Abstract
Staphylococcus aureus is responsible for numerous chronic and recurrent infections, which are frequently associated with the emergence of small-colony variants (SCVs) that lack a functional electron transport chain. SCVs exhibit enhanced expression of fibronectin-binding protein (FnBP) and greatly reduced hemolysin production, although the basis for this is unclear. One hypothesis is that these phenotypes are a consequence of the reduced Agr activity of SCVs, while an alternative is that the lack of a functional electron transport chain and the resulting reduction in ATP production are responsible. Disruption of the electron transport chain of S. aureus genetically (hemB and menD) or chemically, using 2-n-heptyl-4-hydroxyquinoline N-oxide (HQNO), inhibited both growth and Agr activity and conferred an SCV phenotype. Supplementation of the culture medium with synthetic autoinducing peptide (sAIP) significantly increased Agr expression in both hemB mutant strains and S. aureus grown with HQNO and significantly reduced staphylococcal adhesion to fibronectin. However, sAIP did not promote hemolysin expression in hemB mutant strains or S. aureus grown with HQNO. Therefore, while Agr regulates fibronectin binding in SCVs, it cannot promote hemolysin production in the absence of a functional electron transport chain.
INTRODUCTION
Staphylococcus aureus is responsible for a broad range of infections, including furunculitis, abscesses, bacteremia, infective endocarditis, osteomyelitis, septic arthritis, pneumonia, and meningitis, and for colonization of the airways of people with cystic fibrosis (CF) (1–3).
The ability of S. aureus to cause such diverse infections is underpinned by a huge array of different virulence factors and immune evasion molecules, which are tightly regulated by a number of global regulators, including the quorum-sensing master virulence regulator Agr (accessory gene regulator) (2, 4–7). The Agr locus encodes two divergent promoters: P2, which transcribes RNAII, and P3, which transcribes RNAIII. P2 controls the expression of a four-gene operon, agrBDCA, which encodes the quorum-sensing agr autoactivation circuit. Specifically, agrD encodes the quorum-sensing molecule autoinducing peptide (AIP), which is processed and secreted by AgrB. AgrC and AgrA constitute a two-component signaling system, which detects AIP and completes the quorum-sensing part of the Agr system. The secreted AIP binds the transmembrane-bound histidine kinase signal receptor AgrC and activates it; it subsequently undergoes transautophosphorylation and transfers the phosphate to AgrA, the response regulator. The phosphorylated AgrA activates the P2 promoter, thereby completing the autoinduced activation circuit (6). Therefore, Agr is activated as the S. aureus population density increases, up to a maximum level in stationary phase. In addition to quorum sensing, there is also evidence that Agr acts as a diffusion-sensing system to signal bacterial uptake into host cell vacuoles (8). AgrA, in addition to regulating the Agr quorum-sensing system from P2, also drives transcription from the P3 promoter that transcribes the RNAIII transcript, the major effector molecule of the Agr system (6). RNAIII inhibits expression of surface protein (e.g., protein A and fibronectin binding proteins) genes and promotes expression of hemolytic exotoxin genes (e.g., hld and hla) (6, 9, 10).
While a combination of antibiotic therapy and a potent immune response are usually sufficient to resolve most S. aureus infections, a significant number develop a chronic or relapsing course (1, 11–13). Several studies of S. aureus isolated from the sites of chronic and recurrent infections have revealed the presence of slow-growing, weakly pigmented small-colony variants (SCVs) (14–24). As their name suggests, SCVs form small colonies on agar plates, have significantly reduced hemolytic activity, and are often resistant to aminoglycoside or sulfonamide antibiotics (14, 15, 20, 24).
SCVs typically arise from wild-type bacteria via mutations in genes associated with biosynthetic pathways, including those of menaquinone, heme, and thymidine, and are a normal part of the S. aureus life cycle (25–29). The loss of menaquinone or heme production results in loss of the electron transport chain, leading to decreased membrane potential and reduced ATP production and growth rate (20, 30, 31). Additionally, the loss of heme biosynthesis may impact other aspects of S. aureus biology, for example, the production of catalase (20).
The ability of SCVs to survive in host tissues for extended periods is believed to be due, at least in part, to their ability to persist within host cells without triggering apoptosis, which shields them from immune surveillance and therapeutic antibiotics (23, 32, 33). Invasion of, and persistence within, host cells is enabled by the high level of fibronectin-binding protein (FnBP) expression and decreased cytolysin (hemolysin) expression exhibited by SCVs (33–36). However, the regulation of these two key virulence factors in SCVs is unclear (20). Both of these phenotypic properties might conceivably be explained by the significantly reduced Agr activity in SCVs (6, 37). Alternatively, there is overwhelming evidence that the metabolic state of bacteria, which is dramatically altered in SCVs relative to the wild type, can have dramatic effects on virulence factor production (38–40). Therefore, it is possible that the loss of the electron transport chain and associated shift in the metabolic profile, rather than reduced Agr activity, are responsible for the altered virulence factor profile of SCVs. Indeed, Vaudaux et al. provided evidence that the enhanced expression of fibronectin-binding protein in SCVs was agr independent (36), while Proctor et al. showed that chemical inhibition of the electron transport chain abrogated hemolysin production (41).
Therefore, the aim of this study was to determine whether the virulence factor profile of electron transport chain-defective S. aureus is due to reduced Agr activity or the loss of a functional electron transport chain.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
The bacterial strains and plasmids used in this study are listed in Table 1. S. aureus SH1000 was chosen for the study because it has intact agr and sigB regulatory elements (42). USA300 was also employed to demonstrate that key phenotypic effects of loss of a functional electron transport chain occur in a clinically relevant strain (43). S. aureus was cultured either in 3 ml tryptic soy broth (TSB) in 30-ml universal tubes at 37°C with orbital shaking (180 rpm) for phenotypic analyses or in 200 μl TSB in microtiter plates for growth and Agr activity analyses (see below) (44). In some assays, S. aureus was grown in the presence of 2-n-heptyl-4-hydroxyquinoline N-oxide (HQNO) (10 μg ml−1; Santa-Cruz Biotechnology) (45); synthetic type 1 AIP, as described previously (46) (1 to 10 μM; Peptide Synthetics); hemin (1 μg ml−1; Sigma-Aldrich); or menadione (1 μg ml−1; Sigma-Aldrich). The concentration of synthetic AIP (sAIP) used was based on previous measurements of the concentration of active (unoxidized) AIP (∼10 μM) in stationary-phase S. aureus culture supernatants (46). Where appropriate, the following antibiotics were included in the medium: chloramphenicol (10 μg ml−1) and erythromycin (10 μg ml−1).
TABLE 1.
Bacterial strains and plasmids used in this study
| Bacterial strain or plasmid | Relevant characteristics | Source or reference |
|---|---|---|
| S. aureus | ||
| SH1000 | Functional rsbU+ strain repaired for rsbU from parental laboratory strain NCTC8325-4 | 42 |
| SH1001 (agr mutant) | SH1000 agr::tet | 42 |
| SH1000 hemB::Tn | SH1000 transduced with DNA from a USA300 hemB::Tn mutant; Ermr; heme auxotroph | This study |
| SH1001 hemB::Tn | SH1001 transduced with DNA from a USA300 hemB::Tn mutant; Ermr; heme auxotroph | This study |
| SH1000-P3 | pCL55agr IR P3-GFP integrated into the geh locus of SH1000; reports P3 activity | 44 |
| SH1000-P2 | pCL55agr IR P2-GFP integrated into the geh locus of SH1000; reports P2 activity | 44 |
| SH1001-P3 | pCL55agr IR P3-GFP integrated into the geh locus of SH1001; reports P3 activity | 44 |
| SH1001-P2 | pCL55agr IR P2-GFP integrated into the geh locus of SH1001; reports P2 activity | 44 |
| SH1000 hemB::Tn-P3 | SH1000-P3 transduced with DNA from a USA300 hemB::Tn mutant; Ermr; heme auxotroph; reports P3 activity | This study |
| SH1000 hemB::Tn-P2 | SH1000-P2 transduced with DNA from a USA300 hemB::Tn mutant; Ermr; heme auxotroph; reports P2 activity | This study |
| SH1001 hemB::Tn-P3 | SH1001-P3 transduced with DNA from a USA300 hemB::Tn mutant; Ermr; heme auxotroph; reports P3 activity | This study |
| SH1001 hemB::Tn-P2 | SH1001-P2 transduced with DNA from a USA300 hemB::Tn mutant; Ermr; heme auxotroph; reports P3 activity | This study |
| USA300 LAC | CA-MRSA strain of the USA300 lineage | 43 |
| USA300 hemB | USA300 in which hemB has been deleted | This study |
| USA300 menD | USA300 in which menD has been deleted | This study |
| USA300 hemB-P3 | USA300 hemB; reports P3 activity | This study |
| USA300 menD-P3 | USA300 menD; reports P3 activity | This study |
| E. coli | ||
| DC10B | Δdcm in DH10B (ΔhsdRMS endA1 recA1) dam methylation only; high-efficiency cloning strain that bypasses the restriction modification system of S. aureus | 46 |
| Plasmids | ||
| pCL55agr IR P3-GFP | pCL55 Ar Cmr containing the agr P2-P3 intergenic region with gfp expression under the control of P3 | 44 |
| pCL55agr IR P2-GFP | pCL55 Ar Cmr containing the agr P2-P3 intergenic region with gfp expression under the control of P2 | 44 |
Construction of strains.
SH1000 hemB::Tn and SH1001 (Δagr mutant) hemB::Tn were produced via transduction of DNA from USA300 hemB::Tn using Φ11 and selection for erythromycin resistance. Transductants had a classic SCV phenotype, including slow growth, small colonies on TS agar, lack of pigmentation, and resistance to gentamicin (see Fig. S1 in the supplemental material) (45). A wild-type phenotype was restored upon supplementation of the culture medium with hemin (see Fig. S1 in the supplemental material) (reference 45 and data not shown). USA300 hemB and menD mutations were generated using pIMAY (47), resulting in deletion of nucleotides 159 to 901 from hemB and 72 to 1554 from menD. Mutations were confirmed by DNA sequencing. Both the hemB and menD mutants displayed a typical SCV appearance, as described previously (27, 29, 48, 49) (see Fig. S1 in the supplemental material).
Reporter constructs, consisting of the agr P2-P3 intergenic region fused with gfp under the control of either the P2 or P3 promoter, in pCL55 were passaged through Escherichia coli DC10B and transformed directly into S. aureus strains by electroporation (44). Transformants were selected for by using chloramphenicol, and insertion of the construct into the geh locus was confirmed by PCR (44).
Growth and Agr expression reporter assays.
Growth and Agr expression reporter assays were performed as described previously (44). Briefly, stationary-phase cultures were diluted 1:50 in a final volume of 200 μl TSB (with or without HQNO, hemin, or sAIP, as described above) and added to the wells of black microtiter plates with clear bottoms (Corning). The plates were incubated at 37°C with shaking (500 rpm) in a Polarstar Omega multiwell plate reader. Bacterial growth was measured via optical density at 600 nm (OD600) readings, and green fluorescent protein (GFP) expression from the P2 or P3 promoter was measured as relative fluorescence units (excitation filter, 485 nm; emission filter, 520 nm) simultaneously every 30 min for a total of 17 h (44).
Real-time PCR analysis of RNAII and RNAIII transcripts.
RNA extraction and real-time (RT) PCR were performed as described previously (44). Briefly, S. aureus SH1000 wild type or hemB::Tn was grown to either mid-logarithmic or stationary phase, samples were taken, and bacterial cells were lysed using lysostaphin and SDS. RNA was stabilized using TRIzol (Life Technologies), purified using a Qiagen RNeasy kit, and used to generate cDNA with a Qiagen Omniscript reverse transcription kit and random hexanucleotides (Promega). The reaction conditions and details of primers for the amplification of hld, agrA, and gyrA have been described previously (44).
Fibronectin binding.
Bacterial attachment to immobilized human plasma fibronectin was determined as described previously (50, 51). Briefly, fibronectin (1 μg well−1) was immobilized onto plastic Nunc Maxisorp Immuno modules in PBS at 4°C for 16 h. The remaining binding sites were blocked with 300 μl 3% (wt/vol) bovine serum albumin. Bacteria were cultured in 3 ml TSB to stationary phase, washed twice in PBS, and adjusted to equivalent densities in PBS (∼108 ml−1) before 100 μl of each suspension was added to wells and incubated statically for 1 h at 37°C. The wells were then washed thrice with PBS before adherent bacteria were fixed with 0.25% (wt/vol) paraformaldehyde and stained with crystal violet (0.5% [wt/vol]) for 1 min. Excess dye was removed by 3 rounds of PBS washing, and the bound dye was solubilized with 7% (vol/vol) acetic acid (100 μl). Solubilized crystal violet was quantified by absorbance (A595) measurement using a microplate reader, and the values were converted to bacterial numbers by the use of standard plots (52).
Determination of hemolytic activity.
Hemolysis of sheep erythrocytes was employed as a measure of hemolytic activity, as these cells can be lysed by at least two major S. aureus hemolysins (alpha and delta toxins) (53). The hemolytic activity of culture supernatants was determined essentially as described previously (54). Bacteria were removed from spent culture medium by centrifugation (13,000 × g; 10 min), and the supernatant was diluted in 2-fold steps using fresh TSB. Each dilution (100 μl) was mixed with a 2% sheep blood suspension in PBS and incubated statically at 37°C for 1 h. Unlysed blood cells were removed by centrifugation, and the supernatant was transferred to the wells of a new microtiter plate. Erythrocyte lysis was determined by measuring the A450 of the supernatant (54). Erythrocytes incubated with fresh TSB or TSB containing 1% TX-100 served as negative and positive controls, respectively. All values were related to the A450 reading of undiluted supernatant from wild-type cultures (i.e., represented as relative percent hemolysis). For experiments using sAIP, TSB (1 ml) was inoculated with ∼2 × 109 CFU S. aureus and incubated at 37°C with shaking in the absence or presence of HQNO and/or sAIP (10 μM). This ensured that all cultures had sufficient numbers of bacterial cells to reasonably expect detection of hemolytic activity.
Determination of fibronectin-binding protein expression.
Expression of fibronectin-binding proteins was determined by blot overlay of total protein extracts using human plasma fibronectin (Sigma). Wild-type S. aureus SH1000 bacteria grown in the absence or presence of HQNO, hemB::Tn, or SH1001 were grown in the absence or presence of 10 μM sAIP for 16 h. The bacteria were washed three times by alternate rounds of centrifugation and resuspension in PBS before adjustment to equivalent cell numbers via OD600 measurements. Approximately 109 CFU of each strain from each set of growth conditions was lysed with lysostaphin. Insoluble material was removed by centrifugation, and soluble protein was separated by SDS-PAGE. The separated proteins were blotted onto nitrocellulose membranes, and the remaining protein binding sites were blocked with 5% skim milk powder in Tris-buffered saline (TBS) as described previously (44). Fibronectin-binding proteins A and B were detected by overlaying blots with human plasma fibronectin (5 μg ml−1 in TBS). The blots were then washed three times with TBS-Tween (0.1%) before detection of bound fibronectin using an anti-human fibronectin polyclonal antiserum raised in rabbit (Sigma), followed by horseradish peroxidase-conjugated antibodies against rabbit IgG. Bound antibody was detected using enhanced chemiluminescence (ECL) detection as described previously (44).
RESULTS
Disruption of the electron transport chain inhibits growth and reduces Agr activity.
Previous work has shown that the Pseudomonas exoproduct HQNO blocks the electron transport chain of wild-type S. aureus and confers an SCV-like phenotype, including slow growth and aminoglycoside resistance (45). Therefore, this provided an ideal tool to delineate the relative contributions of electron transport chain deficiency and lack of Agr activity to the virulence phenotype of SCVs.
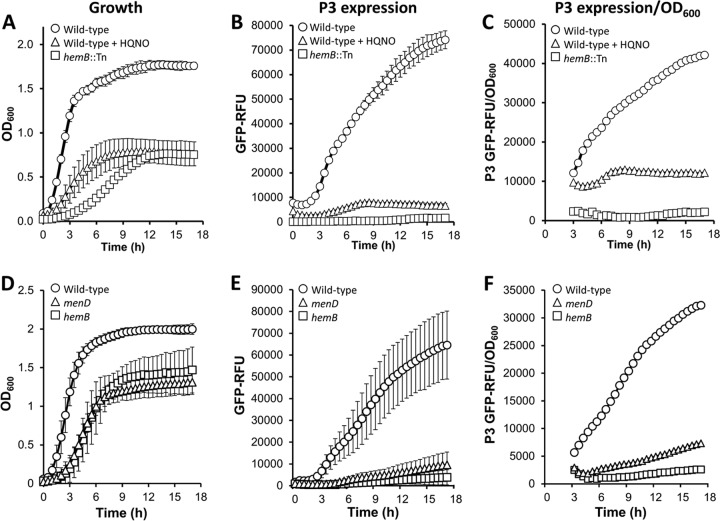
Growth of wild-type S. aureus SH1000 in the presence of HQNO significantly reduced both the growth rate and final density relative to the wild type grown in the absence of HQNO. In comparison, the SH1000 hemB::Tn mutant had an even lower rate of growth, but the final density was identical to that of the wild type grown with HQNO (Fig. 1A). Similarly, USA300 mutants lacking hemB or menD had significantly reduced growth rates and final densities in stationary phase (Fig. 1D).
FIG 1.
Loss of the electron transport chain reduces growth and Agr expression. Shown are growth (A and D), P3 expression (B and E), and P3 expression corrected for growth (C and F) of S. aureus SH1000 (A to C) and USA300 (D to F) and derived strains. (A to C) SH1000 grown in the absence or presence of HQNO and a hemB::Tn mutant in TSB as determined by the OD600 (growth) and A520 (GFP fluorescence) measurements. (D to F) USA300 wild type, ΔhemB, and ΔmenD grown in TSB. The measurements are as described for panels A to C. The data represent the means of at least 4 independent experiments, each in triplicate. The error bars represent the standard deviations of the mean. RFU, relative fluorescence units.
We then employed a fluorescent reporter construct to measure expression from the P3 promoter of the Agr operon during bacterial growth (Fig. 1B and E) (44). Because the strength of the fluorescent signal is affected by the total number of bacteria present, we accounted for differences in culture densities between strains and culture conditions by plotting fluorescence over the OD600 readings (Fig. 1C and F) (44). As reported previously, P3 expression in both wild-type S. aureus strains increased rapidly as the culture entered stationary phase (Fig. 1B and E) (44). In contrast, P3 activity in the hemB::Tn (SH1000) and ΔhemB (USA300) mutants was barely detectable (Fig. 1B and E). Similar results were seen for the ΔmenD mutant (USA300) (Fig. 1E). P3 activity in wild-type SH1000 cultures exposed to HQNO were also very low, although slightly greater than that seen in hemB::Tn mutant cultures (Fig. 1B and C). The very low level of P3 activity in mutants and bacteria grown with HQNO was not simply an artifact of the reduced cell density, as shown in Fig. 1C and F. Therefore, even allowing for differences in bacterial cell density (Fig. 1C and F), Agr activity is reduced in response to loss of a functional electron transport chain, regardless of whether this occurs chemically (HQNO) or genetically (hemB and menD).
To ensure that reduced fluorescence was not simply a consequence of the impaired metabolic status associated with loss of the electron transport chain, we measured RNAIII (hld) transcript by RT-PCR. This demonstrated significantly lower levels of RNAIII transcript in the SH1000 hemB::Tn mutant than in the wild type (see Fig. S2 in the supplemental material). In addition, and in keeping with our previous findings (44), compared with agr-defective strains that harbored reporter constructs but did not express GFP, we could not detect a GFP-induced growth defect in our reporter strains (see Fig. S3 in the supplemental material).
To determine whether the Agr activity data in Fig. 1 correlated with the virulence factor phenotype of each strain, we measured both fibronectin binding and hemolytic activity. In SH1000, as expected from the P3 expression data, the hemB::Tn mutant and the wild-type S. aureus bacteria grown in the presence of HQNO bound strongly to fibronectin-coated plastic, attaching at levels similar to those of an agr-defective mutant (Fig. 2A). In contrast, wild-type SH1000 grown in the absence of HQNO bound fibronectin weakly (Fig. 2A). Similar results were seen with USA300 strains, with the hemB and menD mutants attaching to fibronectin-coated wells at significantly higher levels than the wild type (Fig. 2B).
FIG 2.
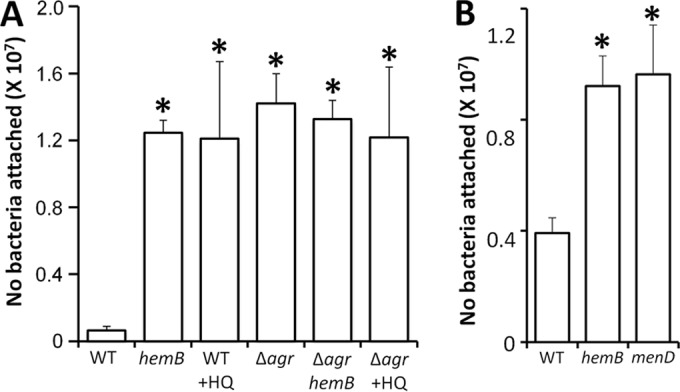
Loss of the electron transport chain results in enhanced fibronectin binding. (A) Attachment of wild-type (WT) S. aureus SH1000 grown in the absence or presence (+HQ) of HQNO and a hemB::Tn mutant (hemB) to immobilized human fibronectin. Also shown are values relating to the attachment of agr-defective SH1001 (Δagr) grown in the absence or presence of HQNO and of a hemB Δagr strain. (B) Attachment of WT USA300 and hemB and menD mutants. The values represent the mean averages of at least 4 experiments done in triplicate, and the error bars represent the standard deviations of the mean. Values that are significantly greater (P = 0.05 by Student's t test) than that of the wild type are indicated by asterisks.
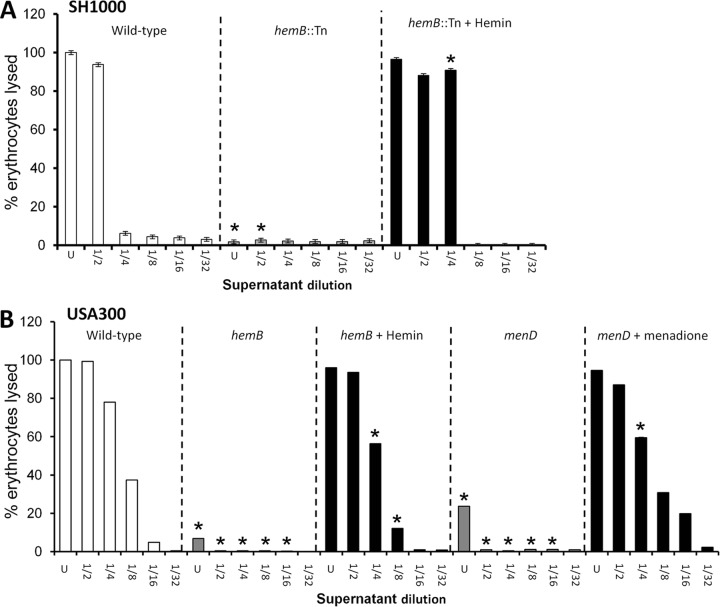
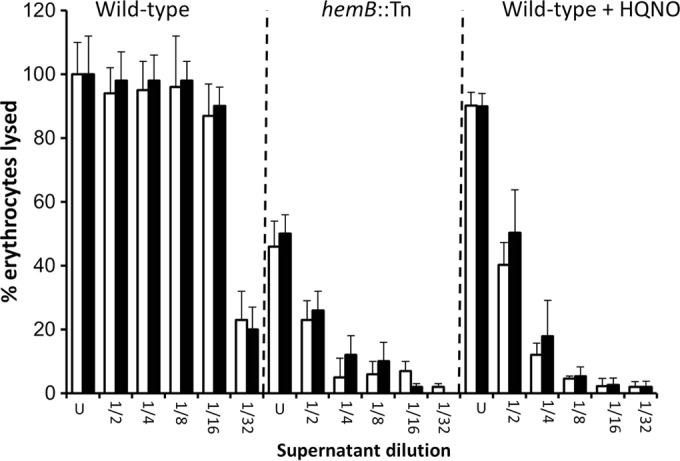
In contrast to the adhesion assay, strong hemolytic activity was detected in culture supernatant from wild-type S. aureus SH1000 grown in the absence of HQNO but was barely detectable in spent medium from the hemB::Tn mutant or the wild type grown with HQNO (Fig. 3A). Similarly, wild-type USA300 had high hemolytic activity while very weak activity was seen in the culture supernatants of hemB and menD mutants (Fig. 3B). Therefore, both the strong fibronectin binding and weak hemolytic activity of bacteria without functional electron transport chains were consistent with the Agr activity data (6, 9, 10, 14, 20, 36).
FIG 3.
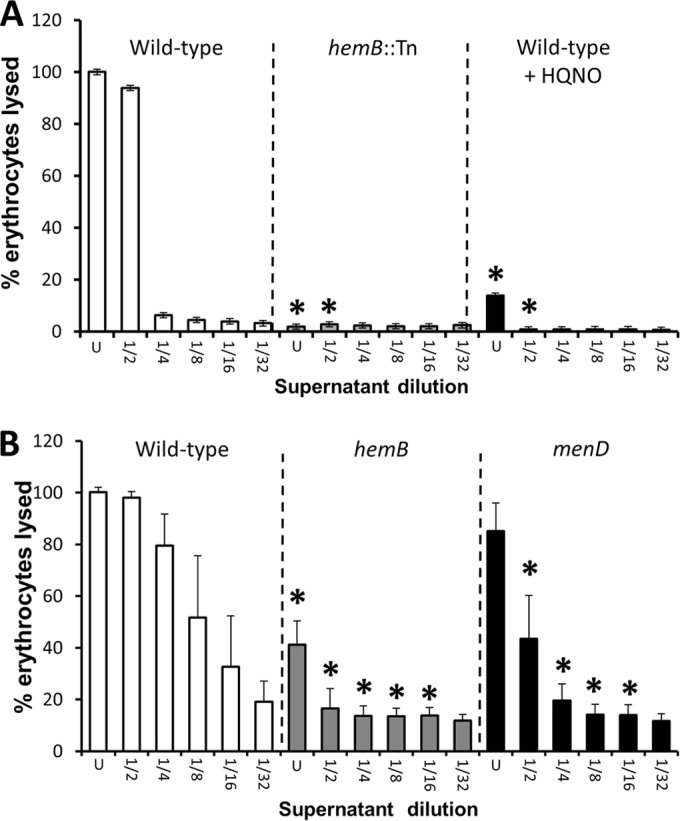
Loss of the electron transport chain results in significantly reduced hemolytic activity. Shown are hemolytic activities of culture supernatants of wild-type S. aureus SH1000 and derived strains (A) and USA300 and derived mutants (B). Culture supernatants were used undiluted (U) or after serial 2-fold dilutions, as indicated. The data represent the means of at least 4 independent experiments, each in triplicate. The error bars represent the standard deviations of the mean. Values that are significantly different (P < 0.05 by Student's t test) from those of the wild type at equivalent dilutions are indicated by asterisks.
sAIP activates Agr expression in the absence of the electron transport chain.
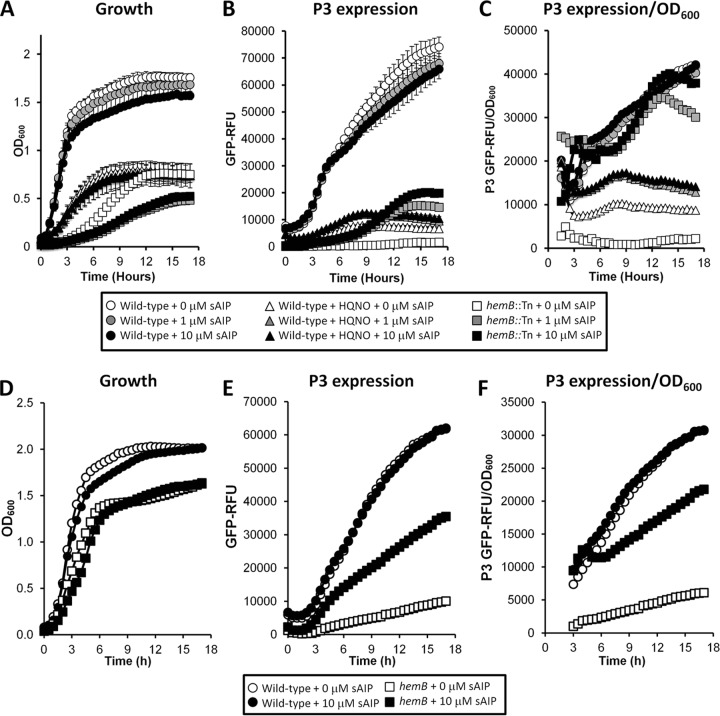
If Agr alone controls virulence factor expression in the absence of the electron transport chain, then modulation of Agr activity should influence both fibronectin binding and hemolytic activity. To determine whether electron transport-deficient S. aureus could respond to AIP, we added sAIP to the culture medium of S. aureus SH1000 hemB::Tn or the wild type in the absence or presence of HQNO and measured both growth and expression from the P3 promoter of Agr. In the wild-type grown without HQNO, AIP caused a slight, dose-dependent reduction in bacterial density (but not the growth rate) in stationary phase and, as a consequence, also a slight decrease in P3 expression levels (Fig. 4A and B). However, when fluorescence was related to the OD600, readings confirmed that the overall fluorescence per unit of biomass was unchanged in the wild type by the addition of sAIP (Fig. 4B and C). This suggests that expression from P3 is maximal in wild-type S. aureus and that exogenous sAIP cannot enhance it without adversely affecting growth. Synthetic AIP caused a much more significant decrease in the growth of the hemB::Tn mutant. Notably, the 1 μM and 10 μM doses caused identical inhibition of the hemB::Tn growth rate and final density in stationary phase (Fig. 4A). Exogenous sAIP triggered Agr expression in the hemB::Tn mutant, and when corrected for differences in bacterial density (OD600), Agr expression in the presence of sAIP was similar to that seen in the wild type (Fig. 4B and C).
FIG 4.
Synthetic AIP enhances agr expression in the absence of a functional electron transport chain. Shown are growth (A and D), P3 expression (B and E), and P3 expression corrected for growth (C and F) of SH1000 and derived strains (A to C) and USA300 and derived strains (D to F). (A to C) Wild-type S. aureus grown in the absence or presence of HQNO and that of a hemB::Tn mutant in TSB only or TSB supplemented with 1 μM or 10 μM sAIP. (D to F) Wild-type S. aureus and a hemB mutant grown in the absence or presence of 10 μM sAIP. The data represent the means of at least 4 independent experiments, each in triplicate. The error bars were omitted to enhance clarity but were all within 5% of the mean.
In contrast, exogenous sAIP did not significantly affect the growth of S. aureus in the presence of HQNO. This is explained by P3 reporter data, which show that while sAIP promoted Agr activity, it was not to levels seen in the wild type or the hemB::Tn mutant when adjusted for OD600 measurements (Fig. 4C). Therefore, while the hemB::Tn mutant is extremely sensitive to the presence of sAIP, S. aureus grown in the presence of HQNO does not respond as strongly.
The effect of sAIP on wild-type USA300 was similar to that seen with SH1000, with the quorum-sensing molecule causing a slight growth defect but no significant alteration in P3 activity (Fig. 4D, E, and F). Growth of USA300 hemB was not significantly affected by sAIP at 10 μM despite P3 activity increasing significantly, although not to the same levels as seen in SH1000 hemB::Tn (Fig. 4D, E, and F).
To investigate the apparently high degree of sensitivity of the SH1000 hemB::Tn mutant to sAIP, we determined the expression of the RNAII transcript, which encodes the quorum-sensing circuit. Analysis of expression from P2 in the hemB::Tn mutant using a gfp reporter construct revealed very low levels of activity (see Fig. S4 in the supplemental material). These findings were confirmed by RT-PCR analysis, which showed that agrA expression in the hemB::Tn strain was significantly lower than that of the wild type in exponential phase (see Fig. S4 in the supplemental material). Therefore, the potent response of the hemB::Tn mutant to sAIP is not due to elevated RNAII and thus to expression of the AgrC sensor kinase that detects AIP.
Finally, to ensure that sAIP-mediated growth inhibition was solely due to activation of Agr expression, rather than any off-target effects of sAIP, we replicated this experiment with strains lacking agr. No inhibition of growth was seen for any of the agr-defective strains when grown with sAIP (see Fig. S5 in the supplemental material).
Exogenous sAIP results in reduced binding of electron transport chain-defective S. aureus to fibronectin.
To determine whether Agr was responsible for the regulation of fibronectin binding in the absence of the electron transport chain, wild-type S. aureus grown with or without HQNO and the hemB::Tn mutant were cultured with or without sAIP, and their abilities to bind to fibronectin were determined. As described above, attachment of wild-type S. aureus to fibronectin was weak in the absence of sAIP, while bacteria grown in the presence of sAIP exhibited even weaker binding (Fig. 5A). The addition of sAIP to the culture media of both the hemB::Tn mutant and the wild type grown with HQNO led to a significant, dose-dependent decrease in fibronectin binding to levels similar to those seen for the wild type (Fig. 5A). To ensure that sAIP itself did not directly inhibit adhesion to fibronectin, these experiments were repeated with agr-negative mutants. All Agr-defective strains bound fibronectin strongly, and this was not affected by the presence of sAIP, ruling out a direct inhibitory effect (Fig. 5A). In addition, we examined the expression levels of fibronectin-binding proteins A and B in bacteria grown in the absence or presence of sAIP using a blot overlay approach. In keeping with the adhesion data, wild-type S. aureus SH1000 grown in either the absence or presence of sAIP had very weak expression of FnBPs (see Fig. S6 in the supplemental material). Both the hemB::Tn mutant and the wild type grown in the presence of HQNO had very high levels of FnBP expression, which was dramatically reduced in bacteria grown with sAIP (see Fig. S6 in the supplemental material). Finally, an agr mutant had moderately high levels of fibronectin-binding protein, which was unaffected by the presence of sAIP in the culture medium. Therefore, FnBP expression in the absence of the electron transport chain is regulated by Agr. However, additional regulators may enhance FnBP expression in the absence of an electron transport chain beyond that seen in an agr mutant with a competent electron transport chain. In addition, protein A expression varied identically to FnBP expression, demonstrating that this immune evasin is also subject to regulation by Agr in the absence of a functional electron transport chain (see Fig. S6 in the supplemental material).
FIG 5.
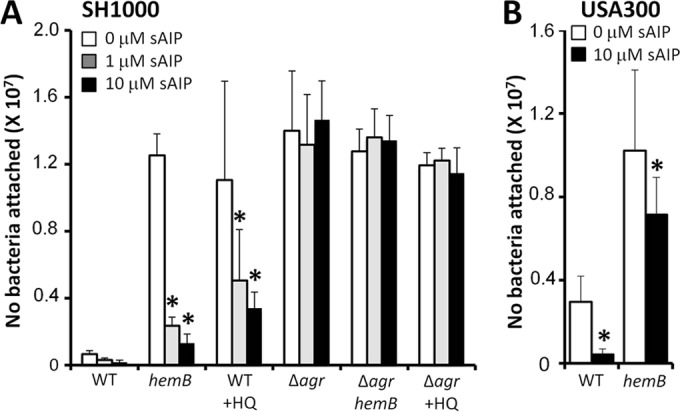
Agr regulates fibronectin binding in both the presence and absence of the electron transport chain. (A) Fibronectin binding of wild-type S. aureus SH1000 grown in the absence (WT) or presence (WT + HQ) of HQNO and a hemB::Tn mutant (hemB). Also shown are the fibronectin-binding levels of agr-deficient mutants grown in the absence (Δagr) or presence (Δagr + HQ) of HQNO or in the hemB::Tn mutant background (Δagr hemB). The culture medium consisted of TSB only or TSB supplemented with 1 μM sAIP or 10 μM sAIP. (B) Fibronectin binding of wild-type S. aureus USA300 and a hemB mutant grown in the absence or presence of 10 μM sAIP. Experiments were repeated 4 times in triplicate. Values that are significantly different (P < 0.05 by Student's t test) from those seen in the absence of sAIP are indicated by asterisks.
Finally, fibronectin-binding results similar to those of SH1000 were seen with USA300, with attachment of both the wild-type and hemB strains to fibronectin being significantly reduced after growth in the presence of sAIP (Fig. 5B).
Taken together, these data demonstrate that fibronectin binding is under the control of Agr in S. aureus in both the presence and absence of a functional electron transport chain.
Exogenous sAIP does not trigger hemolysin production in S. aureus in the absence of the electron transport chain.
Next, we sought to determine whether reduced Agr expression was also directly responsible for the lack of hemolysin production in the absence of the electron transport chain. Initially we examined the hemolytic activity of culture supernatants from bacteria grown in the presence or absence of sAIP at 10 μM, which triggered maximal P3 activity in all strains (Fig. 4). In contrast to the fibronectin-binding assays shown in Fig. 5, the addition of sAIP to cultures of wild-type SH1000 in either the absence or presence of HQNO had no significant effect on hemolytic activity (Fig. 6A). Similarly, sAIP had no effect on the hemolytic activity of SH1000 hemB::Tn (Fig. 6A). In keeping with these findings, the hemolytic activity of both wild-type USA300 and the hemB mutant was unaffected by growth in the presence of sAIP at 10 μM (Fig. 6B).
FIG 6.
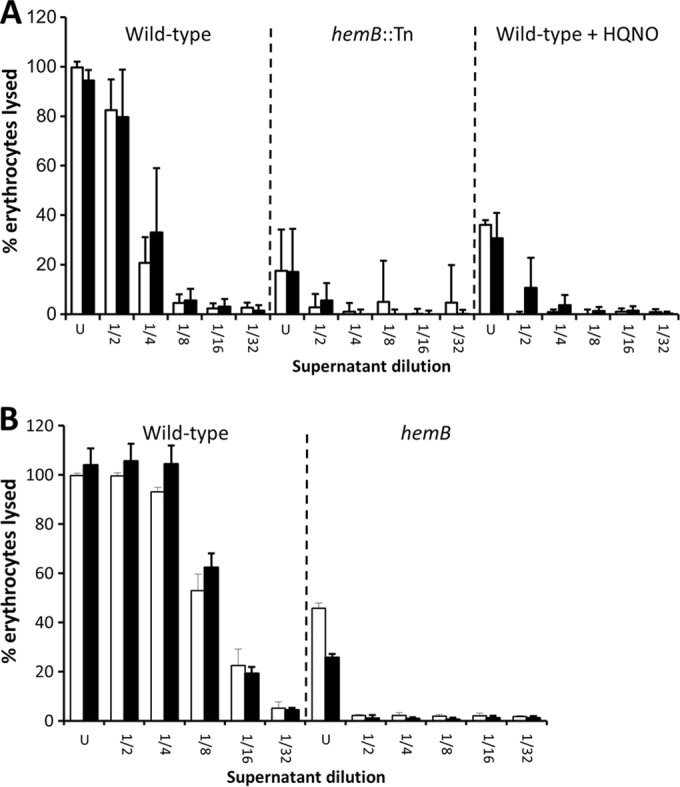
Agr activation by sAIP does not promote hemolytic activity in the absence of a functional electron transport chain. (A) Hemolytic activities of culture supernatants of wild-type SH1000 grown in the absence or presence of HQNO and of a hemB::Tn mutant in the absence (open bars) or presence (filled bars) of 10 μM sAIP. (B) Hemolytic activities of culture supernatants of wild-type USA300 and a hemB mutant in the absence (open bars) or presence (filled bars) of 10 μM sAIP. The data represent the mean averages of 4 experiments performed in triplicate, and the error bars represent the standard deviations of the mean. None of the values obtained from cultures containing sAIP were significantly different from those without sAIP at identical dilutions.
Because the level of hemolytic activity in culture supernatants can be affected by the density of cells in the culture, this was addressed by adjustment of all strains to ∼2 × 109 CFU ml−1 in TSB, with or without HQNO or sAIP, and incubation for 16 h at 37°C. The addition of sAIP to cultures of wild-type S. aureus had no effect on the already strong hemolytic activity, suggesting that native AIP levels trigger maximal hemolysin production (Fig. 7). In contrast, neither the hemB::Tn mutant nor wild-type S. aureus cultured with HQNO produced significantly higher levels of hemolytic activity in the presence of sAIP, despite increased Agr expression and high bacterial densities (Fig. 4B and 7).
FIG 7.
Agr activation by sAIP does not promote hemolytic activity in concentrated cultures in the absence of a functional electron transport chain. Wild-type SH1000 and a hemB::Tn mutant were concentrated to equivalent bacterial densities and cultured in TSB in the absence or presence of HQNO and the absence (open bars) or presence (filled bars) of 10 μM sAIP. The data represent the mean averages of 4 experiments performed in triplicate, and the error bars represent the standard deviations of the mean. None of the values obtained from cultures containing sAIP were significantly different from those without sAIP at identical dilutions.
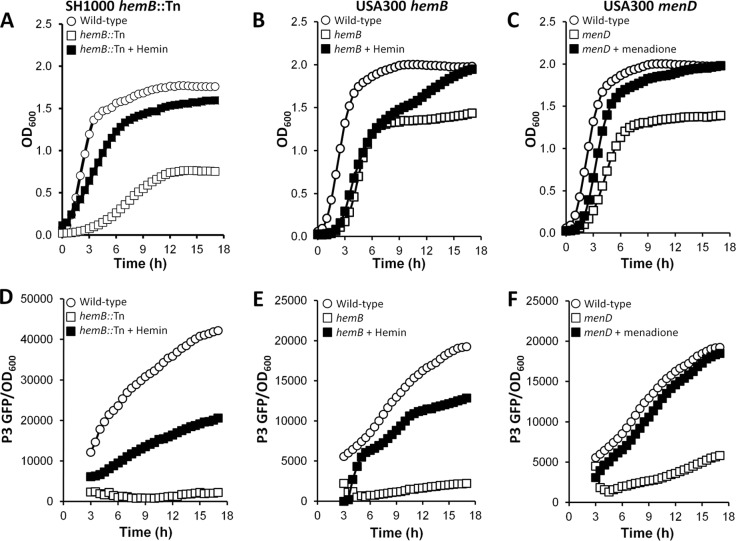
Restoration of the electron transport chain in the hemB or menD mutant via supplementation of the growth medium with hemin or menadione almost completely restored the growth phenotype and partially restored Agr activity (Fig. 8). However, despite the incomplete restoration of Agr activity, hemolytic activity was completely restored (Fig. 9A and B). Therefore, hemolysin production does not require maximal Agr activity but does require a functional electron transport chain.
FIG 8.
Hemin and menadione restore growth and Agr activity in hemB and menD mutants, respectively. (A, B, D, and E) SH1000 hemB::Tn (A and D) and USA300 hemB (B and E) were grown in the absence or presence of 1 μg ml−1 hemin, and growth (A and B) was measured via OD600 readings, while P3 activity (corrected for OD600 values) was measured to determine Agr activity (D and E). (C and F) USA300 menD was grown in the absence or presence of 1 μg ml−1 menadione, and growth (C) and P3 activity (F) were measured as described for the hemB mutants. The data represent the means of at least 4 independent experiments, each in triplicate. The error bars were omitted to enhance clarity but were all within 5% of the mean.
FIG 9.
Hemin and menadione restore hemolytic activity in hemB and menD mutants, respectively. (A) SH1000 hemB::Tn was grown in the absence or presence of 1 μg ml−1 hemin, and the hemolytic activity of the culture supernatant was determined. Culture supernatant from wild-type SH1000 is included for comparison. (B) USA300 hemB and menD were grown in the absence or presence of 1 μg ml−1 hemin or menadione, and the hemolytic activity of the culture supernatant was determined. Culture supernatant from wild-type USA300 is included for comparison. The data represent the means of 4 experiments done in triplicate, and the error bars represent the standard deviations of the mean. Values that are significantly different (P < 0.05 by Student's t test) from those of the wild type at the same dilution are indicated by asterisks.
DISCUSSION
During the course of infection, S. aureus frequently acquires mutations that affect the expression of virulence factors (55–59). However, the mechanisms by which these mutations influence the expression of specific virulence factors are not always clear. This is particularly true for small-colony variants, where mutations in biosynthetic-pathway genes result in disruption of the electron transport chain and loss of Agr activity (25–29).
In this report, we dissected the relative contributions of electron transport chain deficiency and reduced Agr expression to both fibronectin binding and hemolysis. The elevated fibronectin binding of electron transport chain-deficient bacteria was directly due to reduced Agr expression. In contrast, reduced hemolysin production was not simply a consequence of low Agr activity, and we therefore conclude that hemolysin production requires a functional electron transport chain, in addition to Agr expression.
The data in Fig. 4 indicate that Agr expression and growth are finely balanced in wild-type bacteria to maximize both growth and hemolysin production; exogenous sAIP caused a growth defect without increasing hemolytic activity. The sAIP-induced growth defect was even more pronounced in the hemB::Tn mutant, indicating that S. aureus lacking a functional electron transport chain can sense and respond to AIP despite low levels of RNAII transcript. Although sAIP enhanced Agr activation in S. aureus grown in the presence of HQNO, the effect on growth was minor compared to that in the hemB::Tn mutant. This may conceivably be due to interference between HQNO and sAIP, but we were unable to test this.
The mechanism(s) by which S. aureus detects and allocates resources to growth versus virulence factor production is unclear. However, in recent years, a number of gene products have been described which respond to metabolic signals and regulate virulence factor production accordingly (e.g., Rsh, CodY, CcpA, and SrrAB) (39, 40, 59–65). Of particular relevance, SrrAB has been shown to directly monitor electron transport chain activity and is capable of modulating both growth and the expression of several virulence factors 59, 60, 65). In addition, previous work has shown that the lack of Agr expression in electron transport chain-deficient SCVs is, at least in part, due to suppression by the alternative sigma factor SigB, which exhibits constitutive activity during all growth phases (66, 67). In a sigB mutant defective in menaquinone biosynthesis, Agr activity was significantly elevated, providing convincing evidence that regulation by SigB, rather than low growth density, is responsible for decreased activity of Agr in bacteria lacking the electron transport chain (67). Furthermore, the elevated SigB activity in SCVs may also explain the particularly high level of fibronectin-binding proteins seen in S. aureus lacking functional electron transport chains, above that seen in an agr-deficient mutant (see Fig. S6 in the supplemental material) (67).
However, our data demonstrate that this SigB-mediated suppression of Agr expression in SCVs can be overcome by exogenous sAIP, and this may have important implications for virulence factor expression in vivo. During infection, electron transport chain-deficient SCVs emerge from wild-type populations, and SCVs and wild-type bacteria are often coisolated from the same infection site (14–16, 18, 20–22, 24). This makes it likely that, in vivo, SCVs are exposed to AIP generated by wild-type S. aureus. Therefore, the phenotype of electron transport chain-deficient SCVs in the presence of wild-type bacteria will be very different from those observed in SCV monocultures, at least with respect to surface protein expression.
The role of SigB in shaping the phenotype of S. aureus lacking a functional electron transport chain may explain the apparent discrepancy between our data and those of Vaudaux et al. (36). The strain used by Vaudaux and coworkers, 8325, is defective in SigB production due to a mutation in rsbU (42). One of the strains used in this report, SH1000, is derived from 8325 but has a repaired copy of rsbU, restoring SigB activity (42). Furthermore, the undefined spontaneous mutant strain used by Vaudaux et al., which lacked production of RNAIII, was unusual in that it did not display enhanced fnb transcript or fibronectin-binding relative to the parent strain (36). This is at odds with studies using defined agr-deficient mutants, including in the 8325 background, which show significantly enhanced fibronectin binding, and suggests the possibility of additional mutations or pleiotropic effects (68–70). Therefore, the data presented in this report contribute to our growing understanding of the link between metabolism and the regulation of specific virulence factors by providing compelling evidence that decreased Agr expression is directly responsible for elevated fibronectin-binding protein expression in both electron transport chain-defective and -competent S. aureus. In contrast, the Agr-mediated regulation of hemolysin production is dependent upon a functional electron transport chain.
Supplementary Material
ACKNOWLEDGMENTS
A.M.E. acknowledges funding from the Royal Society and the Department of Medicine, Imperial College London. S.W. acknowledges funding from the BBSRC and Wellcome Trust. K.L.P. is supported by a Ph.D. studentship from the Faculty of Medicine, Imperial College London.
Malcolm Horsburgh (University of Liverpool) is gratefully acknowledged for supplying S. aureus strains SH1000 and SH1001. The USA300 hemB::Tn mutant was supplied through the Network on Antimicrobial Resistance in Staphylococcus aureus (NARSA) Program under NIAID/NIH contract no. HHSN272200700055C. Angela Nobbs (University of Bristol) is acknowledged for comments on the manuscript.
Footnotes
Published ahead of print 4 August 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.02254-14.
REFERENCES
- 1. Lowy FD. 1998. Staphylococcus aureus infections. N. Engl. J. Med. 339:520–532. 10.1056/NEJM199808203390806 [DOI] [PubMed] [Google Scholar]
- 2. Gordon RJ, Lowy FD. 2008. Pathogenesis of methicillin-resistant Staphylococcus aureus infection. Clin. Infect. Dis. 5:S350–S359. 10.1086/533591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dasenbrook EC. 2011. Update on methicillin-resistant Staphylococcus aureus in cystic fibrosis. Curr. Opin. Pulm. Med. 17:437–441. 10.1097/MCP.0b013e32834b95ed [DOI] [PubMed] [Google Scholar]
- 4. Recsei P, Kreiswirth B, O'Reilly M, Schlievert P, Gruss A, Novick RP. 1986. Regulation of exoprotein gene expression in Staphylococcus aureus by agr. Mol. Gen. Genet. 202:58–61. 10.1007/BF00330517 [DOI] [PubMed] [Google Scholar]
- 5. Foster TJ. 2005. Immune evasion by staphylococci. Nat. Rev. Microbiol. 3:948–958. 10.1038/nrmicro1289 [DOI] [PubMed] [Google Scholar]
- 6. Novick RP, Geisinger E. 2008. Quorum sensing in staphylococci. Annu. Rev. Genet. 42:541–564. 10.1146/annurev.genet.42.110807.091640 [DOI] [PubMed] [Google Scholar]
- 7. Foster TJ, Geoghegan JA, Ganesh VK, Höök M. 2014. Adhesion, invasion and evasion: the many functions of the surface proteins of Staphylococcus aureus. Nat. Rev. Microbiol. 12:49–62. 10.1038/nrmicro3161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shompole S, Henon KT, Liou LE, Dziewanowska K, Bohach GA, Bayles KW. 2003. Biphasic intracellular expression of Staphylococcus aureus virulence factors and evidence for Agr-mediated diffusion sensing. Mol. Microbiol. 49:919–927. 10.1046/j.1365-2958.2003.03618.x [DOI] [PubMed] [Google Scholar]
- 9. Janzon L, Arvidson S. 1990. The role of the delta-lysin gene (hld) in the regulation of virulence genes by the accessory gene regulator (agr) in Staphylococcus aureus. EMBO J. 9:1391–1399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Novick RP, Ross HF, Projan SJ, Kornblum J, Kreiswirth B, Moghazeh S. 1993. Synthesis of staphylococcal virulence factors is controlled by a regulatory RNA molecule. EMBO J. 12:3967–3975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chen CJ, Su LH, Lin TY, Huang YC. 2010. Molecular analysis of repeated methicillin-resistant Staphylococcus aureus infections in children. PLoS One 5:e14431. 10.1371/journal.pone.0014431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sreeramoju P, Porbandarwalla NS, Arango J, Latham K, Dent DL, Stewart RM, Patterson JE. 2011. Recurrent skin and soft tissue infections due to methicillin-resistant Staphylococcus aureus requiring operative debridement. Am. J. Surg. 201:216–220. 10.1016/j.amjsurg.2009.12.024 [DOI] [PubMed] [Google Scholar]
- 13. Peyrani P, Allen M, Seligson D, Roberts C, Chen A, Haque N, Zervos M, Wiemken T, Harting J, Christensen D, Ramirez R. 2012. Clinical outcomes of osteomyelitis patients infected with methicillin-resistant Staphylococcus aureus USA-300 strains. Am. J. Orthop. 41:117–122 [PubMed] [Google Scholar]
- 14. Proctor RA, van Langevelde P, Kristjansson M, Maslow JN, Arbeit RD. 1995. Persistent and relapsing infections associated with small-colony variants of Staphylococcus aureus. Clin. Infect. Dis. 20:95–102. 10.1093/clinids/20.1.95 [DOI] [PubMed] [Google Scholar]
- 15. Kahl B, Herrmann M, Everding AS, Koch HG, Becker K, Harms E, Proctor RA, Peters G. 1998. Persistent infection with small colony variant strains of Staphylococcus aureus in patients with cystic fibrosis. J. Infect. Dis. 177:1023–1029. 10.1086/515238 [DOI] [PubMed] [Google Scholar]
- 16. Seifert H, von Eiff C, Fätkenheuer G. 1999. Fatal case due to methicillin-resistant Staphylococcus aureus small colony variants in an AIDS patient. Emerg. Infect. Dis. 5:450–453. 10.3201/eid0503.990319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Abele-Horn M, Schupfner B, Emmerling P, Waldner H, Göring H. 2000. Persistent wound infection after herniotomy associated with small-colony variants of Staphylococcus aureus. Infection 2:53–54. 10.1007/s150100050014 [DOI] [PubMed] [Google Scholar]
- 18. Looney WJ. 2000. Small-colony variants of Staphylococcus aureus. Br. J. Biomed. Sci. 57:317–322 [PubMed] [Google Scholar]
- 19. von Eiff C, Becker K, Metze D, Lubritz G, Hockmann J, Schwarz T, Peters G. 2001. Intracellular persistence of Staphylococcus aureus small-colony variants within keratinocytes: a cause for antibiotic treatment failure in a patient with Darier's disease. Clin. Infect. Dis. 32:1643–1647. 10.1086/320519 [DOI] [PubMed] [Google Scholar]
- 20. Proctor RA, von Eiff C, Kahl BC, Becker K, McNamara P, Herrmann M, Peters G. 2006. Small colony variants: a pathogenic form of bacteria that facilitates persistent and recurrent infections. Nat. Rev. Microbiol. 4:295–305. 10.1038/nrmicro1384 [DOI] [PubMed] [Google Scholar]
- 21. Sendi P, Rohrbach M, Graber P, Frei R, Ochsner PE, Zimmerli W. 2006. Staphylococcus aureus small colony variants in prosthetic joint infection. Clin. Infect. Dis. 43:961–967. 10.1086/507633 [DOI] [PubMed] [Google Scholar]
- 22. Agarwal H, Verrall R, Singh SP, Tang YW, Wilson G. 2007. Small colony variant Staphylococcus aureus multiorgan infection. Pediatr. Infect. Dis. J. 26:269–271. 10.1097/01.inf.0000256749.29244.67 [DOI] [PubMed] [Google Scholar]
- 23. Tuchscherr L, Medina E, Hussain M, Völker W, Heitmann V, Niemann S, Holzinger D, Roth J, Proctor RA, Becker K, Peters G, Löffler B. 2011. Staphylococcus aureus phenotype switching: an effective bacterial strategy to escape host immune response and establish a chronic infection. EMBO Mol. Med. 3:129–141. 10.1002/emmm.201000115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kahl BC. 2014. Small colony variants (SCVs) of Staphylococcus aureus: a bacterial survival strategy. Infect. Genet. Evol. 21:515–522. 10.1016/j.meegid.2013.05.016 [DOI] [PubMed] [Google Scholar]
- 25. Besier S, Ludwig A, Ohlsen K, Brade V, Wichelhaus TA. 2007. Molecular analysis of the thymidine-auxotrophic small colony variant phenotype of Staphylococcus aureus. Int. J. Med. Microbiol. 297:217–225. 10.1016/j.ijmm.2007.02.003 [DOI] [PubMed] [Google Scholar]
- 26. Chatterjee I, Kriegeskorte A, Fischer A, Deiwick S, Theimann N, Proctor RA, Peters G, Herrmann M, Kahl BC. 2008. In vivo mutations of thymidylate synthase (encoded by thyA) are responsible for thymidine dependency in clinical small-colony variants of Staphylococcus aureus. J. Bacteriol. 190:834–842. 10.1128/JB.00912-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lannergård J, von Eiff C, Sander G, Cordes T, Seggewiss J, Peters G, Proctor RA, Becker K, Hughes D. 2008. Identification of the genetic basis for clinical menadione-auxotrophic small-colony variant isolates of Staphylococcus aureus. Antimicrob. Agents Chemother. 52:4017–4022. 10.1128/AAC.00668-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Edwards AM. 2012. Phenotype switching is a natural consequence of Staphylococcus aureus replication. J. Bacteriol. 194:5404–5412. 10.1128/JB.00948-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dean MA, Olsen RJ, Long SW, Rosato AE, Musser JM. 2014. Identification of point mutations in clinical Staphylococcus aureus strains that produce small colony variants auxotrophic for menadione. Infect. Immun. 82:1600–1605. 10.1128/IAI.01487-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Baumert N, von Eiff C, Schaaff F, Peters G, Proctor RA, Sahl HG. 2002. Physiology and antibiotic susceptibility of Staphylococcus aureus small colony variants. Microb. Drug Resist. 8:253–260. 10.1089/10766290260469507 [DOI] [PubMed] [Google Scholar]
- 31. Seggewiss J, Becker K, Kotte O, Eisenacher M, Yazdi MR, Fischer A, McNamara P, Al Laham N, Proctor R, Peters G, Heinemann M, von Eiff C. 2006. Reporter metabolite analysis of transcriptional profiles of a Staphylococcus aureus strain with normal phenotype and its isogenic hemB mutant displaying the small-colony-variant phenotype. J. Bacteriol. 188:7765–7777. 10.1128/JB.00774-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sadowska B, Bonar A, von Eiff C, Proctor RA, Chmiela M, Rudnicka W, Róźalska B. 2002. Characteristics of Staphylococcus aureus, isolated from airways of cystic fibrosis patients, and their small colony variants. FEMS Immunol. Med. Microbiol. 32:191–197. 10.1111/j.1574-695X.2002.tb00553.x [DOI] [PubMed] [Google Scholar]
- 33. Tuchscherr L, Heitmann V, Hussain M, Viemann D, Roth J, von Eiff C, Peters G, Becker K, Löffler B. 2010. Staphylococcus aureus small-colony variants are adapted phenotypes for intracellular persistence. J. Infect. Dis. 202:1031–1040. 10.1086/656047 [DOI] [PubMed] [Google Scholar]
- 34. Wesson CA, Liou LE, Todd KM, Bohach GA, Trumble WR, Bayles KW. 1998. Staphylococcus aureus Agr and Sar global regulators influence internalization and induction of apoptosis. Infect. Immun. 66:5238–5243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Menzies BE, Kourteva I. 2000. Staphylococcus aureus alpha-toxin induces apoptosis in endothelial cells. FEMS Immunol. Med. Microbiol. 29:39–45. 10.1111/j.1574-695X.2000.tb01503.x [DOI] [PubMed] [Google Scholar]
- 36. Vaudaux P, Francois P, Bisognano C, Kelley WL, Lew DP, Schrenzel J, Proctor RA, McNamara PJ, Peters G, Von Eiff C. 2002. Increased expression of clumping factor and fibronectin-binding proteins by hemB mutants of Staphylococcus aureus expressing small colony variant phenotypes. Infect. Immun. 70:5428–5437. 10.1128/IAI.70.10.5428-5437.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Senn MM, Bischoff M, von Eiff C, Berger-Bächi B. 2005. SigmaB activity in a Staphylococcus aureus hemB mutant. J. Bacteriol. 187:7397–7406. 10.1128/JB.187.21.7397-7406.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yarwood JM, McCormick JK, Schlievert PM. 2001. Identification of a novel two-component regulatory system that acts in global regulation of virulence factors of Staphylococcus aureus. J. Bacteriol. 183:1113–1123. 10.1128/JB.183.4.1113-1123.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Somerville GA, Proctor RA. 2009. At the crossroads of bacterial metabolism and virulence factor synthesis in Staphylococci. Microbiol. Mol. Biol. Rev. 73:233–248. 10.1128/MMBR.00005-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Geiger T, Francois P, Liebeke M, Fraunholz M, Goerke C, Krismer B, Schrenzel J, Lalk M, Wolz C. 2012. The stringent response of Staphylococcus aureus and its impact on survival after phagocytosis through the induction of intracellular PSMs expression. PLoS Pathog. 8:e1003016. 10.1371/journal.ppat.1003016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Proctor RA, Dalal SC, Kahl B, Brar D, Peters G, Nichols WW. 2002. Two diarylurea electron transport inhibitors reduce Staphylococcus aureus hemolytic activity and protect cultured endothelial cells from lysis. Antimicrob. Agents Chemother. 46:2333–2336. 10.1128/AAC.46.8.2333-2336.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Horsburgh MJ, Aish JL, White IJ, Shaw L, Lithgow JK, Foster SJ. 2002. SigmaB modulates virulence determinant expression and stress resistance: characterization of a functional rsbU strain derived from Staphylococcus aureus 8325-4. J. Bacteriol. 184:5457–5467. 10.1128/JB.184.19.5457-5467.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Diep BA, Gill SR, Chang RF, Phan TH, Chen JH, Davidson MG, Lin F, Lin J, Carleton HA, Mongodin EF, Sensabaugh GF, Perdreau-Remington F. 2006. Complete genome sequence of USA300, an epidemic clone of community-acquired meticillin-resistant Staphylococcus aureus. Lancet 367:731–739. 10.1016/S0140-6736(06)68231-7 [DOI] [PubMed] [Google Scholar]
- 44. James EH, Edwards AM, Wigneshweraraj S. 2013. Transcriptional downregulation of agr expression in Staphylococcus aureus during growth in human serum can be overcome by constitutively active mutant forms of the sensor kinase AgrC. FEMS Microbiol. Lett. 349:153–162. 10.1111/1574-6968.12309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hoffman LR, Déziel E, D'Argenio DA, Lépine F, Emerson J, McNamara S, Gibson RL, Ramsey BW, Miller SI. 2006. Selection for Staphylococcus aureus small-colony variants due to growth in the presence of Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. U. S. A. 103:19890–19895. 10.1073/pnas.0606756104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. McDowell P, Affas Z, Reynolds C, Holden MT, Wood SJ, Saint S, Cockayne A, Hill PJ, Dodd CE, Bycroft BW, Chan WC, Williams P. 2001. Structure, activity and evolution of the group I thiolactone peptide quorum-sensing system of Staphylococcus aureus. Mol. Microbiol. 41:503–512. 10.1046/j.1365-2958.2001.02539.x [DOI] [PubMed] [Google Scholar]
- 47. Monk IR, Shah IM, Xu M, Tan MW, Foster TJ. 2012. Transforming the untransformable: application of direct transformation to manipulate genetically Staphylococcus aureus and Staphylococcus epidermidis. mBio 3:e00277–11. 10.1128/mBio.00277-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. von Eiff C, Heilmann C, Proctor RA, Woltz C, Peters G, Götz F. 1997. A site-directed Staphylococcus aureus hemB mutant is a small-colony variant which persists intracellularly. J. Bacteriol. 179:4706–4012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bates DM, von Eiff C, McNamara PJ, Peters G, Yeaman MR, Bayer AS, Proctor RA. 2003. Staphylococcus aureus menD and hemB mutants are as infective as the parent strains, but the menadione biosynthetic mutant persists within the kidney. J. Infect. Dis. 187:1654–1661. 10.1086/374642 [DOI] [PubMed] [Google Scholar]
- 50. Peacock SJ, Day NP, Thomas MG, Berendt AR, Foster TJ. 2000. Clinical isolates of Staphylococcus aureus exhibit diversity in fnb genes and adhesion to human fibronectin. J. Infect. 41:23–31. 10.1053/jinf.2000.0657 [DOI] [PubMed] [Google Scholar]
- 51. Jakubovics NS, Strömberg N, van Dolleweerd CJ, Kelly CG, Jenkinson HF. 2005. Differential binding specificities of oral streptococcal antigen I/II family adhesins for human or bacterial ligands. Mol. Microbiol. 55:1591–1605. 10.1111/j.1365-2958.2005.04495.x [DOI] [PubMed] [Google Scholar]
- 52. Edwards AM, Potts JR, Josefsson E, Massey RC. 2010. Staphylococcus aureus host cell invasion and virulence in sepsis is facilitated by the multiple repeats within FnBPA. PLoS Pathog. 6:e1000964. 10.1371/journal.ppat.1000964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Herbert S, Ziebandt AK, Ohlsen K, Schäfer T, Hecker M, Albrecht D, Novick R, Götz F. 2010. Repair of global regulators in Staphylococcus aureus 8325 and comparative analysis with other clinical isolates. Infect. Immun. 78:2877–2889. 10.1128/IAI.00088-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Fenno JC, Hannam PM, Leung WK, Tamura M, Uitto VJ, McBride BC. 1998. Cytopathic effects of the major surface protein and the chymotrypsinlike protease of Treponema denticola. Infect. Immun. 66:1869–1877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Lattar SM, Tuchscherr LP, Caccuri RL, Centrón D, Becker K, Alonso CA, Barberis C, Miranda G, Buzzola FR, von Eiff C, Sordelli DO. 2009. Capsule expression and genotypic differences among Staphylococcus aureus isolates from patients with chronic or acute osteomyelitis. Infect. Immun. 77:1968–1975. 10.1128/IAI.01214-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Oliver A, Mena A. 2010. Bacterial hypermutation in cystic fibrosis, not only for antibiotic resistance. Clin. Microbiol. Infect. 16:798–808. 10.1111/j.1469-0691.2010.03250.x [DOI] [PubMed] [Google Scholar]
- 57. McAdam PR, Holmes A, Templeton KE, Fitzgerald JR. 2011. Adaptive evolution of Staphylococcus aureus during chronic endobronchial infection of a cystic fibrosis patient. PLoS One 6:e24301. 10.1371/journal.pone.0024301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hirschhausen N, Block D, Bianconi I, Bragonzi A, Birtel J, Lee JC, Dübbers A, Küster P, Kahl J, Peters G, Kahl BC. 2013. Extended Staphylococcus aureus persistence in cystic fibrosis is associated with bacterial adaptation. Int. J. Med. Microbiol. 303:685–692. 10.1016/j.ijmm.2013.09.012 [DOI] [PubMed] [Google Scholar]
- 59. Pragman AA, Yarwood JM, Tripp TJ, Schlievert PM. 2004. Characterization of virulence factor regulation by SrrAB, a two-component system in Staphylococcus aureus. J. Bacteriol. 186:2430–2438. 10.1128/JB.186.8.2430-2438.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Pragman AA, Ji Y, Schlievert PM. 2007. Repression of Staphylococcus aureus SrrAB using inducible antisense srrA alters growth and virulence factor transcript levels. Biochemistry 46:314–321. 10.1021/bi0603266 [DOI] [PubMed] [Google Scholar]
- 61. Seidl K, Stucki M, Ruegg M, Goerke C, Wolz C, Harris L, Berger-Bächi B, Bischoff M. 2006. Staphylococcus aureus CcpA affects virulence determinant production and antibiotic resistance. Antimicrob. Agents Chemother. 50:1183–1194. 10.1128/AAC.50.4.1183-1194.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Pohl K, Francois P, Stenz L, Schlink F, Geiger T, Herbert S, Goerke C, Schrenzel J, Wolz C. 2009. CodY in Staphylococcus aureus: a regulatory link between metabolism and virulence gene expression. J. Bacteriol. 191:2953–2963. 10.1128/JB.01492-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Rivera FE, Miller HK, Kolar SL, Stevens SM, Jr, Shaw LN. 2012. The impact of CodY on virulence determinant production in community-associated methicillin-resistant Staphylococcus aureus. Proteomics 12:263–268. 10.1002/pmic.201100298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Roux A, Todd DA, Velázquez JV, Cech NB, Sonenshein AL. 2014. CodY-mediated regulation of the Staphylococcus aureus Agr system integrates nutritional and population density signals. J. Bacteriol. 196:1184–1196. 10.1128/JB.00128-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kinkel TL, Roux CM, Dunman PM, Fang FC. 2013. The Staphylococcus aureus SrrAB two-component system promotes resistance to nitrosative stress and hypoxia. mBio 4:e00696–13. 10.1128/mBio.00696-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Moisan H, Brouillette E, Jacob CL, Langlois-Bégin P, Michaud S, Malouin F. 2006. Transcription of virulence factors in Staphylococcus aureus small-colony variants isolated from cystic fibrosis patients is influenced by SigB. J. Bacteriol. 188:64–76. 10.1128/JB.188.1.64-76.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Mitchell G, Fugère A, Pépin Gaudreau K, Brouillette E, Frost EH, Cantin AM, Malouin F. 2013. SigB is a dominant regulator of virulence in Staphylococcus aureus small-colony variants. PLoS One 8:e65018. 10.1371/journal.pone.0065018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Cheung AL, Koomey JM, Butler CA, Projan SJ, Fischetti VA. 1992. Regulation of exoprotein expression in Staphylococcus aureus by a locus (sar) distinct from agr. Proc. Natl. Acad. Sci. U. S. A. 89:6462–6466. 10.1073/pnas.89.14.6462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Cheung AL, Eberhardt KJ, Chung E, Yeaman MR, Sullam PM, Ramos M, Bayer AS. 1994. Diminished virulence of a sar-/agr- mutant of Staphylococcus aureus in the rabbit model of endocarditis. J. Clin. Invest. 94:1815–1822. 10.1172/JCI117530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Saravia-Otten P, Müller HP, Arvidson S. 1997. Transcription of Staphylococcus aureus fibronectin binding protein genes is negatively regulated by agr and an agr-independent mechanism. J. Bacteriol. 179:5259–5263 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.