Abstract
Oritavancin exhibited in vitro activity against 169 strains of vancomycin-susceptible, methicillin-resistant Staphylococcus aureus (MRSA) with MICs ranging from 0.03 to 1 μg/ml and against vancomycin-intermediate MRSA (VISA; n = 29), heterogeneous vancomycin-intermediate MRSA (hVISA; n = 5), and vancomycin-resistant MRSA (n = 5) strains, with MICs ranging from 0.12 to 4 μg/ml. For 10 MRSA isolates comprising 5 VISA and 5 hVISA strains, synergy between oritavancin and gentamicin, linezolid, or rifampin was observed against most of the strains tested using a time-kill method.
TEXT
Methicillin-resistant S. aureus (MRSA) is a global concern, with the majority of strains (and some methicillin-susceptible [MSSA] strains) also exhibiting resistance to currently available quinolones. Furthermore, the appearance and rapid spread of community-associated MRSA in the United States pose clinical challenges. Treatment of MRSA strains in certain types of infections, such as osteomyelitis and bacteremia, with glycopeptides has undoubtedly increased selective pressure, leading to nonsusceptible organisms such as heterogeneous vancomycin-intermediate S. aureus (hVISA), vancomycin-intermediate S. aureus (VISA) (1, 2), and vancomycin-resistant S. aureus (VRSA) (3).
The mechanism of glycopeptide resistance in VISA strains is due to cell wall thickening; some of these strains also exhibit reduced susceptibility to daptomycin (4, 5). Among Hershey MRSA strains isolated between August 2006 and February 2007 from patients that had received prior vancomycin therapy, we determined the incidence of hVISA and VISA to be approximately 1% (1).
Oritavancin, a lipoglycopeptide in late clinical development, has potent in vitro activity against MRSA (6, 7). Synergy between oritavancin and gentamicin, linezolid, or rifampin against 1 methicillin-susceptible strain and 2 MRSA strains (1 VISA and 1 VRSA strain) has been described (8). In this study, we examined the in vitro activities of oritavancin and comparators against 200 recent MRSA isolates. Furthermore, we used time-kill studies to assess synergy between oritavancin and gentamicin, linezolid, or rifampin against 5 VISA and 5 hVISA isolates.
Two hundred three recent MRSA isolates were used in this study, including 127 community-associated organisms (MRSA is defined as community-associated if the MRSA-positive specimen was obtained outside a hospital setting or within 2 days of hospital admission) and 42 hospital-associated organisms isolated from 25 geographically diverse regions in the United States. Two hVISA, 4 VISA, and 1 VRSA isolate were from the Hershey Medical Center. Four VRSA, one hVISA, and 22 VISA isolates were obtained from the Network on Antimicrobial Resistance in Staphylococcus aureus (NARSA) (2). Two hVISA and 127 community-associated MRSA isolates were obtained from JMI Laboratories (Liberty City, IA).
Ten S. aureus strains (5 VISA and 5 hVISA strains) were tested in the time-kill studies. For synergy time-kill testing, which was performed according to CLSI guidelines and as previously described (4, 9, 10), oritavancin was combined with gentamicin, linezolid, or rifampin. Kill kinetics of each drug alone were determined by incubating an initial inoculum of 5 × 105 to 5 × 106 CFU/ml with drug concentrations at the MIC (see Table 2 for isolates' initial MICs used for time-kill testing) and 2 dilutions above (2× and 4× MIC) and 3 dilutions below the MIC (1/2×, 1/4×, and 1/8× MIC). Viability counts were performed at 0, 3, 6, 12, and 24 h of incubation at 35°C in a shaking water bath by plating undiluted and 10-fold serially diluted samples. Concentrations of drugs used in synergy time-kill studies were selected such that one of the two drugs yielded a growth curve similar to that of the drug-free control, while the other drug may have been more active. To prevent drug carryover, aliquots were added to an equal volume of a 25-mg/ml sterile activated charcoal suspension (in 0.9% saline) and then serially diluted (11). Synergy was defined as a ≥2-log10 decrease in CFU/ml between the combination and its most active constituent after 3, 6, 12, and 24 h, with a decrease of ≥2 log10 CFU/ml between the number of surviving organisms and the starting inoculum. Bacteriostatic and bactericidal activities were defined as <3-log10 and ≥3-log10 reductions in CFU/ml, respectively, relative to the starting inoculum. The definitions of synergy and bacteriostatic and bactericidal activities were from the Antimicrobial Agents and Chemotherapy instructions to authors regarding in vitro susceptibility tests (12).
TABLE 2.
MICs for strains tested in time-kill and time-kill synergy assaysa
| Strain | Type | MIC (μg/ml)b |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ORI | GEN | LZD | RIF | VAN | DAP | MIN | Q/D | TEI | T/S | FA | ||
| S. aureus | ||||||||||||
| 504 | VISA | 2 (2) | >16 (256) | 2 (2) | >4 (128) | 4 | 4 | 16 | 0.5 | 8 | 0.12 | ≤0.06 |
| 506 | VISA | 2 (1) | 0.5 (1) | 2 (2) | >4 (256) | 4 | 4 | 0.12 | 0.5 | 8 | 0.12 | ≤0.06 |
| 507 | VISA | 1 (0.5) | >16 (>1,024) | 2 (2) | 2 (4) | 2 | 4 | 4 | 1 | 4 | >4 | ≤0.06 |
| 508 | VISA | 1 (1) | >16 (>1,024) | 2 (2) | 0.008 (0.016) | 4 | 2 | 4 | 0.25 | 8 | >4 | >0.5 |
| 770 | VISA | 0.5 (0.5) | 0.5 (0.5) | 4 (2) | 0.008 (0.016) | 2c | 1 | 0.25 | 1 | 4 | 0.25 | 0.25 |
| 710 | hVISA | 0.5 (0.5) | >16 (512) | 2 (2) | 0.008 (0.016) | 1 | 2 | 16 | 1 | 4 | 0.12 | 0.12 |
| 618 | hVISA | 0.25 (0.25) | ≤0.25 (0.25) | 4 (4) | 0.008 (0.016) | 0.5 | 2 | 0.25 | 1 | 1 | 0.5 | 0.25 |
| 873 | hVISA | 0.25 (0.25) | ≤0.25 (0.25) | 2 (2) | 0.008 (0.03) | 1 | 1 | 0.25 | 1 | 1 | 0.25 | 0.12 |
| 2222 | hVISA | 0.12 (0.12) | 0.5 (1) | 4 (4) | ≤0.004 (0.008) | 0.5 | 1 | 0.25 | 0.5 | 0.5 | 0.5 | 0.12 |
| 2223 | hVISA | 0.25 (0.25) | 16 (8) | 2 (2) | >4 (>1,024) | 1 | 2 | 4 | 1 | 1 | >4 | 0.12 |
| ATCC 29213 | QC | 0.12 (0.12) | 1 (0.5) | 4 (4) | ≤0.004 (0.016) | 0.5 | 1 | 0.5 | 0.25 | 0.5 | 0.12 | 0.12 |
| Enterococcus faecalis ATCC 29212 | QC | 0.03 (0.03) | 16 (8) | 2 (2) | 0.5 (1) | 2 | 8 | 8 | >4 | 8 | 0.06 | >0.5 |
QC, quality control, ORI, oritavancin; GEN, gentamicin; LZD, linezolid; RIF, rifampin; VAN, vancomycin; DAP, daptomycin; MIN, minocycline; Q/D, quinupristin-dalfopristin; TEI, teicoplanin; T/S, trimethoprim-sulfamethoxazole; FA, fusidic acid.
Values are broth microdilution MICs; values in parentheses are macrobroth MICs.
Initial vancomycin susceptibility determination and Etest showed MICs of ≥4 μg/ml, and macro-Etest showed vancomycin-teicoplanin MICs of 6/24 μg/ml.
Drug panels in microtiter trays for susceptibility tests were provided by The Medicines Company (Parsippany, NJ). Broth microdilution and macrodilution MICs were determined in cation-adjusted Muller-Hinton broth (CAMHB; BD Diagnostics, Sparks, MD) according to CLSI guidelines (13, 14); MICs of quality control strains were verified during each determination. For all testing involving oritavancin, CAMHB was supplemented with 0.002% polysorbate-80 (Sigma-Aldrich).
The MIC ranges and MIC50 and MIC90 for all strains tested are presented in Table 1. Oritavancin showed potent activity against 127 community- and 42 hospital-associated vancomycin-susceptible MRSA strains with MICs ranging from 0.03 to 1 μg/ml, and MIC50 and MIC90 values of 0.12 and 0.25 μg/ml, respectively. An identical MIC90 for oritavancin (0.25 μg/ml) was obtained in a prior study of community-associated MRSA from the New York/New Jersey area (7).
TABLE 1.
Microdilution MIC ranges, MIC50s, and MIC90s for tested antimicrobialsa
| Drug | MIC (μg/ml) for (no. of isolates): |
||||||
|---|---|---|---|---|---|---|---|
| Community-acquired MRSA (127) |
Hospital-acquired MRSA (42) |
VISA (24) + hVISA (5) |
VRSA (5)b |
||||
| 50% | 90% | 50% | 90% | 50% | 90% | Range | |
| Oritavancin | 0.12 | 0.25 | 0.12 | 0.25 | 0.5 | 1 | 0.12–1 |
| Vancomycin | 1 | 1 | 1 | 1 | 4 | 8 | 128–>256 |
| Teicoplanin | 0.5 | 0.5 | 0.5 | 1 | 2 | 8 | 2–8 |
| Linezolid | 2 | 2 | 2 | 4 | 2 | 4 | 2 |
| Daptomycin | 0.5 | 0.5 | 0.5 | 1 | 2 | 4 | 0.25–1 |
| Tigecycline | 0.25 | 0.5 | 0.25 | 0.5 | 0.25 | 1 | 0.12–0.5 |
| Quinu/dalfo | 0.5 | 0.5 | 0.5 | 1 | 0.5 | 1 | 0.5–1 |
| Minocycline | 0.12 | 0.12 | 0.06 | 0.12 | 0.12 | 8 | 0.06–1 |
| Rifampin | 0.008 | 0.016 | 0.008 | 0.016 | 1 | >4 | ≤0.004–>4 |
| Trimeth/sulf | 0.12 | 0.25 | 0.12 | 0.5 | 0.5 | >4 | 0.12–>4 |
| Fusidic acid | 0.12 | 0.25 | 0.12 | 0.25 | 0.12 | 0.5 | ≤0.06–0.25 |
MRSA, methicillin-resistant S. aureus; VISA, vancomycin-intermediate S. aureus; hVISA, heterogeneous vancomycin-intermediate S. aureus; VRSA, vancomycin-resistant S. aureus; Quinu/dalfo, quinupristin-dalfopristin; Trimeth/sulf, trimethoprim-sulfamethoxazole.
MIC ranges instead of MIC50 and MIC90 are presented for VRSA isolates, as the number of strains is less than 10.
Against 5 hVISA and 24 VISA strains, oritavancin had an MIC range of 0.12 to 2 μg/ml, with MIC50 and MIC90 values of 0.5 and 1 μg/ml. When tested against five VRSA strains, oritavancin had an MIC range of 0.12 to 1 μg/ml. Seventeen of the 24 VISA strains were nonsusceptible to daptomycin (MIC > 1 μg/ml). Previous reports (5, 15) confirmed higher daptomycin MICs against VISA strains and the propensity of daptomycin therapy to select for such phenotypes. Using CLSI breakpoints (14), 13 (6%), 2 (1.0%), and 12 (6%) of all tested strains were resistant to trimethoprim-sulfamethoxazole, minocycline, and rifampin, respectively.
MIC results by broth micro- and macrodilutions are presented in Table 2. Broth macrodilution MICs were similar to those obtained by broth microdilution. Two strains with gentamicin MICs of >1,024 μg/ml and one strain with a rifampin MIC of >1,024 μg/ml were not tested in synergy time-kill assays in combination with these agents. Also, no VISA isolate from an endocarditis patient treated with daptomycin was tested in the synergy study.
Figures 1 and 2 are graphic representations of synergy observed when oritavancin was combined with each of the tested antimicrobials at subinhibitory concentrations against an hVISA and a VISA strain, respectively. With the combination of oritavancin and gentamicin, synergy was observed against 2 of the 8 strains tested by 3 h (Table 3). By 6 h, synergy was observed against 7 strains. Although synergy was observed for all 8 strains by 12 and 24 h, for 3 of these strains it required concentrations of gentamicin considered too high to be clinically achievable (64 to 128 μg/ml).
FIG 1.
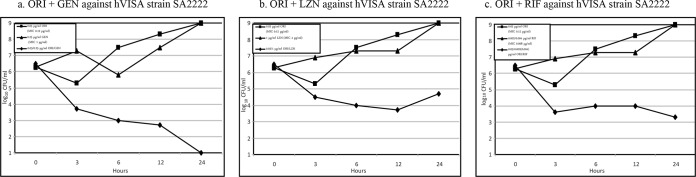
Time-kill curves of synergy at subinhibitory concentrations of oritavancin when combined with each antimicrobial tested for hVISA strain 2222. Abbreviations: ORI, oritavancin; LZD, linezolid; RIF, rifampin.
FIG 2.
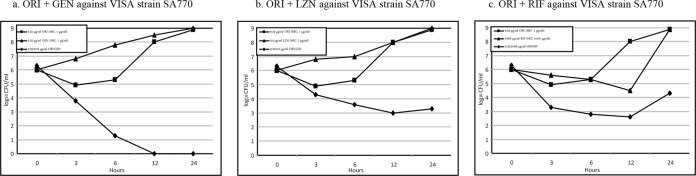
Time-kill curves of synergy at subinhibitory concentrations of oritavancin when combined with each antimicrobial tested for VISA strain 770. Abbreviations: ORI, oritavancin; LZD, linezolid; RIF, rifampin.
TABLE 3.
Synergy time-kill results of oritavancin combined with gentamicin, linezolid, and rifampina
| Strain | Type | MIC (μg/ml)b |
Concn μg/ml at which synergy was observed |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Oritavancin + gentamicin |
Oritavancin + linezolid |
Oritavancin + rifampin |
|||||||||||||||
| ORI | GEN | LZD | RIF | 3 h | 6 h | 12 h | 24 h | 3 h | 6 h | 12 h | 24 h | 3 h | 6 h | 12 h | 24 h | ||
| 504 | VISA | 2 | 256 | 2 | 128 | NS | 1/128 | 0.5/64 | 0.5/64 | NS | NS | 0.5/0.5 | 0.5/0.5 | NS | NS | NS | NS |
| 506 | VISA | 1 | 1 | 2 | 256 | NS | 0.25/0.25 | 0.25/0.25 | 0.25/0.25 | NS | NS | NS | 0.25/1 | NS | NS | NS | NS |
| 507 | VISA | 0.5 | >1,024 | 2 | 4 | NT | NT | NT | NT | NS | NS | 0.25/1 | 0.25/1 | NS | NS | 0.25/1 | 0.12/2 |
| 508 | VISA | 1 | >1,024 | 2 | 0.016 | NT | NT | NT | NT | NS | NS | 0.5/1 | 0.5/1 | NS | NS | 0.5/0.004 | 0.5/0.008 |
| 770 | VISA | 1 | 1 | 2 | 0.016 | NS | 0.25/0.25 | 0.25/0.25 | 0.25/0.25 | NS | NS | 0.25/0.5 | 0.25/0.5 | NS | 0.25/0.008 | NS | 0.25/0.008 |
| 710 | hVISA | 0.5 | 512 | 2 | 0.016 | NS | NS | 0.125/128 | 0.125/128 | NS | NS | NS | 0.25/0.5 | NS | NS | 0.12/0.004 | 0.12/0.008 |
| 618 | hVISA | 0.25 | 1 | 4 | 0.016 | 0.06/0.25 | 0.06/0.25 | 0.06/0.25 | 0.06/0.25 | NS | NS | 0.12/1 | 0.12/1 | 0.06/0.008 | 0.06/0.004 | 0.06/0.004 | 0.06/0.008 |
| 873 | hVISA | 0.5 | 1 | 2 | 0.03 | 0.125/0.5 | 0.125/0.25 | 0.125/0.25 | 0.125/0.25 | NS | NS | 0.12/0.5 | 0.12/0.5 | 0.12/0.008 | 0.12/0.008 | 0.12/0.008 | 0.12/0.008 |
| 2222 | hVISA | 0.12 | 1 | 4 | 0.008 | NS | 0.03/0.25 | 0.03/0.25 | 0.03/0.25 | NS | 0.03/1 | 0.03/1 | 0.03/2 | NS | 0.03/0.002 | 0.03/0.002 | 0.03/0.004 |
| 2223 | hVISA | 0.5 | 32 | 4 | >1,024 | NS | 0.12/8 | 0.12/8 | 0.12/8 | NS | 0.12/1 | 0.12/1 | 0.12/1 | NT | NT | NT | NT |
ORI, oritavancin; GEN, gentamicin; LZD, linezolid; RIF, rifampin; Syn, synergy; VISA, vancomycin-intermediate S. aureus; hVISA, heterogeneous vancomycin-intermediate S. aureus; NS, no synergy; NT, not tested in combination because either the gentamicin or rifampin MIC for the isolate was >1,024 μg/ml.
MIC reading at 24 h of single agents directly from the deep-well plate under conditions used in time-kill assays. These values correspond to 1× MIC in the synergy time-kill experiments.
The combination of oritavancin and linezolid yielded synergy against all 10 strains tested; synergy was observed in 2 strains by 6 h, and the other 8 strains by 12 h (Table 3). For all 10 strains, the linezolid concentration used in the combination was below the CLSI susceptibility breakpoint (≤4 μg/ml) (14).
Oritavancin and rifampin in combination exhibited synergy against 7 of 9 strains tested by 24 h, with synergy observed against 2 and 4 strains by 3 h and 6 h, respectively (Table 3). Against the 7 strains, the rifampin concentration used in the combination was below the CLSI susceptibility breakpoint (≤1 μg/ml) (14).
In summary, oritavancin showed excellent in vitro potency by MIC against all MRSA strains tested, including community- and hospital-associated and vancomycin-susceptible and non-vancomycin-susceptible strains. Only 3 of all 203 strains tested had oritavancin MICs of >1 μg/μl, and all 3 were VISA isolates. Oritavancin also exhibited bactericidal activity against the 5 hVISA and 5 VISA (oritavancin MICs ranging from 0.12 to 2 μg/μl) strains tested by time-kill assays in this study. A similar bactericidal activity of oritavancin against VISA strains has been described previously (8, 16). Synergistic activity of oritavancin in combination with gentamicin or linezolid was observed in all the tested strains. For the combination of oritavancin and rifampin, synergy was observed in 7 of the 9 strains tested. No antagonism was found with any drug combination. Oritavancin concentrations observed in this synergy study were within concentrations predicted to be clinically achievable following a single 1,200-mg dose (17, 18). The marked cross-resistance between oritavancin and daptomycin (19, 20) in non-daptomycin-susceptible VISA isolates was not evaluated in this study.
Antimicrobial combinations that include oritavancin have potential to improve antimicrobial activity against VISA and hVISA isolates.
ACKNOWLEDGMENT
This study was supported by a research grant from The Medicine Company (Parsippany, NJ).
Footnotes
Published ahead of print 14 July 2014
REFERENCES
- 1.Kosowska-Shick K, Ednie LM, McGhee P, Smith K, Todd CD, Wehler A, Appelbaum PC. 2008. Incidence and characteristics of vancomycin nonsusceptible strains of methicillin-resistant Staphylococcus aureus at Hershey Medical Center. Antimicrob. Agents Chemother. 52:4510–4513. 10.1128/AAC.01073-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.BEI Resources. 2014. Network on Antimicrobial Resistance in Staphylococcus aureus (NARSA). http://www.narsa.net/control/member/allapprovedisolates
- 3.Howden BP, Davies JK, Johnson PD, Stinear TP, Grayson ML. 2010. Reduced vancomycin susceptibility in Staphylococcus aureus, including vancomycin-intermediate and heterogeneous vancomycin-intermediate strains: resistance mechanisms, laboratory detection, and clinical implications. Clin. Microbiol. Rev. 23:99–139. 10.1128/CMR.00042-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Credito K, Lin G, Appelbaum PC. 2007. Activity of daptomycin alone and in combination with rifampin and gentamicin against Staphylococcus aureus assessed by time-kill methodology. Antimicrob. Agents Chemother. 51:1504–1507. 10.1128/AAC.01455-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Julian K, Kosowska-Shick K, Whitener C, Roos M, Labischinski H, Rubio A, Parent L, Ednie L, Koeth L, Bogdanovich T, Appelbaum PC. 2007. Characterization of a daptomycin-nonsusceptible vancomycin-intermediate Staphylococcus aureus strain in a patient with endocarditis. Antimicrob. Agents Chemother. 51:3445–3448. 10.1128/AAC.00559-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arhin FF, Draghi DC, Pillar CM, Parr TR, Jr, Moeck G, Sahm DF. 2009. Comparative in vitro activity profile of oritavancin against recent gram-positive clinical isolates. Antimicrob. Agents Chemother. 53:4762–4771. 10.1128/AAC.00952-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arhin FF, Kurepina N, Sarmiento I, Parr TR, Jr, Moeck G, Kreiswirth B. 2010. Comparative in vitro activity of oritavancin against recent, genetically diverse, community-associated methicillin-resistant Staphylococcus aureus (MRSA) isolates. Int. J. Antimicrob. Agents 35:93–94. 10.1016/j.ijantimicag.2009.07.012 [DOI] [PubMed] [Google Scholar]
- 8.Belley A, Neesham-Grenon E, Arhin FF, McKay GA, Parr TR, Jr, Moeck G. 2008. Assessment by time-kill methodology of the synergistic effects of oritavancin in combination with other antimicrobial agents against Staphylococcus aureus. Antimicrob. Agents Chemother. 52:3820–3822. 10.1128/AAC.00361-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin G, Pankuch GA, Ednie LM, Appelbaum PC. 2010. Antistaphylococcal activities of telavancin tested alone and in combination by time-kill assay. Antimicrob. Agents Chemother. 54:2201–2205. 10.1128/AAC.01143-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.National Committee for Clinical Laboratory Standards. 1999. Methods for determining bactericidal activity of antimicrobial agents. Approved guideline M26 NCCLS, Wayne, PA [Google Scholar]
- 11.Arhin FF, Belley A, McKay GA, Moeck G. 2010. Characterization of the in vitro activity of novel lipoglycopeptide antibiotics. Curr. Protoc. Microbiol. Chapter 17:Unit 17.1. 10.1002/9780471729259.mc1701s16 [DOI] [PubMed] [Google Scholar]
- 12.American Society for Microbiology. 2014. Instructions to authors. American Society for Microbiology, Washington, DC [Google Scholar]
- 13.Clinical and Laboratory Standards Institute. 2012. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard M07-A9 Ninth ed. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 14.Clinical and Laboratory Standards Institute. 2012. Performance standards for antimicrobial susceptibility testing. Approved standard M100-S22 Twenty-second informational supplement Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 15.Kaatz GW, Lundstrom TS, Seo SM. 2006. Mechanisms of daptomycin resistance in Staphylococcus aureus. Int. J. Antimicrob. Agents 28:280–287. 10.1016/j.ijantimicag.2006.05.030 [DOI] [PubMed] [Google Scholar]
- 16.McKay GA, Beaulieu S, Arhin FF, Belley A, Sarmiento I, Parr T, Jr, Moeck G. 2009. Time-kill kinetics of oritavancin and comparator agents against Staphylococcus aureus, Enterococcus faecalis and Enterococcus faecium. J. Antimicrob. Chemother. 63:1191–1199. 10.1093/jac/dkp126 [DOI] [PubMed] [Google Scholar]
- 17.Belley A, Arhin FF, Sarmiento I, Deng H, Rose W, Moeck G. 2013. Pharmacodynamics of a simulated single 1200 mg dose of oritavancin in an in vitro pharmacokinetic/pharmacodynamic model of methicillin-resistant Staphylococcus aureus infection. Antimicrob. Agents Chemother. 57:205–211. 10.1128/AAC.01428-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ambrose PG, Drusano GL, Craig WA. 2012. In vivo activity of oritavancin in animal infection models and rationale for a new dosing regimen in humans. Clin. Infect. Dis. 54(Suppl 3):S220–S228. 10.1093/cid/cis001 [DOI] [PubMed] [Google Scholar]
- 19.Saravolatz LD, Pawlak J, Johnson LB. 2010. In vitro activity of oritavancin against community-associated meticillin-resistant Staphylococcus aureus (CA-MRSA), vancomycin-intermediate S. aureus (VISA), vancomycin-resistant S. aureus (VRSA) and daptomycin-non-susceptible S. aureus (DNSSA). Int. J. Antimicrob. Agents 36:69–72. 10.1016/j.ijantimicag.2010.02.023 [DOI] [PubMed] [Google Scholar]
- 20.Arhin FF, Sarmiento I, Parr TR, Jr, Moeck G. 2009. Comparative in vitro activity of oritavancin against Staphylococcus aureus strains that are resistant, intermediate or heteroresistant to vancomycin. J. Antimicrob. Chemother. 64:868–870. 10.1093/jac/dkp286 [DOI] [PubMed] [Google Scholar]


