Ramanamurthy B, Saha B. 2004. Reciprocal CD40 signals through p38MAPK and ERK-1/2 induce counteracting immune responses. Nat. Med. 10:540–544. 10.1038/nm1045 [DOI] [PubMed] [Google Scholar]
- 45. Lee BO, Haynes L, Eaton SM, Swain SL, Randall TD. 2002. The biological outcome of CD40 signaling is dependent on the duration of CD40 ligand expression: reciprocal regulation by interleukin (IL)-4 and IL-12. J. Exp. Med. 196:693–704. 10.1084/jem.20020845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Butcher BA, Kim L, Panopoulos AD, Watowich SS, Murray PJ, Denkers EY. 2005. Cutting edge: IL-10-independent STAT3 activation by Toxoplasma gondii mediates suppression of IL-12 and TNF-α in host macrophages. J. Immunol. 174:3148–3152. 10.4049/jimmunol.174.6.3148 [DOI] [PubMed] [Google Scholar]
- 47. Lang C, Hildebrandt A, Brand F, Opitz L, Dihazi H, Lüder CGK. 2012. Impaired chromatin remodelling at STAT1-regulated promoters leads to global unresponsiveness of Toxoplasma gondii-infected macrophages to IFN-γ. PLoS Pathog. 8:e1002483. 10.1371/journal.ppat.1002483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Niedelman W, Gold DA, Rosowski EE, Sprokholt JK, Lim D, Farid Arenas A, Melo MB, Spooner E, Yaffe MB, Saeij JPJ. 2012. The rhoptry proteins ROP18 and ROP5 mediate Toxoplasma gondii evasion of the murine, but not the human, interferon-gamma response. PLoS Pathog. 8:e1002784. 10.1371/journal.ppat.1002784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Virreira Winter S, Niedelman W, Jensen KD, Rosowski EE, Julien L, Spooner E, Caradonna K, Burleigh BA, Saeij JPJ, Ploegh HL, Frickel EM. 2011. Determinants of GBP recruitment to Toxoplasma gondii vacuoles and the parasitic factors that control it. PLoS One 6:e24434. 10.1371/journal.pone.0024434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Fentress SJ, Behnke MS, Dunay IR, Mashayekhi M, Rommereim LM, Fox BA, Bzik DJ, Taylor GA, Turk BE, Lichti CF, Townsend RR, Qiu W, Hui R, Beatty WL, Sibley LD. 2010. Phosphorylation of immunity-related GTPases by a Toxoplasma gondii-secreted kinase promotes macrophage survival and virulence. Cell Host Microbe 8:484–495. 10.1016/j.chom.2010.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Butcher BA, Fox BA, Rommereim LM, Kim SG, Maurer KJ, Yarovinsky F, Herbert DR, Bzik DJ, Denkers EY. 2011. Toxoplasma gondii rhoptry kinase ROP16 activates STAT3 and STAT6 resulting in cytokine inhibition and arginase-1-dependent growth control. PLoS Pathog. 7:e1002236. 10.1371/journal.ppat.1002236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Reese ML, Zeiner GM, Saeij JPJ, Boothroyd JC, Boyle JP. 2011. Polymorphic family of injected pseudokinases is paramount in Toxoplasma virulence. Proc. Natl. Acad. Sci. U. S. A. 108:9625–9630. 10.1073/pnas.1015980108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Saeij JPJ, Coller S, Boyle JP, Jerome ME, White MW, Boothroyd JC. 2007. Toxoplasma co-opts host gene expression by injection of a polymorphic kinase homologue. Nature 445:324–327. 10.1038/nature05395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Peixoto L, Chen F, Harb OS, Davis PH, Beiting DP, Brownback CS, Ouloguem D, Roos DS. 2010. Integrative genomic approaches highlight a family of parasite-specific kinases that regulate host responses. Cell Host Microbe 8:208–218. 10.1016/j.chom.2010.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Jensen KDC, Wang Y, Wojno EDT, Shastri AJ, Hu K, Cornel L, Boedec E, Ong Y-C, Chien Y-H, Hunter CA, Boothroyd JC, Saeij JPJ. 2011. Toxoplasma polymorphic effectors determine macrophage polarization and intestinal inflammation. Cell Host Microbe 9:472–483. 10.1016/j.chom.2011.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kim SK, Karasov A, Boothroyd JC. 2007. Bradyzoite-specific surface antigen SRS9 plays a role in maintaining Toxoplasma gondii persistence in the brain and in host control of parasite replication in the intestine. Infect. Immun. 75:1626–1634. 10.1128/IAI.01862-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Yang N, Farrell A, Niedelman W, Melo M, Lu D, Julien L, Marth GT, Gubbels M-J, Saeij JPJ. 2013. Genetic basis for phenotypic differences between different Toxoplasma gondii type I strains. BMC Genomics 14:467. 10.1186/1471-2164-14-467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Brenier-Pinchart MP, Bertini RL, Maubon D, Pelloux H. 2010. In vitro differential phenotypic characteristics among Type-II Toxoplasma gondii strains from congenital toxoplasmosis in humans. J. Parasitol. 96:798–799. 10.1645/GE-2405.1 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.