Abstract
Clostridium difficile-associated disease (CDAD) constitutes a large majority of nosocomial diarrhea cases in industrialized nations and is mediated by the effects of two secreted toxins, toxin A (TcdA) and toxin B (TcdB). Patients who develop strong antitoxin antibody responses can clear C. difficile infection and remain disease free. Key toxin-neutralizing epitopes have been found within the carboxy-terminal receptor binding domains (RBDs) of TcdA and TcdB, which has generated interest in developing the RBD as a viable vaccine target. While numerous platforms have been studied, very little data describes the potential of DNA vaccination against CDAD. Therefore, we created highly optimized plasmids encoding the RBDs from TcdA and TcdB in which any putative N-linked glycosylation sites were altered. Mice and nonhuman primates were immunized intramuscularly, followed by in vivo electroporation, and in these animal models, vaccination induced significant levels of both anti-RBD antibodies (blood and stool) and RBD-specific antibody-secreting cells. Further characterization revealed that sera from immunized mice and nonhuman primates could detect RBD protein from transfected cells, as well as neutralize purified toxins in an in vitro cytotoxicity assay. Mice that were immunized with plasmids or given nonhuman-primate sera were protected from a lethal challenge with purified TcdA and/or TcdB. Moreover, immunized mice were significantly protected when challenged with C. difficile spores from homologous (VPI 10463) and heterologous, epidemic (UK1) strains. These data demonstrate the robust immunogenicity and efficacy of a TcdA/B RBD-based DNA vaccine in preclinical models of acute toxin-associated and intragastric, spore-induced colonic disease.
INTRODUCTION
Clostridium difficile infection (CDI) is the leading cause of nosocomial antibiotic-associated diarrhea in developed countries, with 500,000 new infections and 20,000 deaths occurring annually in the United States alone (1). The primary cause of C. difficile-associated disease (CDAD) is antibiotic disruption of the gastrointestinal microflora, followed by overgrowth of C. difficile. Morbidity and mortality associated with CDAD have risen over the past decade (2–4), due most likely to an increased prevalence of relapsing disease and emerging hypervirulent strains (2, 3). CDAD is mediated by the effects of two secreted toxins, toxin A (TcdA) and toxin B (TcdB), both of which disrupt the actin cytoskeleton in the gastrointestinal epithelium, leading to fluid accumulation and inflammation (5). Treating the disease is inherently difficult, given the persistence of C. difficile spores within the hospital environment and the lack of standard and effective therapy for recurrent disease. Therefore, preventing morbidity and mortality associated with new infections and recurrent disease may require a prophylactic treatment that can effectively prevent toxin-mediated cytopathology.
Expression of either TcdA or TcdB alone can cause CDAD in hamsters (6, 7); however, the majority of clinical isolates of C. difficile express both TcdA and TcdB (8). Consequently, the outcome of CDAD in hamsters and humans correlates well with the development of host-antibody responses to both TcdA and TcdB (9–11). In the hamster model, moreover, immunotherapy with antibodies recognizing both toxins reduces CDAD more effectively than antibodies targeting the toxins individually (10, 12, 13). Therefore, a vaccine that targets both virulence factors is most desirable.
TcdA and TcdB share functionally similar C-terminal receptor binding domains (RBDs) that mediate the binding of toxins to carbohydrate receptors on the surfaces of epithelial target cells (14). Toxins lacking the RBD are not cytopathic in vitro (15), and antibodies recognizing epitopes within the RBD are capable of neutralizing the toxin in vitro and in vivo (12, 16, 17). Several studies have identified the RBD as a suitable target for a vaccine or immunotherapy. Parenteral delivery of TcdA RBD protein, or a monoclonal antibody directed against the region, protected mice from a lethal dose of TcdA (18). Second, human RBD-specific monoclonal antibodies prevented C. difficile-induced mortality in hamsters (12) and reduced the number of recurrent infections in humans (19). Despite their efficacy, these approaches have drawbacks that may limit their usefulness in the clinic. For example, protein-based vaccines may suffer from shorter in vivo half-lives, while monoclonal antibodies are expensive and time-consuming to mass produce.
These drawbacks highlight the need to develop alternative vaccines strategies, such as DNA-based immunization against C. difficile toxins. Advantages supporting this platform as an alternative vaccine strategy include ease of manipulation, low production costs, stability, and lack of a cold-chain requirement (20, 21). Moreover, DNA vaccines can induce robust humoral responses, in addition to strong cellular responses, with the use of appropriate adjuvants or delivery techniques. Taken together, these advantages make newer, synthetic DNA-based immunizations a desirable vaccine modality for C. difficile. In support of this idea, optimized plasmids encoding the C-terminal RBD from TcdA (22) or the N-terminal enzymatic domains of TcdA and TcdB (23) have been reported to be immunogenic and to protect mice from lethal toxin challenges. In the latter study, however, a plasmid encoding the RBD from TcdB failed to elicit an antigen-specific humoral response. Considering that TcdB is essential for C. difficile virulence (7) as well as the strong association between recurrent disease and low serum aTcdB RBD antibodies (11), we believe it to be imperative to develop a vaccine that contains both TcdA RBD- and TcdB RBD-expressing plasmids.
In the present study, synthetic inserts encoding the RBD of C. difficile TcdA and TcdB were evaluated for the ability to elicit toxin-specific neutralizing antibodies (nAbs). Our findings show that the RBD vaccine induces a robust multi-isotype humoral response in mice and nonhuman primates (NHPs) that is able to neutralize toxin in vitro. Mice that were immunized with our plasmids or NHP sera were protected from C. difficile toxin and spore challenges. Overall, our work demonstrates that a synthetic DNA vaccine encoding the toxin RBDs is able to provide robust neutralizing and protective immune responses in small- and large-animal models.
MATERIALS AND METHODS
Ethics statement.
In vivo electroporation of DNA vaccines in mice was conducted in accordance with the guidelines set forth by the National Institutes of Health and performed under protocols approved by the Institutional Animal Care and Use Committee (IACUC) at Drexel University College of Medicine (IACUC and Biosafety protocol 18489). Indian rhesus macaques (Macaca mulatta) were housed at the Tulane National Primate Research Center (Covington, LA) according to the standards and guidelines set forth in the Animal Welfare Act of 1966 and the AAALAC Guide for the Care and Use of Laboratory Animals, in addition to the animal care standards deemed acceptable by the Association for the Assessment and Accreditation of Laboratory Animal Care International (TNPRC IACUC P0040R). All animal work was carried out in accordance with the guidelines of and was approved by the Army Medical Research and Materiel Command (USAMRMC) Animal Care and Use Review Office (ACURO), as required by the U.S. Department of Defense.
Cell culture.
HEK-293T/17 (American Type Culture Collection [ATCC] CRL-11268) and Vero 76 (ATCC CRL-1587) cells were cultured in complete growth medium (Dulbecco's modified Eagle medium supplemented with 10% fetal bovine serum and 1% antibiotic-antimycotic). Cells were incubated in a 5% CO2 humidified incubator at 37°C.
DNA vaccine construction and confirmation of antigenic protein expression.
Plasmids expressing the TcdA RBD or TcdB RBD were constructed as described previously (23). Sequences for the TcdA RBD (residues 1848 to 2710) and TcdB RBD (residues 1851 to 2366) from C. difficile strain VPI 10463 were obtained from GenBank (accession numbers P16154.2 and P18177.3, respectively). RBD sequences underwent RNA optimization in order to enhance protein expression and were constructed with a Homo sapiens codon bias (GeneArt; Life Technologies, Carlsbad, CA). Within the RBD sequence, putative N-linked glycosylation sites were disrupted by substituting a glutamine for the initial asparagine residue at each site. Therefore, two constructs were synthesized for each RBD antigen: unmodified (wild type [wt]) and modified (N→Q). Constructs for TcdA RBD and TcdB RBD were independently inserted into the pVAX1 expression vector (GeneArt). The resulting constructs are referred to as pARBD-wt, pARBD-NQ, pBRBD-wt, and pBRBD-NQ.
In vitro expression of plasmids was verified by transfecting HEK-293T cells (3.0 × 105 cells) using Lipofectamine 2000 (Life Technologies). Forty-eight hours after transfection, cellular lysates and supernatants were harvested and fractionated using sodium dodecyl sulfate-10% polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene fluoride membranes (Millipore, Billerica, MA). Immunodetection of vaccine antigens in vitro was performed with specific mouse antiserum, and the expressed proteins were visualized with horseradish peroxidase (HRP)-conjugated rabbit anti-mouse IgG (Santa Cruz Biotechnology, Santa Cruz, CA) using an enhanced-chemiluminescence detection system (Pierce, Rockford, IL). For analysis of glycosylation status, aliquots of lysates and supernatants were digested with 500 U of the peptide N-glycosidase F (PNGase F) (New England BioLabs, Ipswich, MA) for 1 h at 37°C and deactivated at 65°C for 15 min. Samples were subjected to SDS-PAGE and immunodetection as described above.
Generation of recombinant TcdA RBD and TcdB RBD for use as coating antigens in enzyme-linked immunosorbent assays (ELISAs).
The RBD regions of TcdA and TcdB were amplified from either pARBD-NQ or pBRBD-NQ using primers designed to facilitate subcloning into the ligation-independent cloning prokaryotic expression vector pETHSUL as described previously (24). TcdA and TcdB RBD proteins were overproduced in Escherichia coli and purified using the subtractive purification strategy outlined by Zentner et al. (25).
Mouse strains, plasmid immunization, and in vivo electroporation in mice.
Six- to 8-week-old female C57BL/6 mice (Jackson Laboratories, Bar Harbor, ME) were housed in a temperature-controlled, light-cycled, specific-pathogen-free facility at Drexel University College of Medicine. Plasmids were formulated in 0.25% bupivacaine-HCl (Sigma, St. Louis, MO) in isotonic citrate buffer. Initial dosing experiments consisted of groups containing either (i) 25 μg of the control plasmid (pVAX1), (ii) 25 μg of antigenic plasmid (pARBD-NQ or pBRBD-NQ), or (iii) a mixture of 10 μg of antigenic plasmid and 15 μg of pVAX1, so that each group received a total of 25 μg DNA. Later experiments involved 10 μg of each plasmid, delivered either independently or in combination. The vaccine was administered to isoflurane-anesthetized mice (n = 5/group) (the immunizations were 2 weeks apart for a total of 3 immunizations). All immunizations (volume = 20 μl) were administered into the right tibialis anterior muscle using an insulin syringe needle (28 gauge), immediately followed by in vivo electroporation (Cellectra 2000; Inovio Pharmaceuticals, Blue Bell, PA), which entails placing a triangular, three-pronged array directly into the tibialis anterior muscle, followed by two pulses of 0.2 A each delivered for 52 ms/pulse and separated by 1 s.
Splenocyte isolation and enzyme-linked immunospot (ELISpot) assays.
At endpoints designated in the figure legends, animals were sedated using isoflurane. Following sacrifice, spleens from each mouse were harvested and crushed into a single-cell suspension using a Stomacher 80 (Seward Laboratory Systems, Inc., Bohemia, NY). The resultant suspension was filtered through a 40-μm cell strainer (BD Biosciences, Franklin Lakes, NJ), washed, and incubated for 5 min at room temperature in ammonium-chloride-potassium lysing buffer (Gibco, Life Technologies) to induce hemolysis. All cells were washed, resuspended in medium (RPMI 1640 plus 10% fetal bovine serum and 1% antibiotic-antimycotic), and counted (cell viability was determined using trypan blue stain) using a Countess automated cell counter (Life Technologies).
B cell ELISpots were carried out as described previously (26–29) with some modifications as described below. Briefly, 96-well plates (Mabtech, Inc., Cincinnati, OH) were coated with 0.5 μg/ml of toxoid A or toxoid B (List Biological Laboratories, Inc., Campbell, CA) overnight at 4°C. The following day, the plates were washed and blocked for at least 2 h with 1% bovine serum albumin (BSA). For detection of antigen-specific spots, 5.0 × 104 splenocytes from each group of mice were added to each well in triplicate and incubated for 5 h at 37°C, 5% CO2. The plates were then washed and incubated with anti-mouse IgG-biotin overnight at 4°C. The following day, the plates were washed and incubated with streptavidin-alkaline phosphatase for 1 h at room temperature. The plates were washed and developed using the substrate 5-bromo-4-chloro-3-indolyl-phosphate–nitroblue tetrazolium (BCIP-NBT) until distinct spots emerged. The plates were then rinsed with distilled water and dried overnight at room temperature, and the spots were enumerated using an automated ELISpot reader (Cellular Technology Limited, Shaker Heights, OH). The data are represented as the number of antigen-specific spots, or antibody-secreting cells (ASCs), per million splenocytes.
Processing of fecal pellets.
Fecal pellets were collected from vaccinated mice. Stool was dissolved in the following buffer at a specific weight/volume ratio: 1 g of thawed stool was dissolved with 4 ml of phosphate-buffered saline (PBS), pH 7.5, supplemented with 0.05% Tween 20, 0.1% BSA, 0.02% sodium azide, and a cocktail of protease inhibitors (Complete protease inhibitor tablets; Roche, Nutley, NJ). The suspension was incubated for 15 min with frequent vortexing, and the sediment was pelleted by centrifugation at 1,200 rpm for 5 min. The fecal supernatant was centrifuged again at 16,000 × g for 15 min. The cleared supernatants were either immediately used for ELISA or frozen at −80°C.
Analysis of antigen-specific IgG in the sera of immunized animals.
An ELISA was used to determine levels of antigen-specific IgG in mouse serum, as described previously (30, 31). Mouse blood samples were harvested by submandibular bleeding, and subsequently, the sera were analyzed individually within each experimental group. Ninety-six-well enzyme immunoassay/radioimmunoassay plates (Costar; Fisher Scientific, Waltham, MA) were coated for 2 h at room temperature or overnight at 4°C with 0.5 μg/ml of coating antigen (toxoid A or toxoid B [List Biologicals] or recombinant TcdA RBD or TcdB RBD [produced as described above]). The plates were washed and blocked against nonspecific binding with 3% bovine serum albumin for at least 2 h at room temperature. Sera from immunized mice were diluted in blocking buffer, added to wells in duplicate, and incubated at room temperature for 2 h or overnight at 4°C. Bound antibodies were detected with horseradish peroxidase-labeled goat anti-mouse IgG, IgG1, IgG2a, IgG2b, IgG3, IgA, and IgM (all from Santa Cruz Biotechnology) and developed with the substrate 3,3′,5,5′-tetramethylbenzidine (TMB) H2O2 (Pierce). The color reaction was stopped with 2 N H2SO4, and the absorbance at 450 nm was read using an EL312 Bio-Kinetics microplate reader (BioTek Instruments, Inc., Winooski, VT).
Nonhuman-primate husbandry and specimen collection schedule.
Rhesus macaques (M. mulatta) were housed at the Tulane National Primate Research Center in accordance with the standards of the American Association for Accreditation of Laboratory Animal Care. The animals were allowed to acclimate for at least 30 days in quarantine prior to any immunization. All protocols were approved by the Tulane National Primate Research Center Animal Care and Use Committee.
Plasmid immunization and in vivo electroporation delivery in nonhuman primates.
Groups of female rhesus macaques (M. mulatta) of Indian origin aged 4 to 8 years (n = 4 per group) were used in the study. For immunizations, animals were anesthetized with ketamine (0.1 ml/kg of body weight) or tiletamine/zolazepam (0.06 to 0.10 ml/kg) and immunized at weeks 0, 6, 12, and 18 with 1.0 mg per construct of pTcdA RBD and pTcdB RBD. DNA was formulated in sterile water for injection and delivered into the quadriceps muscle in a total volume of 0.75 ml per injection, followed by in vivo electroporation using the constant current Cellectra device (Inovio Pharmaceuticals, Inc., Blue Bell, PA).
Collection of peripheral blood from nonhuman primates.
Animals were bled every 2 weeks starting 2 weeks prior to the first immunization. The animals were anesthetized with ketamine (0.1 ml/kg) or tiletamine/zolazepam (0.06 to 0.10 ml/kg), and blood samples were collected from the femoral vein using the Sarstedt S-Monovette collection system (Sarstedt, Nümbrecht, Germany) and placed into serum gel tubes to allow whole blood to coagulate. Specimens were shipped on cool packs overnight to Drexel University College of Medicine. Upon receipt, the serum gel tubes were spun at 2,000 × g for 15 min to separate the serum from the coagulated blood plug. Serum obtained after centrifugation was aliquoted and frozen until it was tested in ELISAs.
Detection of nonhuman-primate serum anti-toxin IgG by ELISA.
To determine serum antibody titers against TcdA and TcdB, 96-well high-binding polystyrene plates (Corning, Lowell, MA) were coated overnight at 4°C with 0.5 μg/ml of coating antigen (toxoid A or toxoid B [List Biologicals] or recombinant TcdA RBD or TcdB RBD [produced as described above]). The plates were washed and blocked against nonspecific binding with 3% bovine serum albumin for at least 2 h at room temperature. Then, sera from immunized rhesus macaques were diluted in blocking buffer, added to wells in duplicate, and incubated at room temperature for 2 h or overnight at 4°C. Bound IgG antibodies were detected with goat anti-macaque IgG-HRP (Nordic) at a dilution of 1:10,000 and developed with the substrate TMB H2O2 (Pierce). The color reaction was stopped with 2 N H2SO4, and the absorbance at 450 nm was read using an EL312 Bio-Kinetics microplate reader (BioTek Instruments, Inc., Winooski, VT).
In vitro toxin neutralization.
Before each experiment, the dose of purified toxin (List Biologicals) that induced 100% cell rounding was determined using Vero cells. The Vero cells (5.0 × 104) were seeded into 96-well plates 24 h before the beginning of the assay. The next day, serial dilutions of mouse serum were made in growth medium. To each dilution, toxin was added so that the final concentration of toxin was twice that which was needed to yield 100% cell rounding. This mixture was placed at 37°C, 5% CO2 for 1 h before being applied to the Vero cell monolayer. After 20 to 24 h, cell rounding was visualized using phase-contrast microscopy, and the data were represented as the percentage of total cells displaying cytopathic effects (CPE) averaged from five separate fields per well. All samples were tested in duplicate.
Challenge studies in mice.
For challenge studies involving purified C. difficile toxin, mice were immunized with pARBD-NQ or pBRBD-NQ (10 or 25 μg) as described above. Five weeks after the final immunization, the mice were challenged intraperitoneally with 200 μl of toxin diluted in 1× Hanks' buffered saline solution (HBSS). pARBD-NQ-immunized mice received 300 ng of TcdA, while pBRBD-NQ-immunized mice received 150 ng each of TcdA and TcdB. Alternatively, a 1:20 dilution of sera from immunized NHPs was diluted in sterile HBSS and heat inactivated at 55°C for 30 min. This was added to lethal amounts of TcdA plus TcdB (the 100% lethal dose [LD100] determined prior to the experiment) as described above and delivered intraperitoneally to naive mice. All challenged mice were monitored daily for signs of morbidity (hunched posture, ruffled fur, abdominal hardening, and hypothermia) and were sacrificed when at least three signs of morbidity were observed.
For challenge studies involving C. difficile spores, mice were immunized with both pARBD-NQ and pRBD-NQ either twice or four times (see Fig. 7A). Control animals were either naive or immunized with an equivalent amount of empty vector (pVAX1). After resting, the animals were made susceptible to C. difficile infection by treatment with a broad-spectrum antibiotic cocktail (32) for 7 days and subsequently challenged via oral gavage with 105 CFU of spores of strain VPI 10463, a ribotype 087 strain, or UK1, a ribotype 027 strain, prepared as described previously (33). The infected animals were monitored daily for signs of sickness (e.g., diarrhea, hunched posture, lethargy, and weight loss), and moribund animals were euthanized based on a rubric developed and approved by the IACUC. Therefore, death was not an endpoint, as animals were euthanized if they displayed signs of disease/distress as determined by the rubric, although in rare cases, animals might succumb to infection prior to our twice-daily checks for signs of rubric morbidity.
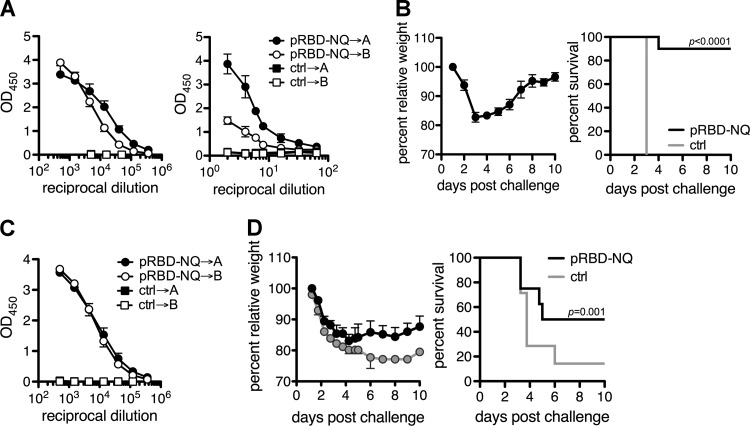
FIG 7.
(A) Mice were immunized at days 0 and 14 with 10 μg each of pRBD-NQ constructs, and anti-RBD IgG levels were measured in the serum and stool 10 days after the final immunization by ELISA. (B) Mice were challenged 4 months following the last immunization with C. difficile strain VPI 10463, and relative weight loss (left) and survival (right) are shown. (C) Mice were immunized with pRBD-NQ at days 0, 14, 28, and 403 with 10 μg each of pRBD-NQ constructs, and anti-RBD IgG levels were measured in the serum after the final immunization. Experiments were performed with 7 to 10 mice/group, and circles represent means ± standard errors of the means from one experiment. (D) Mice were challenged 4 months later with C. difficile strain UK1, and relative weight loss (left) and survival (right) are shown. Statistical differences were determined using a log rank test. P values of ≤0.05 were considered to be significant.
Statistical analysis.
Statistical comparisons were performed using PASW SPSS v20 (IBM Corporation, Armonk, NY). All data were nonparametric; therefore, statistical differences were assessed between immunization groups using either a Mann-Whitney U or Kruskal-Wallis test. To assess differences within groups over time, we applied a Wilcoxon matched-pairs test. A log rank analysis was performed to determine significant differences between groups within the challenge studies. All data are presented as the median plus the range calculated from the averages of duplicate or triplicate wells for each animal. A P value of ≤0.05 was considered to be significantly different.
RESULTS
Construction and expression of synthetic DNA vaccines expressing the RBDs from TcdA and TcdB.
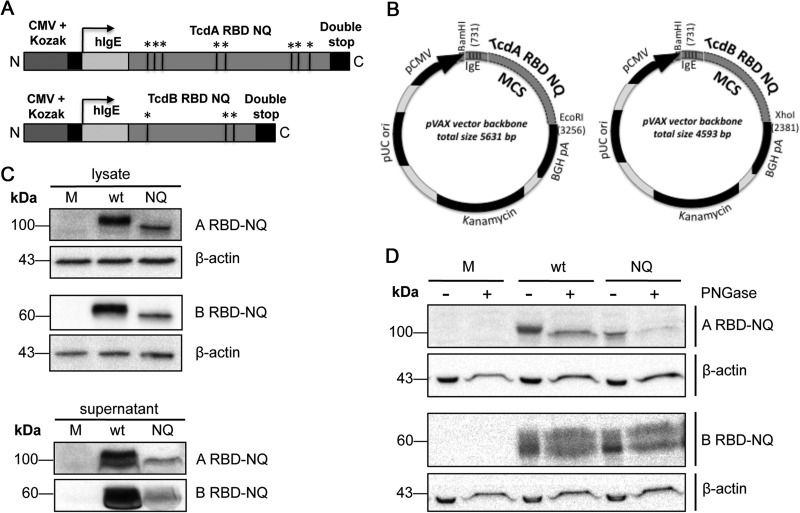
The C-terminal RBDs of TcdA and TcdB are important for receptor-mediated endocytosis of the toxins (34, 35), and several studies have demonstrated the utility of the RBD as a vaccine candidate (12, 18, 19). In this study, we designed highly optimized plasmids based on the DNA sequence that defines the RBDs of TcdA and TcdB (36, 37) from the reference strain of C. difficile (VPI 10463). This was backtranslated in silico with the objective of introducing gene modifications that would enhance protein expression, including RNA and codon optimization (for H. sapiens), introduction of both a Kozak element and an N-terminal IgE leader sequence, and removal of cis-acting motifs/RNA secondary structures that impede translation (21).
In order to avoid glycosylation of the expressed antigens, which could potentially mask key neutralization epitopes, we disrupted any putative N-linked glycosylation sites by introducing an Asn→Gln substitution at each site. This yielded totals of eight and three alterations within the TcdA RBD and TcdB RBD sequences, respectively (Fig. 1A). These modified sequences were submitted for commercial synthesis and ligated into a pVAX1 vector, yielding plasmids that contained RBD inserts with either wild-type (RBD-wt) or altered (RBD-NQ) sequences (Fig. 1B). The expression of RBD-NQ antigens was verified in transiently transfected 293T cells, where RBD protein was detected in both cell lysates and supernatants (albeit at a lower level than for the wild-type protein) using antiserum raised against ARBD-wt or BRBD-wt (Fig. 1C). To assess the glycosylation status of RBD-NQ protein in vitro, we transfected 293T cells with either pARBD-NQ, pBRBD-NQ, pARBD-wt, or pBRBD-wt, and the cell lysates were collected (Fig. 1D). Digestion with PNGase F, which cleaves posttranslational sugar modifications, resulted in a decreased molecular weight for RBD-wt but not for RBD-NQ protein. Taken together, these data demonstrate that pARBD-NQ and pBRBD-NQ are well expressed in a mammalian cell line and that the Asn→Gln substitution does not interfere with recognition by polyclonal RBD-wt serum. Moreover, RBD-NQ protein is not sensitive to N-linked glycosylation in vitro.
FIG 1.
Construction and expression of a DNA vaccine encoding the RBDs from TcdA and TcdB. (A) ARBD-NQ and BRBD-NQ constructs contain a cytomegalovirus promoter with a Kozak sequence, a human IgE leader, and either the TcdA RBD or TcdB RBD followed by two stop codons. Within the RBD sequence, the black lines indicate putative N-linked glycosylation sites that were altered. Black lines and asterisks refer to putative N-linked glycosylation sites. (B) The inserts were cloned into pVAX1, creating four plasmids: pARBD-wt, pARBD-NQ, pBRBD-wt, and pBRBD-NQ. MCS, multiple cloning site. (C) pARBD-NQ and pBRBD-NQ expression was confirmed in transfected HEK-293T cells. Forty-eight hours after transfection, immunodetection of RBD protein was performed on the lysates (30 μg) and supernatants (100 μg for TcdA RBD and 150 μg for TcdB RBD) using mouse RBD antiserum. (D) Similar amounts of lysates and supernatants were treated with PNGase F and subjected to SDS-PAGE in order to assess the glycosylation of RBD proteins in vitro. M, mock.
Expression and immunogenicity of pARBD-NQ and pBRBD-NQ in mice.
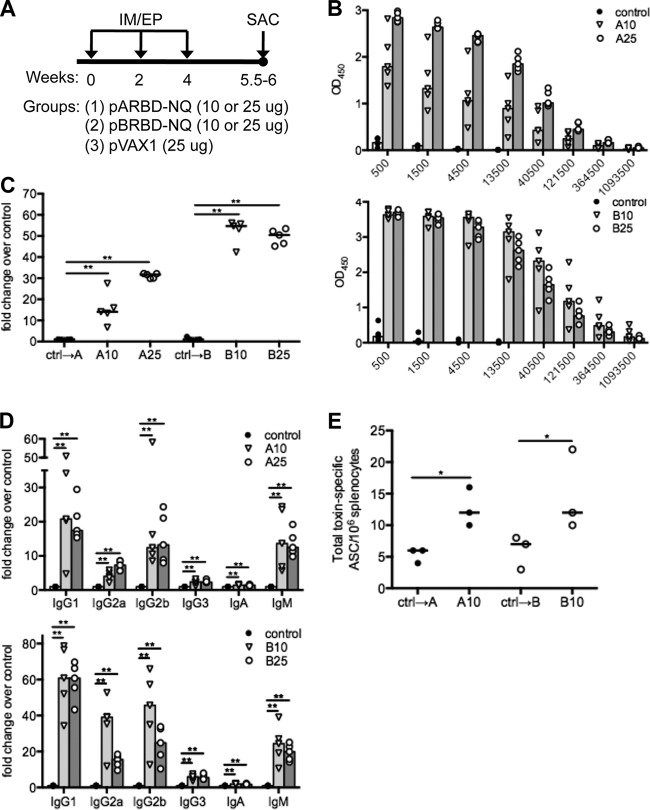
The immunogenicity of pARBD-NQ and pBRBD-NQ constructs was verified by analysis of sera from mice that were immunized three times intramuscularly, followed by electroporation (IM-EP) (Fig. 2A). Following the third IM-EP immunization, all mice displayed elevated levels of RBD-specific serum IgG (Fig. 2B), demonstrating increases over controls as high as 30- and 55-fold for pARBD-NQ and pBRBD-NQ, respectively (Fig. 2C). As expected, animals immunized with empty vector (pVAX1) displayed negligible antigen-specific responses. Because lower titers of TcdA-specific IgM, IgG2, and IgG3 are characteristic of patients who have relapsing CDAD (38, 39), we investigated the isotype of the humoral immune response. Of note, a significant increase in absorbance for antigen-specific IgM, IgG1, IgG2a, and IgG2b, but not IgG3 or IgA, was observed in the sera of immunized mice compared with controls (Fig. 2D). To further confirm the immunogenicity of the constructs, we screened for RBD-specific ASCs in the spleens of immunized animals. After three IM-EP immunizations, there was a significant increase in the number of antigen-specific ASCs compared with control-immunized animals (Fig. 2E). Considering that toxin nAbs are thought to be important for the control of CDAD (19), we tested the abilities of vaccine-induced antibodies to neutralize toxin. Importantly, sera from immunized mice neutralized the cytopathic effects of TcdA and TcdB in a sensitive in vitro neutralization assay (Fig. 3). Taken together, these data demonstrate that immunization with either pARBD-NQ or pBRBD-NQ elicits antigen-specific and toxin-neutralizing humoral immune responses.
FIG 2.
RBD DNA vaccination induces strong humoral responses in mice. (A) C57BL/6 mice (n = 5) were immunized three times (via intramuscular electroporation) with 10 or 25 μg of either pARBD-NQ (denoted A) or pBRBD-NQ (denoted B). Animals immunized with pVAX-1 are referred to as “control.” (B and C) After the third immunization, total serum anti-RBD IgG responses were measured by ELISA. (C) Fold change in OD450 values were compared at a 1/4,500 dilution. (D) An ELISA was used to determine the isotypes of vaccine-induced RBD-specific antibodies; post-third-immunization sera were subjected to a similar analysis. (E) Spleens from immunized animals were isolated 10 days after the third immunization. Pooled splenocytes were added to RBD- or IgG-coated ELISpot plates, and the numbers of antigen-specific ASCs were determined. The bars (B and D) and horizontal lines (C and E) indicate the medians of the groups. OD450, optical density at 450 nm. *, P ≤ 0.05; **, P ≤ 0.01.
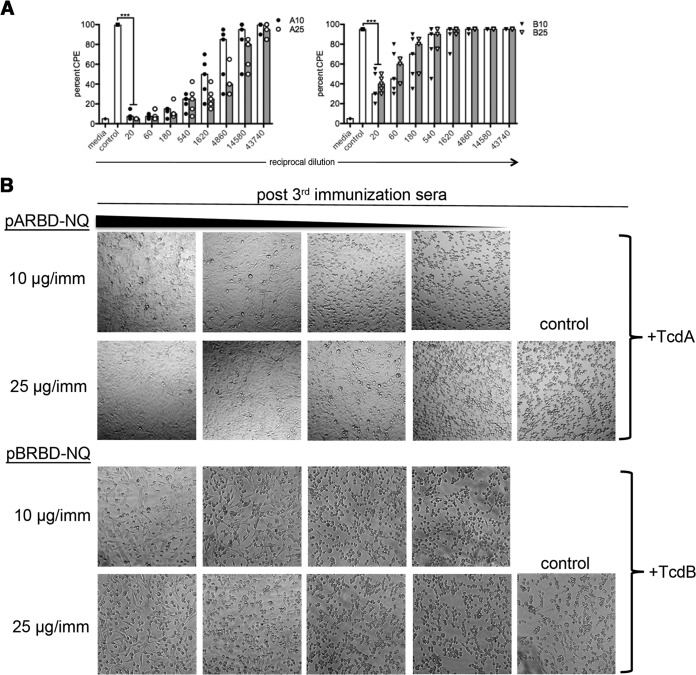
FIG 3.
Induction of toxin-neutralizing antibodies in mice. (A) Systemic TcdA-specific and TcdB-specific nAbs from immunized mice assessed using an in vitro toxin neutralization assay. Vero cells were exposed to mouse sera preincubated with either TcdA or TcdB, and the average CPE across two wells was assessed under ×10 magnification. “Media” represents the effect of toxin in the absence of serum. The bars indicate the medians for the groups. (B) Representative images displaying immune serum neutralization of the cytopathic effect of toxin. ***, P ≤ 0.001.
Protection of immunized mice following toxin challenge.
Given that hyperimmune serum could neutralize C. difficile toxins in vitro, we next addressed whether immunization with pARBD-NQ and/or pBRBD-NQ could confer protective immunity in vivo. A lethal toxin challenge model was employed to directly assay for toxin-neutralizing antibodies within the serum. TcdA and/or TcdB was delivered intraperitoneally to naive mice, and a lethal dose of TcdA was determined (LD100 = 300 ng [unpublished data from our laboratory]). No mortality was observed when the same dose of TcdB was administered alone, which is in accordance with previously published data (40). However, combining two sublethal doses of TcdA and TcdB (150 ng each) was lethal, and this regimen was used to challenge immunized mice.
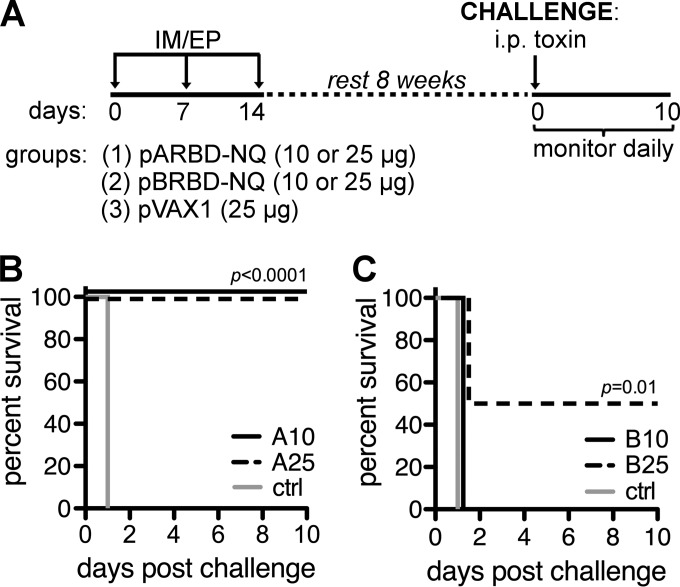
Toxin-challenged mice were monitored daily for 7 days after the challenge, and the outcome was based on morbidity (e.g., lethargy and hunched posture) and mortality associated with the challenge model (32). Acute morbidities were observed in the majority of challenged animals within 72 h of challenge. Compared with controls, all of which succumbed to challenge, 10/10 (100%) of the animals immunized with 10 or 25 μg of pARBD-NQ were protected against TcdA challenge (Fig. 4A). Interestingly, only those animals that received 25 μg of pBRBD-NQ were protected completely (10/10) from the dual-toxin challenge (Fig. 4B).
FIG 4.
Survival of immunized mice challenged with TcdA and TcdB. (A) An immunization and challenge schedule for mice is shown. C57BL/6 mice (n = 10/group) were immunized as described in the text and rested for 8 weeks before being challenged intraperitoneally with a lethal dose of C. difficile toxin. (B and C) Animals immunized with pARBD-NQ (B) or pBRBD-NQ (C) were challenged with 300 ng of TcdA or 150 ng of both TcdA and TcdB, respectively. i.p., intraperitoneal; ctrl, control. *, P ≤ 0.05; ***, P ≤ 0.001.
Immunogenicity of DNA vaccination in nonhuman primates.
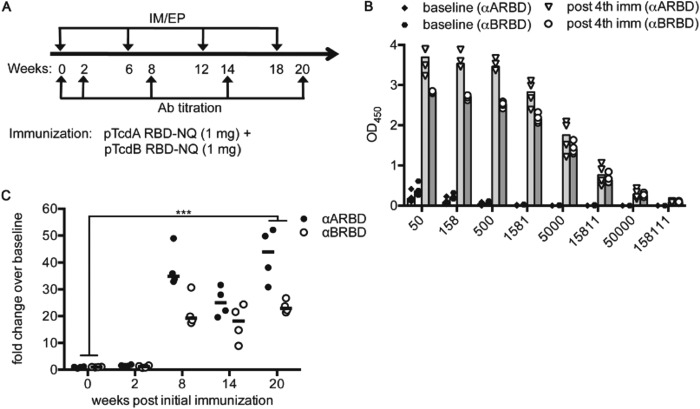
To determine whether pA/B RBD vaccination is immunogenic in a larger-animal model, we performed NHP studies to detect postvaccination humoral immune responses. Four rhesus macaques were immunized IM-EP with both pARBD-NQ and pBRBD-NQ (Fig. 5A). Compared with their respective baseline time points, all four animals showed detectable levels of RBD-specific IgG in the serum as measured by ELISA (Fig. 5B and C). Moreover, robust nAb responses to both TcdA and TcdB were observed in the sera (Fig. 6, bottom left). In contrast, all control animals remained negative throughout the course of the study.
FIG 5.
pRBD-NQ immunization elicits strong humoral responses in nonhuman primates. (A) An immunization schedule for NHPs is shown. Female rhesus macaques (n = 4) were given 1.0 mg of both pARBD-NQ and pBRBD-NQ by IM-EP. NHPs received four immunizations spaced 6 weeks apart. (B and C) RBD-specific IgG was analyzed in post-fourth-immunization sera (post 4th imm) (B) and IgG responses at week 20 and week 0 were compared at a 1/5,000 dilution 2 weeks after each immunization (C). The bars (B) and horizontal lines (C) indicate the medians for the groups. ***, P ≤ 0.001.
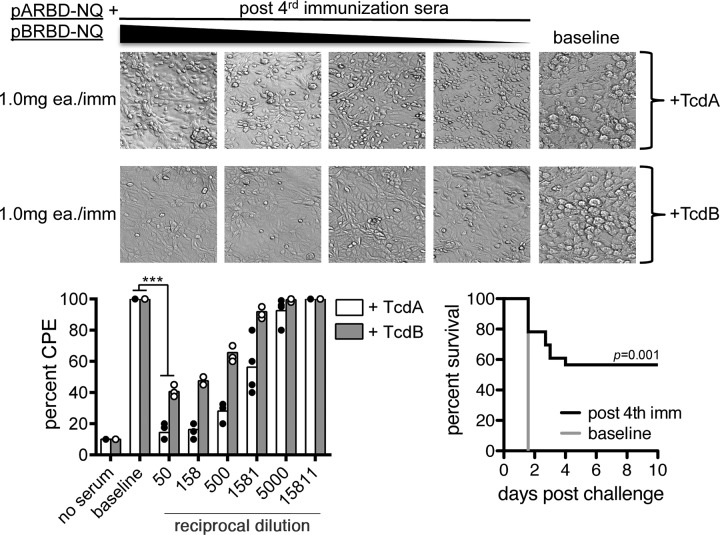
FIG 6.
Sera from immunized nonhuman primates neutralizes toxin in vitro and protects mice from a lethal intraperitoneal toxin challenge. (Bottom left) Systemic TcdA-specific and TcdB-specific nAbs from immunized mice assessed using an in vitro toxin neutralization assay. The abilities to neutralize TcdA and TcdB were assessed independently. The bars indicate the medians for the groups. (Top) Sample images of neutralization. (Bottom right) NHP sera harvested after the final immunization were diluted (1/20), heat inactivated, and combined with a lethal dose of TcdA plus TcdB. This was delivered intraperitoneally to naive animals, and survival was measured over the course of 5 days. Control animals received baseline NHP sera. **, P ≤ 0.01; ***, P ≤ 0.001.
We next tested the ability of the NHP immune sera to passively protect mice in an in vivo toxin neutralization assay. Sera collected 2 weeks after the fourth immunization were preincubated with a lethal dose of C. difficile toxin and delivered intraperitoneally to naive mice. As seen in Fig. 6, bottom right, 56.5% (n = 13/23) of mice survived challenge compared with controls. These data indicate that coimmunization with pARBD-NQ and pBRBD-NQ constructs is immunogenic in NHPs and that serum antibodies can neutralize toxin and protect mice from toxin-associated mortality.
Protection of immunized mice following spore challenge.
Inducing CDAD in mice and hamsters requires pretreatment of the animals with a cocktail of broad-spectrum antibiotics (32). This model mimics the fecal-oral route of transmission through intragastric delivery of purified C. difficile spores. Similar to what is observed during human infection, sickly mice display symptoms of CDAD (e.g., watery stool and intestinal pathology) that may require euthanasia if the symptoms become too severe. To test our vaccine against a spore challenge using a clinically applicable vaccination schedule, we immunized animals twice and administered a lethal dose of spores from the homologous vaccine strain (VPI 10463). We found that after two immunizations with pARB-NQ and pRBD-NQ (10 μg each), we could detect robust RBD-specific IgG responses within the blood (Fig. 7A) and stool (Fig. 7B) that could neutralize toxin cytopathology in vitro (data not shown). After treating these mice with antibiotics, we observed 90% (n = 9/10) protection from a homologous challenge compared with naive controls (Fig. 7D). All surviving animals experienced acute weight loss, peaking between days 3 and 5, followed by weight gain that stabilized by day 8 (Fig. 7C). Finally, we were unable to prevent the onset of CDAD in antibiotic-treated mice by increasing the amount of DNA/immunization (data not shown).
Because various toxin isoforms have been identified within clinical isolates, and given the increasing prevalence of infections with hypervirulent strains, we thought to test our DNA vaccine against a clinically relevant, heterologous strain (UK1; B1/NAP1/027). As expected, pVAX-immunized mice, which were seronegative for RBD IgG (Fig. 7E), responded poorly to challenge with UK1 spores. All the animals developed signs of disease, and 14.3% (n = 1/7) of the animals were euthanized before the end of the experiment (Fig. 7F and G). In contrast, after four immunizations with RBD-NQ plasmids, we observed RBD-specific serum antibody and 50% survival (n = 4/8) after challenge. All animals in this experiment lost weight, but unlike the controls, weight loss in pRBD-immunized animals had either stabilized or begun to reverse by day 7 postinfection. Importantly, the protection observed in these experiments was seen at least 4 months after the final immunization, indicating that a strong neutralizing memory response is maintained for at least several months using the DNA/electroporation platform.
DISCUSSION
C. difficile-associated disease has emerged as a primary health concern worldwide (41). Recently, an increased prevalence of infections has been observed among traditionally low-risk people, which is potentially attributable to the emergence of hypervirulent strains (42, 43). Considering that the majority of C. difficile clinical isolates express both TcdA and TcdB (44), the presence of both anti-TcdA and anti-TcdB antibodies would be optimal for providing robust protection from CDAD. Indeed, lower anti-toxin antibody responses are associated with an elevated risk of infection and greater disease severity. Providing a strong humoral immune response has been the focus of several active and passive immunization approaches that are currently in clinical development. However, cost and stability issues limit their effectiveness in domestic and foreign clinical settings. Alternative vaccination platforms, such as DNA vaccination, which are cost-effective and demonstrate a favorable safety profile in humans, should be the focus of current and future efforts to prevent CDAD.
In the current study, we designed plasmids expressing the C-terminal RBD regions of both toxins. In order to improve immunogenicity, the antigens were modified to disrupt putative N-linked glycosylation sites that could mask key neutralizing epitopes within the RBD. A key finding from our study is that these modified RBDs can serve as excellent immunogens, effective at producing a strong neutralizing antibody response that can prevent toxin-associated cytopathology in vitro, as well as provide both active and passive protection of mice from challenges with lethal doses of TcdA and TcdB. Furthermore, our group is the first to report on a modified TcdB RBD-expressing plasmid that is immunogenic in both small- and large-animal models. We believe this discovery will not only enhance the success of these plasmids in future clinical trials, but also improve the efficacy of any current or next-generation vaccines and therapies for CDAD.
Other groups have described plasmids expressing optimized TcdA RBDs with an N-terminal tissue plasminogen activator (tPA) signal peptide sequence (22, 23). As expected, our plasmids, which encode N-linked-glycan-null RBDs located downstream of a human IgE leader sequence, were expressed well based on ELISA and Western blot analysis. RBD proteins generated from plasmids expressing wild-type inserts resolved at a higher molecular weight, which decreased upon treatment with PNGase F. Thus, wild-type RBD proteins possess bona fide N-linked glycosylation sites; however, due to the lower relative expression of RBD-NQ constructs, some of these sites may be needed to maintain a native structural conformation. Immunization with either construct elicited a multi-isotype antigen-specific antibody response. A wider range of toxin-specific isotypes is advantageous for a C. difficile vaccine, considering that a multi-isotype response may be more prevalent in asymptomatic carriers or nonrecurrent cases (1). Interestingly, we noticed significant induction of antigen-specific IgG2a for both constructs. The presence of antigen-specific IgG2a suggests the involvement of a T cell component, given that gamma interferon (IFN-γ) is required to drive IgG2a class switching in activated murine B cells (45). Cellular immunity, however, is not known to be essential for control of CDAD, and future studies will be required to better understand the importance of T cells during infection. We do not believe that this response is due to an inherent quality associated with the RBD antigen. Instead, it is most likely a result of the potent adjuvant properties of either EP, which can promote a broader range of isotypes to various antigens (46–49), or plasmid-incorporated cytosine phosphate guanosine nucleotide sequences, which signal through Toll-like receptor 9 and scavenger receptors.
In agreement with the ELISA data, we noticed that across all doses, sera from pARBD-NQ-immunized animals contained a more impressive level of toxin nAbs than sera from pBRBD-NQ-immunized animals. The lower immunogenicity observed for pBRBD-NQ may be a result of lower secretion of BRBD-NQ protein (Fig. 1C, supernatant); however, this was not reflected in the sera of immunized mice. In order to assess the effectiveness of an RBD DNA vaccine, a challenge model is needed. To this end, delivery of purified C. difficile toxins intraperitoneally, either individually or in combination, is lethal in mice (50). However, systemic toxin is not indicative of a normal infection scenario, since the majority of clinical manifestations of CDAD are self-limiting within the intestine (51). In life-threatening cases, however, systemic complications have been documented (52–56), and entry of the toxin into circulation is thought to be a possible cause (57). Therefore, challenging immunized mice with intraperitoneal toxin represents a stringent method for assaying the nAb response. Immunization with pARBD-NQ, at both doses, elicited sterilizing immunity to TcdA challenge, which is consistent with survival data for a previously described TcdA RBD DNA vaccine (22). In contrast, immunization with 25 μg of pBRBD-NQ was required to elicit significant protection from challenge compared to controls. This may be reflective of the higher nAb response observed for animals in this immunization group. Therefore, administration of a higher dose of pBRBD-NQ may be required to generate a titer of nAbs comparable to that of pARBD-NQ immunization.
In both mouse and hamster infection models of CDI, preventing infection-associated mortality is seen as an important metric of vaccine efficacy (12, 13, 22, 23, 58–66). Upon challenging pRBD-NQ-immunized animals with a lethal dose of spores, we observed 90% and 50% protection against homologous and heterologous strains, respectively. The partial protection seen with the heterologous UK1 challenge is likely due to a strain-dependent variation in toxin sequences. In fact, sequence alignments between VPI 10463 and hypervirulent strains (e.g., UK1) reveal that TcdA remains relatively well conserved while the majority of heterology exists within the RBD of TcdB (67, 68). This creates a pattern of unique neutralizing epitopes, so that polyclonal serum raised against TcdB RBD from VPI 10463 cannot cross-neutralize TcdB from UK1 in vitro (67). Although our challenge data do not agree with this report, we believe that during an ongoing UK1 infection within our immunized animals, either (i) TcdA is neutralized, leaving insufficient TcdBto cause lethal disease, or (ii) TcdA and TcdB are both sufficiently neutralized, indicating that in vitro toxin neutralization assays do not accurately represent toxin-associated pathology within the infected intestinal environment. Since the individual roles of TcdA and TcdB during a UK1 infection are unknown, future studies utilizing genetically modified UK1 will aid in answering this question.
Because we noticed a strong neutralizing antibody response and protection in mice, we next wanted to assess whether the RBD DNA vaccines were immunogenic in NHPs. After four immunizations, the NHP cohort displayed a robust level of RBD-specific serum IgG, similar to mice. Serum nAb responses for TcdA RBD were similar to what was observed in mice. Importantly, in NHP serum compared with mouse serum, TcdB RBD nAbs seemed to prevent more CPE at similar dilutions (Fig. 6). We further tested the nAb response of the NHPs in an in vivo toxin neutralization assay. Hyperimmune NHP sera that were preincubated with toxin protected a significant portion of mice (14/23) from challenge. While these challenge studies were performed with immune sera taken after four immunizations, high RBD-specific IgG levels were noted as early as two immunizations, which may be important for clinical translation. Taken together, these data demonstrate that our RBD DNA vaccine is immunogenic in an NHP model and that it can produce titers of nAbs that are protective in mice.
The ability of TcdA and TcdB to independently cause disease in animal models of infection has highlighted the importance of targeting both toxins to prevent CDAD. Since this discovery, several groups have attempted to incorporate both toxins in various vaccine modalities. Recently, Jin et al. attempted to create a TcdB RBD-expressing plasmid, but it failed to elicit immune responses after four immunizations with electroporation and 100 μg/mouse (23). There are several differences in the design and delivery of pBRBD-NQ that may account for differences in antigen expression: (i) inclusion of a larger segment of the TcdB C terminus (526 amino acids [aa] versus 515 aa), (ii) use of different N-terminal signal peptide sequences (IgE versus tPA), and (iii) use of different in vivo electroporation delivery systems. Since the crystal structure of the TcdB RBD has not been resolved and there is a lack of comparative studies between the leader sequences and electroporation devices used in these studies, it is difficult to discern why BRBD-NQ is more immunogenic. However, we feel that the use of both a human IgE leader sequence and a potentially superior electroporation system, which have been proven clinically (69), will increase the success of these plasmids in future clinical trials. Specifically, utilizing the Cellectra 2000 in vivo electroporation delivery method (Inovio Pharmaceuticals, Inc.), a DNA vaccine delivery platform that is currently being used in phase I clinical trials for HIV (PENNVAX) and influenza prophylactic strategies and in phase II clinical trials for HPV therapy (VGX-3100), is a strength of the work presented here.
The results from this study establish the immunogenicity and protective efficacy of RBD-NQ and demonstrate that the immunogenicity of both ARBD-NQ and BRBD-NQ can be improved through codelivery. In particular, levels of total antigen-specific and nAb responses to RBD-NQ were higher than in previously described RBD DNA vaccines (22, 23). This is especially important for preventing primary CDAD, which can manifest within 2 to 4 days after infection in animal models. For this reason, a shorter vaccination regimen, reliant upon boosting through either immunization or natural infection, would be ideal for preventing the onset of CDAD in high-risk patients. Such a vaccine strategy may be more attainable by utilizing a DNA prime-heterologous boost strategy, which has demonstrated superior immunogenicity profiles for several antigens in animal models and humans (70).
ACKNOWLEDGMENTS
We acknowledge Linc Sonenshein for allowing M.A.K. and S.M.B. to train in his laboratory on working in an anaerobic chamber to properly propagate and quantify C. difficile bacteria and spores for use in our studies. We also thank L. Sonenshein for his expertise and guidance in working with C. difficile and for providing the spore stocks for these studies. We also acknowledge Rafi Ahmed, Shane Crotty, and Rachael Aubert for assay guidance for the murine B cell ELISpot assay. We thank Jason Dufour and the Division of Veterinary Medicine for animal care and services at Tulane National Primate Research Center. We acknowledge Diana Winters from Drexel University College of Medicine Academic Publishing Services for her editorial, formatting, and journal submission expertise.
We acknowledge the Drexel University College of Medicine Office of Faculty Affairs and Professional Development for funding a Professional Enrichment and Growth grant so that M.A.K. and S.M.B. could travel and spend an extended amount of time in the Sonenshein laboratory to train. The PEG grant also partially funded the purchase of the anaerobic chamber. This work was funded by a Congressionally Directed Medical Research Grant, W81XWH-09-1-0382 (http://cdmrp.army.mil/). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. This work was supported in part with federal funds from the National Center for Research Resources and the Office of Research Infrastructure Programs (ORIP) of the National Institutes of Health through grant number P51 RR00164 to the Tulane National Primate Research Center.
D.B.W. has grant funding, participates in industry collaborations, and has received speaking honoraria and fees for consulting. This service includes serving on scientific review committees and advisory boards (SAB). Remuneration includes direct payments or stock/stock options, and in the interest of disclosure, therefore, he notes potential conflicts associated with this work, in particular with Inovio, where he serves on the SAB, as well as with Pfizer, Bristol Myers Squibb, Merck, Aldevron, Roche, Ferring Pharma, and possibly others. We declare no other competing financial interests.
Footnotes
Published ahead of print 14 July 2014
REFERENCES
- 1. Rupnik M, Wilcox MH, Gerding DN. 2009. Clostridium difficile infection: new developments in epidemiology and pathogenesis. Nat. Rev. Microbiol. 7:526–536. 10.1038/nrmicro2164 [DOI] [PubMed] [Google Scholar]
- 2. McDonald LC, Killgore GE, Thompson A, Owens RC, Jr, Kazakova SV, Sambol SP, Johnson S, Gerding DN. 2005. An epidemic, toxin gene-variant strain of Clostridium difficile. N. Engl. J. Med. 353:2433–2441. 10.1056/NEJMoa051590 [DOI] [PubMed] [Google Scholar]
- 3. Bartlett JG. 2002. Clostridium difficile-associated enteric disease. Curr. Infect. Dis. Rep. 4:477–483. 10.1007/s11908-002-0032-0 [DOI] [PubMed] [Google Scholar]
- 4. Redelings MD, Sorvillo F, Mascola L. 2007. Increase in Clostridium difficile-related mortality rates, United States, 1999–2004. Emerg. Infect. Dis. 13:1417–1419. 10.3201/eid1309.061116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Carter GP, Rood JI, Lyras D. 2010. The role of toxin A and toxin B in Clostridium difficile-associated disease: past and present perspectives. Gut Microbes 1:58–64. 10.4161/gmic.1.1.10768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kuehne SA, Cartman ST, Heap JT, Kelly ML, Cockayne A, Minton NP. 2010. The role of toxin A and toxin B in Clostridium difficile infection. Nature 467:711–713. 10.1038/nature09397 [DOI] [PubMed] [Google Scholar]
- 7. Lyras D, O'Connor JR, Howarth PM, Sambol SP, Carter GP, Phumoonna T, Poon R, Adams V, Vedantam G, Johnson S, Gerding DN, Rood JI. 2009. Toxin B is essential for virulence of Clostridium difficile. Nature 458:1176–1179. 10.1038/nature07822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lyerly DM, Krivan HC, Wilkins TD. 1988. Clostridium difficile: its disease and toxins. Clin. Microbiol. Rev. 1:1–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Aboudola S, Kotloff KL, Kyne L, Warny M, Kelly EC, Sougioultzis S, Giannasca PJ, Monath TP, Kelly CP. 2003. Clostridium difficile vaccine and serum immunoglobulin G antibody response to toxin A. Infect. Immun. 71:1608–1610. 10.1128/IAI.71.3.1608-1610.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kink JA, Williams JA. 1998. Antibodies to recombinant Clostridium difficile toxins A and B are an effective treatment and prevent relapse of C. difficile-associated disease in a hamster model of infection. Infect. Immun. 66:2018–2025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Leav BA, Blair B, Leney M, Knauber M, Reilly C, Lowy I, Gerding DN, Kelly CP, Katchar K, Baxter R, Ambrosino D, Molrine D. 2010. Serum anti-toxin B antibody correlates with protection from recurrent Clostridium difficile infection (CDI). Vaccine 28:965–969. 10.1016/j.vaccine.2009.10.144 [DOI] [PubMed] [Google Scholar]
- 12. Babcock GJ, Broering TJ, Hernandez HJ, Mandell RB, Donahue K, Boatright N, Stack AM, Lowy I, Graziano R, Molrine D, Ambrosino DM, Thomas WD., Jr 2006. Human monoclonal antibodies directed against toxins A and B prevent Clostridium difficile-induced mortality in hamsters. Infect. Immun. 74:6339–6347. 10.1128/IAI.00982-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kim PH, Iaconis JP, Rolfe RD. 1987. Immunization of adult hamsters against Clostridium difficile-associated ileocecitis and transfer of protection to infant hamsters. Infect. Immun. 55:2984–2992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Greco A, Ho JG, Lin SJ, Palcic MM, Rupnik M, Ng KK. 2006. Carbohydrate recognition by Clostridium difficile toxin A. Nat. Struct. Mol. Biol. 13:460–461. 10.1038/nsmb1084 [DOI] [PubMed] [Google Scholar]
- 15. Olling A, Goy S, Hoffmann F, Tatge H, Just I, Gerhard R. 2011. The repetitive oligopeptide sequences modulate cytopathic potency but are not crucial for cellular uptake of Clostridium difficile toxin A. PLoS One 6:e17623. 10.1371/journal.pone.0017623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lyerly DM, Phelps CJ, Toth J, Wilkins TD. 1986. Characterization of toxins A and B of Clostridium difficile with monoclonal antibodies. Infect. Immun. 54:70–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Frey SM, Wilkins TD. 1992. Localization of two epitopes recognized by monoclonal antibody PCG-4 on Clostridium difficile toxin A. Infect. Immun. 60:2488–2492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sauerborn M, Leukel P, von Eichel-Streiber C. 1997. The C-terminal ligand-binding domain of Clostridium difficile toxin A (TcdA) abrogates TcdA-specific binding to cells and prevents mouse lethality. FEMS Microbiol. Lett. 155:45–54. 10.1111/j.1574-6968.1997.tb12684.x [DOI] [PubMed] [Google Scholar]
- 19. Lowy I, Molrine DC, Leav BA, Blair BM, Baxter R, Gerding DN, Nichol G, Thomas WD, Jr, Leney M, Sloan S, Hay CA, Ambrosino DM. 2010. Treatment with monoclonal antibodies against Clostridium difficile toxins. N. Engl. J. Med. 362:197–205. 10.1056/NEJMoa0907635 [DOI] [PubMed] [Google Scholar]
- 20. Hokey DA, Weiner DB. 2006. DNA vaccines for HIV: challenges and opportunities. Springer Semin. Immunopathol. 28:267–279. 10.1007/s00281-006-0046-z [DOI] [PubMed] [Google Scholar]
- 21. Kutzler MA, Weiner DB. 2008. DNA vaccines: ready for prime time? Nat. Rev. Genet. 9:776–788. 10.1038/nrg2432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gardiner DF, Rosenberg T, Zaharatos J, Franco D, Ho DD. 2009. A DNA vaccine targeting the receptor-binding domain of Clostridium difficile toxin A. Vaccine 27:3598–3604. 10.1016/j.vaccine.2009.03.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jin K, Wang S, Zhang C, Xiao Y, Lu S, Huang Z. 2013. Protective antibody responses against Clostridium difficile elicited by a DNA vaccine expressing the enzymatic domain of toxin B. Hum. Vaccin. Immunother. 9:63–73. 10.4161/hv.22434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Weeks SD, Drinker M, Loll PJ. 2007. Ligation independent cloning vectors for expression of SUMO fusions. Protein Expr. Purif. 53:40–50. 10.1016/j.pep.2006.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zentner I, Sierra LJ, Fraser AK, Maciunas L, Mankowski MK, Vinnik A, Fedichev P, Ptak RG, Martin-Garcia J, Cocklin S. 2013. Identification of a small-molecule inhibitor of HIV-1 assembly that targets the phosphatidylinositol (4,5)-bisphosphate binding site of the HIV-1 matrix protein. ChemMedChem 8:426–432. 10.1002/cmdc.201200577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Slifka MK, Ahmed R. 1996. Limiting dilution analysis of virus-specific memory B cells by an ELISPOT assay. J. Immunol. Methods 199:37–46. 10.1016/S0022-1759(96)00146-9 [DOI] [PubMed] [Google Scholar]
- 27. Brown KA, Kriss JA, Moser CA, Wenner WJ, Offit PA. 2000. Circulating rotavirus-specific antibody-secreting cells (ASCs) predict the presence of rotavirus-specific ASCs in the human small intestinal lamina propria. J. Infect. Dis. 182:1039–1043. 10.1086/315808 [DOI] [PubMed] [Google Scholar]
- 28. Crotty S, Aubert RD, Glidewell J, Ahmed R. 2004. Tracking human antigen-specific memory B cells: a sensitive and generalized ELISPOT system. J. Immunol. Methods 286:111–122. 10.1016/j.jim.2003.12.015 [DOI] [PubMed] [Google Scholar]
- 29. Qadri F, Ryan ET, Faruque AS, Ahmed F, Khan AI, Islam MM, Akramuzzaman SM, Sack DA, Calderwood SB. 2003. Antigen-specific immunoglobulin A antibodies secreted from circulating B cells are an effective marker for recent local immune responses in patients with cholera: comparison to antibody-secreting cell responses and other immunological markers. Infect. Immun. 71:4808–4814. 10.1128/IAI.71.8.4808-4814.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ogawa T, Tarkowski A, McGhee ML, Moldoveanu Z, Mestecky J, Hirsch HZ, Koopman WJ, Hamada S, McGhee JR, Kiyono H. 1989. Analysis of human IgG and IgA subclass antibody-secreting cells from localized chronic inflammatory tissue. J. Immunol. 142:1150–1158 [PubMed] [Google Scholar]
- 31. Mestecky J, Jackson S, Moldoveanu Z, Nesbit LR, Kulhavy R, Prince SJ, Sabbaj S, Mulligan MJ, Goepfert PA. 2004. Paucity of antigen-specific IgA responses in sera and external secretions of HIV-type 1-infected individuals. AIDS Res. Hum. Retrovir. 20:972–988. 10.1089/aid.2004.20.972 [DOI] [PubMed] [Google Scholar]
- 32. Chen X, Katchar K, Goldsmith JD, Nanthakumar N, Cheknis A, Gerding DN, Kelly CP. 2008. A mouse model of Clostridium difficile-associated disease. Gastroenterology 135:1984–1992. 10.1053/j.gastro.2008.09.002 [DOI] [PubMed] [Google Scholar]
- 33. Sorg JA, Dineen SS. 2009. Laboratory maintenance of Clostridium difficile. Curr. Protoc. Microbiol. Chapter 9:Unit9A 1. 10.1002/9780471729259.mc09a01s12 [DOI] [PubMed] [Google Scholar]
- 34. Frisch C, Gerhard R, Aktories K, Hofmann F, Just I. 2003. The complete receptor-binding domain of Clostridium difficile toxin A is required for endocytosis. Biochem. Biophys. Res. Commun. 300:706–711. 10.1016/S0006-291X(02)02919-4 [DOI] [PubMed] [Google Scholar]
- 35. Florin I, Thelestam M. 1986. Lysosomal involvement in cellular intoxication with Clostridium difficile toxin B. Microb. Pathog. 1:373–385. 10.1016/0882-4010(86)90069-0 [DOI] [PubMed] [Google Scholar]
- 36. Dove CH, Wang SZ, Price SB, Phelps CJ, Lyerly DM, Wilkins TD, Johnson JL. 1990. Molecular characterization of the Clostridium difficile toxin A gene. Infect. Immun. 58:480–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. von Eichel-Streiber C, Laufenberg-Feldmann R, Sartingen S, Schulze J, Sauerborn M. 1992. Comparative sequence analysis of the Clostridium difficile toxins A and B. Mol. Gen. Genet. 233:260–268. 10.1007/BF00587587 [DOI] [PubMed] [Google Scholar]
- 38. Kyne L, Warny M, Qamar A, Kelly CP. 2001. Association between antibody response to toxin A and protection against recurrent Clostridium difficile diarrhoea. Lancet 357:189–193. 10.1016/S0140-6736(00)03592-3 [DOI] [PubMed] [Google Scholar]
- 39. Katchar K, Taylor CP, Tummala S, Chen X, Sheikh J, Kelly CP. 2007. Association between IgG2 and IgG3 subclass responses to toxin A and recurrent Clostridium difficile-associated disease. Clin. Gastroenterol. Hepatol. 5:707–713. 10.1016/j.cgh.2007.02.025 [DOI] [PubMed] [Google Scholar]
- 40. Gill DM. 1982. Bacterial toxins: a table of lethal amounts. Microbiol. Rev. 46:86–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kuntz JL, Chrischilles EA, Pendergast JF, Herwaldt LA, Polgreen PM. 2011. Incidence of and risk factors for community-associated Clostridium difficile infection: a nested case-control study. BMC Infect. Dis. 11:194. 10.1186/1471-2334-11-194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kyne L, Hamel MB, Polavaram R, Kelly CP. 2002. Health care costs and mortality associated with nosocomial diarrhea due to Clostridium difficile. Clin. Infect. Dis. 34:346–353. 10.1086/338260 [DOI] [PubMed] [Google Scholar]
- 43. Blondeau JM. 2009. What have we learned about antimicrobial use and the risks for Clostridium difficile-associated diarrhoea? J. Antimicrob. Chemother. 63:238–242. 10.1093/jac/dkn477 [DOI] [PubMed] [Google Scholar]
- 44. Voth DE, Ballard JD. 2005. Clostridium difficile toxins: mechanism of action and role in disease. Clin. Microbiol. Rev. 18:247–263. 10.1128/CMR.18.2.247-263.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Stavnezer J. 1996. Immunoglobulin class switching. Curr. Opin. Immunol. 8:199–205. 10.1016/S0952-7915(96)80058-6 [DOI] [PubMed] [Google Scholar]
- 46. Xing X, Sha S, Li Y, Zong L, Jiang T, Cao Y. 2012. Immunization with a new DNA vaccine for Alzheimer's disease elicited Th2 immune response in BALB/c mice by in vivo electroporation. J. Neurol. Sci. 313:17–21. 10.1016/j.jns.2011.09.040 [DOI] [PubMed] [Google Scholar]
- 47. Wu CJ, Lee SC, Huang HW, Tao MH. 2004. In vivo electroporation of skeletal muscles increases the efficacy of Japanese encephalitis virus DNA vaccine. Vaccine 22:1457–1464. 10.1016/j.vaccine.2003.10.011 [DOI] [PubMed] [Google Scholar]
- 48. Rath A, Batra D, Kaur R, Vrati S, Gupta SK. 2003. Characterization of immune response in mice to plasmid DNA encoding dog zona pellucida glycoprotein-3. Vaccine 21:1913–1923. 10.1016/S0264-410X(02)00824-1 [DOI] [PubMed] [Google Scholar]
- 49. Tollefsen S, Tjelle T, Schneider J, Harboe M, Wiker H, Hewinson G, Huygen K, Mathiesen I. 2002. Improved cellular and humoral immune responses against Mycobacterium tuberculosis antigens after intramuscular DNA immunisation combined with muscle electroporation. Vaccine 20:3370–3378. 10.1016/S0264-410X(02)00289-X [DOI] [PubMed] [Google Scholar]
- 50. Taylor NS, Thorne GM, Bartlett JG. 1981. Comparison of two toxins produced by Clostridium difficile. Infect. Immun. 34:1036–1043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Jawa RS, Mercer DW. 2012. Clostridium difficile-associated infection: a disease of varying severity. Am. J. Surg. 204:836–842. 10.1016/j.amjsurg.2012.05.025 [DOI] [PubMed] [Google Scholar]
- 52. Cunney RJ, Magee C, McNamara E, Smyth EG, Walshe J. 1998. Clostridium difficile colitis associated with chronic renal failure. Nephrol. Dial. Transplant. 13:2842–2846. 10.1093/ndt/13.11.2842 [DOI] [PubMed] [Google Scholar]
- 53. Dobson G, Hickey C, Trinder J. 2003. Clostridium difficile colitis causing toxic megacolon, severe sepsis and multiple organ dysfunction syndrome. Intensive Care Med. 29:1030. 10.1007/s00134-003-1754-7 [DOI] [PubMed] [Google Scholar]
- 54. Jacob SS, Sebastian JC, Hiorns D, Jacob S, Mukerjee PK. 2004. Clostridium difficile and acute respiratory distress syndrome. Heart Lung 33:265–268. 10.1016/j.hrtlng.2004.04.003 [DOI] [PubMed] [Google Scholar]
- 55. Johnson S, Kent SA, O'Leary KJ, Merrigan MM, Sambol SP, Peterson LR, Gerding DN. 2001. Fatal pseudomembranous colitis associated with a variant clostridium difficile strain not detected by toxin A immunoassay. Ann. Intern. Med. 135:434–438. 10.7326/0003-4819-135-6-200109180-00012 [DOI] [PubMed] [Google Scholar]
- 56. Sakurai T, Hajiro K, Takakuwa H, Nishi A, Aihara M, Chiba T. 2001. Liver abscess caused by Clostridium difficile. Scand. J. Infect. Dis. 33:69–70. 10.1080/003655401750064112 [DOI] [PubMed] [Google Scholar]
- 57. Hamm EE, Voth DE, Ballard JD. 2006. Identification of Clostridium difficile toxin B cardiotoxicity using a zebrafish embryo model of intoxication. Proc. Natl. Acad. Sci. U. S. A. 103:14176–14181. 10.1073/pnas.0604725103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Permpoonpattana P, Hong HA, Phetcharaburanin J, Huang JM, Cook J, Fairweather NF, Cutting SM. 2011. Immunization with Bacillus spores expressing toxin A peptide repeats protects against infection with Clostridium difficile strains producing toxins A and B. Infect. Immun. 79:2295–2302. 10.1128/IAI.00130-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. O'Brien JB, McCabe MS, Athie-Morales V, McDonald GS, Ni Eidhin DB, Kelleher DP. 2005. Passive immunisation of hamsters against Clostridium difficile infection using antibodies to surface layer proteins. FEMS Microbiol. Lett. 246:199–205. 10.1016/j.femsle.2005.04.005 [DOI] [PubMed] [Google Scholar]
- 60. Ni Eidhin DB, O'Brien JB, McCabe MS, Athie-Morales V, Kelleher DP. 2008. Active immunization of hamsters against Clostridium difficile infection using surface-layer protein. FEMS Immunol. Med. Microbiol. 52:207–218. 10.1111/j.1574-695X.2007.00363.x [DOI] [PubMed] [Google Scholar]
- 61. Sandolo C, Pechine S, Monnier AL, Hoys S, Janoir C, Coviello T, Alhaique F, Collignon A, Fattal E, Tsapis N. 2011. Encapsulation of Cwp84 into pectin beads for oral vaccination against Clostridium difficile. Eur. J. Pharm. Biopharm. 79:566–573. 10.1016/j.ejpb.2011.05.011 [DOI] [PubMed] [Google Scholar]
- 62. Pechine S, Deneve C, Le Monnier A, Hoys S, Janoir C, Collignon A. 2011. Immunization of hamsters against Clostridium difficile infection using the Cwp84 protease as an antigen. FEMS Immunol. Med. Microbiol. 63:73–81. 10.1111/j.1574-695X.2011.00832.x [DOI] [PubMed] [Google Scholar]
- 63. Seregin SS, Aldhamen YA, Rastall DP, Godbehere S, Amalfitano A. 2012. Adenovirus-based vaccination against Clostridium difficile toxin A allows for rapid humoral immunity and complete protection from toxin A lethal challenge in mice. Vaccine 30:1492–1501. 10.1016/j.vaccine.2011.12.064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Wang H, Sun X, Zhang Y, Li S, Chen K, Shi L, Nie W, Kumar R, Tzipori S, Wang J, Savidge T, Feng H. 2012. A chimeric toxin vaccine protects against primary and recurrent Clostridium difficile infection. Infect. Immun. 80:2678–2688. 10.1128/IAI.00215-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Yang Z, Schmidt D, Liu W, Li S, Shi L, Sheng J, Chen K, Yu H, Tremblay JM, Chen X, Piepenbrink KH, Sundberg EJ, Kelly CP, Bai G, Shoemaker CB, Feng H. 2014. A novel multivalent, single-domain antibody targeting TcdA and TcdB prevents fulminant Clostridium difficile infection in mice. J. Infect. Dis. 210:964–972. 10.1093/infdis/jiu196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kuehne SA, Collery MM, Kelly ML, Cartman ST, Cockayne A, Minton NP. 2014. Importance of toxin A, toxin B, and CDT in virulence of an epidemic Clostridium difficile strain. J. Infect. Dis. 209:83–86. 10.1093/infdis/jit426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Lanis JM, Heinlen LD, James JA, Ballard JD. 2013. Clostridium difficile 027/BI/NAP1 encodes a hypertoxic and antigenically variable form of TcdB. PLoS Pathog. 9:e1003523. 10.1371/journal.ppat.1003523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Stabler RA, He M, Dawson L, Martin M, Valiente E, Corton C, Lawley TD, Sebaihia M, Quail MA, Rose G, Gerding DN, Gibert M, Popoff MR, Parkhill J, Dougan G, Wren BW. 2009. Comparative genome and phenotypic analysis of Clostridium difficile 027 strains provides insight into the evolution of a hypervirulent bacterium. Genome Biol. 10:R102. 10.1186/gb-2009-10-9-r102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kalams SA, Parker SD, Elizaga M, Metch B, Edupuganti S, Hural J, De Rosa S, Carter DK, Rybczyk K, Frank I, Fuchs J, Koblin B, Kim DH, Joseph P, Keefer MC, Baden LR, Eldridge J, Boyer J, Sherwat A, Cardinali M, Allen M, Pensiero M, Butler C, Khan AS, Yan J, Sardesai NY, Kublin JG, Weiner DB. 2013. Safety and comparative immunogenicity of an HIV-1 DNA vaccine in combination with plasmid interleukin 12 and impact of intramuscular electroporation for delivery. J. Infect. Dis. 208:818–829. 10.1093/infdis/jit236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Woodland DL. 2004. Jump-starting the immune system: prime-boosting comes of age. Trends Immunol. 25:98–104. 10.1016/j.it.2003.11.009 [DOI] [PubMed] [Google Scholar]