Abstract
Chloroquine (CQ) is used to treat Plasmodium vivax malaria in areas where CQ resistance has not been reported. The use of artemisinin (ART)-based combination therapies (ACTs) to treat CQ-sensitive P. vivax infections is effective and convenient but may promote the emergence and worsening of ART resistance in sympatric Plasmodium falciparum populations. Here, we show that CQ effectively treats P. vivax malaria in Pursat Province, western Cambodia, where ART-resistant P. falciparum is highly prevalent and spreading. (This study has been registered at ClinicalTrials.gov under registration no. NCT00663546.)
TEXT
Half of the population of Cambodia is at risk of acquiring malaria (1). In response to the emergence of artemisinin (ART)-resistant Plasmodium falciparum in multiple provinces (2–4), Cambodia has developed a strategic plan to combat malaria (5). Its goals are to eliminate ART-resistant P. falciparum by 2015, P. falciparum malaria deaths by 2020, and all forms of malaria (including Plasmodium vivax) by 2025. This approach recognizes that as control measures become more effective, P. vivax will cause an increasingly greater proportion of malaria cases. This is because P. vivax hypnozoites survive blood-stage antimalarials and are not routinely treated with primaquine due to the 14% prevalence of G6PD deficiency in Cambodia (6); therefore, they can cause relapsing malaria (7).
Chloroquine (CQ) is the frontline treatment for P. vivax malaria in areas where resistance has not been reported, while ART-based combination therapies (ACTs) are the recommended treatments in areas where resistance is prevalent (8), especially where ACTs are used as frontline treatments for P. falciparum malaria (9). CQ-resistant P. vivax malaria was first described in Papua New Guinea in 1989 (10) and has since been documented in parts of Southeast Asia and the Americas (11–14). Only two studies have measured the efficacy of CQ in treating P. vivax malaria in Cambodia: Poravuth et al. (15) reported a 98% cure rate in Pailin Province (western Cambodia) in 2007 to 2008, and Leang et al. (16) reported 96%, 88%, and 83% cure rates in Pailin, Preah Vihear (northern Cambodia), and Ratanakiri (eastern Cambodia), respectively, in 2009. In response to the declining efficacy of CQ in Preah Vihear and Ratanakiri, Cambodia changed its frontline treatment for P. vivax malaria from CQ to dihydroartemisinin-piperaquine (DHA-PPQ) in all 24 provinces in 2011. In this study (registered at ClinicalTrials.gov under registration no. NCT00663546), we investigated the efficacy of CQ for treating acute P. vivax malaria in Pursat (western Cambodia), where ART-resistant P. falciparum is highly prevalent (2–4).
We based our study on the standardized World Health Organization (WHO) protocol for assessing the therapeutic response to chloroquine (17). From August 2012 to January 2013, we screened 1,929 individuals seeking treatment for fever at Sampov Meas Referral Hospital in Pursat. Of these, 1,518 patients had a negative rapid diagnostic test (RDT) (First Response [pLDH/HRP2 combo]; Premier Medical Corp. Ltd., India) for malaria parasitemia. Giemsa-stained smears of RDT-positive blood samples showed that 178 patients had P. falciparum infection, 42 had mixed P. falciparum and P. vivax infection, and 191 had P. vivax malaria (monoinfection, any parasite density, and axillary temperature of ≥37.5°C or history of fever in the previous 2 days). Of these 191 patients, 87 met additional inclusion criteria: age of >2 years, hematocrit of ≥25%, and a willingness to complete treatment in the hospital and receive follow-up visits at home. Patients were excluded if they were pregnant or used any antimalarial in the previous 2 months. No patient had signs of severe malaria, malnutrition, concomitant or chronic disease, or reported hypersensitivity to CQ. The adult patients or the parents of the child patients gave written informed consent. The study was approved by the Cambodian National Ethics Committee for Health Research and the National Institute of Allergy and Infectious Diseases (NIAID) institutional review board.
The patient characteristics on admission are presented in Table 1. Most patients were male (79.3%), febrile (≥37.5°C; 86.2%), and reported multiple symptoms: headache (86.2%), body aches (75.9%), malaise (73.6%), vomiting (19.5%), shivering (99.0%), chills (99.0%), and sweats (85.0%). A minority (13.8%) of patients reported experiencing a classic paroxysm. The median hematocrit was 39% (interquartile range [IQR], 35 to 43%). Just prior to the drug administration, the median asexual parasite density was 5,784/μl (IQR, 2,600 to 11,902/μl), and the median gametocyte density was 349/μl (IQR, 112 to 1,131/μl). Only 6 (7%) patients had a parasite density below the WHO-recommended cutoff of 250/μl as an inclusion criterion (17). On admission, the asexual parasite density correlated significantly with axillary temperature (r = 0.4739, P < 0.0001, Spearman correlation test; GraphPad Software, La Jolla, CA) and gametocyte density (r = 0.3945, P = 0.0002). Neither asexual parasite density (r = 0.1916, P = 0.0754) nor gametocyte density (r = 0.1536, P = 0.1555) correlated with age.
TABLE 1.
Baseline characteristics of 87 P. vivax malaria patients
| Parameter | Values |
|---|---|
| Male (%) | 79.3 |
| Age (yr) | |
| Median | 26 |
| IQR | 18–32 |
| Range | 4–68 |
| Wt (kg) | |
| Median | 53 |
| IQR | 46–59 |
| Range | 11–69 |
| Temp (°C) | |
| Median | 38.0 |
| IQR | 38.0–38.5 |
| Range | 37.5–40.0 |
| Hematocrit (%) | |
| Median | 39 |
| IQR | 35–43 |
| Range | 28–54 |
| Asexual parasite density (/μl) | |
| Median | 5,784 |
| IQR | 2,600-11,902 |
| Range | 64–73,333 |
| Gametocyte density (/μl) | |
| Median | 349 |
| IQR | 112-1,131 |
| Range | 0–5,553 |
All patients were hospitalized and treated orally with 10 mg/kg of body weight CQ phosphate (250 mg salt/tablet; Ranbaxy Pharmaceuticals, Jacksonville, FL) at 0 and 24 h, and with 5 mg/kg at 48 h. We specifically withheld primaquine therapy to avoid inducing hemolytic anemia in patients with G6PD deficiency. The axillary temperatures were measured and parasite densities estimated as described previously (2) every 24 h until asexual parasitemia was undetectable (i.e., no asexual parasites observed per 500 leukocytes) on one blood smear instead of two consecutive blood smears, as recommended by the WHO (17). The asexual parasite densities dropped significantly at 24 h (median, 5,784/μl to 32/μl; 99.4% reduction; P < 0.0001, Wilcoxon matched-pairs signed-rank test) (Fig. 1A). The gametocyte densities also dropped at 24 h (median, 349/μl to 16/μl; 96.4% reduction; P < 0.0001) (Fig. 1B). The proportions of patients with asexual parasitemia at 0, 24, 48, and 72 h were 100, 79.3, 4.60, and 0%, respectively; the proportions with gametocytemia were 85.0, 58.6, 11.5, and 2.30%, respectively; and the proportions with fever (≥37.5°C) were 86.2, 21.8, 0, and 0%. Nearly all (90.8%) patients, however, were given paracetamol for fever.
FIG 1.
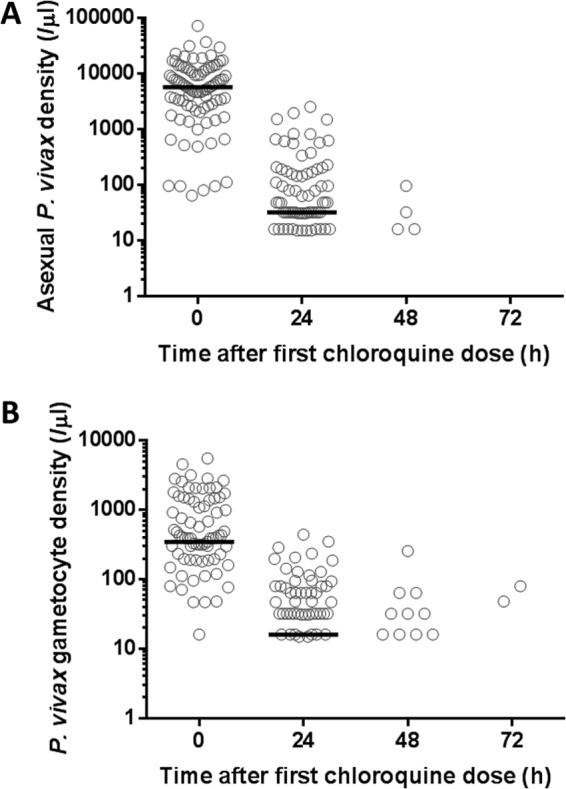
Asexual parasite densities (A) and gametocyte densities (B) following chloroquine treatment at 0, 24, and 48 h. The median values are indicated by the horizontal black lines. (A) There are 18, 65, and 4 zero values at 24, 48, and 72 h, respectively, which are not shown on the logarithmic scale. (B) There are 36, 59, and 2 zero values at 24, 48, and 72 h, respectively, which are also not shown.
The patients were followed up on days 7, 14, 21, and 28. At each visit, their axillary temperature was measured and blood smears examined for recurrent malaria parasitemia. The data from 3 patients who were lost to follow-up were not analyzed, but the data from 8 patients who missed 1, 2, or 3 follow-up visits were analyzed, since all were seen on day 28 and none reported fever since discharge. Among 84 patients with adequate follow-up, 1 had asymptomatic P. vivax parasitemia (60/μl) on day 28, 1 had asymptomatic P. falciparum parasitemia (402/μl) on day 28, and 1 had P. falciparum malaria (16,149/μl, 40.1°C) on day 21. Thus, the cumulative risk of recurrent P. vivax parasitemia at 28 days was 1.2% (1/84). Whether this recurrent parasitemia episode was due to relapse, recrudescence, or reinfection has not been determined.
In Pursat in 2012, our use of RDTs and expert microscopy accurately detected P. vivax monoinfections, which were treated effectively with CQ. The lingering presence of CQ at levels above the minimally effective concentration likely prevented the detection of relapse infections, which have a high probability of occurring within 28 days. In other provinces where ART-resistant P. falciparum is prevalent, programs that accurately diagnose and monitor the clearance of P. vivax monoinfections may help to minimize the use of ACTs. Since the proportion of patients with detectable parasitemia at 72 h was 0%, an increase in this proportion may be a useful phenotypic marker for emerging CQ resistance in Pursat. The day 3 positivity rates, which estimate this proportion, have been associated with CQ resistance in Preah Vihear (3.3%) and Ratanakiri (4.3%), as well as with CQ sensitivity in Pailin (0%) (16). The baseline data for each province might be useful in future surveillance and elimination programs in Cambodia. Nearly all patients had undetectable gametocytemia at 72 h, suggesting that CQ also remains effective in reducing P. vivax transmission in Pursat.
ACKNOWLEDGMENTS
This work was funded by the Intramural Research Program of the NIAID, NIH.
We thank our patients for participating in this study and Sokea Chim, Teang Eam, Robert Gwadz, Pech Eng Ly, Vunsokserey Ou, Koeuth Savuth, Malis Tith, Thomas Wellems, Vanna Yek, and the staff of the Sampov Meas Referral Hospital for their efforts in support of this work.
Footnotes
Published ahead of print 21 July 2014
REFERENCES
- 1.World Health Organization. 2013. World malaria report. World Health Organization, Geneva, Switzerland: http://www.who.int/malaria/publications/world_malaria_report_2013/report/en/ [Google Scholar]
- 2.Amaratunga C, Sreng S, Suon S, Phelps ES, Stepniewska K, Lim P, Zhou C, Mao S, Anderson JM, Lindegardh N, Jiang H, Song J, Su XZ, White NJ, Dondorp AM, Anderson TJ, Fay MP, Mu J, Duong S, Fairhurst RM. 2012. Artemisinin-resistant Plasmodium falciparum in Pursat province, western Cambodia: a parasite clearance rate study. Lancet Infect. Dis. 12:851–858. 10.1016/S1473-3099(12)70181-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ariey F, Witkowski B, Amaratunga C, Beghain J, Langlois AC, Khim N, Kim S, Duru V, Bouchier C, Ma L, Lim P, Leang R, Duong S, Sreng S, Suon S, Chuor CM, Bout DM, Ménard S, Rogers WO, Genton B, Fandeur T, Miotto O, Ringwald P, Le Bras J, Berry A, Barale JC, Fairhurst RM, Benoit-Vical F, Mercereau-Puijalon O, Ménard D. 2014. A molecular marker of artemisinin-resistant Plasmodium falciparum malaria. Nature 505:50–55. 10.1038/nature12876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ashley EA, Dhorda M, Fairhurst RM, Amaratunga C, Lim P, Suon S, Sreng S, Anderson JM, Mao S, Sam B, Sopha C, Char MC, Nguon C, Sovannaroth S, Pukrittayakamee S, Jittamala P, Chotivanich K, Chutasmit K, Suchatsoonthorn C, Runcharoen R, Hien TT, Nguyen TTN, Ngo VT, Nguyen HP, Htut Y, Han KT, Aye KH, Mokuolu OA, Olaosebikan RR, Folaranmi OO, Mayxay M, Khanthavong M, Hongvanthong B, Newton PN, Onyamboko MA, Fanello CI, Tshefu AK, Mishra N, Valecha N, Phyo AP, Nosten F, Yi P, Tripura R, Borrmann S, Bashraheil M, Peshu J, Faiz MA, Ghose A, Hossain MA, Samad R, et al. 2014. Spread of artemisinin resistance in Plasmodium falciparum malaria. N. Engl. J. Med. 371:411–423. 10.1056/NEJMoa1314981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Royal Government of Cambodia. 2011. National strategic plan for elimination of malaria in the Kingdom of Cambodia, 2011–2025. National Malaria Center, Phnom Penh, Cambodia: http://www.cnm.gov.kh/userfiles/file/N%20Strategy%20Plan/National%20Strtegyin%20English.pdf [Google Scholar]
- 6.Khim N, Benedet C, Kim S, Kheng S, Siv S, Leang R, Lek S, Muth S, Chea N, Chuor CM, Duong S, Kerleguer A, Tor P, Chim P, Canier L, Witkowski B, Taylor WR, Ménard D. 2013. G6PD deficiency in Plasmodium falciparum and Plasmodium vivax malaria-infected Cambodian patients. Malar. J. 12:171. 10.1186/1475-2875-12-171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mendis K, Sina BJ, Marchesini P, Carter R. 2001. The neglected burden of Plasmodium vivax malaria. Am. J. Trop. Med. Hyg. 64:97–106 [DOI] [PubMed] [Google Scholar]
- 8.Gogtay N, Kannan S, Thatte UM, Olliaro PL, Sinclair D. 2013. Artemisinin-based combination therapy for treating uncomplicated Plasmodium vivax malaria. Cochrane Database Syst. Rev. 10:CD008492. 10.1002/14651858.CD008492.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization. 2010. Guidelines for the treatment of malaria–2nd edition. World Health Organization, Geneva, Switzerland: http://apps.who.int/medicinedocs/documents/s19105en/s19105en.pdf [Google Scholar]
- 10.Rieckmann KH, Davis DR, Hutton DC. 1989. Plasmodium vivax resistance to chloroquine? Lancet ii:1183–1184 [DOI] [PubMed] [Google Scholar]
- 11.Fryauff DJ, Tuti S, Mardi A, Masbar S, Patipelohi R, Leksana B, Kain KC, Bangs MJ, Richie TL, Baird JK. 1998. Chloroquine-resistant Plasmodium vivax in transmigration settlements of West Kalimantan, Indonesia. Am. J. Trop. Med. Hyg. 59:513–518 [DOI] [PubMed] [Google Scholar]
- 12.Baird JK, Caneta-Miguel E, Masbar S, Bustos DG, Abrenica JA, Layawen AV, Calulut JM, Leksana B, Wignall FS. 1996. Survey of resistance to chloroquine of falciparum and vivax malaria in Palawan, the Philippines. Trans. R Soc. Trop. Med. Hyg. 90:413–414. 10.1016/S0035-9203(96)90528-3 [DOI] [PubMed] [Google Scholar]
- 13.de Santana Filho FS, Arcanjo AR, Chehuan YM, Costa MR, Martinez-Espinosa FE, Vieira JL, Barbosa Md, Alecrim WD, Alecrim Md. 2007. Chloroquine-resistant Plasmodium vivax, Brazilian Amazon. Emerg. Infect. Dis. 13:1125–1126. 10.3201/eid1307.061386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Phyo AP, Lwin KM, Price RN, Ashley EA, Russell B, Sriprawat K, Lindegardh N, Singhasivanon P, White NJ, Nosten F. 2011. Dihydroartemisinin-piperaquine versus chloroquine in the treatment of Plasmodium vivax malaria in Thailand: a randomized controlled trial. Clin. Infect. Dis. 53:977–984. 10.1093/cid/cir631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Poravuth Y, Socheat D, Rueangweerayut R, Uthaisin C, Pyae Phyo A, Valecha N, Rao BH, Tjitra E, Purnama A, Borghini-Fuhrer I, Duparc S, Shin CS, Fleckenstein L. 2011. Pyronaridine-artesunate versus chloroquine in patients with acute Plasmodium vivax malaria: a randomized, double-blind, non-inferiority trial. PLoS One 6:e14501. 10.1371/journal.pone.0014501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leang R, Barrette A, Bouth DM, Menard D, Abdur R, Duong S, Ringwald P. 2013. Efficacy of dihydroartemisinin-piperaquine for treatment of uncomplicated Plasmodium falciparum and Plasmodium vivax in Cambodia, 2008 to 2010. Antimicrob. Agents Chemother. 57:818–826. 10.1128/AAC.00686-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.World Health Organization. 2009. Methods for surveillance of antimalarial drug efficacy. World Health Organization, Geneva, Switzerland: http://whqlibdoc.who.int/publications/2009/9789241597531_eng.pdf [Google Scholar]


