Abstract
Streptococcus pneumoniae is a globally significant pathogen that causes a range of diseases, including pneumonia, sepsis, meningitis, and otitis media. Its ability to cause disease depends upon the acquisition of nutrients from its environment, including transition metal ions such as zinc. The pneumococcus employs a number of surface proteins to achieve this, among which are four highly similar polyhistidine triad (Pht) proteins. It has previously been established that these proteins collectively aid in the delivery of zinc to the ABC transporter substrate-binding protein AdcAII. Here we have investigated the contribution of each individual Pht protein to pneumococcal zinc homeostasis by analyzing mutant strains expressing only one of the four pht genes. Under conditions of low zinc availability, each of these mutants showed superior growth and zinc accumulation profiles relative to a mutant strain lacking all four genes, indicating that any of the four Pht proteins are able to facilitate delivery of zinc to AdcAII. However, optimal growth and zinc accumulation in vitro and pneumococcal survival and proliferation in vivo required production of all four Pht proteins, indicating that, despite their overlapping functionality, the proteins are not dispensable without incurring a fitness cost. We also show that surface-attached forms of the Pht proteins are required for zinc recruitment and that they do not contribute to defense against extracellular zinc stress.
INTRODUCTION
Streptococcus pneumoniae causes a wide range of human diseases, including pneumonia, sepsis, meningitis, and otitis media, and these diseases result in huge morbidity and mortality globally (1). Among the many surface proteins that contribute to pneumococcal pathogenicity are the polyhistidine triad (Pht) proteins, a family consisting of four members (PhtA, PhtB, PhtD, and PhtE) (2). A number of functions have been ascribed to these proteins, including binding to the complement regulator factor H (3) and adherence to host epithelial cells (4, 5). However, to date, the majority of attention has focused on their contribution to metal ion homeostasis.
The defining feature of the Pht proteins is the presence of multiple copies of a histidine triad (HXXHXH) motif. Each motif within each of the Pht proteins is potentially capable of binding one Zn2+ ion, depending on protein conformational considerations (6). Zinc acquisition is critical for the survival of S. pneumoniae during infection and occurs via the ATP-binding cassette (ABC) transporter AdcBC after delivery of the metal via the substrate-binding proteins (SBPs) AdcA and/or AdcAII (7–11). There is now substantial evidence supporting the hypothesis that an important role of the Pht proteins is to facilitate Zn2+ acquisition via AdcAII (6, 9, 12). The phtD and adcAII genes are cotranscribed, and their expression is coregulated by cellular Zn2+ abundance. The two proteins have been shown to interact with one another by in vitro cross-linking and immunoprecipitation experiments (6, 12). Recently we showed that the growth of a ΔphtABDE ΔadcA mutant was highly attenuated in media containing a low concentration of available Zn2+ compared to the growth of the ΔadcA strain, and the in vivo fitness of a ΔphtABDE ΔadcA mutant was significantly less than that of the ΔphtABDE ΔadcAII strain (9). Collectively, these data are consistent with AdcA being able to acquire Zn2+ independently, whereas AdcAII requires the Pht proteins to be present for optimal functionality (9).
However, an important, yet unanswered, question is whether each of the four Pht proteins is able to aid in the delivery of Zn2+ to AdcAII or whether this function is confined to only certain members of the protein family. This issue has significance for our understanding of pneumococcal Zn2+ homeostasis, and it also has implications for vaccine development. The Pht proteins, and PhtD in particular, are targets of several novel protein-based pneumococcal vaccines currently in development. The efficacy of these formulations may depend upon stimulation of an opsonophagocytic antibody response against these surface antigens as well as blockade of their function by antibody binding (13–16). As a consequence, if the Pht proteins are redundant, cross-reactive antibodies that bind to all four proteins would be required to elicit protection based on blockade of the function(s) of the proteins, whereas if they are not redundant, such a protective effect may be derived from single Pht protein-specific antibodies. While the Pht proteins share high sequence similarity, suggesting that they may all be able to perform the same function, only phtD is cotranscribed with adcAII. Thus, it is plausible that PhtD alone may contribute to Zn2+ acquisition. A limited number of previous studies have shed some light on the issue of redundancy among the protein family. Ogunniyi et al. found that deletion of all four pht genes led to increased complement deposition on the pneumococcal surface and increased survival time after intraperitoneal challenge in a mouse model of disease relative to the wild type (3). However, strains with one or two pht genes deleted did not show significant differences from the wild type, leading to the conclusion that the Pht proteins are functionally redundant (3). However, triple pht mutants (i.e., strains with only one pht gene) were not tested in this study, limiting the strength of the conclusions that could be drawn. In another study, Rioux et al. reported that deletion of all four pht genes was necessary to observe a phenotype of impaired growth in chemically defined medium deficient in cations, whereas single, double, or triple (ΔphtBDE) mutants behaved similarly to the wild type (17). However, deletion of individual pht genes has been shown to cause a significant reduction in pneumococcal adherence to nasopharyngeal and lung cell lines, suggesting that the Pht proteins are not fully redundant in this role (4, 5).
The aim of this study was to further investigate potential functional redundancy in the Pht protein family, with specific emphasis on their role in Zn2+ homeostasis. In order to resolve earlier conflicting evidence regarding Pht protein redundancy, we assessed their functionality in a more comprehensive manner than has been performed previously by generating and examining the phenotypes of all four possible pht triple mutants (ΔphtBDE, ΔphtADE, ΔphtABE, and ΔphtABD), allowing examination of the ability of each Pht protein to contribute to Zn2+acquisition in the absence of the three other Pht proteins.
MATERIALS AND METHODS
Strains and growth media.
Defined, nonpolar deletion replacement or unmarked pneumococcal mutants of Streptococcus pneumoniae strain D39 lacking phtA, phtB, phtD, phtE or adcA had been generated previously (3, 9). Combination mutants used in this study were generated by amplifying the relevant loci from the preexisting mutants by PCR and using these loci to transform the appropriate background strain using established methods (18, 19). PCR, DNA sequencing, and immunoblotting were used to confirm that the desired mutations had been introduced. A complete list of strains used in this study is shown in Table 1. Opaque phase variants were used in all experiments. Bacteria were routinely grown at 37°C in a CO2-enriched atmosphere on Columbia agar supplemented with 5% (vol/vol) horse blood. If the bacteria would be used to challenge mice, they were grown in serum broth (10% heat-inactivated horse serum in nutrient broth). Alternatively, cation-defined medium (CDM) (prepared as described previously [9, 20]) was used. To assess the effect of Zn2+ starvation, the chelating agent N,N,N′,N′-tetrakis(2-pyridylmethyl)ethylenediamine (TPEN) was supplemented at an equimolar concentration to Zn2+ determined to be in CDM by inductively coupled plasma mass spectrometry (ICP-MS) to provide CDM-TPEN. A single batch of CDM was used for all experiments in this study, and it was supplemented with 13 μM TPEN to yield CDM-TPEN when required.
TABLE 1.
S. pneumoniae strains used in this study
| S. pneumoniae strain | Description or characteristic(s) | Source or reference |
|---|---|---|
| D39 | Serotype 2 | NCTC7466 |
| ΔadcA mutant | 9 | |
| ΔphtABDE ΔadcAII mutant | 9 | |
| ΔphtABDE ΔadcA mutant | 9 | |
| ΔphtBDE ΔadcA mutant | Expresses only PhtA of the four Pht proteins | This study |
| ΔphtADE ΔadcA mutant | Expresses only PhtB of the four Pht proteins | This study |
| ΔphtABE ΔadcA mutant | Expresses only PhtD of the four Pht proteins | This study |
| ΔphtABD ΔadcA mutant | Expresses only PhtE of the four Pht proteins | This study |
| PhtD 3 Ala ΔadcA mutant | D39 ΔphtABDE mutant complemented with phtD with amino acid substitutions R26A, H27A, and Q28A; adcA replaced by chloramphenicol acetyltransferase gene | 19 |
| PhtE 3 Ala ΔadcA mutant | D39 ΔphtABDE mutant complemented with phtE with amino acid substitutions Q27A, H28A, and R29A; adcA replaced by chloramphenicol acetyltransferase gene | 19 |
| ΔadcAII mutant | 9 |
Growth analysis.
For growth curve measurements, wild-type S. pneumoniae D39 and mutant strains were grown in CDM with 1 μM MnSO4 until they reached an optical density at 600 nm (OD600) of 0.4. They were then subcultured into 200 μl CDM, CDM-TPEN, or CDM supplemented with ZnSO4. The bacteria were incubated at 37°C, and growth was monitored by measurement of the OD600 at 30-min intervals.
Whole-cell metal ion accumulation.
S. pneumoniae strains were grown in CDM-TPEN to an OD600 of 0.4. The bacteria were washed three times with phosphate-buffered saline (PBS) containing 5 mM EDTA and then washed twice with PBS, with centrifugation at 12,000 × g for 10 min at 4°C between each wash. Bacterial pellets were desiccated by heating to 95°C overnight. The dry mass of cellular material was recorded and then resuspended in 35% HNO3. The metal ion content of the dissolved cellular material was measured on an Agilent 7500cx inductively coupled plasma mass spectrometer (Adelaide Microscopy).
Quantitative immunoblot and qRT-PCR analyses.
Wild-type and mutant S. pneumoniae bacteria were grown under the same conditions as for the whole-cell metal ion accumulation experiments. After the cultures reached an OD600 of 0.4, they were split into samples to be processed for protein and RNA extraction. For protein extraction, cells were incubated with 0.1% sodium deoxycholate (Sigma-Aldrich) at 37°C for 60 min to induce lysis. Protein concentrations were determined (DC Bio-Rad protein assay; Bio-Rad), and 20 μg of total protein was loaded into each lane. After electrophoretic separation by SDS-PAGE, proteins were transferred to a nitrocellulose membrane using the iBlot (Life Technologies) system. The blots were incubated with previously generated murine antisera against the relevant Pht protein (16). The blots were then incubated with anti-mouse IRDye 800 and scanned using an Odyssey infrared imaging system (LI-COR Biosciences). Band intensities were measured using the manufacturer's application software. For measurement of absolute molar quantities of protein, a standard curve with a range of quantities of the relevant purified Pht protein was included in the Western blot and used to calibrate the band intensities measured for cell lysates against absolute amounts, and the predicted molecular weights of the proteins were used to convert to molar quantities (16).
For RNA extraction and reverse transcription-PCR (RT-PCR) analysis, 500 μl of culture was mixed with 1 ml of RNA protect (Qiagen). RNA was extracted and purified using an RNeasy Protect Bacteria minikit (Qiagen) after enzymatic lysis using lysozyme and mutanolysin, all according to the manufacturer's instructions. The total RNA samples were treated with DNase I (Roche), and quantitative RT-PCR (qRT-PCR) was carried out using a SuperScript III one-step RT-PCR kit (Invitrogen) on an LC480 real-time cycler (Roche). Transcription levels of genes analyzed were normalized to those obtained for 16S rRNA. Primer sequences are shown in Table 2.
TABLE 2.
Oligonucleotide primers used for qRT-PCR in this study
| Primera | Sequence (5′→3′) |
|---|---|
| 16s_F | CATGCAAGTAGAACGCTGAA |
| 16s_R | TGTCATGCAACATCCACTCT |
| phtA_F | AGTCTGAAAGCCAATGCAACAG |
| phtA_R | CCTTACTTACAGATGAAGGATTAC |
| phtB_F | CCAACAGAGGAACCAGAAGAAG |
| phtB_R | TAATTGGATTCTGGATTTTTCC |
| phtD_F | GTATTAGACAAAATGCTGTGGAG |
| phtD_R | CTGTATAGGAGTCGGTTGACTTTC |
| phtE_F | GGAACAGTTGAGAACCAACCA |
| phtE_R | TAGGGTCACTCCCCACATTC |
The direction of the primer is indicated by the letter at the end of the primer designation as follows: F, forward; R, reverse.
Assessment of bacterial fitness by in vivo competition experiments.
Outbred 5- to 6-week-old female CD1 (Swiss) mice were used in all animal experiments. Mice were anesthetized by intraperitoneal injection of pentobarbital sodium (Nembutal; Rhone-Merieux) at a dose of 66 μg per g of body weight, followed by intranasal administration of 50 μl of bacterial suspension containing an equal mixture of the two mutant strains, with a total of approximately 1 × 107 CFU. The challenge dose and input ratio were confirmed retrospectively by serial dilution and plating on blood agar followed by patching of colonies onto blood agar plates with and without antibiotic to allow discrimination of the two strains.
At 24 and 48 h postchallenge, mice were euthanized by CO2 asphyxiation. Blood was collected from the posterior vena cava by using a syringe. The pleural cavity was lavaged with 1 ml sterile PBS containing 2 mM EDTA introduced through the diaphragm. Pulmonary vasculature was perfused by infusion of sterile PBS through the heart. The lungs were subsequently excised. The trachea was then exposed, and 1 ml of PBS containing 0.5% (wt/vol) trypsin (Life Technologies) was inserted into the tracheal end of the upper respiratory tract and collected from the nose (nasal wash). Last, the nasopharynx/upper palate was excised (nasal tissue). Tissues were homogenized, and all samples were serially diluted and plated on blood agar. Colonies were subsequently patched onto blood plates with and without the relevant antibiotic to allow discrimination of the two mutant strains and determination of output ratios. Competitive indices (the ratio of one mutant strain to the other mutant strain relative to the input ratio) were then calculated and compared to a theoretical value of 1 (which would indicate no difference in fitness between strains) by one-sample t tests.
All procedures performed in this study were conducted with a view to minimizing the discomfort of the animals and used the minimum numbers of animals to generate reproducible and statistically significant data. All experiments were approved by the University of Adelaide Animal Ethics Committee (Animal Welfare Assurance number A5491-01; project approval number S-2013-053) and were performed in strict adherence to guidelines dictated by the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes.
RESULTS
Growth of pht-deficient strains under conditions of low Zn2+ availability.
In order to examine the contributions of the individual Pht proteins to Zn2+ homeostasis, mutant strains were constructed lacking all possible combinations of three out of the four pht genes (ΔphtBDE, ΔphtADE, ΔphtABE, and ΔphtABD mutants). Our prior study showed that the contribution of the Pht proteins to Zn2+ acquisition by AdcAII was most pronounced when the other Zn2+-recruiting substrate-binding protein (SBP), AdcA, was absent (9). Consequently, we also deleted the adcA gene from all strains in order to obviate its effect on Zn2+ uptake. Therefore, the resultant mutant strains were entirely reliant upon AdcAII and either PhtA, PhtB, PhtD, or PhtE alone for Zn2+ import. A complete list of strains used in this study is shown in Table 1.
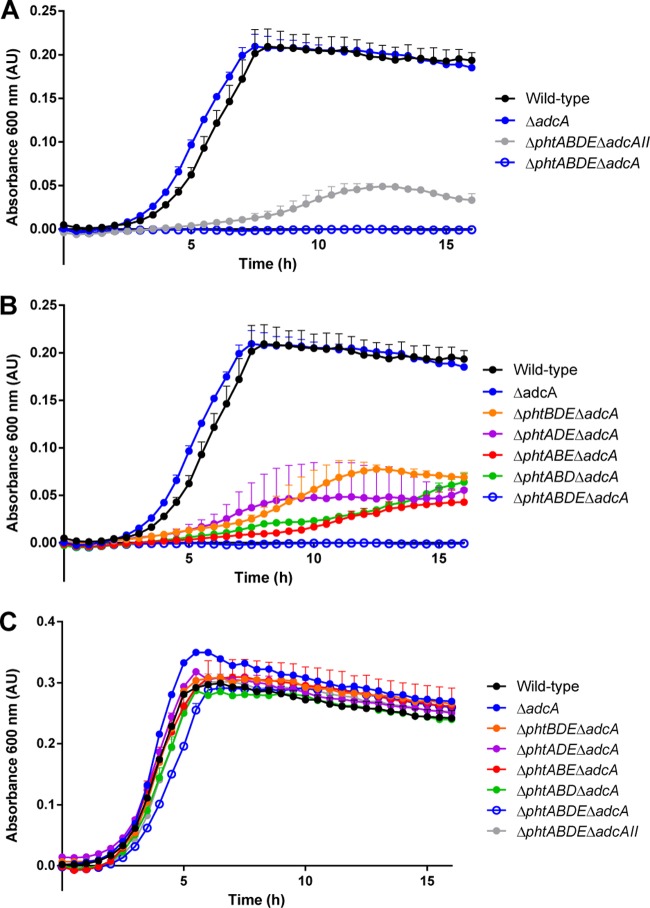
For growth experiments, cation-defined medium (CDM) was prepared, and its concentration of Zn2+ was measured by ICP-MS. As Zn2+ cannot be completely removed from any culture medium, we then restricted the bioavailability of Zn2+ by use of the chelating agent TPEN at an equimolar concentration to Zn2+, as determined by ICP-MS of the CDM, to yield the culture medium CDM-TPEN, as described previously (9, 20). CDM-TPEN contains negligible bioavailable Zn2+, as TPEN has a KD (equilibrium dissociation constant) for Zn2+ of ∼2.6 × 10−16 M. As a consequence, bacteria grown in this medium are critically dependent on bacterial high-affinity Zn2+-recruiting proteins, which have experimentally reported KDs for Zn2+ in the nanomolar range. Initially, growth of the wild-type, ΔadcA, ΔphtABDE ΔadcAII, and ΔphtABDE ΔadcA strains was compared (Fig. 1A). Of the latter two strains, only the ΔphtABDE ΔadcAII mutant was able to grow (albeit to a much lesser extent than the wild type), consistent with our previous observation that AdcAII is more reliant on the Pht proteins to obtain Zn2+ than AdcA is (9). We then examined strains with only one of the four pht genes in the absence of AdcA (Fig. 1B). These mutant strains displayed a much lower rate of growth and lower final cell density than the ΔadcA mutant, which grew in a manner indistinguishable from the wild type. However, the mutant strains containing a single pht gene all showed superior growth compared to the ΔphtABDE ΔadcA strain, which was not able to grow in CDM-TPEN. There was no defect in the growth of any of the strains in the absence of TPEN (Fig. 1C), indicating that the differences observed were caused by the lack of available Zn2+ in the medium. Therefore, these results show that all four of the Pht proteins are capable of contributing to Zn2+ recruitment by AdcAII. However, these results also show that the Pht proteins are not fully redundant, in the sense of being dispensable, as strains producing only one Pht protein did not grow as effectively as when all four proteins were present.
FIG 1.
In vitro growth measurements of pneumococcal strains. (A and B) The indicated strains were grown in CDM-TPEN (i.e., under Zn2+-restricted conditions) at 37°C, and growth was monitored by OD600 measurements every 30 min. The absorbance at 600 nm or OD600 is shown in arbitrary units (AU). (C) Control growth in unsupplemented CDM (i.e., Zn2+-replete conditions). Data are representative mean (plus standard error of the mean [SEM] [error bar]) OD600 measurements from three independent biological experiments.
Surface attachment of Pht proteins is required for Zn2+ recruitment.
In a previous study, we identified amino acids in PhtD and PhtE that are critical for the proteins' surface attachment. We further showed that a significant proportion (up to 50% in strain D39) of the total amount of each Pht protein produced was released into the culture medium during planktonic growth, even in wild-type strains (19). It is not clear what biological role, if any, this material performs. As a consequence, we sought to investigate whether surface attachment of the Pht proteins was required for Zn2+ acquisition. To assess this, we used strains containing only a single Pht protein, into which the specific amino acid substitutions R26A, H27A, and Q28A in PhtD or Q27A, H28A, and R29A in PhtE had been introduced, resulting in complete loss of surface attachment of the proteins (19). These mutant strains were also generated without adcA to elucidate the impact of surface attachment of PhtD or PhtE on AdcAII Zn2+ uptake in this background. The growth of the resultant strains (PhtD 3 Ala ΔadcA and PhtE 3 Ala ΔadcA) in CDM-TPEN was compared to the ΔphtABE ΔadcA and ΔphtABD ΔadcA strains. The results, shown in Fig. 2, demonstrate that the PhtD 3 Ala ΔadcA and PhtE 3 Ala ΔadcA strains were unable to grow in CDM-TPEN, indicating that surface attachment of PhtD and PhtE is essential for their contribution to Zn2+ acquisition.
FIG 2.
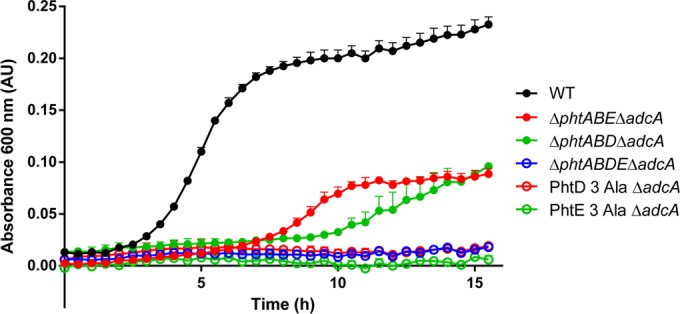
In vitro growth comparison of strains with or without surface-attached Pht proteins. Bacteria were grown in CDM-TPEN (i.e., under Zn2+-restricted conditions) at 37°C, and growth was monitored by OD600 measurements every 30 min. Data are representative mean (plus SEM) OD600 measurements from three independent biological experiments. WT, wild type.
The Pht proteins do not aid in defense against extracellular Zn2+ stress.
The results of this work and prior studies support the involvement of the Pht proteins in facilitating Zn2+ acquisition from the environment under conditions where its availability is tightly restricted (9). However, it is also plausible that they could help defend the pneumococcus when an excess of the metal ion is present in the extracellular environment, which exerts a toxic effect on the bacteria by interfering with the physiological function of the ABC permease, PsaBCA, in importing the essential transition metal ion manganese (20, 21). It has been speculated that the Pht proteins may aid in protecting the PsaBCA permease from extracellular Zn2+ by binding excess ions and acting as a “sink” (12, 22). To test this hypothesis, wild-type and ΔphtABDE pneumococci were first grown in CDM-TPEN to induce maximal expression of pht genes (6, 9) and then subcultured into CDM supplemented with increasing concentrations of ZnSO4 (Fig. 3). As expected, high extracellular concentrations of Zn2+ impaired bacterial growth and resulted in reduced final cell densities. However, no phenotypic differences were apparent between the wild-type and ΔphtABDE strains in any of the culturing conditions, indicating that the Pht proteins did not protect the pneumococcus against extracellular Zn2+ stress under the conditions examined.
FIG 3.
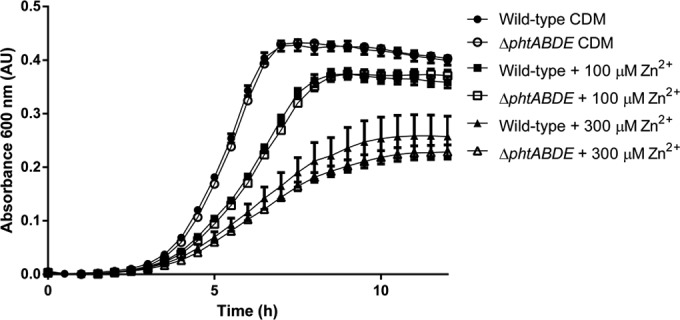
In vitro growth comparison of wild-type and ΔphtABDE strains grown under conditions of high extracellular Zn2+ stress. Bacteria were grown in CDM supplemented with ZnSO4 at 37°C, and growth was monitored by OD600 measurements every 30 min. Data are representative mean (plus SEM) OD600 measurements from three independent biological experiments.
Metal ion accumulation of pht-deficient strains under conditions of low Zn2+ availability.
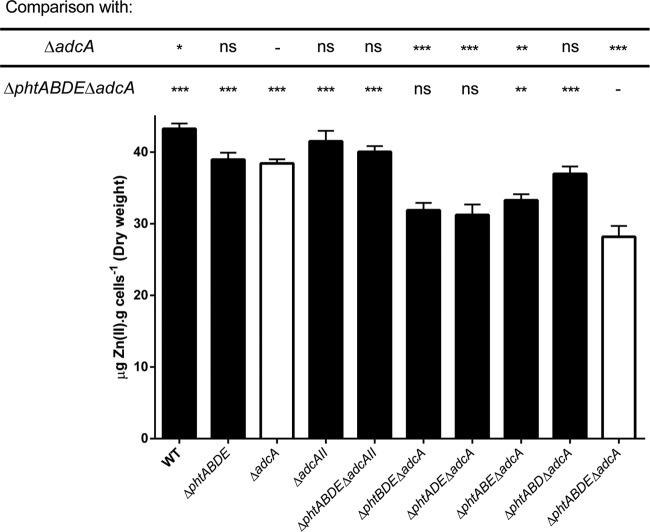
To further examine the impact of possessing only one pht gene, whole-cell metal accumulation was measured for all relevant mutant strains after growth in CDM-TPEN to an OD600 of 0.4 (Fig. 4). It should be noted that all mutants required approximately 5 h to reach this density, except for the ΔphtABDE ΔadcA control strain, which took approximately 8 h. This strain also had the smallest amount of intracellular Zn2+, and since Zn2+ acquisition was the major limiting factor for its growth, we infer that the 30 μg of Zn2+ per g cells (dry weight) it was found to contain represents the minimum required for pneumococcal viability. In contrast, Zn2+ acquisition in the ΔphtABDE ΔadcAII strain, where AdcA was the only protein present responsible for uptake of the metal, was impaired to a lesser extent, consistent with our prior results (9). In the strains carrying only one of the four pht genes, Zn2+ accumulation was more than for the ΔphtABDE ΔadcA strain. This showed that all of the Pht proteins were capable of aiding in Zn2+ acquisition via AdcAII, consistent with the phenotypic growth experiments (Fig. 1B). Intriguingly, the ΔphtABD ΔadcA strain (i.e., AdcAII and PhtE present) showed more Zn2+ accumulation than the other strains with a single pht gene. Statistical analysis by one-way analysis of variance (ANOVA) with Dunnett's posttest to compare all strains to the ΔphtABDE ΔadcA mutant showed significant differences in all cases except for the ΔphtBDE ΔadcA and ΔphtADE ΔadcA strains. The same test was also applied to compare all strains to the ΔadcA mutant; of the strains with only one pht gene, only the ΔphtABD ΔadcA mutant did not show a significant difference. Taken together, these results show that production of only one Pht protein allows more Zn2+ accumulation than when all four Pht proteins are absent but less than when the four Pht proteins are present.
FIG 4.
Whole-cell Zn2+ accumulation of wild-type and pht or adc mutant strains determined by ICP-MS. Error bars indicate SEMs. All strains were compared to the ΔadcA and ΔphtABDE ΔadcA mutants (white bars) by one-way ANOVA using Dunnett's posttest, and statistical significance is indicated as follows: ns, not significant; *, P < 0.05; **, P < 0.01; ***, P < 0.001. Data are means (plus SEMs) from a minimum of three biological replicates.
Expression levels of individual pht genes in pht-deficient strains.
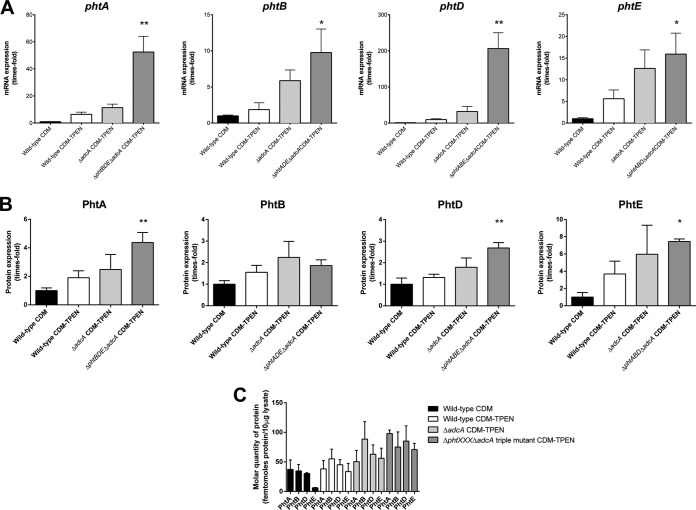
To complement the above analyses, changes in expression levels of each of the Pht proteins in Zn2+-replete and Zn2+-starved conditions were measured. We hypothesized that strains lacking adcA and three out of the four pht genes would show greater expression levels of their one remaining pht gene under Zn2+-starved conditions than the adcA mutant, since as shown by the previous experiments, they cannot accumulate as much Zn2+ and face a greater challenge to grow in CDM-TPEN. Conversely, it was also feasible that there would be no difference in pht expression between these strains if pht expression were already maximally derepressed in the ΔadcA mutant. To investigate this, strains were grown in CDM-TPEN, and mRNA and protein expression levels of each of the pht genes and proteins was measured by qRT-PCR and Western blotting, respectively, and compared to that of the wild type grown in CDM (Fig. 5). The results reveal that, in general, pht expression was greatest in strains lacking adcA and three out of four pht genes, consistent with the difficulty these strains experience in obtaining sufficient quantities of Zn2+ to grow. Furthermore, phtA and phtD transcripts were more abundant in their respective triple pht mutant strains than in the strain lacking adcA alone. In contrast, expression levels of phtB and phtE were similar in the ΔadcA mutant and the relevant ΔadcA triple pht mutant, although a minor but statistically significant difference was observed for phtE. For a control, the expression of the remaining intact Pht proteins in the triple mutants was measured after growth in CDM, and no differences were found from that of the wild type, indicating that the induction of expression was specifically caused by the addition of TPEN to the medium (data not shown).
FIG 5.
Analysis of Pht expression levels in wild-type (white bars), ΔadcA (light gray bars), or ΔphtXXX (XXX represents three pht loci) ΔadcA (dark gray bars) strains grown in CDM-TPEN, compared to the wild-type strain grown in CDM (black bars). (A and B) mRNA (A) and protein (B) expression levels are shown, normalized to levels in the wild type grown in CDM. For mRNA, measurements were made relative to 16S rRNA. Error bars depict SEMs. Strains were compared to the wild type grown in CDM by one-way ANOVA using Dunnett's posttest, and statistically significant differences where present are indicated as follows: *, P < 0.05; **, P < 0.01. (C) Absolute protein expression levels of PhtA, PhtB, PhtD, and PhtE, expressed as femtomoles per 10 μg of bacterial lysate. Results are representative of three independent experiments.
To complement this analysis, we also quantified the absolute level of protein expression of each of the Pht proteins, since if one of the proteins were much more abundant in molar terms than any of the others, it could be speculated that it would play a more important role in Zn2+ import. However, as shown in Fig. 5C, the amounts of the Pht protein were largely similar in the given mutant strains, with the exception of PhtE in the wild type grown in CDM. The expression of this protein appears to be more strongly repressed in this medium, allowing for a greater fold increase when strains are grown in CDM-TPEN.
Collectively, these data show that expression of PhtB and PhtE was already maximally derepressed in the ΔadcA mutant and could not be further increased even when the degree of Zn2+ starvation was increased by deletion of the other three pht genes. In contrast, PhtA and PhtD could be further derepressed under these circumstances.
Redundancy of Pht proteins in vivo: four Phts are better than one.
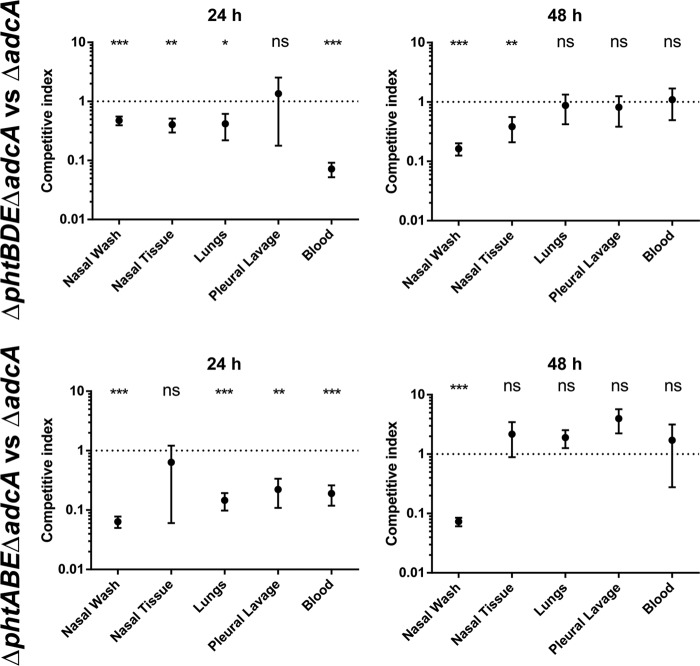
To compare the fitness of the pht-deficient strains in vivo, a series of competition experiments were performed in which mice were infected intranasally with a mixed culture of two mutant strains. After 24 and 48 h, mice were euthanized, and bacteria were enumerated from the indicated niches. Results are displayed as competitive indices, calculated as the ratio of the number of antibiotic-resistant bacteria to the control strain relative to the input ratio (Fig. 6).
FIG 6.
In vivo competition between the ΔadcA mutant and strains lacking three out of four pht genes (ΔphtBDE ΔadcA [top graphs] and ΔphtABE ΔadcA [bottom graphs] mutants). Eight mice were used per time point per group. Results are shown as mean (±SEM) competitive indices (the ratio of ΔphtXXX ΔadcA bacteria to ΔadcA bacteria corrected for the input ratio). The mean competitive index was compared to a theoretical mean of 1 (dotted line) by one-sample t tests, and statistical significance is indicated as follows: *, P < 0.05; **, P < 0.01; ***, P < 0.001; ns, not significant.
We first sought to examine the impact of loss of three out of four pht genes in vivo, so the ΔadcA mutant was used in competition with the ΔphtBDE ΔadcA mutants and with the ΔphtABE ΔadcA mutants. The experiments were restricted to these mutant strains due to ethical considerations regarding the number of mice required to assess each strain. The “PhtA-only” and “PhtD-only” strains were selected as PhtD is the leading vaccine candidate and thus of the greatest interest, while PhtA is encoded at a separate gene locus from the gene encoding PhtD and may provide a different insight into the functional roles of these proteins. After 24 h, the strain expressing only PhtA (ΔphtBDE ΔadcA mutant) was significantly less competitive than the control ΔadcA mutant in all niches except for pleural lavage fluid, while the strain expressing only PhtD (ΔphtABE ΔadcA mutant) was significantly less competitive in all niches except for nasal tissue at this time point. At 48 h, the strain expressing only PhtA was significantly attenuated in the nasal wash fluid and nasal tissue, and the strain expressing only PhtD was significantly attenuated in the nasal wash fluid alone. These results suggest that the Pht proteins are not fully redundant in vivo, since the strains containing only one out of the four pht genes were significantly less competitive in a number of instances compared to the ΔadcA mutant, which had all four pht genes intact. It is also apparent that production of all four Pht proteins may be of greatest importance at the early stage of infection, as in most niches, the differences present at 24 h were no longer apparent after 48 h.
The Pht proteins have roles outside facilitating Zn2+ acquisition through AdcAII.
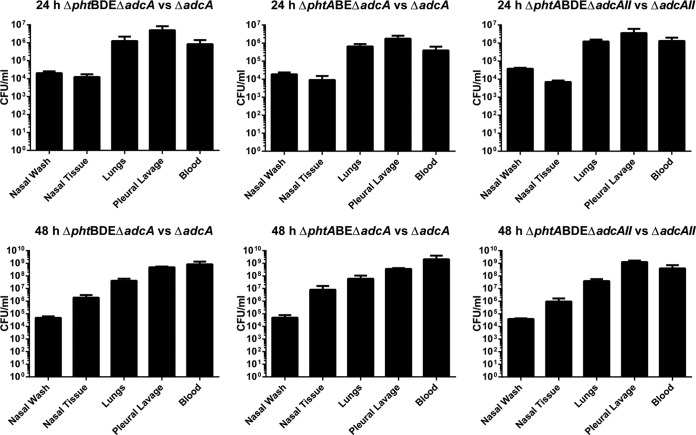
It was also of interest to assess whether the Pht proteins had role(s) outside facilitating Zn2+ acquisition through AdcAII. To achieve this, a competition experiment was performed using the ΔadcAII and ΔphtABDE ΔadcAII strains. The results of the competition experiment are shown in Fig. 7. The ΔadcAII mutant was significantly more competitive after 24 h in the lungs, pleural lavage fluid, and blood, indicating that Pht proteins do contribute to pathogenesis independently of AdcAII at this time point. However, this was not the case after 48 h. Interestingly, the strain lacking pht genes was significantly more competitive in the nasal wash fluid at this time point. For reference, the absolute numbers of CFU recovered from all animal model experiments (as opposed to competitive indices) are shown in Fig. 8.
FIG 7.
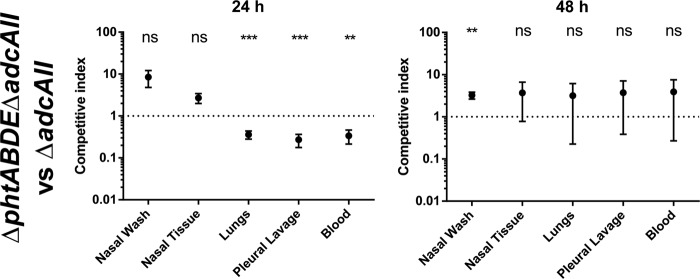
In vivo competition between the ΔphtABDE ΔadcAII and ΔadcAII mutants. Eight mice were used per time point. Results are shown as mean (±SEM) competitive indices (the ratio of ΔphtABDE ΔadcAII bacteria to ΔadcAII bacteria corrected for the input ratio). The mean competitive index was compared to a theoretical mean of 1 (dotted line) by one-sample t tests, and statistical significance is indicated as follows: *, P < 0.05; **, P < 0.01; ***, P < 0.001; ns, not significant.
FIG 8.
Numbers of CFU recovered from animal model experiments. Values are shown as means plus SEMs.
DISCUSSION
The Pht proteins are an important family of surface-associated virulence factors with potential to be used in novel pneumococcal protein-based vaccines (13–16). In this work we have investigated whether the four Pht proteins act redundantly in contributing to Zn2+ homeostasis. Under conditions of low Zn2+ availability, the presence of any one of the four proteins permitted superior growth and increased intracellular Zn2+ accumulation relative to when all four were absent. These findings demonstrate that all four of the Pht proteins aid in facilitating pneumococcal Zn2+ acquisition by AdcAII. Notably, they also clarify the debate over the contribution of PhtE, the most divergent member of the family in terms of primary structure (32% identity with the other family members, compared to over 60% identity shared between PhtA, PhtB, and PhtD [2]), to Zn2+ homeostasis. Indeed, the ΔphtABD ΔadcA strain showed the greatest whole-cell accumulation of Zn2+ among the strains producing only one Pht protein. However, despite this observation, the growth experiments did not reveal any consistent differences between the four mutant strains with a single pht gene. Thus, these findings indicate that each Pht protein has a similar capacity to facilitate Zn2+ import via AdcAII. We also observed that surface attachment is required for the proteins to play this role, thereby ruling out the possibility of a siderophore-like mechanism for Zn2+ recruitment. Furthermore, our data also indicate that the Pht proteins do not contribute to defense against extracellular Zn2+ stress.
A recent study concluded that Zn2+ transfer from PhtD to AdcAII involves a direct interaction (12). Since the metal-binding site of AdcAII is buried ∼10 to 15 Å beneath the surface of the protein (23), free Zn2+ ions may not be easily acquired by this site. A direct interaction with a Zn2+-loaded Pht protein may enable binding of the metal ion by inducing a conformational change in AdcAII to present the Zn2+ to the metal-binding site. This interaction may serve an analogous role to the proposed capture of Zn2+ in AdcA by its ZinT-like domain and histidine-rich loop region (9). While the possibility of a direct interaction of AdcAII with PhtA, PhtB, and PhtE has not been examined, we may speculate that they would also operate to deliver Zn2+ to AdcAII in this direct manner. This would presumably predominantly involve the highly conserved N termini of the Pht proteins, which are also closer to the cell membrane (and thus to AdcAII) than the more surface-exposed C-terminal regions. However, the immunoprecipitation experiments described by Bersch et al. (12) used purified cell membranes, which may not accurately represent the conditions found in intact cells; furthermore, a stable complex between PhtD and AdcAII could not be detected by nuclear magnetic resonance (NMR) spectroscopy in their study (12). Previous cross-linking evidence purportedly showing that the two purified proteins interact with one another in vitro was also somewhat weak, since only a small proportion of the purified PhtD became associated with AdcAII, in spite of the latter being present at 8-fold molar excess (6). Therefore, the possibility that transfer of Zn2+ to AdcAII by the Pht proteins may occur in the absence of a physical interaction cannot be discounted. The transfer of Zn2+ to AdcAII may instead simply rely upon the Pht proteins increasing the metal's local concentration near the cell membrane, thereby increasing the frequency of the metal encountering AdcAII. Further experimental investigation will be required to discriminate between the direct and indirect models of Zn2+ delivery to AdcAII by the Pht proteins.
Previous studies have either pointed to complete redundancy among the Pht proteins (3, 17) or observed a phenotypic impact when only one pht gene was deleted, consistent with the proteins being nonredundant (4). In this study, we observed partial redundancy, whereby strains producing only one Pht protein showed growth phenotypes and Zn2+ accumulation levels intermediate between those of strains producing either all four Pht proteins or no Pht protein. Simply put, in terms of Zn2+ acquisition, production of one Pht protein was better than none, but not as effective as having all four. Thus, the Pht proteins have overlapping functionality, and while the data reflect that they all aid in Zn2+ acquisition via AdcAII, they are not redundant in the sense of being dispensable, as deletion of three out of four of the genes led to a fitness cost both in vitro and in vivo.
This was also reflected in the observed levels of expression of each of the Pht proteins, which in general terms increased in proportion to the severity of the challenge of obtaining a sufficient quantity of Zn2+ experienced by the particular strain under examination. Thus, strains that lacked three of the four pht genes (as well as adcA) showed the greatest levels of pht expression when grown in CDM-TPEN. However, differences were observed between the Pht proteins with respect to when their expression became maximally derepressed. PhtB and PhtE protein expression appeared to be fully derepressed under Zn2+ restriction irrespective of whether the other pht genes were present. In contrast, expression of PhtA and PhtD showed further derepression in the absence of the other Pht proteins. Thus, there are differences in the degree of regulation of the pht genes in response to Zn2+ restriction and the ability of the pneumococcus to acquire it. This may indicate that carrying four pht genes could be of benefit to pneumococcal Zn2+ acquisition by allowing several “layered” increases in the expression of proteins required for this process, depending on the precise extent of the challenge the bacteria face in acquiring a sufficient quantity of the metal. This observation also mirrors our previous findings that the fold change in expression of AdcAII is greater than that of AdcA when Zn2+-restricted and Zn2+-replete conditions are compared. That finding led to the conclusion that the role of AdcA is to provide a baseline level of Zn2+ recruitment, with AdcAII providing a greater enhancement in Zn2+ acquisition when the ion is particularly scarce (9). It is interesting to note that PhtE showed a greater fold change in protein levels than the other Pht proteins, and this may be linked to the slightly elevated Zn2+ accumulation in the “PhtE-only” ΔphtABD ΔadcA mutant compared to the other mutants lacking three out of four pht genes. The presence of six histidine triad motifs in PhtE, which is one more than is found in the other three pneumococcal Pht proteins, may also contribute to the increased Zn2+ accumulation in this mutant. Furthermore, these results further support the notion of overlapping functionality of the Pht proteins, in that they suggest that the impairment in growth of the strains lacking three pht genes is due to a lower total amount of Pht protein expressed in each strain. Intriguingly, a degree of cross-reactivity was observed in the Western blots between different Pht family members for each antiserum (the antisera were generated against individual Pht proteins [16]), as has been observed previously (13). Immunization with only one Pht protein may therefore be sufficient to induce protective immunity targeting multiple members of the protein family.
The results of the animal experiments were in agreement with the conclusion from the in vitro work that the Pht proteins are all capable of performing the same role in Zn2+ uptake, but they are not dispensable without incurring a fitness cost. A clear attenuation in fitness in vivo was observed for strains producing either PhtA or PhtD alone compared to the ΔadcA control strain (Fig. 6), consistent with production of more than one Pht protein being beneficial in pneumococcal pathogenesis. We also observed that, in the absence of AdcAII, the Pht proteins do still contribute to pathogenesis at 24 h postinfection. Our experiments thus far have clearly demonstrated a role for the Pht proteins in aiding Zn2+ acquisition by AdcAII but have not definitively determined whether they also contribute to the functionality of AdcA; such a role could explain the results observed here. Alternatively, the proposed adherence and factor H binding properties of the Pht proteins, or a combination of all of these roles, may account for this AdcAII-independent contribution to pathogenesis (3–5). The finding that the ΔphtABDE ΔadcAII mutant was more competitive in the nasal wash after 48 h may also be explained by the Pht proteins playing a role in adherence to host surfaces, since this strain may be expected to adhere more weakly than the ΔadcAII mutant and thus be overrepresented in the nasal wash sample.
Across both sets of animal experiments, it can be seen that in most cases mutants lacking pht genes were significantly attenuated in various niches at 24 h postinfection, but they had recovered by 48 h. This implies that the challenge of acquiring a sufficient amount of Zn2+ is more profound for the pneumococcus at the earlier stages of infection. Indeed, this is supported by our previous observation that single mutants lacking adcA or adcAII are attenuated compared to the wild type in the nasopharynx at 24 but not 48 h postinfection (9). We may speculate that the array of Zn2+-binding proteins employed by S. pneumoniae reflects the severity of this challenge. The pneumococcus is unusual among pathogenic bacteria that infect the nasopharynx in its possession of two Zn2+-specific SBPs. These, in conjunction with the Pht proteins, may provide an advantage over other microorganisms with which the pneumococcus has to compete to colonize the nasopharynx, since they may allow more-effective acquisition of the limited quantities of available Zn2+. We have previously shown that, at 48 h postinfection, host Zn2+ levels are elevated and that this may be an immune mechanism used to expose the pneumococcus to a toxic excess of Zn2+ (20). The release of Zn2+ is consistent with the recovery (in terms of competitive indices) of strains that are deficient in Zn2+ uptake at this time point observed in this study, since the increase in abundance of the metal means its acquisition would no longer pose a significant challenge. Furthermore, such a recovery for strains lacking pht genes is also consistent with the Pht proteins not contributing to defense against Zn2+ toxicity, as we found to be the case in vitro.
In other streptococci, adcAII homologues are often found with only one pht gene in the genome (24). Given that we have shown that all four Pht proteins of S. pneumoniae have overlapping functionality with respect to aiding in delivery of Zn2+ to AdcAII, it is not apparent what the advantage of producing four such proteins is, as opposed to perhaps simply producing a greater quantity of one Pht protein. Further studies will be required to examine whether each Pht protein does make a distinct contribution to pneumococcal fitness, either in terms of Zn2+ uptake or in an alternative virulence role. Such understanding will be crucially important to guide future vaccine development targeting this intriguing family of surface proteins.
ACKNOWLEDGMENTS
This work was supported by the National Health and Medical Research Council (NHMRC) (program grant 565526 to J.C.P. and project grant 1022240 to C.A.M.) and the Australian Research Council (discovery project grants DP120101432 and DP120103957 to J.C.P. and C.A.M., respectively). J.C.P. is an NHMRC Senior Principal Research Fellow.
Footnotes
Published ahead of print 28 July 2014
REFERENCES
- 1. Walker CLF, Rudan I, Liu L, Nair H, Theodoratou E, Bhutta ZA, O'Brien KL, Campbell H, Black RE. 2013. Global burden of childhood pneumonia and diarrhoea. Lancet 381:1405–1416. 10.1016/S0140-6736(13)60222-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Plumptre CD, Ogunniyi AD, Paton JC. 2012. Polyhistidine triad proteins of pathogenic streptococci. Trends Microbiol. 20:485–493. 10.1016/j.tim.2012.06.004 [DOI] [PubMed] [Google Scholar]
- 3. Ogunniyi AD, Grabowicz M, Mahdi LK, Cook J, Gordon DL, Sadlon TA, Paton JC. 2009. Pneumococcal histidine triad proteins are regulated by the Zn2+-dependent repressor AdcR and inhibit complement deposition through the recruitment of complement factor H. FASEB J. 23:731–738. 10.1096/fj.08-119537 [DOI] [PubMed] [Google Scholar]
- 4. Khan MN, Pichichero ME. 2012. Vaccine candidates PhtD and PhtE of Streptococcus pneumoniae are adhesins that elicit functional antibodies in humans. Vaccine 30:2900–2907. 10.1016/j.vaccine.2012.02.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kallio A, Sepponen K, Hermand P, Denoël P, Godfroid F, Melin M. 2014. The role of pneumococcal histidine triad (Pht) proteins in the attachment of Streptococcus pneumoniae to respiratory epithelial cells. Infect. Immun. 82:1683–1691. 10.1128/IAI.00699-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Loisel E, Chimalapati S, Bougault C, Imberty A, Gallet B, Di Guilmi AM, Brown J, Vernet T, Durmort C. 2011. Biochemical characterization of the histidine triad protein PhtD as a cell surface zinc-binding protein of pneumococcus. Biochemistry 50:3551–3558. 10.1021/bi200012f [DOI] [PubMed] [Google Scholar]
- 7. Bayle L, Chimalapati S, Schoehn G, Brown J, Vernet T, Durmort C. 2011. Zinc uptake by Streptococcus pneumoniae depends on both AdcA and AdcAII and is essential for normal bacterial morphology and virulence. Mol. Microbiol. 82:904–916. 10.1111/j.1365-2958.2011.07862.x [DOI] [PubMed] [Google Scholar]
- 8. Lewis VG, Ween MP, McDevitt CA. 2012. The role of ATP-binding cassette transporters in bacterial pathogenicity. Protoplasma 249:919–942. 10.1007/s00709-011-0360-8 [DOI] [PubMed] [Google Scholar]
- 9. Plumptre CD, Eijkelkamp BA, Morey JR, Behr F, Couñago RM, Ogunniyi AD, Kobe B, O'Mara ML, Paton JC, McDevitt CA. 2014. AdcA and AdcAII employ distinct zinc acquisition mechanisms and contribute additively to zinc homeostasis in Streptococcus pneumoniae. Mol. Microbiol. 91:834–851. 10.1111/mmi.12504 [DOI] [PubMed] [Google Scholar]
- 10. Honsa ES, Johnson MDL, Rosch JW. 2013. The roles of transition metals in the physiology and pathogenesis of Streptococcus pneumoniae. Front. Cell. Infect. Microbiol. 3:92. 10.3389/fcimb.2013.00092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shafeeq S, Kuipers OP, Kloosterman TG. 2013. The role of zinc in the interplay between pathogenic streptococci and their hosts. Mol. Microbiol. 88:1047–1057. 10.1111/mmi.12256 [DOI] [PubMed] [Google Scholar]
- 12. Bersch B, Bougault C, Roux L, Favier A, Vernet T, Durmort C. 2013. New insights into histidine triad proteins: solution structure of a Streptococcus pneumoniae PhtD domain and zinc transfer to AdcAII. PLoS One 8:e81168. 10.1371/journal.pone.0081168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Godfroid F, Hermand P, Verlant V, Denoël P, Poolman JT. 2011. Preclinical evaluation of the Pht proteins as potential cross-protective pneumococcal vaccine antigens. Infect. Immun. 79:238–245. 10.1128/IAI.00378-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Adamou JE, Heinrichs JH, Erwin AL, Walsh W, Gayle T, Dormitzer M, Dagan R, Brewah YA, Barren P, Lathigra R, Langermann S, Koenig S, Johnson S. 2001. Identification and characterization of a novel family of pneumococcal proteins that are protective against sepsis. Infect. Immun. 69:949–958. 10.1128/IAI.69.2.949-958.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Seiberling M, Bologa M, Brookes R, Ochs M, Go K, Neveu D, Kamtchoua T, Lashley P, Yuan T, Gurunathan S. 2012. Safety and immunogenicity of a pneumococcal histidine triad protein D vaccine candidate in adults. Vaccine 30:7455–7460. 10.1016/j.vaccine.2012.10.080 [DOI] [PubMed] [Google Scholar]
- 16. Plumptre CD, Ogunniyi AD, Paton JC. 2013. Vaccination against Streptococcus pneumoniae using truncated derivatives of polyhistidine triad protein D. PLoS One 8:e78916. 10.1371/journal.pone.0078916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rioux S, Neyt C, Di Paolo E, Turpin L, Charland N, Labbe S, Mortier MC, Mitchell TJ, Feron C, Martin D, Poolman JT. 2011. Transcriptional regulation, occurrence and putative role of the Pht family of Streptococcus pneumoniae. Microbiology 157:336–348. 10.1099/mic.0.042184-0 [DOI] [PubMed] [Google Scholar]
- 18. McAllister LJ, Tseng HJ, Ogunniyi AD, Jennings MP, McEwan AG, Paton JC. 2004. Molecular analysis of the psa permease complex of Streptococcus pneumoniae. Mol. Microbiol. 53:889–901. 10.1111/j.1365-2958.2004.04164.x [DOI] [PubMed] [Google Scholar]
- 19. Plumptre CD, Ogunniyi AD, Paton JC. 2013. Surface association of Pht proteins of Streptococcus pneumoniae. Infect. Immun. 81:3644–3651. 10.1128/IAI.00562-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. McDevitt CA, Ogunniyi AD, Valkov E, Lawrence MC, Kobe B, McEwan AG, Paton JC. 2011. A molecular mechanism for bacterial susceptibility to zinc. PLoS Pathog. 7:e1002357. 10.1371/journal.ppat.1002357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Eijkelkamp BA, Morey JR, Ween MP, Ong C-LY, McEwan AG, Paton JC, McDevitt CA. 2014. Extracellular zinc competitively inhibits manganese uptake and compromises oxidative stress management in Streptococcus pneumoniae. PLoS One 9:e89427. 10.1371/journal.pone.0089427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lisher JP, Giedroc DP. 2013. Manganese acquisition and homeostasis at the host-pathogen interface. Front. Cell. Infect. Microbiol. 3:91. 10.3389/fcimb.2013.00091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Loisel E, Jacquamet L, Serre L, Bauvois C, Ferrer JL, Vernet T, Di Guilmi AM, Durmort C. 2008. AdcAII, a new pneumococcal Zn-binding protein homologous with ABC transporters: biochemical and structural analysis. J. Mol. Biol. 381:594–606. 10.1016/j.jmb.2008.05.068 [DOI] [PubMed] [Google Scholar]
- 24. Shao Z-Q, Zhang Y-M, Pan X-Z, Wang B, Chen J-Q. 2013. Insight into the evolution of the histidine triad protein (HTP) family in Streptococcus. PLoS One 8:e60116. 10.1371/journal.pone.0060116 [DOI] [PMC free article] [PubMed] [Google Scholar]