Abstract
Pathogen-induced reactive oxygen species (ROS) play a crucial role in host innate immune responses through regulating the quality and quantity of inflammatory mediators. However, the underlying molecular mechanisms of this effect have yet to be clarified. In this study, we examined the mechanism of action of ROS stimulated by Porphyromonas gingivalis in gingival epithelial cells. P. gingivalis induced the rapid production of ROS, which lead to the phosphorylation of JAK2 and increased levels of secreted proinflammatory cytokines interleukin-6 (IL-6) and IL-1β. Neutralization of ROS by N-acetyl-l-cysteine (NAC) abrogated the phosphorylation of JAK2 and suppressed the production of IL-6 and IL-1β. ROS-mediated phosphorylation of JAK2 induced the phosphoactivation of c-Jun amino-terminal protein kinase (JNK) and the downstream transcriptional regulator c-Jun. Inhibition of JAK2, either pharmacologically or by small interfering RNA (siRNA), reduced both the phosphorylation of these molecules and the production of proinflammatory cytokines in response to P. gingivalis. Furthermore, pharmacological inhibition or siRNA-mediated gene silencing of JNK or c-Jun mimicked the effect of JAK2 inhibition to suppress P. gingivalis-induced IL-6 and IL-1β levels. The results show that ROS-mediated activation of JAK2 is required for P. gingivalis-induced inflammatory cytokine production and that the JNK/c-Jun signaling axis is involved in the ROS-dependent regulation of IL-1β and IL-6 production.
INTRODUCTION
Reactive oxygen species (ROS) are a group of chemically reactive molecules consisting of radical and nonradical oxygen species formed by the partial reduction of oxygen. Accumulating evidence implicates ROS in the pathogenesis of many diseases, such as rheumatoid arthritis, chronic pulmonary disease, atherosclerosis, and, more recently, periodontitis (1–4). In addition to direct tissue-destructive properties, ROS have been established as secondary signaling molecules with a key role in diverse physiological processes, including cellular proliferation, senescence, cell death and survival, and immune responses (5, 6). Cellular ROS can be generated either through the process of mitochondrial oxidative phosphorylation or upon challenge by exogenous factors, including bacteria and their virulence factors (7, 8). Overwhelming ROS activity, caused by either an increase in ROS levels or a decrease in antioxidant capacity, induces oxidative stress and results in collateral damage to nucleic acids, proteins, and lipids (9, 10). In contrast, restrained ROS production plays an important role in microbial host defense, as ROS can regulate diverse signaling pathways and consequently modify inflammatory responses (11, 12). However, the early signaling events which are impacted by ROS and the molecular mechanisms by which ROS affect inflammatory responses have yet to be fully characterized.
Periodontitis is a chronic immune inflammatory disease which afflicts a large percentage of the adult population and is characterized by the destruction of periodontal tissues, resorption of alveolar bone, and eventual exfoliation of the teeth (13, 14). Porphyromonas gingivalis, a Gram-negative black-pigmented anaerobe, is a major pathogen in the initiation and progression of periodontitis (15–18). While P. gingivalis can induce robust inflammatory responses, including the generation of a wide variety of cytokines and chemokines, the organism is also capable of subverting and stalling host immunity depending on the context (15, 19–24). Moreover, phagocytosed P. gingivalis is resistant to oxidative burst killing by polymorphonuclear neutrophils, in part by producing antioxidant enzymes such as superoxide dismutase, thiol peroxidase, and rubrerythrin (25–28). Additionally, P. gingivalis accumulates a hemin layer on the cell surface that provides oxidative stress protection (29). P. gingivalis can also invade and survive within epithelial cells (30–33), and recent evidence suggests that within gingival epithelial cells P. gingivalis not only upregulates the antioxidant glutathione (GSH) response to create an environment conducive to intracellular growth but also induces the production of ROS in the early stages of infection (34). P. gingivalis-induced ROS production requires extracellular ATP, which acts through a complex consisting of P2X4, P2X7, and pannexin-1 (35). However, the functional role of P. gingivalis-induced ROS in epithelial cells and the underlying molecular mechanisms that lead to the induction of inflammatory responses are unknown.
JAK2 is one of the four Janus tyrosine kinase members in mammalian cells and is a critical component of signaling pathways involved in cellular survival, proliferation, differentiation, and apoptosis (36). In particular, JAK2 is thought to play a central role in the immune system by regulating the nature and magnitude of inflammatory cytokines. JAK2 both can act as a component of Toll-like receptor (TLR)-initiated signaling pathways and thus directly control cytokine production and can be involved in autocrine signaling stimulated by TLR-induced cytokines (37, 38). Recent studies suggest that various ROS components induced by bacteria can phosphoactivate JAK2 signaling and thereby control inflammatory cytokine production in both myeloid and epithelial cells (39, 40). We hypothesized, therefore, that P. gingivalis-induced ROS control subsequent inflammatory cytokine production through regulating JAK2 and its downstream signaling cascade. In this study, we show that P. gingivalis stimulation of gingival epithelial cells induces the rapid production of ROS, causing phosphoactivation of JAK2. Furthermore, we found that JAK2 activated by ROS in turn positively regulates the production of the inflammatory cytokines interleukin-1β (IL-1β) and IL-6 through downstream c-Jun amino-terminal protein kinase (JNK)-mediated c-Jun activation. These findings characterize the functional role of P. gingivalis-induced ROS and elucidate the molecular mechanism by which ROS are involved in the P. gingivalis-mediated production of inflammatory cytokines in gingival epithelial cells.
MATERIALS AND METHODS
Bacteria, eukaryotic cells, and infection conditions.
Wild-type (WT) P. gingivalis ATCC 33277 and the isogenic ΔfimA mutant (33) were cultured anaerobically in Trypticase soy broth supplemented with yeast extract (1 mg ml−1), hemin (5 μg ml−1), and menadione (1 μg ml−1) at 37°C. Telomerase-immortalized gingival epithelial cells (TIGKs) (41) were maintained in supplemented keratinocyte-SFM (Invitrogen) (19). TIGKs were challenged with P. gingivalis at a multiplicity of infection (MOI) of 10. The JAK2 inhibitor AG490 (25 μM) or the antioxidant N-acetyl-l-cysteine (NAC) (20 mM), were used for 2 h prior to challenge with P. gingivalis. Control cells were pretreated for 2 h with 0.01% dimethyl sulfoxide (DMSO) (solvent control).
Antibodies and reagents.
AG490 was from LC Laboratories. NAC and aminophenyl fluorescein (APF) were from Sigma-Aldrich. Lipofectamine and RNAiMax were from Invitrogen. All small interfering RNAs (siRNAs) were from Dharmacon. Cytokine enzyme-linked immunosorbent assay (ELISA) kits were from eBioscience. All antibodies were from Cell Signaling Technology, with the exceptions of phospho-JAK2 (Tyr 221; AssayBiotech) and anti-rabbit IgG-phycoerythrin (PE) (eBioscience).
Flow cytometry.
TIGKs were pretreated with the fluorescent oxidative indicator dye APF (1 μM) for 60 min, washed twice with phosphate-buffered saline (PBS) to remove unincorporated dye, and then stimulated with P. gingivalis in the presence or absence of the antioxidant NAC. Cells were harvested, washed twice with 2 ml of fluorescence-activated cell sorting (FACS) buffer (PBS containing 2% fetal bovine serum [FBS] and 0.01% sodium azide), and fixed with formaldehyde at a final concentration of 4% in PBS for 10 min at room temperature. Cells were washed twice in PBS containing 2% FBS and analyzed immediately by flow cytometry.
Transfection.
TIGKs were transfected with siRNA (50 nM) using Lipofectamine RNAiMax for 24 h in transfection medium (Invitrogen). The medium was then replaced with regular culture medium, which was left for a further 48 h. The levels of target molecules were assessed by Western blotting.
Western blotting.
TIGKs were lysed with radioimmunoprecipitation assay (RIPA) buffer (Sigma), and protein concentrations were determined using the bicinchoninic acid protein assay kit (Thermo). Samples were separated on NuPage Novex 4 to 12% bis-Tris polyacrylamide gels (Invitrogen) and electroblotted onto polyvinylidene difluoride (PVDF) membranes (Millipore). Primary antibody was used at 1 μg/ml at 4°C overnight. Antigen-antibody binding was detected using horseradish peroxidase-conjugated species-specific secondary antibodies followed by ECL Western blotting detection reagents (Thermo). Blots were stripped and probed with β-actin antibodies as a loading control. Images were acquired and analyzed using a GE LAS 4010 Image Station system.
Cytokine analysis.
Cytokine levels in cell-free supernatants were determined by ELISA according to the manufacturer's instructions.
Statistical analysis.
Statistical significance between groups was evaluated by analysis of variance (ANOVA) and the Tukey multiple-comparison test using the InStat program (GraphPad). Differences between groups were considered significant at a P value of <0.05.
RESULTS
P. gingivalis induces rapid production of ROS in TIGKs.
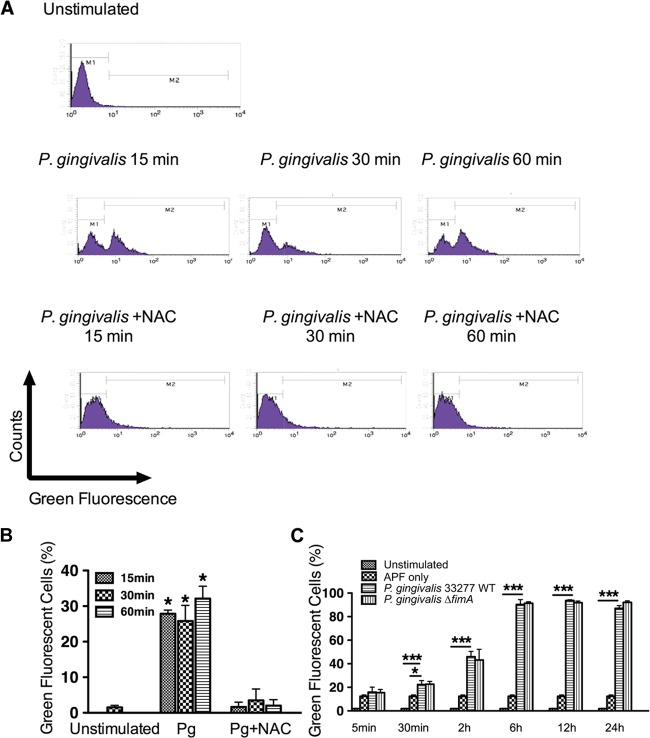
The kinetics of ROS production in TIGKs in response to P. gingivalis were investigated by flow cytometry with green-fluorescent APF staining. Stimulation with P. gingivalis for 15, 30, and 60 min caused an increase in the levels of ROS (Fig. 1A and B). To confirm the specificity of APF, the antioxidant NAC was used to neutralize ROS and the fluorescence of APF was determined. Addition of NAC abrogated the detection of ROS in P. gingivalis-stimulated TIGKs (Fig. 1A and B). To investigate the extent to which the ROS response to P. gingivalis is sustained, TIGKs were examined by flow cytometry up to 24 h after challenge with P. gingivalis. As shown in Fig. 1C, ROS levels remained elevated through 24 h. P. gingivalis rapidly invades gingival epithelial cells in high numbers in a fimbria-dependent manner (31, 33). Hence, we investigated the role of invasion in the generation of ROS by examination of a mutant of P. gingivalis lacking the structural subunit protein (FimA) of the major fimbriae. The fimbria-deficient mutant of P. gingivalis showed no difference in induction of ROS compared to the parental strain (Fig. 1C), indicating that an intracellular location is not required by P. gingivalis to incite ROS generation.
FIG 1.
P. gingivalis induces production of ROS in TIGKs. TIGKs stimulated with P. gingivalis 33277 (WT) or the ΔfimA mutant with or without NAC as indicated were stained with APF, and ROS levels were determined by flow cytometry. (A) Typical fluorescence plots of flow cytometry. (B and C) Percentages of cells expressing ROS. Results are means ± standard errors (SE) from 3 independent experiments. *, P < 0.05; *** P < 0.01.
Phosphoactivation of JAK2 is dependent on ROS in P. gingivalis-stimulated cells.
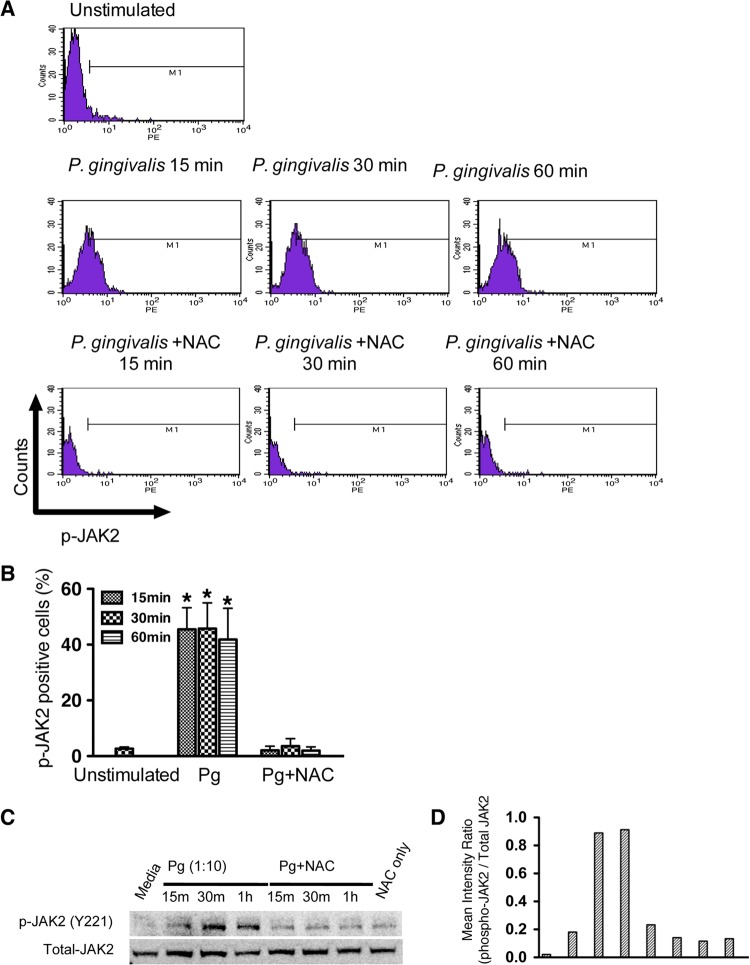
Flow cytometry was used to examine the involvement of JAK2 in P. gingivalis-induced ROS signaling. Infection with P. gingivalis stimulated the phosphorylation of JAK2 in TIGKs over times (15 to 60 min) that ROS levels are increased (Fig. 2A and B). Moreover, neutralization of ROS with NAC attenuated the phosphorylation of JAK2. Western blotting corroborated phosphoactivation of JAK2 by P. gingivalis and further showed that neutralization of ROS suppresses the phosphorylation of JAK2 over 15 to 60 min (Fig. 2C and D). Taken together, these results establish that P. gingivalis-mediated phosphoactivation of JAK2 in TIGKs requires the production of ROS.
FIG 2.
Phosphoactivation of JAK2 is dependent on ROS in P. gingivalis-stimulated TIGKs. TIGKs were stimulated with P. gingivalis for the times indicated in the presence or absence of NAC, and phospho (p)-JAK2 was measured by flow cytometry or Western blotting. (A) Typical fluorescence plots of flow cytometry. (B) Percentages of cells expressing p-JAK2. Results are means ± SE from 3 independent experiments. *, P < 0.05. (C) Western blot of TIGK lysates probed with antibodies to p-JAK2 (Y221). The blot is representative of 3 independent experiments. (D) Densitometric analysis of the image in panel C.
Inhibition of JAK2 or ROS suppresses P. gingivalis-induced IL-6 and IL-1β production.
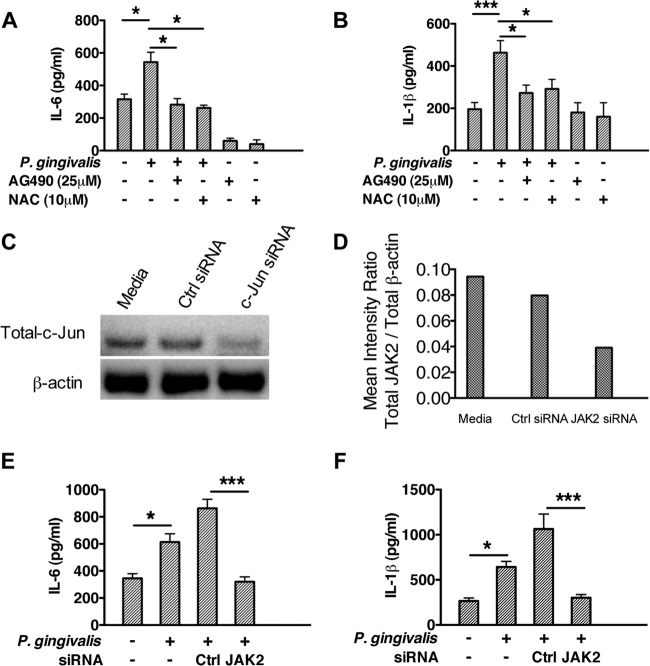
To determine if ROS-mediated JAK2 activation plays a functional role in P. gingivalis-mediated inflammatory cytokine production in epithelial cells, an antioxidant (NAC) and a pharmacological inhibitor (AG490) were used to suppress activation of ROS and JAK2, respectively. As shown in Fig. 3A and B, neutralization of ROS by NAC or inhibition of JAK2 by AG490 significantly reduced P. gingivalis-mediated IL-6 and IL-1β production. Since AG490 has been reported to have nonspecific effects on other kinases, we next used siRNA-mediated gene silencing to confirm the functional role of JAK2 in P. gingivalis-mediated production of IL-6 and IL-1β. JAK2 was knocked down with siRNA, which reduced JAK2 levels by >60% compared with those in nontransfected cells or cells transfected with control siRNA (Fig. 3C and D). Reduced JAK2 levels significantly diminished P. gingivalis-induced IL-6 (Fig. 3E) and IL-1β (Fig. 3F) secretion, data that support the role of JAK2 in the production of these proinflammatory cytokines.
FIG 3.
Inhibition of JAK2 or ROS suppresses P. gingivalis-induced IL-6 and IL-1β production. (A and B) TIGKs were pretreated with JAK2 inhibitor AG490 or ROS neutralizer NAC for 2 h and then stimulated with P. gingivalis for 4 h. Cell-free supernatants were collected, and the levels of IL-6 (A) and IL-1β (B) were determined by ELISA. Results are means (n = 3) with standard deviations and are representative of 3 biological replicates. (C to F) TIGKs were pretreated with JAK2-specific siRNA or nontarget siRNA for 72 h and stimulated with P. gingivalis for 4 h. Whole-cell lysates and cell-free supernatants were collected to determine the transfection efficiency and cytokine levels, respectively. siRNA-mediated knockdown of JAK2 and total β-actin levels were assessed by Western blotting (C), and the ratio of total JAK2 to total β-actin was determined by densitometry (D). P. gingivalis-induced production of IL-6 (E) an IL-1β (F) was suppressed by siRNA-mediated JAK2 inhibition. Results are means (n = 3) with standard deviations and are representative of 3 biological replicates. *, P < 0.05; *** P < 0.005.
Influence of JAK2 or ROS inhibition on P. gingivalis-mediated phosphorylation of STATs, JNK, and c-Jun.
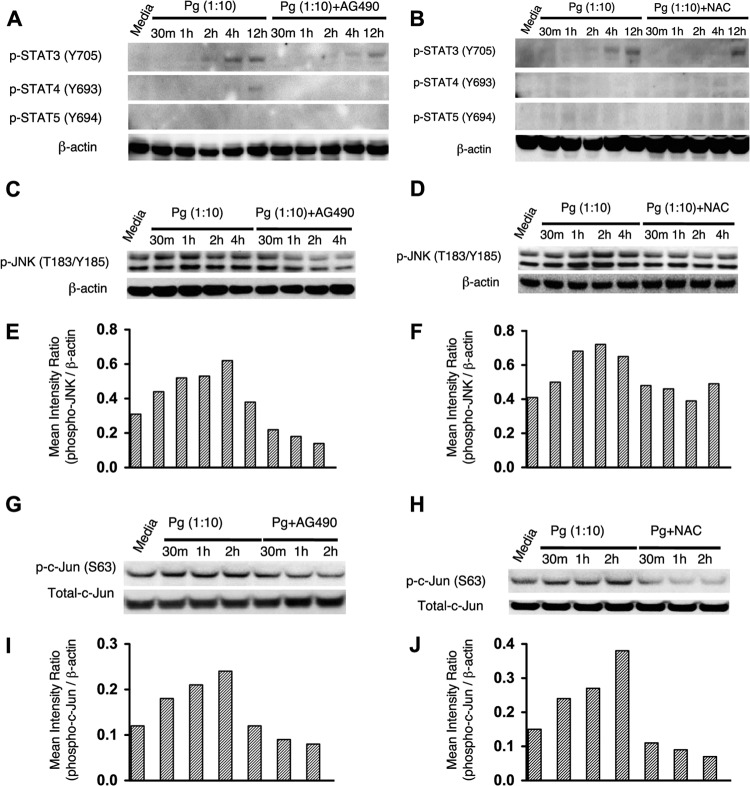
Downstream of JAK are the prototypical STAT (signal transducer and activator of transcription) and mitogen-activated protein kinase (MAPK) pathways (42). To identify the effectors of ROS-JAK2-mediated inflammatory cytokine production, we monitored the effect of P. gingivalis on the phosphorylation status of STATs 3, 4, and 5, along with c-Jun amino-terminal kinase (JNK), a member of the MAPK family that is known to be activated by P. gingivalis (43). As shown in Fig. 4A and B, STAT 5 was not phosphorylated in response to P. gingivalis challenge, and STAT 4 was only weakly phosphorylated after prolonged exposure (12 h). STAT 3 was phosphorylated after 2 h of infection with P. gingivalis, a time frame that would indicate autocrine activation rather than direct JAK2-mediated activation. Collectively these results suggest that STATs 3, 4, and 5 do not directly regulate inflammatory cytokine production in gingival epithelial cells. In contrast, stimulation by P. gingivalis resulted in the phosphorylation of JNK in TIGKs at multiple time points (Fig. 4C to F). Pretreatment with either antioxidant (NAC) or JAK2 inhibitor (AG490) reduced phosphorylation levels of JNK upon P. gingivalis stimulation, confirming that ROS and JAK2 are epistatic to JNK. As JNK-mediated phosphorylation of c-Jun has been defined as an essential regulator in the transcription of IL-6 and IL-1β, we next tested the phosphorylation of c-Jun in P. gingivalis-stimulated TIGKs. The Western blot analyses in Fig. 4G to J show that P. gingivalis challenge enhanced the phosphorylation of c-Jun at all of the time points tested, and the activation of c-Jun was reversed by inhibition of ROS or JAK2. Collectively, these results demonstrate that induction of ROS by P. gingivalis causes phosphoactivation of inflammatory signaling pathways that include c-Jun in a JAK2-dependent manner.
FIG 4.
Influence of JAK2 or ROS inhibition on P. gingivalis-mediated phosphorylation of STATs (3, 4, and 5), JNK, and c-Jun. Western blots of TIGKs stimulated with P. gingivalis with or without SG490 or NAC and probed with antibodies to phospho (p)-STAT 3, 4, or 5 (A and B), antibodies to p-JNK (C and D), or antibodies to p-c-Jun and total c-Jun (G and H) are shown. Antibodies to β-actin were used as a loading control. Panels E and F and panels I and J are densitometric analyses of the blots in panels C and D and panels G and H, respectively. Data are representative of 3 independent experiments.
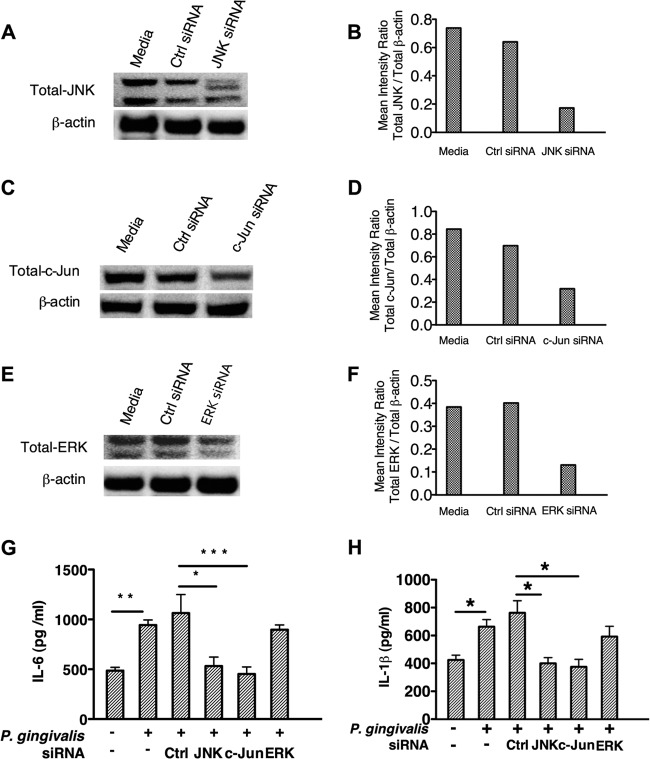
Gene silencing of c-Jun mimics the effect of JAK2 or ROS inhibition on the production of IL-6 and IL-1β in P. gingivalis-stimulated TIGKs.
Our results established that the P. gingivalis-initiated ROS-JAK2 axis phosphoactivates JNK and its downstream c-Jun signaling pathways in TIGKs. Therefore, we next wanted to determine the role of these molecules in P. gingivalis-mediated inflammatory cytokine production. To this end, siRNAs were used to silence JNK or c-Jun in TIGKs. siRNA-mediated gene silencing reduced the expression of JNK and c-Jun by more than 60% (Fig. 5A to D) compared to that in nontransfected cells or cells transfected with control siRNA. The reduced amounts of JNK and c-Jun abrogated the production of IL-6 and IL-1β in P. gingivalis-stimulated TIGKs (Fig. 5G and H). Furthermore, knockdown of extracellular signal-regulated kinase (ERK) (Fig. 5E and F), which is not activated by P. gingivalis in gingival epithelial cells (43), did not exhibit a significant influence on P. gingivalis-mediated IL-6 and IL-1β production. These results suggest that the JNK–c-Jun pathway exerts significant transcriptional control over epithelial cell inflammatory responses to P. gingivalis-mediated ROS signals.
FIG 5.
Gene silencing of JNK or c-Jun inhibits the production of IL-6 and IL-1β in P. gingivalis-stimulated TIGKs. TIGKs were pretreated with ERK, JNK, c-Jun, or nontarget (Ctrl) siRNA for 72 h and stimulated with P. gingivalis for 4 h. Whole-cell lysates and cell-free supernatant were collected to determine the transfection efficiency and cytokine levels, respectively. siRNA-mediated knockdown of c-JNK (A and B), c-Jun (C and D), or ERK (E and F) was assessed by Western blotting and densitometric analysis. P. gingivalis-induced production of IL-6 (G) an IL-1β (H) in TIGKs with JNK, c-Jun, or ERK knockdown was determined by ELISA. Results are means (n = 3) with standard deviations and are representative of 3 independent experiments. *, P < 0.05; ** P < 0.01; *** P < 0.005.
DISCUSSION
The epithelial cells that line the gingival crevice constitute an interactive interface that senses bacterial colonization and signals the presence of organisms to the underlying cells of the immune system, primarily through the production of cytokines and chemokines. Dysregulation of these inflammatory responses by pathogens such P. gingivalis is considered an important component in the development of periodontitis (16, 17). P. gingivalis engages in a delicately balanced interaction with gingival epithelial cells and can suppress production of neutrophil and T-cell chemokines while also inciting secretion of proinflammatory cytokines such as IL-1β and IL-6 (19, 20, 44). This “dual personality” with regard to innate immune activation is accomplished through targeted intervention of signaling pathways within epithelial cells. For example, the SerB serine phosphatase of P. gingivalis specifically dephosphorylates the p65 subunit of NF-κB, thus inhibiting IL-8 production (19).
In this study, we investigated the proinflammatory nature of P. gingivalis with regard to the stimulation of IL-1β and IL-6 production and secretion from gingival epithelial cells. We found that P. gingivalis induces the production of ROS rapidly after infection and that ROS are required for the initiation of subsequent JAK2-mediated signaling pathways, which in turn control the production of IL-1β and IL-6. These findings provide the first documentation of the functional role of P. gingivalis-mediated ROS in inflammation and elucidate a molecular mechanism for P. gingivalis-induced inflammatory cytokine production by epithelial cells. P. gingivalis expresses several microbe-associated molecular patterns (MAMPs), such as lipopolysaccharide (LPS) and fimbriae, which are recognized by TLRs and lead to the production of proinflammatory cytokines (45, 46). The results presented here expand on these fundamental studies and show that the generation of ROS upon P. gingivalis infection is necessary for maximal secretion of IL-1β and IL-6. Of relevance to the pathogenesis of periodontitis is that both IL-1β and IL-6 are capable of upregulating osteoclastic activity via RANK/RANKL/OPG pathways and can also induce alveolar bone loss through RANK-independent pathways (47). Furthermore, IL-1β and IL-6 can contribute to tissue degradation through the induction of matrix metalloproteinases and other inflammatory mediators (48–50). A number of studies have demonstrated increased IL-1β and IL-6 levels in the crevicular fluid and periodontal tissues of patients with periodontitis (51, 52), and the application of antagonists to IL-1 reduces the severity of periodontitis in experimental animals (50).
In gingival epithelial cells, ATP stimulation results in both NADPH-induced and mitochondrially derived ROS generation through ligation of P2X7 receptors, indicating that NADPH oxidase and mitochondria produce ROS synergistically (34). Although the underlying mechanism by which P. gingivalis stimulates ROS production is yet to be determined, an intracellular location is not required, as an invasion-defective mutant of P. gingivalis was equally as effective as the parental strain at increasing ROS amounts. P. gingivalis can also induce the production of antioxidants, including glutathione (GSH) and glutathione peroxidase, to suppress the oxidization effect of ROS (34); however, the timing of this process may be dependent on the degree of bacterial challenge. In the current study, using an MOI of 10, ROS levels in response to P. gingivalis were sustained through 24 h, whereas with an MOI of 100, previous work has shown a reduction in ROS at 6 h postinfection (34). The number of P. gingivalis organisms associated with gingival epithelial cells is likely to vary according to stage and severity of infection, and thus in vivo ROS production and suppression may be delicately balanced. The function of P. gingivalis-induced ROS at early stages of infection has not hitherto been addressed, and we show here that P. gingivalis-induced ROS lead to phosphoactivation of JAK2. Activated JAK2 can phosphorylate tyrosine residues within the JAK2-associated receptor, which allows for the recruitment of downstream signaling molecules. These recruited molecules then relay JAK2-mediated signaling to transcription factors. The most well described cell signaling pathway involving JAK2 is phosphorylation and activation of members of the STAT family. P. gingivalis activated STAT 3 in TIGKs, consistent with previous studies with primary cultures of gingival epithelial cells (53), and to a lesser extent STAT 4. However, STAT activation was delayed in relation to ROS induction, indicating that the STAT pathway is not the primary mechanism for P. gingivalis-induced ROS control of cytokine expression. JAK2 is also involved in the activation of other signaling pathways such as those involving JNK and activating protein 1 (AP-1)/c-Jun (54, 55). JNK has been shown to be phosphorylated in response to P. gingivalis infection of primary gingival epithelial cells, and such activation is necessary for efficient internalization by the organism (43). The current results corroborate the activation of JNK by P. gingivalis in TIGKs and show that maximal phosphorylation is dependent on ROS and JAK2. A nuclear substrate of JNK is the proto-oncogene product c-Jun, a component of the AP-1 transcription factor family that has been shown to control cytokine production in immune cells (56). In this study, we show that c-Jun was primarily responsible for the upregulation of IL-1β and IL-6 in response to P. gingivalis-induced ROS. The literature contains examples of both JAK2/STAT and JAK2/MAPK signal transduction pathways linking microbe-induced ROS-JAK2 with cytokine expression. For example, Helicobacter pylori induces an increase in ROS and IL-8 expression through the activation of MAPKs and AP-1/c-Jun in gastric epithelial cells (57). However, in pulmonary epithelial cells stimulated with lipid-associated membrane proteins from Mycoplasma pneumoniae, ROS mediate the phosphorylation of JAK2/STAT3 and the consequent expression of IL-8 (39). Hence the nature of the signaling induced by ROS is likely to depend on a number of factors, such as the nature and duration of the stimulus along with the cell type.
The role of JAK2 in multiple downstream pathways indicates that P. gingivalis-mediated activation of JAK2 through ROS could impact a number of epithelial cell processes, a topic that requires further investigation. In the case of IL-1β, secretion of the mature cytokine requires cleavage of immature pro-IL-1β by capsase-1, an enzyme that is activated in the inflammasome. Assembly of the inflammasome occurs in response to MAMPs and also endogenous danger-associated molecular patterns (DAMPs) such as ATP. ROS can act as secondary signaling molecules for the formation of the NALP3 inflammasome (58), and P. gingivalis-induced ROS production in gingival epithelial cells can activate the NLRP3 inflammasome and caspase-1, leading to IL-1β secretion (35). Hence, ROS may play a role in both transcriptional and posttranslational regulation of IL-1β by P. gingivalis.
In summary, we have demonstrated that P. gingivalis-induced ROS is required for the phosphoactivation of JAK2, which in turn activates downstream signaling pathways including JNK and c-Jun in gingival epithelial cells. Moreover, activation of the transcription factor c-Jun regulates production of the inflammatory cytokines IL-6 and IL-1β. These findings suggest that ROS-JAK2 signaling represents a potential therapeutic target in the control of inflammatory diseases such as periodontitis.
ACKNOWLEDGMENTS
This work was supported by NIH NIDCR grants DE017921, DE011111, and DE014372 (R.J.L.), DE023633 (H.W.), and DE017680 and DE019826 (D.A.S.).
Footnotes
Published ahead of print 21 July 2014
REFERENCES
- 1. Filippin LI, Vercelino R, Marroni NP, Xavier RM. 2008. Redox signalling and the inflammatory response in rheumatoid arthritis. Clin. Exp. Immunol. 152:415–422. 10.1111/j.1365-2249.2008.03634.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Soory M. 2007. Periodontal diseases and rheumatoid arthritis: a coincident model for therapeutic intervention? Curr. Drug Metab. 8:750–757. 10.2174/138920007782798162 [DOI] [PubMed] [Google Scholar]
- 3. Tavakoli S, Asmis R. 2012. Reactive oxygen species and thiol redox signaling in the macrophage biology of atherosclerosis. Antioxid. Redox Signal. 17:1785–1795. 10.1089/ars.2012.4638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Waddington RJ, Moseley R, Embery G. 2000. Reactive oxygen species: a potential role in the pathogenesis of periodontal diseases. Oral Dis. 6:138–151. 10.1111/j.1601-0825.2000.tb00325.x [DOI] [PubMed] [Google Scholar]
- 5. Thannickal VJ, Fanburg BL. 2000. Reactive oxygen species in cell signaling. Am. J. Physiol. Lung Cell. Mol. Physiol. 279:L1005–L1028 [DOI] [PubMed] [Google Scholar]
- 6. Padgett LE, Broniowska KA, Hansen PA, Corbett JA, Tse HM. 2013. The role of reactive oxygen species and proinflammatory cytokines in type 1 diabetes pathogenesis. Ann. N. Y. Acad. Sci. 1281:16–35. 10.1111/j.1749-6632.2012.06826.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sareila O, Kelkka T, Pizzolla A, Hultqvist M, Holmdahl R. 2011. NOX2 complex-derived ROS as immune regulators. Antioxid. Redox Signal. 15:2197–2208. 10.1089/ars.2010.3635 [DOI] [PubMed] [Google Scholar]
- 8. Gallois A, Klein JR, Allen LA, Jones BD, Nauseef WM. 2001. Salmonella pathogenicity island 2-encoded type III secretion system mediates exclusion of NADPH oxidase assembly from the phagosomal membrane. J. Immunol. 166:5741–5748. 10.4049/jimmunol.166.9.5741 [DOI] [PubMed] [Google Scholar]
- 9. Circu ML, Aw TY. 2010. Reactive oxygen species, cellular redox systems, and apoptosis. Free Radic. Biol. Med. 48:749–762. 10.1016/j.freeradbiomed.2009.12.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chang SP, Shen SC, Lee WR, Yang LL, Chen YC. 2011. Imatinib mesylate induction of ROS-dependent apoptosis in melanoma B16F0 cells. J. Dermatol. Sci. 62:183–191. 10.1016/j.jdermsci.2011.03.001 [DOI] [PubMed] [Google Scholar]
- 11. Finkel T. 2011. Signal transduction by reactive oxygen species. J. Cell Biol. 194:7–15. 10.1083/jcb.201102095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lau AT, Wang Y, Chiu JF. 2008. Reactive oxygen species: current knowledge and applications in cancer research and therapeutic. J. Cell. Biochem. 104:657–667. 10.1002/jcb.21655 [DOI] [PubMed] [Google Scholar]
- 13. Armitage GC. 2004. Periodontal diagnoses and classification of periodontal diseases. Periodontol. 2000 34:9–21. 10.1046/j.0906-6713.2002.003421.x [DOI] [PubMed] [Google Scholar]
- 14. Eke PI, Dye BA, Wei L, Thornton-Evans GO, Genco RJ. 2012. Prevalence of periodontitis in adults in the United States: 2009 and 2010. J. Dent. Res. 91:914–920. 10.1177/0022034512457373 [DOI] [PubMed] [Google Scholar]
- 15. Lamont RJ, Jenkinson HF. 1998. Life below the gum line: pathogenic mechanisms of Porphyromonas gingivalis. Microbiol. Mol. Biol. Rev. 62:1244–1263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hajishengallis G, Lamont RJ. 2012. Beyond the red complex and into more complexity: the polymicrobial synergy and dysbiosis (PSD) model of periodontal disease etiology. Mol. Oral Microbiol. 27:409–419. 10.1111/j.2041-1014.2012.00663.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hajishengallis G, Lamont RJ. 2014. Breaking bad: manipulation of the host response by Porphyromonas gingivalis. Eur. J. Immunol. 44:328–338. 10.1002/eji.201344202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Byrne SJ, Dashper SG, Darby IB, Adams GG, Hoffmann B, Reynolds EC. 2009. Progression of chronic periodontitis can be predicted by the levels of Porphyromonas gingivalis and Treponema denticola in subgingival plaque. Oral Microbiol. Immunol. 24:469–477. 10.1111/j.1399-302X.2009.00544.x [DOI] [PubMed] [Google Scholar]
- 19. Takeuchi H, Hirano T, Whitmore SE, Morisaki I, Amano A, Lamont RJ. 2013. The serine phosphatase SerB of Porphyromonas gingivalis suppresses IL-8 production by dephosphorylation of NF-kappaB RelA/p65. PLoS Pathog. 9:e1003326. 10.1371/journal.ppat.1003326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jauregui CE, Wang Q, Wright CJ, Takeuchi H, Uriarte SM, Lamont RJ. 2013. Suppression of T-cell chemokines by Porphyromonas gingivalis. Infect. Immun. 81:2288–2295. 10.1128/IAI.00264-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pollreisz A, Huang Y, Roth GA, Cheng B, Kebschull M, Papapanou PN, Schmidt AM, Lalla E. 2010. Enhanced monocyte migration and pro-inflammatory cytokine production by Porphyromonas gingivalis infection. J. Periodont. Res. 45:239–245. 10.1111/j.1600-0765.2009.01225.x [DOI] [PubMed] [Google Scholar]
- 22. Brown J, Wang H, Hajishengallis GN, Martin M. 2011. TLR-signaling networks: an integration of adaptor molecules, kinases, and cross-talk. J. Dent. Res. 90:417–427. 10.1177/0022034510381264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Darveau RP, Belton CM, Reife RA, Lamont RJ. 1998. Local chemokine paralysis, a novel pathogenic mechanism for Porphyromonas gingivalis. Infect. Immun. 66:1660–1665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Handfield M, Baker HV, Lamont RJ. 2008. Beyond good and evil in the oral cavity: insights into host-microbe relationships derived from transcriptional profiling of gingival cells. J. Dent. Res. 87:203–223. 10.1177/154405910808700302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bostanci N, Belibasakis GN. 2012. Porphyromonas gingivalis: an invasive and evasive opportunistic oral pathogen. FEMS Microbiol. Lett. 333:1–9. 10.1111/j.1574-6968.2012.02579.x [DOI] [PubMed] [Google Scholar]
- 26. Sztukowska M, Bugno M, Potempa J, Travis J, Kurtz DM., Jr 2002. Role of rubrerythrin in the oxidative stress response of Porphyromonas gingivalis. Mol. Microbiol. 44:479–488. 10.1046/j.1365-2958.2002.02892.x [DOI] [PubMed] [Google Scholar]
- 27. Mydel P, Takahashi Y, Yumoto H, Sztukowska M, Kubica M, Gibson FC, III, Kurtz DM, Jr, Travis J, Collins LV, Nguyen KA, Genco CA, Potempa J. 2006. Roles of the host oxidative immune response and bacterial antioxidant rubrerythrin during Porphyromonas gingivalis infection. PLoS Pathog. 2:e76. 10.1371/journal.ppat.0020076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kikuchi Y, Ohara N, Sato K, Yoshimura M, Yukitake H, Sakai E, Shoji M, Naito M, Nakayama K. 2005. Novel stationary-phase-upregulated protein of Porphyromonas gingivalis influences production of superoxide dismutase, thiol peroxidase and thioredoxin. Microbiol. 151:841–853. 10.1099/mic.0.27589-0 [DOI] [PubMed] [Google Scholar]
- 29. Smalley JW, Birss AJ, Silver J. 2000. The periodontal pathogen Porphyromonas gingivalis harnesses the chemistry of the μ-oxo bishaem of iron protoporphyrin IX to protect against hydrogen peroxide. FEMS Microbiol. Lett. 183:159–164. 10.1111/j.1574-6968.2000.tb08951.x [DOI] [PubMed] [Google Scholar]
- 30. Belton CM, Izutsu KT, Goodwin PC, Park Y, Lamont RJ. 1999. Fluorescence image analysis of the association between Porphyromonas gingivalis and gingival epithelial cells. Cell. Microbiol. 1:215–223. 10.1046/j.1462-5822.1999.00022.x [DOI] [PubMed] [Google Scholar]
- 31. Lamont RJ, Chan A, Belton CM, Izutsu KT, Vasel D, Weinberg A. 1995. Porphyromonas gingivalis invasion of gingival epithelial cells. Infect. Immun. 63:3878–3885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tribble GD, Mao S, James CE, Lamont RJ. 2006. A Porphyromonas gingivalis haloacid dehalogenase family phosphatase interacts with human phosphoproteins and is important for invasion. Proc. Natl. Acad. Sci. U. S. A. 103:11027–11032. 10.1073/pnas.0509813103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yilmaz O, Watanabe K, Lamont RJ. 2002. Involvement of integrins in fimbriae-mediated binding and invasion by Porphyromonas gingivalis. Cell. Microbiol. 4:305–314. 10.1046/j.1462-5822.2002.00192.x [DOI] [PubMed] [Google Scholar]
- 34. Choi CH, Spooner R, DeGuzman J, Koutouzis T, Ojcius DM, Yilmaz O. 2013. Porphyromonas gingivalis-nucleoside-diphosphate-kinase inhibits ATP-induced reactive-oxygen-species via P2X7 receptor/NADPH-oxidase signalling and contributes to persistence. Cell. Microbiol. 15:961–976. 10.1111/cmi.12089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hung SC, Choi CH, Said-Sadier N, Johnson L, Atanasova KR, Sellami H, Yilmaz O, Ojcius DM. 2013. P2X4 assembles with P2X7 and pannexin-1 in gingival epithelial cells and modulates ATP-induced reactive oxygen species production and inflammasome activation. PLoS One 8:e70210. 10.1371/journal.pone.0070210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ghoreschi K, Laurence A, O'Shea JJ. 2009. Janus kinases in immune cell signaling. Immunol. Rev. 228:273–287. 10.1111/j.1600-065X.2008.00754.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Page TH, Smolinska M, Gillespie J, Urbaniak AM, Foxwell BM. 2009. Tyrosine kinases and inflammatory signalling. Curr. Mol. Med. 9:69–85. 10.2174/156652409787314507 [DOI] [PubMed] [Google Scholar]
- 38. Novogrodsky A, Vanichkin A, Patya M, Gazit A, Osherov N, Levitzki A. 1994. Prevention of lipopolysaccharide-induced lethal toxicity by tyrosine kinase inhibitors. Science 264:1319–1322. 10.1126/science.8191285 [DOI] [PubMed] [Google Scholar]
- 39. Choi SY, Lim JW, Shimizu T, Kuwano K, Kim JM, Kim H. 2012. Reactive oxygen species mediate Jak2/Stat3 activation and IL-8 expression in pulmonary epithelial cells stimulated with lipid-associated membrane proteins from Mycoplasma pneumoniae. Inflamm. Res. 61:493–501. 10.1007/s00011-012-0437-7 [DOI] [PubMed] [Google Scholar]
- 40. Forget G, Gregory DJ, Olivier M. 2005. Proteasome-mediated degradation of STAT1α following infection of macrophages with Leishmania donovani. J. Biol. Chem. 280:30542–30549. 10.1074/jbc.M414126200 [DOI] [PubMed] [Google Scholar]
- 41. Moffatt-Jauregui CE, Robinson B, de Moya AV, Brockman RD, Roman AV, Cash MN, Culp DJ, Lamont RJ. 2013. Establishment and characterization of a telomerase immortalized human gingival epithelial cell line. J. Periodont. Res. 48:713–721. 10.1111/jre.12059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Schindler C, Levy DE, Decker T. 2007. JAK-STAT signaling: from interferons to cytokines. J. Biol. Chem. 282:20059–20063. 10.1074/jbc.R700016200 [DOI] [PubMed] [Google Scholar]
- 43. Watanabe K, Yilmaz O, Nakhjiri SF, Belton CM, Lamont RJ. 2001. Association of mitogen-activated protein kinase pathways with gingival epithelial cell responses to Porphyromonas gingivalis infection. Infect. Immun. 69:6731–6737. 10.1128/IAI.69.11.6731-6737.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sandros J, Karlsson C, Lappin DF, Madianos PN, Kinane DF, Papapanou PN. 2000. Cytokine responses of oral epithelial cells to Porphyromonas gingivalis infection. J. Dent. Res. 79:1808–1814. 10.1177/00220345000790101301 [DOI] [PubMed] [Google Scholar]
- 45. Hajishengallis G, Sojar H, Genco RJ, DeNardin E. 2004. Intracellular signaling and cytokine induction upon interactions of Porphyromonas gingivalis fimbriae with pattern-recognition receptors. Immunol. Invest. 33:157–172. 10.1081/IMM-120030917 [DOI] [PubMed] [Google Scholar]
- 46. Hiramine H, Watanabe K, Hamada N, Umemoto T. 2003. Porphyromonas gingivalis 67-kDa fimbriae induced cytokine production and osteoclast differentiation utilizing TLR2. FEMS Microbiol. Lett. 229:49–55. 10.1016/S0378-1097(03)00788-2 [DOI] [PubMed] [Google Scholar]
- 47. Preshaw PM, Taylor JJ. 2011. How has research into cytokine interactions and their role in driving immune responses impacted our understanding of periodontitis? J. Clin. Periodontol. 38(Suppl 11):S60–S84. 10.1111/j.1600-051X.2010.01671.x [DOI] [PubMed] [Google Scholar]
- 48. Birkedal-Hansen H. 1993. Role of cytokines and inflammatory mediators in tissue destruction. J. Periodont. Res. 28:500–510. 10.1111/j.1600-0765.1993.tb02113.x [DOI] [PubMed] [Google Scholar]
- 49. Graves D. 2008. Cytokines that promote periodontal tissue destruction. J. Periodontol. 79:1585–1591. 10.1902/jop.2008.080183 [DOI] [PubMed] [Google Scholar]
- 50. Graves DT, Cochran D. 2003. The contribution of interleukin-1 and tumor necrosis factor to periodontal tissue destruction. J. Periodontol. 74:391–401. 10.1902/jop.2003.74.3.391 [DOI] [PubMed] [Google Scholar]
- 51. Howells GL. 1995. Cytokine networks in destructive periodontal disease. Oral Dis. 1:266–270 [DOI] [PubMed] [Google Scholar]
- 52. Okada H, Murakami S. 1998. Cytokine expression in periodontal health and disease. Crit. Rev. Oral Biol. Med. 9:248–266. 10.1177/10454411980090030101 [DOI] [PubMed] [Google Scholar]
- 53. Moffatt CE, Lamont RJ. 2011. Porphyromonas gingivalis induction of microRNA-203 expression controls suppressor of cytokine signaling 3 in gingival epithelial cells. Infect. Immun. 79:2632–2637. 10.1128/IAI.00082-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Du Z, Wei L, Murti A, Pfeffer SR, Fan M, Yang CH, Pfeffer LM. 2007. Non-conventional signal transduction by type 1 interferons: the NF-kappaB pathway. J. Cell. Biochem. 102:1087–1094. 10.1002/jcb.21535 [DOI] [PubMed] [Google Scholar]
- 55. Hu X, Chen J, Wang L, Ivashkiv LB. 2007. Crosstalk among Jak-STAT, Toll-like receptor, and ITAM-dependent pathways in macrophage activation. J. Leukoc. Biol. 82:237–243. 10.1189/jlb.1206763 [DOI] [PubMed] [Google Scholar]
- 56. Wang H, Garcia CA, Rehani K, Cekic C, Alard P, Kinane DF, Mitchell T, Martin M. 2008. IFN-β production by TLR4-stimulated innate immune cells is negatively regulated by GSK3-β. J. Immunol. 181:6797–6802. 10.4049/jimmunol.181.10.6797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Jang SH, Cho S, Lee ES, Kim JM, Kim H. 2013. The phenyl-thiophenyl propenone RK-I-123 reduces the levels of reactive oxygen species and suppresses the activation of NF-κB and AP-1 and IL-8 expression in Helicobacter pylori-infected gastric epithelial AGS cells. Inflamm. Res. 62:689–696. 10.1007/s00011-013-0621-4 [DOI] [PubMed] [Google Scholar]
- 58. Martinon F. 2010. Signaling by ROS drives inflammasome activation. Eur. J. Immunol. 40:616–619. 10.1002/eji.200940168 [DOI] [PubMed] [Google Scholar]