Abstract
The pathogenesis of malaria is complex, generating a broad spectrum of clinical manifestations. One of the major complications and concerns in malaria is anemia, which is responsible for considerable morbidity in the developing world, especially in children and pregnant women. Despite its enormous health importance, the immunological mechanisms involved in malaria-induced anemia remain incompletely understood. Plasmodium vivax, one of the causative agents of human malaria, is known to induce a strong inflammatory response with a robust production of immune effectors, including cytokines and antibodies. Therefore, it is possible that the extent of the immune response not only may facilitate the parasite killing but also may provoke severe illness, including anemia. In this review, we consider potential immune effectors and their possible involvement in generating this clinical outcome during P. vivax infections.
INTRODUCTION
Malaria remains one of the most important public health problems in the world, with about 3 billion people at risk of contracting the disease and 781,000 deaths estimated annually (1). The global burden of human malaria is caused almost exclusively by two species of parasites: Plasmodium falciparum and Plasmodium vivax. Existing research efforts have largely focused on P. falciparum because of the higher mortality it causes, especially in Africa (2, 3). However, P. vivax remains more widely distributed than P. falciparum and is a major public health threat affecting populous regions in Asia, the horn of Africa, and Central and South America (4). The spectrum of vivax malaria ranges from presentation as a relatively benign disease to severe and sometimes fatal illness, mainly in children (5, 6) and pregnant women (7). The mortality rates among patients presenting P. vivax malaria are comparable to those attributable to P. falciparum malaria, as evidenced by hospital-based studies (6, 8, 9). It has been demonstrated that chloroquine resistance parallels severe disease (especially severe anemia) in some areas (10). In addition to the concerns imposed by increasingly drug-resistant parasites, it should not be forgotten that transmission of P. vivax is harder to control and eliminate than P. falciparum transmission because the former species may cause a relapse after resolution of the primary infection and also due to its early gametocytogenesis. In areas of endemicity, relapse of vivax malaria is an important source of parasite transmission to susceptible vectors and a major cause of malaria in young children (11).
BURDEN OF ANEMIA RELATED TO VIVAX MALARIA
In times of renewed efforts to eradicate malaria, the attention on P. vivax increases in consideration of the fact that infections related to this species are also able to cause severe disease, including anemia as one of the major complications (5, 12, 13). Despite the striking statistics, there are few studies focusing on anemia triggered by P. vivax (5, 6, 14) and most of what is known about that refers to evidence obtained from studies conducted with P. falciparum, leading to the use of proxy pathophysiological processes to explain vivax anemia.
Estimates of rates of severe anemia in vivax malaria range from 1.4% to 32% (15–18). In terms of frequency and severity, the literature points particularly to a greater burden of anemia in young children (9, 15, 19–24) and during pregnancy (7, 25). Cross-sectional studies carried out in the Brazilian Amazon, where P. vivax predominates, showed frequencies of anemia of as high as 80% in children and adolescents (15, 16, 18, 19). P. vivax disease affects only 25% of children from newborns to those 14 years old. Moreover, severe anemia was reported in hospitalized children and adults in need of red blood cell (RBC) transfusions (26).
Severe anemia in pregnancy is an obstetric emergency in regions of falciparum and vivax malaria endemicity (26–28). In areas of concomitant circulation of the two species, the relative frequency of vivax malaria in pregnant women ranges from 30% in Southeast Asia (18, 25, 29) to nearly 80% in Latin America (30–32), pointing to an increasing risk of anemia in the latter region. Severe neonatal anemia was also reported in a study conducted in Colombia (33). Indeed, an extensive evaluation of data from hospitalized newborns in the Indonesian Papua revealed that severe anemia has an important clinical impact on young infants with congenital malaria (18).
OVERVIEW OF THE MAJOR DETERMINANTS OF VIVAX ANEMIA
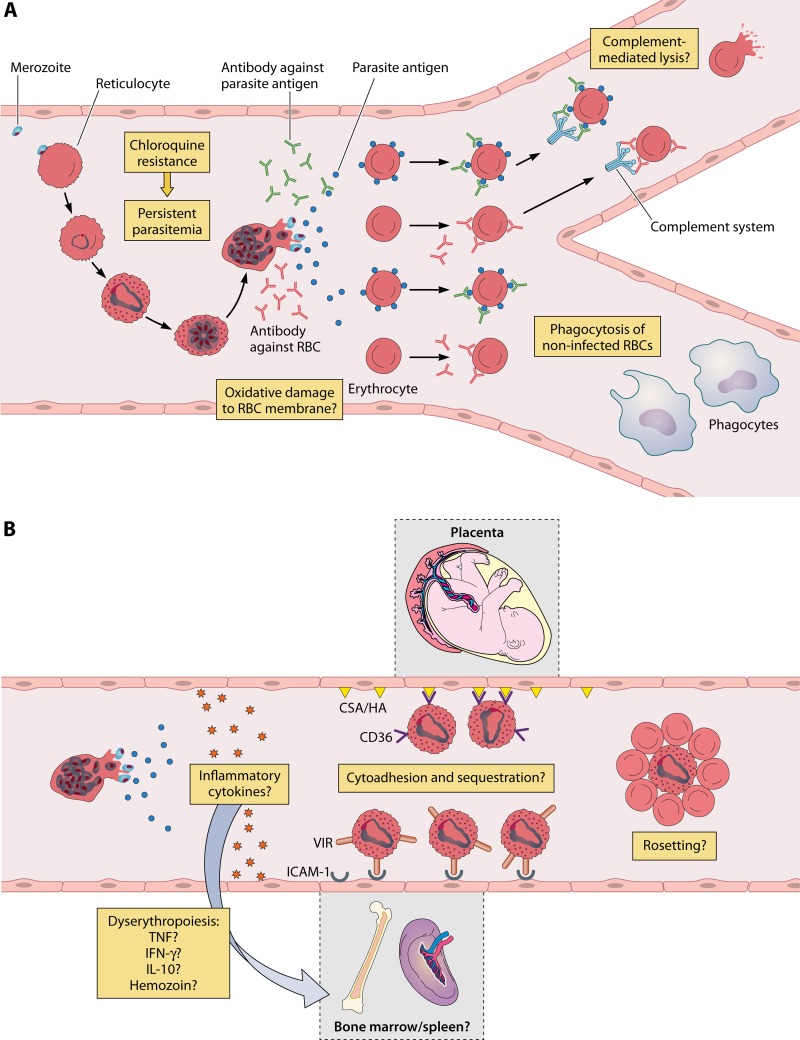
Malaria is an intravascular infection that results essentially from the presence of blood-stage parasites inside RBCs during its intraerythrocytic cycle. Hematological disturbances, therefore, may be caused by the destruction of RBCs, by the release of parasites and RBC debris into the circulation, and finally, by a host reaction to these events (34). Although several mechanisms are likely to participate in the generation of anemia in individuals infected with malarial parasites, they may be grouped into two main categories: (i) destruction of RBCs in the peripheral circulation, spleen, and bone marrow and (ii) dyserythropoiesis (Fig. 1).
FIG 1.
Anemia in Plasmodium vivax malaria and possible immune mechanisms associated with destruction of infected and noninfected red cells. (A) During its intraerythrocytic cycle, P. vivax promotes extensive changes in the host reticulocyte, leading to its rupture. Parasites, antigens, and debris are released into the circulation. In response to these molecules, the host induces a strong immune response which may damage red blood cell (RBC) membranes or still lead to hemolysis or phagocytosis of both noninfected and infected erythrocytes. P. vivax has developed resistance to chloroquine, which may delay parasite clearance, therefore contributing to anemia. (B) Immune mediators may also act in the bone marrow and spleen, causing a toxic effect on erythroid lineages and leading to dyserythropoiesis. Moreover, P. vivax-infected RBCs seem to be able to cytoadhere to endothelial cells from these organs and also to the placental microvasculature. Another possible route of RBC loss is via rosetting. It has been suggested that these RBC aggregates may interfere negatively in erythropoiesis or that the noninfected RBCs attached to the infected one are destroyed in some way. However, the mechanisms that link cytoadherence/rosetting to anemia remain unknown. CSA, chondroitin sulfate A; HA, hyaluronic acid.
Since P. vivax merozoites prefer reticulocytes as host cells (35), as opposed to P. falciparum, which targets all types of RBCs, the density of peripheral parasitemia in vivax malaria is often lower than that detected in falciparum infections (36). Despite this, studies on antimalarial therapy have demonstrated that P. vivax is responsible for a comparable decrease in the RBC mass because P. vivax infection results in a 4-fold-higher removal of noninfected RBCs compared to P. falciparum (37, 38) and, in part, because the invasion of reticulocytes interferes with the supply of mature RBCs (37, 39–41). In P. falciparum infection, 8.5 uninfected RBCs are destroyed per infected RBC (38, 42), while in P. vivax infection, the number of uninfected RBCs that are destroyed is estimated to be around 34 (37). A P. vivax-infected reticulocyte is up to two times larger than a noninfected RBC, and Schüffner's dots associated with caveola-vesicle complexes are seen along the infected RBC plasmalemma (43, 44). In contrast to P. falciparum deformability, RBC deformability seems to be increased in P. vivax infection (45, 46). As a consequence, P. vivax-infected RBCs decrease their clearance during their passage through the spleen sinusoids, making sequestration and obstruction to blood flow unlikely in vivax malaria (47).
Another important contributor to anemia is the reduced deformability of nonparasitized RBCs, as experimentally demonstrated in falciparum malaria. At high shear stresses, erythrocytes increased their rigidity and were removed in the spleen (48). In relation to vivax malaria, it has been demonstrated that after passage under microfluidic conditions simulating splenic filter and fine capillary beds, about 15% of nonparasitized RBCs were lost (46). This observation suggests another possible mechanism for RBC destruction, although how it occurs is still unknown.
Other features of P. vivax parasites that could be associated with the pathogenesis of severe anemia are rosetting (49, 50) and cytoadherence, a phenomenon that has been recently described in vivax malaria (51–54). In vitro studies showed that P. vivax-infected RBCs are able to cytoadhere to endothelial cells from the human lung (54) and also to human placental microvasculature (51, 53). It has also been suggested that in the human spleen, P. vivax attaches to barrier cells to avoid its clearance from circulation, allowing the release of merozoites in a reticulocyte-rich environment (55, 56). Nevertheless, how cytoadherence influences anemia associated with vivax malaria remains to be investigated. In relation to rosetting, this phenomenon was verified in vitro for cells containing parasites with visible malaria pigment (49), and it has been considered a potential contributor to the hypothesized but uncharacterized microvascular obstruction and end-organ pathology described in vivax malaria (57). Recently, another route of normal RBC removal was proposed in a study conducted with Kenyan children presenting with natural P. falciparum infection. According to this work, 4-hydroxynonenal, a biomembrane lipid peroxidation product, is prone to diffuse from P. falciparum-parasitized RBCs to the nonparasitized ones, leading to their clearance by macrophages (58). Notwithstanding, it is unknown whether this process also occurs in P. vivax infection.
It is noteworthy that the overall inflammatory response seems to be stronger in vivax than in falciparum malaria (59, 60), and it is possible that the modifications in the surface of noninfected RBCs may be a direct consequence of cytokine imbalance (61) and oxidative damage (62).
P. vivax infection is accompanied by changes in the host antioxidant defense system which reverse after chloroquine treatment (63). The increase in the level of reactive oxygen species (ROSs) may deplete RBC defense mechanisms, comprising in particular intracellular enzymes, e.g., superoxide dismutase, catalase, and the glutathione system (64, 65). In this manner, alterations in the redox status would play an important role in the pathogenesis of disease, including anemia, as has been proposed for P. falciparum (66).
As host genetic factors may exert some influence on malaria susceptibility, these parameters should be considered important determinants of the anemia onset. However, few studies have focused on investigations of human genetic variants that confer some degree of protection against or resistance to P. vivax and anemia, limiting our understanding of the associations between these polymorphisms and infection (67). It is well established that P. vivax endemicity and estimates of populations at risk are strongly influenced by the proportion of Duffy antigen-negative individuals relatively refractory to the P. vivax infection (4, 68). FY*B/FY*X and FY*A/FY*X genotypes are associated with low parasite density, which may favorably impact hemoglobin levels (69). Observational studies have shown protection against P. vivax infections conferred by a RBC enzyme (glucose-6-phosphate dehydrogenase) deficiency (70–72) and an erythrocyte membrane disorder (Southeast Asian ovalocytosis) by a mechanism that is independent of the Duffy antigen (73). On the other hand, thalassemias, which are disorders of globin synthesis, appear to increase the susceptibility to vivax malaria in carrier populations from different geographic regions (74–77). The reasons why individuals with thalassemias may be more prone to malaria are related to their ineffective erythropoiesis as well as to the shortened survival of their RBCs, which leads to a high cell turnover, increasing their reticulocyte counts and favoring the infection of these cells by P. vivax (76–78). Interestingly, a different association, in which thalassemia would decrease the susceptibility to P. falciparum, conferring protection against severe diseases such as anemia, was observed in falciparum malaria (79). This protection appears to be related to the higher levels of antibodies (Abs) that bind to parasitized erythrocytes (80), allowing their phagocytosis by blood monocytes (81).
DYSERYTHROPOIESIS AND IMMUNE-MEDIATED ANEMIA
During a plasmodial infection, the normal erythropoiesis is disturbed in patients with malaria, reflecting erythropoietic suppression and subsequent dyserythropoiesis. In the acute phase of malaria, the ineffectiveness of erythropoiesis may be evidenced by the presence of normal or reduced cellularity associated with a reduced percentage of erythroblasts. On the other hand, in the chronic phase, it may be deduced by an increase in marrow cellularity and also in erythroblast percentages (82).
A series of studies performed with both P. falciparum and P. vivax infections have shown that a common feature in anemic patients with malaria is the presence of defective erythroblasts exhibiting various abnormalities such as cytoplasmatic vacuolation, nuclei with irregular shape or multinuclearity, intercytoplasmatic bridges, and loss or myelination of parts of nuclear membrane, among others (82–85). These studies have also demonstrated the presence of erythroblasts in different stages of degradation inside the cytoplasm of macrophages from bone marrow, suggesting that erythrophagocytosis was an important mechanism involved in the degradation of injured erythroblasts (86).
Data obtained by transmission electron microscopy revealed two cases of vivax malaria in which parasites were detected in erythroblasts, suggesting that the destruction of these cells by P. vivax could be an underlying mechanism contributing to P. vivax-related anemia (87). Moreover, bone marrow aspirate from a Brazilian Amazon patient with chronic P. vivax infection presenting with splenomegaly and thrombocytopenia showed schizonts inside RBCs, without parallel detection of parasites in the peripheral blood (88).
Recently, an in vitro study conducted with hematopoietic CD34+ cells derived from umbilical cord blood showed that P. vivax could directly inhibit erythroid development. The authors also showed that the presence of the parasite inhibited the growth as well as the differentiation of the erythroid progenitors (41). However, despite the fact that the parasite has perturbed cell division and differentiation, the presence of the parasite did not lead to cell death. Therefore, the importance of erythroblast parasitism in severe P. vivax anemia is still unknown and it is unlikely that the situation occurs in P. falciparum infection, in which there is obstruction of the bone marrow microvasculature by parasitized red blood cells (86).
Besides the direct effects of parasites, the defective erythropoiesis in malaria may also be linked to parasite-derived molecules that cause a toxic effect in erythroblasts or in other erythroid progenitor cells. A second possible factor that may also exert an influence in erythropoiesis is the production of immune mediators, by host cells, as a response to parasite products; when released, these mediators would damage surrounding haematopoietic cells, altering their morphology and function (82). In this regard, it has been demonstrated that the presence of hemozoin, a metabolic product generated during the digestion of hemoglobin, in plasma, leukocytes, or erytroid precursors, was able to inhibit erythropoiesis (89). Studies conducted with bone marrow sections obtained from children who died as a result of severe malaria showed an association between the amount of hemozoin and the proportion of abnormal erythroid cells (90). The negative effects of hemozoin in the erythroid expansion seem to be related to its ability to stimulate the release of cytokines, chemokines, or lipoperoxidases, molecules that inhibit erythropoiesis by bone marrow macrophages (90). Although hemozoin has been considered an important mediator of apoptosis leading to impairment in RBC production during falciparum anemia (90), it remains unknown whether a similar phenomenon also occurs during P. vivax infections.
An imbalance in the production of host immune mediators could be another important factor contributing to anemia, especially in P. vivax malaria, in which the inflammatory response seems to be more intense than that observed in P. falciparum infections with a similar parasite biomass (59, 60, 91, 92). It has been shown that P. vivax patients presenting with moderate to severe anemia exhibited higher concentrations of monocyte chemoattractant protein-1 (MCP-1) (17) It was shown that patients with mild anemia associated with vivax malaria presented higher levels of gamma interferon (IFN-γ) and interleukin-10 (IL-10) (93), as well as tumor necrosis factor (TNF) (61), than the nonanemic ones. IFN-γ, TNF, and IL-10 are some mediators released as a result of T cell activation (82). Elevated levels of TNF alone, or in combination with other cytokines or chemokines, have been associated with the inhibition of the erythroid progenitor cells such as burst-forming unit-erythroid (BFU-E) and CFU-erythroid (CFU-E) cells (94). IFN-γ is another potent inhibitor of erythropoiesis (95). It has been proposed that the negative effects of IFN-γ and TNF in erythropoiesis are related to their ability to induce accelerated apoptosis in the nucleated erythrocyte precursors (96, 97), to their ability to interfere in the expression and regulation of specific transcription factors that control erythroid differentiation (95), and also to their ability to interfere in the production of erythropoietin (98), a hormone which promotes erythropoiesis by stimulating the proliferation, differentiation, and maturation of erythroid progenitors (99). In contrast to IFN-γ and TNF, IL-10 is an anti-inflammatory cytokine that regulates the expression of surface and soluble TNF receptors (100). Since elevated levels of IL-10 seem to limit the TNF effects in neighboring cells, it has been suggested that high IL-10/TNF ratios in plasma from patients with malaria may be associated with protection, while an inverse relation may be indicative of severe anemia (101, 102). Other cytokines that may also be produced during malaria are IL-12, IL-18, and migratory inhibitory factor (MIF). The first two are secreted from macrophages and stimulate natural killer cells as well as B and T cells to produce IFN-γ (34, 103, 104). In B cells, IL-12 also seems to stimulate antibody production. Since it has been believed that IL-12 modulates macrophage activity, which is associated with increased erythrocyte destruction, some studies have demonstrated that higher levels of this cytokine are associated with a better outcome (105–107). MIF is a potent inhibitor of erythroid differentiation, and it may suppress erythropoietin-dependent erythroid colony formation and hemoglobin production (108). The role of MIF in plasma from uncomplicated P. vivax malaria patients has been investigated, and its levels have been positively associated with parasite density but not with hematological parameters (109).
The role of these cytokines in dyserythropoiesis has been most studied in P. falciparum infections, so it is still obscure in vivax malaria and remains to be properly investigated. Recently, a network analysis was attempted to identify the mediators that drive vivax malaria pathogenesis. Levels of a panel composed of different biomarkers of inflammation, tissue damage, and oxidative stress were measured in a large number of individuals, whose data were stratified into different groups according to disease severity and clinical outcome. The results showed that lethality was associated with interactions among markers related to hemolysis-induced damage such as tumor necrosis factor (TNF), hemoxygenase-1 (HO-1), and superoxide dismutase-1 (SOD-1) (110). Since anemia was not included in their analysis, further studies considering this hematological feature will be required to dissect the interactions that lead to RBC loss. Understanding these intricate interactions might be the key that would lead to better and preventive management of P. vivax-associated anemia.
THE COMPLEMENT SYSTEM AND MALARIA
It is known that an important component of the innate immunity is the complement system, which consists of more than 30 fluid-phase or membrane-bound proteins that play an important role in the rapid destruction of invading microorganisms and also of damaged or altered self-tissues (111). The involvement of complement in Plasmodium infections has been widely reported in malaria literature (10, 112, 113) and extensively reviewed elsewhere (114). Different reports have demonstrated that during malaria, complement activation is increased (115, 116). Thus, it is necessary that the host complement regulatory proteins, molecules that protect normal cells from autologous complement-mediated lysis, are expressed in sufficient levels to control complement activation on the cell surface and thereby maintain physiological homeostasis (117). Along these lines, several works have suggested that the erythrocyte complement regulatory proteins may play an important role in the pathogenesis of anemia, protecting nonparasitized RBCs from destruction. This hypothesis was tested by different researchers, who reported that changes in the expression patterns of some complement regulatory proteins such as complement receptor 1 (CR1) (118), decay-accelerating factor (CD55), and membrane inhibitor of reactive lysis (CD59) may render RBCs more susceptible to lysis, increasing their destruction and resulting in anemia (119–124). Studies conducted in areas of P. falciparum endemicity have documented that higher levels of CR1 and CD55 are exhibited in RBCs from children with uncomplicated malaria or who are uninfected than in RBCs from those with severe anemia (119–121). Furthermore, a study conducted on susceptible children in western Kenya demonstrated an association between low levels of complement regulatory proteins on RBC surfaces and increased risk of C3 deposition on their membranes (125). These data suggest that lower expression levels of such biomarkers would contribute to an increased clearance of erythrocytes, leading to anemia in falciparum malaria. It has also been hypothesized that there is age-dependent regulation of the expression pattern of RBC regulatory complement proteins (121). As there is no available information in the literature concerning these aspects of P. vivax infection, it would be a breakthrough to understand if these processes involving the complement system also participate in pathophysiological mechanisms related to this species.
ANTIBODIES AND MALARIA: PROTECTIVE OR PATHOGENIC?
In terms of adaptive immune responses, it is important to emphasize that antibodies are the principal effector molecules that participate in the specific host-parasite interactions. Furthermore, these molecules may act in concert with other factors and it has been known that their protective or pathogenic role is related not only to the magnitude at which they are produced but also to their effector functions.
In malaria, the protective role of antibodies has been well documented by several groups, who demonstrated that the passive transfer of immunoglobulins purified from immune adults to malarial patients can control the infection by reducing parasitemia and protecting individuals against severe disease (126, 127). On the other hand, experiments conducted with P. falciparum have shown that the tagging of the surface of noninfected RBCs by parasite proteins, as well as the tagging of the erythroid precursor cells in bone marrow, may elicit a specific antibody response, triggering phagocytosis and complement activation and inducing the clearance of these cells (128–131). These data suggest that specific immune responses induced by some parasite antigens may contribute to malaria pathogenesis, playing a role in the development of malarial anemia. Data from the past show P. vivax antigens on the surface of infected human RBCs (132). Regarding P. vivax infection, an association between specific antibodies and anemia has also been observed (133). In this scenario, we cannot exclude the possibility of a dual role for specific antibodies against P. vivax. They may participate in both immunity and the pathogenesis of malaria. Taking all these observations into account, further studies are necessary to better elucidate the functional activity of P. vivax-specific antibodies, a vital concern in vivax malaria studies.
AUTOANTIBODIES: A NEGLECTED BUT PROMISING RESEARCH ISSUE
Another element of the adaptive immunity that may also take part in the destruction of noninfected RBCs that occurs in Plasmodium infections is the presence of autoantibodies, molecules induced with regard to autologous components of RBCs. The presence of these circulating immunoglobulins has been well documented both in falciparum and in vivax infection (134–136), and it has been speculated that they are produced in response to cross-reactive antigenicity between parasite and host as well as to normal or altered host proteins. Nevertheless, the relationship between malaria and autoantibodies is still a controversial issue and many hypotheses have been proposed to explain this link. One hypothesis is that infection by Plasmodium parasites induces host autoimmune responses that may be, in part, responsible for some malaria clinical manifestations. Along these lines, it has been shown that, in malaria, autoantibodies may be associated with anemia (137–139) and thrombocytopenia (140) as well as kidney pathology (141). Interestingly, sera from patients with vivax malaria seem to present higher levels of antierythrocyte immunoglobulins compared to sera from patients infected with P. falciparum (136). It is possible that the recognition of surface proteins from noninfected RBCs by autoantibodies or even cross-reacting antibodies, whose levels are increased during P. vivax infection, leads to the opsonization of normal RBCs, facilitating their removal by erythrophagocytosis. The increase in levels of malaria autoantibodies may be associated with molecular mimicry, a mechanism in which a foreign antigen produced by a pathogen shares structural, functional, or immunological similarities with a self-antigen. This strategy may represent an attempt by the parasite to manipulate its host to trigger an immune response directed against autoantigens, facilitating pathogen evasion from the immune system (142). Indeed, it has been demonstrated that two distinct molecules expressed in different Plasmodium life stages, Pf25 and MSP-1, possess epidermal growth factor (EGF) motifs (143–145). In addition, it has been recently shown that a 14-amino-acid motif in PfEMP1 exhibits identity with human vitronectin (146). Considering this information, it is possible to speculate that Plasmodium parasites may also mimic RBC proteins, a hypothesis that should be further evaluated in P. vivax anemia.
RETURNING TO OLD CONCEPTS TO PROPOSE NEW IMMUNE MECHANISMS AGAINST VIVAX ANEMIA
In the challenging task of understanding anemia in vivax malaria, and in order to continue moving forward, an important step that may help the scientific community to better elucidate the mechanisms involved in this hematological feature is to go back to old concepts. This strategy may be a good attempt to answer the following question: how are noninfected RBCs removed from circulation in a P. vivax-infected patient?
Early studies conducted in the 1970s showed that enhanced expression of neoantigens (antigens that arise from changes in components already present in the cell membrane) occurs under physiological conditions during erythrocyte aging. These neoantigens constitute targets for auto-Abs that, in association with enhanced complement components, culminate in phagocytosis (147–150). According to several studies, anti-band 3 antibodies mediate this senescent RBC removal (149, 150). Band 3 is the major integral protein of the RBC membrane, comprising 25% to 30% of its total protein. Band 3 is an anion exchanger protein mediator and is responsible for cell flexibility and shape maintenance (151). In old RBCs, clusters of band 3 are formed and constitute an important target for natural occurring antibodies (152–154). In P. falciparum areas of endemicity, band 3 immune responses seem to be beneficial since malaria-immune children with higher levels of antibodies induced by two conserved band 3 peptides present a lower mean parasite density than nonimmune children (155). Other studies have evidenced that, during P. falciparum infections, synthetic peptides overlapping human band 3 may inhibit not only the RBC invasion but also the cytoadherence/sequestration by antibody-mediated clearance of infected RBCs (156, 157). On the other hand, a different role for anti-band 3 immunoglobulins was proposed (158). According to that work, higher anti-band 3 titers were detected in the high-infection group of children. Interestingly, during the follow-up of these infants, five of them exhibited a significant loss of hemoglobin associated with an increase in anti-band 3 titers (158).
In order to explain the clearance of P. falciparum-parasitized erythrocytes, a band 3/complement RBC removal model was proposed (154). According to literature data, approximately 1 million band 3 molecules are dispersedly expressed on RBC surface (154). Nevertheless, during the aging of erythrocytes, these molecules form band 3 clusters with hemichromes (products derived from hemoglobin degradation) originated by oxidative stress. Thereafter, the immune system recognizes and quickly eliminates those clusters. It has been shown that P. falciparum-infected erythrocytes, in addition to senescent RBCs, also display these clusters. These data lead to the hypothesis that those band 3 antibodies may be involved in the mediation of RBC removal. Taking all this information into account, a question remains: could this model be used to explain the destruction of uninfected RBCs in vivax malaria via extravascular hemolysis?
Recently, it has been proposed that decay-accelerating factor (DAF; a complement receptor that accelerates the decay of C3 and C5 convertases) exerts a crucial role in the RBC recognition by macrophages (159). According to this model, DAF forms a complex on the surface of the RBC membrane through its association with C3b, glycophorin A, and band 3 (DAF-C3b-GPA-Band 3). This complex is thought to alter the viscoelastic properties of the erythrocyte membrane. As a result, RBCs containing such a complex in their membrane become less deformable than normal ones and are cleared from circulation by macrophages in the liver or spleen. By this model, it may be noted that global changes in RBC membrane organization are directly linked to complement activation. We believe that it is important to clarify whether this mechanism is also involved in the destruction of uninfected RBCs during vivax malaria. Further studies are necessary to resolve this issue.
GAPS OF KNOWLEDGE AND FUTURE STUDIES
Considering that some attention was given only recently to the major complications of P. vivax infection, such as severe anemia, data on the pathogenesis of P. vivax-triggered anemia almost do not exist. Therefore, any future study on this issue will be relevant, as seen with P. falciparum. The anemia induced by P. vivax infections is still crudely understood, and the mechanisms that lead to the loss of uninfected RBCs remain unclear. The lack of an appropriate culture method and the difficulties involved in performing in vivo assays limit the tools available to study this parasite. However, the possible involvement of the host immune system in generating anemia may be evaluated using blood samples from different patients living in areas of endemicity. The link between the number of previous malaria episodes and the generation of severe anemia may also provide interesting information. It is still important to investigate what is behind the immune system dysregulation described in the literature and why some individuals residing in areas of endemicity display severe symptoms such as anemia whereas others do not. Furthermore, it is interesting to evaluate whether this difference is due to host polymorphisms and intrinsic divergences between infected patients and their own immunity or also due to polymorphisms between parasite strains that could induce different immune responses. The confirmation of the existence of molecular mimicry by P. vivax and its probable involvement in increasing autoantibody levels, including levels of immunoglobulins against RBC membrane proteins, is another exciting and interesting research field in which proteomic and genomic approaches could give us some important clues. Studies in all these directions may lead to the identification of biomarkers that could serve as prognostic indicators as well as guidelines for a more accurate strategy of treatment and better clinical management of infected patients and severe cases.
Biographies

Thiago Castro-Gomes obtained his master's degree in biochemistry and immunology and his Ph.D. in biochemistry from the Federal University of Minas Gerais, Brazil, studying and characterizing the hemolytic factors produced by the pathogenic protozoan parasite Leishmania amazonensis. He worked as a postdoctoral fellow in the Laboratory of Malaria at the Federal University of Minas Gerais, Brazil. In Dr. Braga's laboratory, he established the basis for the study of the involvement of the host immune system in the generation of anemia in Plasmodium vivax infection. Since 2012, he has been a postdoctoral fellow at the laboratory of Dr. Norma Andrews at the University of Maryland. His current research focuses on mammalian cell biology and the cell biology and biochemistry of plasma membrane repair, a physiological mechanism subverted by intracellular parasites during infections. His fields of interest are the biochemistry and cell biology of parasitic infections and mechanisms of invasion/evasion used by intracellular parasites.

Luiza C. Mourão received her bachelor's degree in biology from the Universidade Federal de Viçosa (UFV), Brazil. She is currently a Ph.D. candidate completing her doctoral degree in parasitology at the Universidade Federal de Minas Gerais (UFMG). She works in the laboratory of Dr. Érika Braga, where she has investigated the associations between host immune responses induced by Plasmodium vivax and morbidity in malaria. Her current research interest is to understand how noninfected red blood cells are destroyed during P. vivax infections leading to anemia, focusing on host immune responses.

Gisely C. Melo is an Assistant Professor of Parasitology at the Faculty of Medicine, University of the Amazonas State (UEA), in Manaus (Brazilian Amazon). She has experience in human parasitology, working mainly with Plasmodium vivax-intestinal parasite coinfections and chloroquine resistance in vivax malaria. Recently, she has dedicated her attention to the study of molecular markers of chloroquine resistance in vivax malaria. She earned her bachelor's degree in pharmacy and biochemistry from Maringá State University in 2004. She received her master's degree in tropical diseases from the Amazonas State University (UEA), in Manaus (2009). Currently, she is a Ph.D. candidate in tropical diseases in this same university.

Wuelton Monteiro is a Professor of Epidemiology of the Faculty of Medicine, University of the Amazonas State (UEA), in Manaus (Brazilian Amazon) and has been a Researcher at the Tropical Medicine Foundation Heitor Vieira Dourado (FMT-HVD) since 2011. He has experience in the area of the epidemiology of neglected parasitic diseases, working mainly in Chagas disease and malaria. Recently, he has been dedicated more specifically to the study of the epidemiology of Plasmodium vivax malaria. In 2004, he earned his bachelor's degree in pharmacy and biochemistry at the Maringá State University. He received his master's degree in clinical analyses from this university in 2006. In 2011, he obtained his Ph.D. degree in tropical diseases from the UEA at Manaus.

Marcus Lacerda is Professor of Infectious Diseases at the University of the Amazonas State (UEA), in Manaus (Brazilian Amazon). As a malaria researcher at the FMT-HVD, he is an expert in infectious diseases and is responsible for coordinating the International Center for Clinical Malaria Research. He currently serves as the Director of Learning and Research of the FMT-HVD and is an affiliate member of the Brazilian Academy of Sciences. Born in Taguatinga, Brazil, Dr. Lacerda obtained his Medical Degree at the University of Brasília (1999) and completed his residency in infectious diseases at the Tropical Medicine Foundation under the mentorship of Dr. Heitor Vieira Dourado (FMT-HVD) (2002). He completed his Ph.D. in Tropical Medicine at the University of Brasília in partnership with the New York University (2007).

Erika Martins Braga is a Full Professor of Parasitology at the Universidade Federal de Minas Gerais (UFMG), Brazil. She received her B.A. degree in biology in 1990 and her Ph.D. in parasitology from the same university in 1997. She has been the head of the Malaria Laboratory of the Parasitology Department at UFMG since 1997. Her research is focused on two distinct approaches: study of the immune response in human malaria and study of the diversity of avian malaria parasites in wild birds. She has spent the last 20 years studying the humoral and cellular immune responses among different populations in the Brazilian endemic Amazon region. Her current research interest includes the study of immunological mechanisms which determine anemia in patients infected by Plasmodium vivax.
Footnotes
Published ahead of print 4 August 2014
REFERENCES
- 1. WHO. 2013. Word malaria report 2013. WHO, Geneva, Switzerland [Google Scholar]
- 2. Hay SI, Guerra CA, Tatem AJ, Atkinson PM, Snow RW. 2005. Urbanization, malaria transmission and disease burden in Africa. Nat. Rev. Microbiol. 3:81–90. 10.1038/nrmicro1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. WHO. 2000. Severe falciparum malaria. World Health Organization, Communicable Diseases Cluster. Trans. R. Soc. Trop. Med. Hyg. 94(Suppl 1):S1–S90 [PubMed] [Google Scholar]
- 4. Gething PW, Elyazar IR, Moyes CL, Smith DL, Battle KE, Guerra CA, Patil AP, Tatem AJ, Howes RE, Myers MF, George DB, Horby P, Wertheim HF, Price RN, Mueller I, Baird JK, Hay SI. 2012. A long neglected world malaria map: Plasmodium vivax endemicity in 2010. PLoS Negl. Trop. Dis. 6:e1814. 10.1371/journal.pntd.0001814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Genton B, D'Acremont V, Rare L, Baea K, Reeder JC, Alpers MP, Muller I. 2008. Plasmodium vivax and mixed infections are associated with severe malaria in children: a prospective cohort study from Papua New Guinea. PLoS Med. 5:e127. 10.1371/journal.pmed.0050127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tjitra E, Anstey NM, Sugiarto P, Warikar N, Kenangalem E, Karyana M, Lampah DA, Price RN. 2008. Multidrug-resistant Plasmodium vivax associated with severe and fatal malaria: a prospective study in Papua, Indonesia. PLoS Med. 5:e128. 10.1371/journal.pmed.0050128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rodriguez-Morales AJ, Sanchez E, Vargas M, Piccolo C, Colina Arria M, Franco-Paredes C. 2006. Pregnancy outcomes associated with Plasmodium vivax malaria in northeastern Venezuela. Am. J. Trop. Med. Hyg. 74:755–757 [PubMed] [Google Scholar]
- 8. Rogerson SJ, Carter R. 2008. Severe vivax malaria: newly recognised or rediscovered. PLoS Med. 5:e136. 10.1371/journal.pmed.0050136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nurleila S, Syafruddin D, Elyazar IR, Baird JK. 2012. Serious and fatal illness associated with falciparum and vivax malaria among patients admitted to hospital at West Sumba in eastern Indonesia. Am. J. Trop. Med. Hyg. 87:41–49. 10.4269/ajtmh.2012.11-0577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Price RN, Douglas NM, Anstey NM. 2009. New developments in Plasmodium vivax malaria: severe disease and the rise of chloroquine resistance. Curr. Opin. Infect. Dis. 22:430–435. 10.1097/QCO.0b013e32832f14c1 [DOI] [PubMed] [Google Scholar]
- 11. White NJ. 2011. Determinants of relapse periodicity in Plasmodium vivax malaria. Malar. J. 10:297. 10.1186/1475-2875-10-297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Price RN, Tjitra E, Guerra CA, Yeung S, White NJ, Anstey NM. 2007. Vivax malaria: neglected and not benign. Am. J. Trop. Med. Hyg. 77:79–87 [PMC free article] [PubMed] [Google Scholar]
- 13. Kochar DK, Das A, Kochar SK, Saxena V, Sirohi P, Garg S, Kochar A, Khatri MP, Gupta V. 2009. Severe Plasmodium vivax malaria: a report on serial cases from Bikaner in northwestern India. Am. J. Trop. Med. Hyg. 80:194–198 [PubMed] [Google Scholar]
- 14. Melo GC, Reyes-Lecca RC, Vitor-Silva S, Monteiro WM, Martins M, Benzecry SG, Alecrim MG, Lacerda MV. 2010. Concurrent helminthic infection protects schoolchildren with Plasmodium vivax from anemia. PLoS One 5:e11206. 10.1371/journal.pone.0011206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rodríguez-Morales AJ, Sánchez E, Vargas M, Piccolo C, Colina R, Arria M. 2006. Anemia and thrombocytopenia in children with Plasmodium vivax malaria. J. Trop. Pediatr. 52:49–51. 10.1093/tropej/fmi069 [DOI] [PubMed] [Google Scholar]
- 16. Ventura AMRS. 2010. Anemia da malária por Plasmodium vivax: estudo clínico e laboratorial em crianças e adolescentes. Instituto Oswaldo Cruz, Belém, Brazil [Google Scholar]
- 17. Fernandes AA, Carvalho LJ, Zanini GM, Ventura AM, Souza JM, Cotias PM, Silva-Filho IL, Daniel-Ribeiro CT. 2008. Similar cytokine responses and degrees of anemia in patients with Plasmodium falciparum and Plasmodium vivax infections in the Brazilian Amazon region. Clin. Vaccine Immunol. 15:650–658. 10.1128/CVI.00475-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Poespoprodjo JR, Fobia W, Kenangalem E, Lampah DA, Hasanuddin A, Warikar N, Sugiarto P, Tjitra E, Anstey NM, Price RN. 2009. Vivax malaria: a major cause of morbidity in early infancy. Clin. Infect. Dis. 48:1704–1712. 10.1086/599041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ventura AM, Pinto AY, Silva RS, Calvosa VS, Silva Filho MG, Souza JM. 1999. Plasmodium vivax malaria in children and adolescents - epidemiological, clinical and laboratory features. J. Pediatr. (Rio J) 75:187–194 (In Portuguese.) 10.2223/JPED.295 [DOI] [PubMed] [Google Scholar]
- 20. Jat KR, Guglani V, Khairwa A. 2012. Severe and complicated Plasmodium vivax malaria in children. Trop. Doct. 42:185–187. 10.1258/td.2012.120174 [DOI] [PubMed] [Google Scholar]
- 21. Mahgoub H, Gasim GI, Musa IR, Adam I. 2012. Severe Plasmodium vivax malaria among Sudanese children at New Halfa Hospital, eastern Sudan. Parasit. Vectors 5:154. 10.1186/1756-3305-5-154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sharma R, Gohain S, Chandra J, Kumar V, Chopra A, Chatterjee S, Aneja S, Dutta AK. 2012. Plasmodium vivax malaria admissions and risk of mortality in a tertiary-care children's hospital in North India. Paediatr. Int. Child. Health 32:152–157. 10.1179/2046905512Y.0000000012 [DOI] [PubMed] [Google Scholar]
- 23. Kaushik JS, Gomber S, Dewan P. 2012. Clinical and epidemiological profiles of severe malaria in children from Delhi, India. J. Health Popul. Nutr. 30:113–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lança EF, Magalhães BM, Vitor-Silva S, Siqueira AM, Benzecry SG, Alexandre MA, O'Brien C, Bassat Q, Lacerda MV. 2012. Risk factors and characterization of Plasmodium vivax-associated admissions to pediatric intensive care units in the Brazilian Amazon. PLoS One 7:e35406. 10.1371/journal.pone.0035406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nosten F, McGready R, Simpson JA, Thwai KL, Balkan S, Cho T, Hkirijaroen L, Looareesuwan S, White NJ. 1999. Effects of Plasmodium vivax malaria in pregnancy. Lancet 354:546–549. 10.1016/S0140-6736(98)09247-2 [DOI] [PubMed] [Google Scholar]
- 26. Alexandre MA, Ferreira CO, Siqueira AM, Magalhaes BL, Mourao MP, Lacerda MV, Alecrim M. 2010. Severe Plasmodium vivax malaria, Brazilian Amazon. Emerg. Infect. Dis. 16:1611–1614. 10.3201/eid1610.100685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Matteelli A, Donato F, Shein A, Muchi JA, Leopardi O, Astori L, Carosi G. 1994. Malaria and anemia in pregnant women in urban Zanzibar, Tanzania. Ann. Trop. Med. Parasitol. 88:475–483 [DOI] [PubMed] [Google Scholar]
- 28. Smereck J. 2011. Malaria in pregnancy: update on emergency management. J. Emerg. Med. 40:393–396. 10.1016/j.jemermed.2010.04.029 [DOI] [PubMed] [Google Scholar]
- 29. Singh N, Mehra RK, Srivastava N. 2001. Malaria during pregnancy and infancy, in an area of intense malaria transmission in central India. Ann. Trop. Med. Parasitol. 95:19–29. 10.1080/00034980020035889 [DOI] [PubMed] [Google Scholar]
- 30. Chagas EC, do Nascimento CT, de Santana Filho FS, Botto-Menezes CH, Martinez-Espinosa FE. 2009. Impact of malaria during pregnancy in the Amazon region. Rev. Panam. Salud Publica 26:203–208 (In Portuguese.) 10.1590/S1020-49892009000900003 [DOI] [PubMed] [Google Scholar]
- 31. Gómez E, López E, Ache A. 2009. Malaria and pregnancy. San Isidro parish, municipality Sifontes, state of Bolivar, Venezuela, 2005–2006. Invest. Clin. 50:455–464 (In Spanish.) [PubMed] [Google Scholar]
- 32. Almeida LB, Barbosa M, Martinez-Espinosa FE. 2010. Malaria among women aged 10 to 49 years, according to SIVEP-Malaria, Manaus, State of Amazonas, 2003–2006. Rev. Soc. Bras. Med. Trop. 43:304–308 (In Portuguese.) 10.1590/S0037-86822010000300018 [DOI] [PubMed] [Google Scholar]
- 33. Piñeros-Jiménez JG, Arboleda M, Jaramillo JC, Blair S. 2008. Report of five cases of severe neonatal Plasmodium vivax malaria in Uraba, Colombia. Biomedica 28:471–479 (In Spanish.) 10.7705/biomedica.v28i4.53 [DOI] [PubMed] [Google Scholar]
- 34. Lamikanra AA, Brown D, Potocnik A, Casals-Pascual C, Langhorne J, Roberts DJ. 2007. Malarial anemia: of mice and men. Blood 110:18–28. 10.1182/blood-2006-09-018069 [DOI] [PubMed] [Google Scholar]
- 35. Kitchen SF. 1938. The infection of reticulocytes by Plasmodium vivax. Am. J. Trop. Med. Hyg. 18:347–359 [Google Scholar]
- 36. McKenzie FE, Jeffery GM, Collins WE. 2002. Plasmodium vivax blood-stage dynamics. J. Parasitol. 88:521–535. 10.1645/0022-3395(2002)088[0521:PVBSD]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Collins WE, Jeffery GM, Roberts JM. 2003. A retrospective examination of anemia during infection of humans with Plasmodium vivax. Am. J. Trop. Med. Hyg. 68:410–412 [PubMed] [Google Scholar]
- 38. Jakeman GN, Saul A, Hogarth WL, Collins WE. 1999. Anemia of acute malaria infections in non-immune patients primarily results from destruction of uninfected erythrocytes. Parasitology 119(Pt 2):127–133. 10.1017/S0031182099004564 [DOI] [PubMed] [Google Scholar]
- 39. McQueen PG, McKenzie FE. 2004. Age-structured red blood cell susceptibility and the dynamics of malaria infections. Proc. Natl. Acad. Sci. U. S. A. 101:9161–9166. 10.1073/pnas.0308256101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. McQueen PG. 2010. Population dynamics of a pathogen: the conundrum of vivax malaria. Biophys. Rev. 2:111–120. 10.1007/s12551-010-0034-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Panichakul T, Payuhakrit W, Panburana P, Wongborisuth C, Hongeng S, Udomsangpetch R. 2012. Suppression of erythroid development in vitro by Plasmodium vivax. Malar. J. 11:173. 10.1186/1475-2875-11-173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Price RN, Simpson JA, Nosten F, Luxemburger C, Hkirijaroen L, ter Kuile F, Chongsuphajaisiddhi T, White NJ. 2001. Factors contributing to anemia after uncomplicated falciparum malaria. Am. J. Trop. Med. Hyg. 65:614–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Aikawa M, Miller LH, Rabbege J. 1975. Caveola–vesicle complexes in the plasmalemma of erythrocytes infected by Plasmodium vivax and P cynomolgi. Unique structures related to Schüffner's dots. Am. J. Pathol. 79:285–300 [PMC free article] [PubMed] [Google Scholar]
- 44. Barnwell JW, Ingravallo P, Galinski MR, Matsumoto Y, Aikawa M. 1990. Plasmodium vivax: malarial proteins associated with the membrane-bound caveola-vesicle complexes and cytoplasmic cleft structures of infected erythrocytes. Exp. Parasitol. 70:85–99. 10.1016/0014-4894(90)90088-T [DOI] [PubMed] [Google Scholar]
- 45. Suwanarusk R, Cooke BM, Dondorp AM, Silamut K, Sattabongkot J, White NJ, Udomsangpetch R. 2004. The deformability of red blood cells parasitized by Plasmodium falciparum and P. vivax. J. Infect. Dis. 189:190–194. 10.1086/380468 [DOI] [PubMed] [Google Scholar]
- 46. Handayani S, Chiu DT, Tjitra E, Kuo JS, Lampah D, Kenangalem E, Renia L, Snounou G, Price RN, Anstey NM, Russell B. 2009. High deformability of Plasmodium vivax-infected red blood cells under microfluidic conditions. J. Infect. Dis. 199:445–450. 10.1086/596048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gaur D, Mayer DC, Miller LH. 2004. Parasite ligand-host receptor interactions during invasion of erythrocytes by Plasmodium merozoites. Int. J. Parasitol. 34:1413–1429. 10.1016/j.ijpara.2004.10.010 [DOI] [PubMed] [Google Scholar]
- 48. Dondorp AM, Angus BJ, Chotivanich K, Silamut K, Ruangveerayuth R, Hardeman MR, Kager PA, Vreeken J, White NJ. 1999. Red blood cell deformability as a predictor of anemia in severe falciparum malaria. Am. J. Trop. Med. Hyg. 60:733–737 [DOI] [PubMed] [Google Scholar]
- 49. Udomsanpetch R, Thanikkul K, Pukrittayakamee S, White NJ. 1995. Rosette formation by Plasmodium vivax. Trans. R. Soc. Trop. Med. Hyg. 89:635–637. 10.1016/0035-9203(95)90422-0 [DOI] [PubMed] [Google Scholar]
- 50. Chotivanich KT, Pukrittayakamee S, Simpson JA, White NJ, Udomsangpetch R. 1998. Characteristics of Plasmodium vivax-infected erythrocyte rosettes. Am. J. Trop. Med. Hyg. 59:73–76 [DOI] [PubMed] [Google Scholar]
- 51. Carvalho BO, Lopes SC, Nogueira PA, Orlandi PP, Bargieri DY, Blanco YC, Mamoni R, Leite JA, Rodrigues MM, Soares IS, Oliveira TR, Wunderlich G, Lacerda MV, del Portillo HA, Araujo MO, Russell B, Suwanarusk R, Snounou G, Renia L, Costa FT. 2010. On the cytoadhesion of Plasmodium vivax-infected erythrocytes. J. Infect. Dis. 202:638–647. 10.1086/654815 [DOI] [PubMed] [Google Scholar]
- 52. Bernabeu M, Lopez FJ, Ferrer M, Martin-Jaular L, Razaname A, Corradin G, Maier AG, Del Portillo HA, Fernandez-Becerra C. 2012. Functional analysis of Plasmodium vivax VIR proteins reveals different subcellular localizations and cytoadherence to the ICAM-1 endothelial receptor. Cell. Microbiol. 14:386–400. 10.1111/j.1462-5822.2011.01726.x [DOI] [PubMed] [Google Scholar]
- 53. Chotivanich K, Udomsangpetch R, Suwanarusk R, Pukrittayakamee S, Wilairatana P, Beeson JG, Day NP, White NJ. 2012. Plasmodium vivax adherence to placental glycosaminoglycans. PLoS One 7:e34509. 10.1371/journal.pone.0034509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Anstey NM, Handojo T, Pain MCF, Kenangalem E, Tjitra E, Price RN, Maguire GP. 2007. Lung injury in vivax malaria: pathophysiological evidence for pulmonary vascular sequestration and posttreatment alveolar-capillary inflammation. J. Infect. Dis. 195:589–596. 10.1086/510756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Bowdler AJ. 2002. The complete spleen. Structure, function and clinical disorders. Humana Press, Totowa, NJ [Google Scholar]
- 56. del Portillo HA, Lanzer M, Rodriguez-Malaga S, Zavala F, Fernandez-Becerra 2004. Variant genes and the spleen in Plasmodium vivax malaria. Int. J. Parasitol. 34:1547–1554. 10.1016/j.ijpara.2004.10.012 [DOI] [PubMed] [Google Scholar]
- 57. Barnwell JW, MRG 1998. Invasion of vertebrate cells: erythrocytes, p 93–120 In Sherman IW. (ed), Malaria: parasite biology, pathogenesis, and protection. ASM Press, Washington, DC [Google Scholar]
- 58. Uyoga S, Skorokhod OA, Opiyo M, Orori EN, Williams TN, Arese P, Schwarzer E. 2012. Transfer of 4-hydroxynonenal from parasitized to non-parasitized erythrocytes in rosettes. Proposed role in severe malaria anemia. Br. J. Haematol. 157:116–124. 10.1111/j.1365-2141.2011.09015.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Hemmer CJ, Holst FG, Kern P, Chiwakata CB, Dietrich M, Reisinger EC. 2006. Stronger host response per parasitized erythrocyte in Plasmodium vivax or ovale than in Plasmodium falciparum malaria. Trop. Med. Int. Health 11:817–823. 10.1111/j.1365-3156.2006.01635.x [DOI] [PubMed] [Google Scholar]
- 60. Yeo TW, Lampah DA, Tjitra E, Piera K, Gitawati R, Kenangalem E, Price RN, Anstey NM. 2010. Greater endothelial activation, Weibel-Palade body release and host inflammatory response to Plasmodium vivax, compared with Plasmodium falciparum: a prospective study in Papua, Indonesia. J. Infect. Dis. 202:109–112. 10.1086/653211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Andrade BB, Reis-Filho A, Souza-Neto SM, Clarencio J, Camargo LM, Barral A, Barral-Netto M. 2010. Severe Plasmodium vivax malaria exhibits marked inflammatory imbalance. Malar. J. 9:13. 10.1186/1475-2875-9-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Sharma A, Eapen A, Subbarao SK. 2004. Parasite killing in Plasmodium vivax malaria by nitric oxide: implication of aspartic protease inhibition. J. Biochem. 136:329–334. 10.1093/jb/mvh128 [DOI] [PubMed] [Google Scholar]
- 63. Sarin K, Kumar A, Prakash A, Sharma A. 1993. Oxidative stress and antioxidant defence mechanism in Plasmodium vivax malaria before and after chloroquine treatment. Indian J. Malariol. 30:127–133 [PubMed] [Google Scholar]
- 64. Erel O, Kocyigit A, Avci S, Aktepe N, Bulut V. 1997. Oxidative stress and antioxidative status of plasma and erythrocytes in patients with vivax malaria. Clin. Biochem. 30:631–639. 10.1016/S0009-9120(97)00119-7 [DOI] [PubMed] [Google Scholar]
- 65. Bilgin R, Yalcin MS, Yucebilgic G, Koltas IS, Yazar S. 2012. Oxidative stress in vivax malaria. Korean J. Parasitol. 50:375–377. 10.3347/kjp.2012.50.4.375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Keller CC, Kremsner PG, Hittner JB, Misukonis MA, Weinberg JB, Perkins DJ. 2004. Elevated nitric oxide production in children with malarial anemia: hemozoin-induced nitric oxide synthase type 2 transcripts and nitric oxide in blood mononuclear cells. Infect. Immun. 72:4868–4873. 10.1128/IAI.72.8.4868-4873.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Taylor SM, Parobeck CM, Fairhurst RM. 2012. Haemoglobinopathies and the clinical epidemiology of malaria: a systematic review and meta-analysis. Lancet Infect. Dis. 12:457–468. 10.1016/S1473-3099(12)70055-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Howes RE, Patil AP, Piel FB, Nyangiri OA, Kabaria CW, Gething PW, Zimmerman PA, Barnadas C, Beall CM, Gebremedhin A, Menard D, Williams TN, Weatherall DJ, Hay SI. 2011. The global distribution of the Duffy blood group. Nat. Commun. 2:266. 10.1038/ncomms1265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Albuquerque SR, Cavalcante Fde O, Sanguino EC, Tezza L, Chacon F, Castilho L, dos Santos MC. 2010. FY polymorphisms and vivax malaria in inhabitants of Amazonas State, Brazil. Parasitol. Res. 106:1049–1053. 10.1007/s00436-010-1745-x [DOI] [PubMed] [Google Scholar]
- 70. Santana MS, de Lacerda MV, Barbosa M, Alecrim WD, Alecrim M. 2009. Glucose-6-phosphate dehydrogenase deficiency in an endemic area for malaria in Manaus: a cross-sectional survey in the Brazilian Amazon. PLoS One 4:e5259. 10.1371/journal.pone.0005259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Leslie T, Briceno M, Mayan I, Mohammed N, Klinkenberg E, Sibley CH, Whitty CJ, Rowland M. 2010. The impact of phenotypic and genotypic G6PD deficiency on risk of plasmodium vivax infection: a case-control study amongst Afghan refugees in Pakistan. PLoS Med. 7:e1000283. 10.1371/journal.pmed.1000283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Santana MS, Monteiro WM, Siqueira AM, Costa MF, Sampaio Lacerda VMV, Alecrim MG. 2013. Glucose-6-phosphate dehydrogenase deficient variants are associated with reduced susceptibility to malaria in the Brazilian Amazon. Trans. R. Soc. Trop. Med. Hyg. 107:301–306. 10.1093/trstmh/trt015 [DOI] [PubMed] [Google Scholar]
- 73. Rosanas-Urgell A, Lin E, Manning L, Rarau P, Laman M, Senn N, Grimberg BT, Tavul L, Stanisic DI, Robinson LJ, Aponte JJ, Dabod E, Reeder JC, Siba P, Zimmerman PA, Davis TM, King CL, Michon P, Mueller I. 2012. Reduced risk of Plasmodium vivax malaria in Papua New Guinean children with Southeast Asian ovalocytosis in two cohorts and a case-control study. PLoS Med. 9:e1001305. 10.1371/journal.pmed.1001305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Williams TN, Maitland K, Bennett S, Ganczakowski M, Peto TE, Newbold CI, Bowden DK, Weatherall DJ, Clegg JB. 1996. High incidence of malaria in alpha-thalassaemic children. Nature 383:522–525. 10.1038/383522a0 [DOI] [PubMed] [Google Scholar]
- 75. Allen SJ, O'Donnell A, Alexander ND, Alpers MP, Peto TE, Clegg JB, Weatherall DJ. 1997. Alpha+-Thalassemia protects children against disease caused by other infections as well as malaria. Proc. Natl. Acad. Sci. U. S. A. 94:14736–14741. 10.1073/pnas.94.26.14736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. O'Donnell A, Premawardhena A, Arambepola M, Samaranayake R, Allen SJ, Peto TE, Fisher CA, Cook J, Corran PH, Olivieri NF, Weatherall DJ. 2009. Interaction of malaria with a common form of severe thalassemia in an Asian population. Proc. Natl. Acad. Sci. U. S. A. 106:18716–18721. 10.1073/pnas.0910142106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Zimmerman PA, Ferreira MU, Howes RE, Mercereau-Puijalon O. 2013. Red blood cell polymorphism and susceptibility to Plasmodium vivax. Adv. Parasitol. 81:27–76. 10.1016/B978-0-12-407826-0.00002-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Weatherall DJ, Clegg JB. 2001. The thalassemia syndromes. Blackwell Scientific, Oxford, United Kingdom [Google Scholar]
- 79. Wambua S, Mwangi TW, Kortok M, Uyoga SM, Macharia AW, Mwacharo JK, Weatherall DJ, Snow RW, Marsh K, Williams TN. 2006. The effect of α+-thalassaemia on the incidence of malaria and other diseases in children living on the coast of Kenya. PLoS Med. 3:e158. 10.1371/journal.pmed.0030158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Luzzi GA, Merry AH, Newbold CI, Marsh K, Pasvol G, Weatherall DJ. 1991. Surface antigen expression on Plasmodium falciparum-infected erythrocytes is modified in alpha- and beta-thalassemia. J. Exp. Med. 173:785–791. 10.1084/jem.173.4.785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Yuthavong Y, Butthep P, Bunyaratvej A, Fucharoen S, Khusmith S. 1988. Impaired parasite growth and increased susceptibility to phagocytosis of Plasmodium falciparum infected alpha-thalassemia or hemoglobin constant spring red blood cells. Am. J. Clin. Pathol. 89:521–525 [DOI] [PubMed] [Google Scholar]
- 82. Wickramasinghe SN, Abdalla SH. 2000. Blood and bone marrow changes in malaria. Baillieres Best. Pract. Res. Clin. Haematol. 13:277–299. 10.1053/beha.1999.0072 [DOI] [PubMed] [Google Scholar]
- 83. Abdalla S, Weatherall DJ, Wickramasinghe SN, Hughes M. 1980. The anaemia of P. falciparum malaria. Br. J. Haematol. 46:171–183. 10.1111/j.1365-2141.1980.tb05956.x [DOI] [PubMed] [Google Scholar]
- 84. Abdalla SH. 1990. Hematopoiesis in human malaria. Blood Cells 16:401–419 [PubMed] [Google Scholar]
- 85. Wickramasinghe SH, Phillips RE, Looareesuwan S, Warrell DA, Hughes M. 1987. The bone marrow in human cerebral malaria: parasite sequestration within sinusoids. Br. J. Haematol. 66:295–306. 10.1111/j.1365-2141.1987.tb06913.x [DOI] [PubMed] [Google Scholar]
- 86. Wickramasinghe SN, Looareesuwan S, Nagachinta B, White NJ. 1989. Dyserythropoiesis and ineffective erythropoiesis in Plasmodium vivax malaria. Br. J. Haematol. 72:91–99. 10.1111/j.1365-2141.1989.tb07658.x [DOI] [PubMed] [Google Scholar]
- 87. Ru YX, Mao BY, Zhang FK, Pang TX, Zhao SX, Liu JH, Wickramasinghe SN. 2009. Invasion of erythroblasts by Plasmodium vivax: a new mechanism contributing to malarial anemia. Ultrastruct. Pathol. 33:236–242. 10.3109/01913120903251643 [DOI] [PubMed] [Google Scholar]
- 88. Lacerda MVG, Hipólito JR, Passos LNM. 2008. Chronic Plasmodium vivax infection in a patient with splenomegaly and severe thrombocytopenia. Rev. Soc. Bras. Med. Trop. 41:522–523. 10.1590/S0037-86822008000500021 [DOI] [PubMed] [Google Scholar]
- 89. Casals-Pascual C, Kai O, Cheung JO, Williams S, Lowe B, Nyanoti M, Williams TN, Maitland K, Molyneux M, Newton CR, Peshu N, Watt SM, Roberts DJ. 2006. Suppression of erythropoiesis in malarial anemia is associated with hemozoin in vitro and in vivo. Blood 108:2569–2577. 10.1182/blood-2006-05-018697 [DOI] [PubMed] [Google Scholar]
- 90. Lamikanra AA, Theron M, Kooij TW, Roberts DJ. 2009. Hemozoin (malarial pigment) directly promotes apoptosis of erythroid precursors. PLoS One 4:e8446. 10.1371/journal.pone.0008446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Karunaweera ND, Grau GE, Gamage P, Carter R, Mendis KN. 1992. Dynamics of fever and serum levels of tumor necrosis factor are closely associated during clinical paroxysms in Plasmodium vivax malaria. Proc. Natl. Acad. Sci. U. S. A. 89:3200–3203. 10.1073/pnas.89.8.3200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Gonçalves RM, Scopel KK, Bastos MS, Ferreira MU. 2012. Cytokine balance in human malaria: does Plasmodium vivax elicit more inflammatory responses than Plasmodium falciparum? PLoS One 7:e44394. 10.1371/journal.pone.0044394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Jain V, Singh PP, Silawat N, Patel R, Saxena A, Bharti PK, Shukla M, Biswas S, Singh N. 2010. A preliminary study on pro- and anti-inflammatory cytokine profiles in Plasmodium vivax malaria patients from central zone of India. Acta Trop. 113:263–268. 10.1016/j.actatropica.2009.11.009 [DOI] [PubMed] [Google Scholar]
- 94. Roodman GD, Bird A, Hutzeler D, Montgomery W. 1987. Tumor necrosis factor-alpha and hematopoietic progenitors: effects of tumor necrosis factor on the growth of erythroid progenitors CFU-E and BFU-E and the hematopoietic cell lines K562, HL60, and HEL cells. Exp. Hematol. 15:928–935 [PubMed] [Google Scholar]
- 95. Libregts SF, Gutiérrez L, de Bruin AM, Wensveen FM, Papadopoulos P, van Ijcken W, Ozgür Z, Philipsen S, Nolte MA. 2011. Chronic IFN-γ production in mice induces anemia by reducing erythrocyte life span and inhibiting erythropoiesis through an IRF-1/PU.1 axis. Blood 118:2578–2588. 10.1182/blood-2010-10-315218 [DOI] [PubMed] [Google Scholar]
- 96. Dai C, Krantz SB. 1999. Interferon γ induces upregulation and activation of caspases 1, 3, and 8 to produce apoptosis in human erythroid progenitor cells. Blood 93:3309–3316 [PubMed] [Google Scholar]
- 97. Papadaki HA, Kritikos HD, Valatas V, Boumpas DT, Eliopoulos GD. 2002. Anemia of chronic disease in rheumatoid arthritis is associated with increased apoptosis of bone marrow erythroid cells: improvement following anti-tumor necrosis factor-alpha antibody therapy. Blood 100:474–482. 10.1182/blood-2002-01-0136 [DOI] [PubMed] [Google Scholar]
- 98. Jelkmann W. 1998. Proinflammatory cytokines lowering erythropoietin production. J. Interferon Cytokine Res. 18:555–559. 10.1089/jir.1998.18.555 [DOI] [PubMed] [Google Scholar]
- 99. Chang KH, Stevenson MM. 2004. Malarial anaemia: mechanisms and implications of insufficient erythropoiesis during blood-stage malaria. Int. J. Parasitol. 34:1501–1516. 10.1016/j.ijpara.2004.10.008 [DOI] [PubMed] [Google Scholar]
- 100. Dickensheets HL, Freeman SL, Smith MF, Donnelly RP. 1997. Interleukin-10 upregulates tumor necrosis factor receptor type-II (p75) gene expression in endotoxin-stimulated human monocytes. Blood 90:4162–4171 [PubMed] [Google Scholar]
- 101. Othoro C, Lal AA, Nahlen B, Koech D, Orago AS, Udhayakumar V. 1999. A low interleukin-10 tumor necrosis factor-alpha ratio is associated with malaria anemia in children residing in a holoendemic malaria region in western Kenya. J. Infect. Dis. 179:279–282. 10.1086/314548 [DOI] [PubMed] [Google Scholar]
- 102. Thuma PE, van Dijk J, Bucala R, Debebe Z, Nekhai S, Kuddo T, Nouraie M, Weiss G, Gordeuk VR. 2011. Distinct clinical and immunologic profiles in severe malarial anemia and cerebral malaria in Zambia. J. Infect. Dis. 203:211–219. 10.1093/infdis/jiq041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Watford WT, Moriguchi M, Morinobu A, Oshea JJ. 2003. The biology of IL-12: coordinating innate and adaptive immune responses. Cytokine Growth Factor Rev. 14:361–368. 10.1016/S1359-6101(03)00043-1 [DOI] [PubMed] [Google Scholar]
- 104. Malaguarnera L, Pignatelli S, Musumeci M, Simporè J, Musumeci S. 2002. Plasma levels of interleukin-18 and interleukin-12 in Plasmodium falciparum malaria. Parasite Immunol. 24:489–492. 10.1046/j.1365-3024.2002.00485.x [DOI] [PubMed] [Google Scholar]
- 105. Malaguarnera L, Imbesi RM, Pignatelli S, Simporé J, Malaguarnera M, Musumeci S. 2002. Increased levels of interleukin-12 in Plasmodium falciparum malaria: correlation with the severity of disease. Parasite Immunol. 24:387–389. 10.1046/j.1365-3024.2002.00478.x [DOI] [PubMed] [Google Scholar]
- 106. Luty AJ, Perkins DJ, Lell B, Sclunidt D, Weinberg JB, Kremsner PG. 2000. Low interleukin-12 activity in severe Plasmodium falciparum malaria. Infect. Immun. 68:3909–3915. 10.1128/IAI.68.7.3909-3915.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Perkins DJ, Weinberg JB, Kremsner PG. 2000. Reduced interleukin-12 and transforming growth factor-beta 1 in severe childhood malaria: relationship of cytokine balance with disease severity. J. Infect. Dis. 182:988–992. 10.1086/315762 [DOI] [PubMed] [Google Scholar]
- 108. McDevitt MA, Xie J, Shanmugasundaram G, Griffith J, Liu A, McDonald C, Thuma P, Gordeuk VR, Metz CN, Mitchell R, Keefer J, David J, Leng L, Bucala R. 2006. A critical role for the host mediator macrophage migration inhibitory factor in the pathogenesis of malarial anemia. J. Exp. Med. 203:1185–1196. 10.1084/jem.20052398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Han C, Lin Y, Shang G, Zhang Z, Sun X, Wang Z, Wei C, Deng Y, Zhang L, Bu L, Shao D, Wang H. 2010. Plasma concentrations of malaria parasite-derived macrophage migration factor in uncomplicated malaria patients correlates with parasitemia and disease severity. Clin. Vaccine Immunol. 17:1524–1532. 10.1128/CVI.00149-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Mendonça VR, Queiroz AT, Lopes FM, Andrade BB, Barral-Netto M. 2013. Networking the host immune response in Plasmodium vivax malaria. Malar. J. 12:69. 10.1186/1475-2875-12-69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Dunkelberger JR, Song WC. 2010. Complement and its role in innate and adaptive immune responses. Cell Res. 20:34–50. 10.1038/cr.2009.139 [DOI] [PubMed] [Google Scholar]
- 112. Greenwood BM, Brueton MJ. 1974. Complement activation in children with acute malaria. Clin. Exp. Immunol. 18:267–272 [PMC free article] [PubMed] [Google Scholar]
- 113. Adam C, Geniteau M, Gougerot-Pocidalo M, Verroust P, Lebras J, Gibert C, Morel-Maroger L. 1981. Cryoglobulins, circulating immune complexes, and complement activation in cerebral malaria. Infect. Immun. 31:530–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Silver KL, Higgins SJ, McDonald CR, Kain KC. 2010. Complement driven innate immune response to malaria: fuelling severe malarial diseases. Cell. Microbiol. 12:1036–1045. 10.1111/j.1462-5822.2010.01492.x [DOI] [PubMed] [Google Scholar]
- 115. Goka BQ, Kwarko H, Kurtzhals JA, Gyan B, Ofori-Adjei E, Ohene SA, Hviid L, Akanmori BD, Neequaye J. 2001. Complement binding to erythrocytes is associated with macrophage activation and reduced haemoglobin in Plasmodium falciparum malaria. Trans. R. Soc. Trop. Med. Hyg. 95:545–549. 10.1016/S0035-9203(01)90036-7 [DOI] [PubMed] [Google Scholar]
- 116. Roestenberg M, McCall M, Mollnes TE, van Deuren M, Sprong T, Klasen I, Hermsen CC, Sauerwein RW, van der Ven A. 2007. Complement activation in experimental human malaria infection. Trans. R. Soc. Trop. Med. Hyg. 101:643–649. 10.1016/j.trstmh.2007.02.023 [DOI] [PubMed] [Google Scholar]
- 117. Sjöberg AP, Trouw LA, Blom AM. 2009. Complement activation and inhibition: a delicate balance. Trends Immunol. 30:83–90. 10.1016/j.it.2008.11.003 [DOI] [PubMed] [Google Scholar]
- 118. Fernandez-Arias CF, Lopez JP, Hernandez-Perez JN, Bautista-Ojeda MD, Branch O, Rodriguez A. 2013. Malaria inhibits surface expression of complement receptor 1 in monocytes/macrophages, causing decreased immune complex internalization. J. Immunol. 190:3363–3372. 10.4049/jimmunol.1103812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Waitumbi JN, Opollo MO, Muga RO, Misore AO, Stoute JA. 2000. Red cell surface changes and erythrophagocytosis in children with severe Plasmodium falciparum anemia. Blood 95:1481–1486 [PubMed] [Google Scholar]
- 120. Stoute JA, Odindo AO, Owuor BO, Mibei EK, Opollo MO, Waitumbi JN. 2003. Loss of red blood cell-complement regulatory proteins and increased levels of circulating immune complexes are associated with severe malarial anemia. J. Infect. Dis. 187:522–525. 10.1086/367712 [DOI] [PubMed] [Google Scholar]
- 121. Waitumbi JN, Donvito B, Kisserli A, Cohen JH, Stoute JA. 2004. Age-related changes in red blood cell complement regulatory proteins and susceptibility to severe malaria. J. Infect. Dis. 190:1183–1191. 10.1086/423140 [DOI] [PubMed] [Google Scholar]
- 122. Owuor BO, Odhiambo CO, Otieno WO, Adhiambo C, Makawiti DW, Stoute JA. 2008. Reduced immune complex binding capacity and increased complement susceptibility of red cells from children with severe malaria-associated anemia. Mol. Med. 14:89–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Gwamaka M, Fried M, Domingo G, Duffy PE. 2011. Early and extensive CD55 loss from red blood cells supports a causal role in malarial anemia. Malar. J. 10:386. 10.1186/1475-2875-10-386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Mahajan RC, Narain K, Mahanta J. 2011. Anemia & expression levels of CD35, CD55 & CD59 on red blood cells in Plasmodium falciparum malaria patients from India. Indian J. Med. Res. 133:662–664 [PMC free article] [PubMed] [Google Scholar]
- 125. Odhiambo CO, Otieno W, Adhiambo C, Odera MM, Stoute JA. 2008. Increased deposition of C3b on red cells with low CR1 and CD55 in a malaria-endemic region of western Kenya: implications for the development of severe anemia. BMC Med. 6:23. 10.1186/1741-7015-6-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Cohen S, Butcher GA. 1971. Serum antibody in acquired malarial immunity. Trans. R. Soc. Trop. Med. Hyg. 65:125–135. 10.1016/0035-9203(71)90210-0 [DOI] [PubMed] [Google Scholar]
- 127. Sabchareon A, Burnouf T, Ouattara D, Attanath P, Bouharoun-Tayoun H, Chantavanich P, Foucault C, Chongsuphajaisiddhi T, Druilhe P. 1991. Parasitologic and clinical human response to immunoglobulin administration in falciparum malaria. Am. J. Trop. Med. Hyg. 45:297–308 [DOI] [PubMed] [Google Scholar]
- 128. Layez C, Nogueira P, Combes V, Costa FT, Juhan-Vague I, da Silva LH, Gysin J. 2005. Plasmodium falciparum rhoptry protein RSP2 triggers destruction of the erythroid lineage. Blood 106:3632–3638. 10.1182/blood-2005-04-1574 [DOI] [PubMed] [Google Scholar]
- 129. Sterkers Y, Scheidig C, da Rocha M, Lepolard C, Gysin J, Scherf A. 2007. Members of the low-molecular-mass rhoptry protein complex of Plasmodium falciparum bind to the surface of normal erythrocytes. J. Infect. Dis. 196:617–621. 10.1086/519685 [DOI] [PubMed] [Google Scholar]
- 130. Awah NW, Troye-Blomberg M, Berzins K, Gysin J. 2009. Mechanisms of malarial anemia: potential involvement of the Plasmodium falciparum low molecular weight rhoptry-associated proteins. Acta Trop. 112:295–302. 10.1016/j.actatropica.2009.08.017 [DOI] [PubMed] [Google Scholar]
- 131. Awah N, Balogun H, Achidi E, Mariuba LA, Nogueira PA, Orlandi P, Troye-Blomberg M, Gysin J, Berzins K. 2011. Antibodies to the Plasmodium falciparum rhoptry protein RAP-2/RSP-2 in relation to anemia in Cameroonian children. Parasite Immunol. 33:104–115. 10.1111/j.1365-3024.2010.01259.x [DOI] [PubMed] [Google Scholar]
- 132. Mendis KN, Thalamulla RI, David PH. 1988. Diversity of Plasmodim vivax-induced antigens on the surface of infected human erythrocytes. Am. J. Trop. Med. Hyg. 38:42–46 [DOI] [PubMed] [Google Scholar]
- 133. Mourão LC, Morais CG, Bueno LL, Jimenez MC, Soares IS, Fontes CJ, Guimaraes Lacerda MV, Xavier MS, Barnwell JW, Galinski MR, Braga EM. 2012. Naturally acquired antibodies to Plasmodium vivax blood-stage vaccine candidates (PvMSP-119 and PvMSP-3α359–798) and their relationship with hematological features in malaria patients from the Brazilian Amazon. Microbes Infect. 14:730–739. 10.1016/j.micinf.2012.02.011 [DOI] [PubMed] [Google Scholar]
- 134. Facer CA, Bray RS, Brown J. 1979. Direct Coombs antiglobulin reactions in Gambian children with Plasmodium falciparum malaria. I. Incidence and class specificity. Clin. Exp. Immunol. 35:119–127 [PMC free article] [PubMed] [Google Scholar]
- 135. Facer CA. 1980. Direct Coombs antiglobulin reactions in Gambian children with Plasmodium falciparum malaria. II. Specificity of erythrocyte-bound IgG. Clin. Exp. Immunol. 39:279–288 [PMC free article] [PubMed] [Google Scholar]
- 136. Berzins K, Wahlgren M, Perlmann P. 1983. Studies on the specificity of anti-erythrocyte antibodies in the serum of patients with malaria. Clin. Exp. Immunol. 54:313–318 [PMC free article] [PubMed] [Google Scholar]
- 137. Zuckerman A. 1964. Autoimmunization and other types of indirect damage to host cells as factors in certain protozoan diseases. Exp. Parasitol. 15:138–183. 10.1016/0014-4894(64)90014-1 [DOI] [PubMed] [Google Scholar]
- 138. Lefrançois G, Bouvet E, Le Bras J, Vroklans M, Simonneau M, Vachon F. 1981. Anti-erythrocyte autoimmunisation during chronic falciparum malaria. Lancet ii:661–664 [DOI] [PubMed] [Google Scholar]
- 139. Banic DM, Viana-Martins FS, De Souza JM, Peixoto TD, Daniel-Ribeiro C. 1991. Polyclonal B-lymphocyte stimulation in human malaria and its association with ongoing parasitemia. Am. J. Trop. Med. Hyg. 44:571–577 [DOI] [PubMed] [Google Scholar]
- 140. Sørensen PG, Mickley H, Schmidt KG. 1984. Malaria-induced immune thrombocytopenia. Vox Sang. 47:68–72. 10.1111/j.1423-0410.1984.tb01563.x [DOI] [PubMed] [Google Scholar]
- 141. Wozencraft AO, Lloyd CM, Staines NA, Griffiths VJ. 1990. Role of DNA-binding antibodies in kidney pathology associated with murine malaria infections. Infect. Immun. 58:2156–2164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Cusick MF, Libbey JE, Fujinami RS. 2012. Molecular mimicry as a mechanism of autoimmune disease. Clin. Rev. Allergy Immunol. 42:102–111. 10.1007/s12016-011-8294-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Kaslow DC, Quakyi IA, Syin C, Raum MG, Keister DB, Coligan JE, McCutchan TF, Miller LH. 1988. A vaccine candidate from the sexual stage of human malaria that contains EGF-like domains. Nature 333:74–76. 10.1038/333074a0 [DOI] [PubMed] [Google Scholar]
- 144. Han HJ, Park SG, Kim SH, Hwang SY, Han J, Traicoff J, Kho WG, Chung JY. 2004. Epidermal growth factor-like motifs 1 and 2 of Plasmodium vivax merozoite surface protein 1 are critical domains in erythrocyte invasion. Biochem. Biophys. Res. Commun. 320:563–570. 10.1016/j.bbrc.2004.06.008 [DOI] [PubMed] [Google Scholar]
- 145. Blackman MJ, Ling IT, Nicholls SC, Holder AA. 1991. Proteolytic processing of the Plasmodium falciparum merozoite surface protein-1 produces a membrane-bound fragment containing two epidermal growth factor-like domains. Mol. Biochem. Parasitol. 49:29–33. 10.1016/0166-6851(91)90127-R [DOI] [PubMed] [Google Scholar]
- 146. Ludin P, Nilsson D, Maser P. 2011. Genome-wide identification of molecular mimicry candidates in parasites. PLoS One 6:e17546. 10.1371/journal.pone.0017546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Kay MM. 1975. Mechanism of removal of senescent cells by human macrophages in situ. Proc. Natl. Acad. Sci. U. S. A. 72:3521–3525. 10.1073/pnas.72.9.3521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Turrini F, Arese P, Yuan J, Low PS. 1991. Clustering of integral membrane proteins of the human erythrocyte membrane stimulates autologous IgG binding, complement deposition, and phagocytosis. J. Biol. Chem. 266:23611–23617 [PubMed] [Google Scholar]
- 149. Kay M. 2005. Immunoregulation of cellular life span. Ann. N. Y. Acad. Sci. 1057:85–111. 10.1196/annals.1356.005 [DOI] [PubMed] [Google Scholar]
- 150. Dinkla S, Novotny VM, Joosten I, Bosman GJ. 2012. Storage-induced changes in erythrocyte membrane proteins promote recognition by autoantibodies. PLoS One 7:e42250. 10.1371/journal.pone.0042250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Zhang D, Kiyatkin A, Bolin JT, Low PS. 2000. Crystallographic structure and functional interpretation of the cytoplasmic domain of erythrocyte membrane band 3. Blood 96:2925–2933 [PubMed] [Google Scholar]
- 152. Waugh SM, Low PS. 1985. Hemichrome binding to band 3: nucleation of Heinz bodies on the erythrocyte membrane. Biochemistry 24:34–39. 10.1021/bi00322a006 [DOI] [PubMed] [Google Scholar]
- 153. Waugh SM, Walder JA, Low PS. 1987. Partial characterization of the copolymerization reaction of erythrocyte membrane band 3 with hemichromes. Biochemistry 26:1777–1783. 10.1021/bi00380a041 [DOI] [PubMed] [Google Scholar]
- 154. Pantaleo A, Giribaldi G, Mannu F, Arese P, Turrini F. 2008. Naturally occurring anti-band 3 antibodies and red blood cell removal under physiological and pathological conditions. Autoimmun. Rev. 7:457–462. 10.1016/j.autrev.2008.03.017 [DOI] [PubMed] [Google Scholar]
- 155. Hogh B, Petersen E, Crandall I, Gottschau A, Sherman IW. 1994. Immune responses to band 3 neoantigens on Plasmodium falciparum-infected erythrocytes in subjects living in an area of intense malaria transmission are associated with low parasite density and high hematocrit value. Infect. Immun. 62:4362–4366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Crandall I, Collins WE, Gysin J, Sherman IW. 1993. Synthetic peptides based on motifs present in human band 3 protein inhibit cytoadherence/sequestration of the malaria parasite Plasmodium falciparum. Proc. Natl. Acad. Sci. U. S. A. 90:4703–4707. 10.1073/pnas.90.10.4703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Goel VK, Li X, Chen H, Liu SC, Chishti AH, Oh SS. 2003. Band 3 is a host receptor binding merozoite surface protein 1 during the Plasmodium falciparum invasion of erythrocytes. Proc. Natl. Acad. Sci. U. S. A. 100:5164–5169. 10.1073/pnas.0834959100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Luginbühl A, Nikolic M, Beck HP, Wahlgren M, Lutz HU. 2007. Complement factor D, albumin, and immunoglobulin G anti-band 3 protein antibodies mimic serum in promoting rosetting of malaria-infected red blood cells. Infect. Immun. 75:1771–1777. 10.1128/IAI.01514-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Karnchanaphanurach P, Mirchev R, Ghiran I, Asara JM, Papahadjopoulos-Sternberg B, Nicholson-Weller A, Golan DE. 2009. C3b deposition on human erythrocytes induces the formation of a membrane skeleton-linked protein complex. J. Clin. Invest. 119:788–801. 10.1172/JCI36088 [DOI] [PMC free article] [PubMed] [Google Scholar]