Abstract
Highly active antiretroviral therapy (HAART) involves combination treatment with three or more antiretroviral agents. The antiviral effects of combinations of emtricitabine (FTC) plus tenofovir (TFV) plus antiretroviral agents of all the major drug classes were investigated. Combinations of FTC and TFV with a nonnucleoside reverse transcriptase inhibitor (NNRTI) (efavirenz or rilpivirine) or with a protease inhibitor (PI) (atazanavir, lopinavir, or darunavir) showed additive to synergistic anti-HIV-1 activity. FTC-TFV with an HIV-1 integrase strand transfer inhibitor (INSTI) (elvitegravir or raltegravir) showed the strongest synergy. Anti-HIV-1 synergy suggests enhancement of individual anti-HIV-1 activities within cells that may contribute to potent treatment efficacy and open new areas of research into interactions between reverse transcriptase (RT) and integrase inhibitors.
INTRODUCTION
Highly active antiretroviral therapy (HAART) is the current standard of care for HIV infection and involves treatment with a combination of three or more antiretroviral agents. Generally, these are combinations of two or more drug classes which target different steps of the HIV-1 replication cycle. The most extensively studied anti-HIV-1 drug combinations are those of nucleoside/nucleotide reverse transcriptase (RT) inhibitors (NRTIs) and nonnucleoside RT inhibitors (NNRTIs). NRTIs are competitive inhibitors of HIV-1 RT that cause chain termination of viral DNA polymerization and form the two-drug backbone of most regimens. The third agents are chosen from the different drug classes, consisting of NNRTIs (noncompetitive inhibitors of HIV-1 RT), protease inhibitors (PIs), and integrase strand transfer inhibitors (INSTIs). The first single-tablet regimen containing an INSTI was recently approved and consists of the two NRTIs emtricitabine (FTC) and tenofovir (TFV) disoproxil fumarate (TDF), an oral prodrug of TFV; the INSTI elvitegravir (EVG); and the pharmacoenhancer cobicistat (COBI), which increases EVG concentrations (1).
Combinations of antiviral inhibitors can directly affect the antiviral potency of their counterparts in an additive, antagonistic, or synergistic manner. Determination of the in vitro antiviral interactions between inhibitors used together in patients is an important component of the drug development process. Combinations that show antagonism should be avoided, and combinations that show synergy may have added benefit in vivo. There have been a number of studies showing that combinations of NRTIs plus NNRTIs inhibit HIV-1 infection more efficiently than the additive effect expected for the individual drugs studied alone, thus demonstrating synergy in vitro (2–9). For example, combinations of efavirenz (EFV)-TFV, EFV-FTC, rilpivirine (RPV)-TFV, and RPV-FTC have shown moderate to strong antiviral synergy against HIV-1 in cell culture (3, 10). Studies have also shown that some combinations within a drug class, such as two or more NRTIs, can act synergistically in vitro (11–17). In-depth studies have been performed on the combination of FTC and TFV, and these two drugs show synergy (by median-effect analysis, combination index range of 0.52 to 0.56) to strong synergy (by MacSynergy analysis, synergy volumes of 153 to 181 nM2%) against HIV-1 in cell culture (3, 10). This has been partially explained by a positive metabolic interaction between FTC and TFV that leads to higher levels of phosphorylation to the active metabolites when dosed in combination and more efficient trapping of TFV in a dead-end chain-terminated complex (3, 10, 17). Combinations of NRTIs or NNRTIs with INSTIs have also shown additive to synergistic effects in vitro (18, 19).
As combination therapies are the standard of care in HIV treatment, it is important to understand how newer inhibitors in different classes work in combination with existing therapies. This study evaluates the in vitro anti-HIV activity of three-drug combinations of FTC and TFV plus representatives from all the major drug classes—NNRTIs, PIs, and INSTIs.
MATERIALS AND METHODS
Reagents.
TFV, FTC, EVG, atazanavir (ATV), darunavir (DRV), and COBI were synthesized at Gilead Sciences, Inc. Raltegravir (RAL) was purchased from Naeja Pharmaceutical, Inc. (Edmonton, Alberta, Canada). EFV and lopinavir (LPV) were purchased from Toronto Research Chemicals (North York, Ontario, Canada). RPV was synthesized by Janssen Infectious Diseases BVBA (Beerse, Belgium). Ribavirin (RBV) and zidovudine (AZT) were purchased from Sigma-Aldrich (St. Louis, MO). Stavudine (d4T) was provided by Bristol-Myers Squibb (Princeton, NJ).
Susceptibility assays.
MT-2 cells were obtained from the NIH AIDS Research and Reference Reagent Program and were maintained as described previously (10). The cells were infected with the HIV-1 strain IIIb virus (Advanced Biotechnologies, Columbia, MD) or xxLAI virus (20), as described previously (10). TFV, FTC, EVG, RAL, EFV, RPV, ATV, DRV, LPV, RBV, AZT, and d4T were each tested for effective concentrations that inhibited 50% of viral replication (EC50), determined using the GraphPad Prism (La Jolla, CA). After a 5-day incubation period at 37°C, the virus-induced cytopathic effect was determined using an XTT [2,3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide]-based colorimetric cell viability assay (21).
For the three-drug-combination studies, compounds were combined at a 1:1:1 ratio based on their EC50. Eight concentrations of each combination were assayed in triplicate in 96-well plates, using 1.5-fold serial dilutions.
Drug combination data analysis.
Three-drug-combination data were analyzed according to the median-effect principle of Chou and Talalay (22) using CalcuSyn software (version 2.0, Biosoft, Cambridge, United Kingdom). The EC50s obtained with single antiviral agents were compared to the EC50s obtained with the combination drugs. When studying RBV, which has no antiviral effect against HIV-1, a negligible dose response from 0% to 0.2% inhibition was entered into the software in order to model the synergy or antagonism effects seen with this drug. Virus inhibition values between 5% and 99% were used in the analysis; extrapolated values were not included. A combination index (CI) was calculated from the data as a measure of the interaction among drugs. CI values of <0.9 indicate synergy, CI values of 0.9 to 1.1 indicate an additive effect, and CI values of >1.1 indicate antagonism. The degree of synergy is proportional to the CI value; key values noted in this study showed CIs ranging from 0.3 to 0.7 (representing synergy), 0.7 to 0.85 (representing moderate synergy), and 3.3 to 10 (representing strong antagonism). The reported CI value for a combination of drugs is the average of the CI values at EC50, EC75, and EC90 from each replicate experiment. To graph the data, all CI points from the replicate experiments were plotted on one graph for each combination, and the mean and 95% confidence interval lines were determined from these complete data sets.
RESULTS
Three-drug combinations of TFV, FTC, and other antiretroviral agents showed antiviral additivity or synergy against HIV-1 in cell culture.
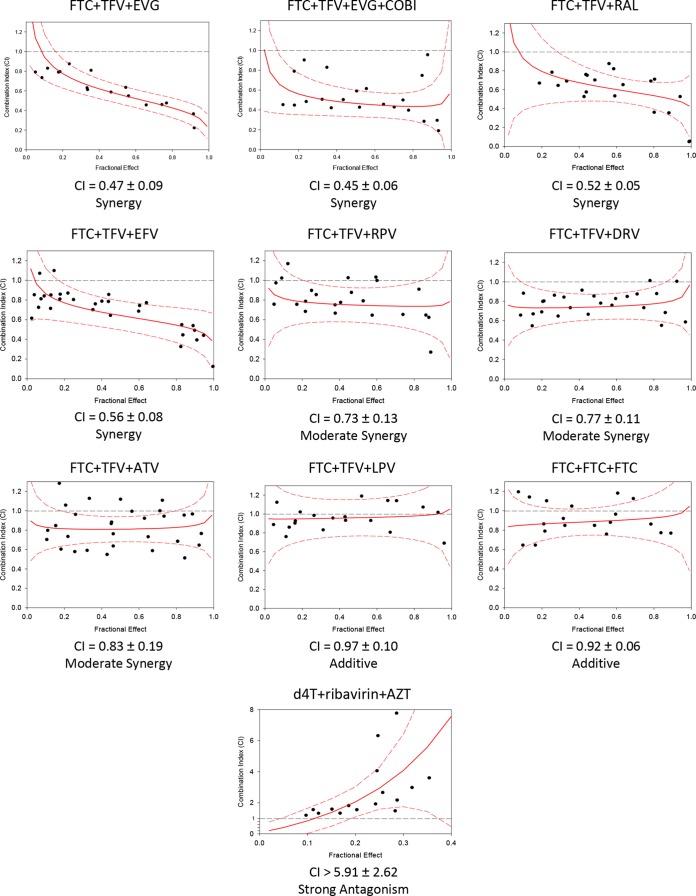
Three-drug-combination studies were performed with FTC-TFV plus representative agents of the other major drug classes—NNRTIs, PIs, and INSTIs (Fig. 1). The two NNRTIs studied were EFV and RPV. The EFV-FTC-TFV combination showed synergy as expected, with a CI value of 0.56 ± 0.08 (3). The RPV-FTC-TFV combination showed moderate synergy (CI, 0.73 ± 0.13) that was not statistically different from the EFV-FTC-TFV combination.
FIG 1.
Three-drug combination and control results. Three-drug antiviral synergy plots of the combination index (CI; synergy score) versus the fractional effect (level of inhibition of viral replication). Dotted black line, additivity line indicating a CI value of 1; solid red line, mean synergy curve fit line; dotted red line, 95% confidence interval. CI values were calculated as described in Materials and Methods, using CalcuSyn. These numbers represent the mean and standard deviation of at least three independent experiments.
The three PIs studied were DRV, ATV, and LPV. The combinations of DRV-FTC-TFV and ATV-FTC-TFV both showed moderate synergy, with CI values of 0.77 ± 0.11 and 0.83 ± 0.19, respectively. The LPV-FTC-TFV combination showed additivity, with a CI value of 0.97 ± 0.10.
The INSTIs studied were EVG and RAL. The EVG-FTC-TFV combination showed synergy, with a mean CI value of 0.47 ± 0.09. The RAL-FTC-TFV combination was tested in parallel and also showed synergy, with a mean CI value of 0.52 ± 0.05. The single-tablet regimen containing EVG, COBI, FTC, and TDF also contains a pharmacokinetic enhancer, COBI, which lacks antiviral activity; the in vitro EVG-FTC-TFV combination was tested with an overlay of 25 μM COBI to determine whether COBI altered the antiviral activity of the triple combination. This combination showed synergy comparable to that of the EVG-FTC-TFV combination, with a mean CI value of 0.45 ± 0.06. The EVG-FTC-TFV combination was significantly more synergistic than the RPV-FTC-TFV combination (P = 0.03) but was only numerically more synergistic than the EFV-FTC-TFV combination (P = 0.15). The EVG-FTC-TFV combination was significantly more synergistic than all three of the combinations of FTC-TFV with PIs (P = 0.013 for ATV, P = 0.009 for DRV, and P = 0.002 for LPV). We also carried out the isobologram analysis (23, 24) for the combinations of FTC-TFV with the representative NNRTIs, PIs, and INSTIs, and found similar synergy results (data not shown).
Control experiments combining FTC, FTC, and FTC yielded the expected additivity, with a mean CI value of 0.92 ± 0.06. The d4T-AZT-RBV combination was tested as an antagonism control, since previous reports have shown RBV-d4T to be strongly antagonistic (25, 26), RBV-AZT to be strongly antagonistic (25), and d4T-AZT to be additive to antagonistic (27, 28). The triple combination d4T-AZT-RBV showed strong antagonism, with a mean CI value of >5.9.
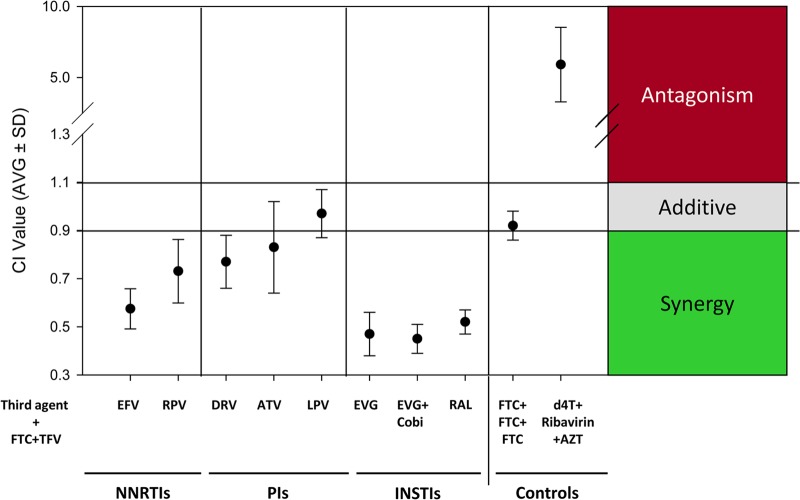
Overall, the combination of TFV-FTC and the INSTIs showed the highest level of synergy, with no evidence of antagonism (Fig. 2). The combinations of FTC-TFV with antiretroviral agents from the NNRTI and PI classes showed synergy or additivity, with no evidence of antagonism.
FIG 2.
Summary of the FTC-TFV three-drug antiviral combinations. Overall CI values of the FTC-TFV three-drug antiviral combinations and controls are shown. CI values of <0.9 indicate synergy; CI values of 0.9 to 1.1 indicate additivity, and CI values of >1.1 indicate antagonism. The numbers represent the mean and standard deviation (SD) of at least three independent experiments.
DISCUSSION
This study investigated the anti-HIV-1 activity of three-drug combinations consisting of FTC and TFV plus a third antiretroviral agent from one of the three major drug classes—NNRTIs, PIs, and INSTIs. The three-drug combination of FTC-TFV with the NNRTIs EFV and RPV or the PIs DRV and ATV all showed antiviral synergy in cell culture. Additive antiviral activity was found for FTC-TFV and LPV. The strongest synergy observed was with the combinations of FTC-TFV with the INSTIs EVG and RAL. Synergy between the NRTI backbone FTC-TFV has been attributed to elevations in the levels of the active metabolites of FTC and TFV when dosed together in cells and to enhanced dead-end complex formation of TFV-terminated DNA and HIV-1 RT in the presence of FTC-triphosphate (TP) (3, 17).
Three-drug combinations of FTC-TFV with two agents from the INSTI class showed comparable and high-level synergy. EVG and RAL have a similar mechanism of action of inhibiting integrase (IN) strand transfer activity, which is required for the integration of HIV-1 DNA into the host genome (29). Previous studies have shown additive to synergistic inhibition of HIV-1 infection using a combination of the integrase inhibitor RAL plus FTC or TFV (30) and EVG plus FTC or TFV (19). Therefore, synergy in vitro was not unexpected.
Clinical studies of INSTIs with or without FTC/TDF have shown durable efficacy (31–39). The INSTIs as a class and by themselves elicit a more rapid viral load decline than that observed for any other drug class. Monotherapies with the EVG or RAL INSTI have shown significant drops in viral load over 10 days, with average decreases of about 2.2 log10 HIV-1 RNA copies/ml (31, 32). Further, when dosed in a combination study of treatment-naive subjects receiving tenofovir and lamivudine (3TC) as a background regimen, subjects taking RAL had a more rapid decline in HIV-1 RNA than subjects taking the EFV–3TC–TDF regimen, although both arms reached the same reduction in viral load by week 24 (40). Clinical studies comparing EVG-COBI-FTC-TDF with EFV-FTC-TDF or ATV-ritonavir-FTC/TDF had the similar result of a transiently more rapid viral load suppression (36, 37). The finding in this study that NRTIs with INSTIs showed the highest level of synergy may contribute to the clinical efficacy of INSTIs with FTC-TDF.
Potential underlying mechanisms of rapid HIV-1 RNA decreases with INSTIs have been proposed (41, 42). The most widely accepted model is that the INSTIs uniquely block HIV-1 replication in cells that have been infected and have produced the viral double-stranded DNA genome but have not yet undergone integration into the host genome (preintegration latency) (41). The first-phase viral load decay rate is longer for INSTIs than for NNRTIs (43), potentially because they act later in the HIV-1 replication cycle (42). Although INSTIs show a rapid viral load decline in monotherapy studies, and the synergy of FTC with TFV is well established, the finding that INSTIs combined with NRTIs showed the highest levels of synergy does not appear solely attributable to simply a combination of two classes of drugs since the NRTIs with PIs were significantly less synergistic. RT and IN are both present in HIV-1 preintegration complexes (PICs) (44), so there may be an interaction between these two enzymes or the drugs that target them. Studies have shown that the IN protein is required for efficient reverse transcription and may interact physically with RT or other components of the RT initiation complex (45, 46). Other studies with noncatalytic site integrase inhibitors (NCINIs) showed that these inhibitors block viral replication by targeting an IN-dependent step during virus production that subsequently causes a defect in reverse transcription in newly infected target cells (47, 48). Additionally, the INSTI drug to HIV-1 double-stranded DNA target ratio may be increased in the presence of NRTIs leading to better inhibition of the virus with INSTIs. This study supports these observations and hypotheses by showing that combinations of FTC-TFV with INSTIs show synergy greater than the combinations of FTC-TFV with other antiretroviral drug classes. These results open new areas of research into interactions between RT and integrase inhibitors, including other possible mechanisms of action to account for the high synergy observed between NRTIs and INSTIs.
In summary, the combinations of FTC-TFV with a third agent from one of the major drug classes, INSTIs, NNRTIs, or PIs, all showed additive to synergistic anti-HIV-1 activity in vitro. The strongest synergy was seen with the combinations of EVG or RAL with FTC-TFV, and this may contribute to the durable clinical antiviral efficacy observed for these drug regimens, which are both recommended as preferred INSTI-based regimens for ART-naive patients (49).
ACKNOWLEDGMENTS
R.K., R.H., D.M.M., M.D.M., and K.L.W. are or were employees of Gilead Sciences, Inc.
We have no other conflicts of interest to disclose.
Footnotes
Published ahead of print 4 August 2014
REFERENCES
- 1.Gilead Sciences Limited. September 2013. Stribild 150 mg/150 mg/200 mg/245 mg film-coated tablets. Summary of product characteristics. Gilead Sciences Limited, County Cork, Ireland: http://www.gilead.com/~/media/Files/pdfs/medicines/hiv/stribild/stribild_pi.pdf [Google Scholar]
- 2.Schader SM, Colby-Germinario SP, Schachter JR, Xu H, Wainberg MA. 2011. Synergy against drug-resistant HIV-1 with the microbicide antiretrovirals, dapivirine and tenofovir, in combination. AIDS 25:1585–1594. 10.1097/QAD.0b013e3283491f89 [DOI] [PubMed] [Google Scholar]
- 3.Feng JY, Ly JK, Myrick F, Goodman D, White KL, Svarovskaia ES, Borroto-Esoda K, Miller MD. 2009. The triple combination of tenofovir, emtricitabine and efavirenz shows synergistic anti-HIV-1 activity in vitro: a mechanism of action study. Retrovirology 6:44. 10.1186/1742-4690-6-44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Richman D, Rosenthal AS, Skoog M, Eckner RJ, Chou TC, Sabo JP, Merluzzi VJ. 1991. BI-RG-587 is active against zidovudine-resistant human immunodeficiency virus type 1 and synergistic with zidovudine. Antimicrob. Agents Chemother. 35:305–308. 10.1128/AAC.35.2.305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pauwels R, Andries K, Debyser Z, Kukla MJ, Schols D, Breslin HJ, Woestenborghs R, Desmyter J, Janssen MA, De Clercq E. 1994. New tetrahydroimidazo[4,5,1-jk][1,4]-benzodiazepin-2(1H)-one and -thione derivatives are potent inhibitors of human immunodeficiency virus type 1 replication and are synergistic with 2′,3′-dideoxynucleoside analogs. Antimicrob. Agents Chemother. 38:2863–2870. 10.1128/AAC.38.12.2863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brennan TM, Taylor DL, Bridges CG, Leyda JP, Tyms AS. 1995. The inhibition of human immunodeficiency virus type 1 in vitro by a non-nucleoside reverse transcriptase inhibitor MKC-442, alone and in combination with other anti-HIV compounds. Antiviral Res. 26:173–187. 10.1016/0166-3542(94)00074-I [DOI] [PubMed] [Google Scholar]
- 7.Chong KT, Pagano PJ. 1997. Inhibition of human immunodeficiency virus type 1 infection in vitro by combination of delavirdine, zidovudine and didanosine. Antiviral Res. 34:51–63. 10.1016/S0166-3542(96)01021-2 [DOI] [PubMed] [Google Scholar]
- 8.Buckheit RW, Jr, Watson K, Fliakas-Boltz V, Russell J, Loftus TL, Osterling MC, Turpin JA, Pallansch LA, White EL, Lee JW, Lee SH, Oh JW, Kwon HS, Chung SG, Cho EH. 2001. SJ-3366, a unique and highly potent nonnucleoside reverse transcriptase inhibitor of human immunodeficiency virus type 1 (HIV-1) that also inhibits HIV-2. Antimicrob. Agents Chemother. 45:393–400. 10.1128/AAC.45.2.393-400.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.King RW, Klabe RM, Reid CD, Erickson-Viitanen SK. 2002. Potency of nonnucleoside reverse transcriptase inhibitors (NNRTIs) used in combination with other human immunodeficiency virus NNRTIs, NRTIs, or protease inhibitors. Antimicrob. Agents Chemother. 46:1640–1646. 10.1128/AAC.46.6.1640-1646.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kulkarni R, Feng JY, Miller MD, White KL. 2014. Dead-end complexes contribute to the synergistic inhibition of HIV-1 RT by the combination of rilpivirine, emtricitabine, and tenofovir. Antiviral Res. 101:131–135. 10.1016/j.antiviral.2013.11.010 [DOI] [PubMed] [Google Scholar]
- 11.Dornsife RE, St Clair MH, Huang AT, Panella TJ, Koszalka GW, Burns CL, Avertett DR. 1991. Anti-human immunodeficiency virus synergism by zidovudine (3′-azidothymidine) and didanosine (dideoxyinosine) contrasts with their additive inhibition of normal human marrow progenitor cells. Antimicrob. Agents Chemother. 35:322–328. 10.1128/AAC.35.2.322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson VA, Merrill DP, Videler JA, Chou TC, Byington RE, Eron JJ, D'Aquila RT, Hirsch MS. 1991. Two-drug combinations of zidovudine, didanosine, and recombinant alpha interferon A inhibit replication of zidovudine-resistant human immunodeficiency virus type 1 synergistically in vitro. J. Infect. Dis. 164:646–655. 10.1093/infdis/164.4.646 [DOI] [PubMed] [Google Scholar]
- 13.Parker WB, Shaddix SC, Bowdon BJ, Rose LM, Vince R, Shannon WM, Bennett LL., Jr 1993. Metabolism of carbovir, a potent inhibitor of human immunodeficiency virus type 1, and its effects on cellular metabolism. Antimicrob. Agents Chemother. 37:1004–1009. 10.1128/AAC.37.5.1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eron JJ, Jr, Johnson VA, Merrill DP, Chou TC, Hirsch MS. 1992. Synergistic inhibition of replication of human immunodeficiency virus type 1, including that of a zidovudine-resistant isolate, by zidovudine and 2′,3′-dideoxycytidine in vitro. Antimicrob. Agents Chemother. 36:1559–1562. 10.1128/AAC.36.7.1559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bridges EG, Dutschman GE, Gullen EA, Cheng Y-C. 1996. Favorable interaction of β-l(-) nucleoside analogues with clinically approved anti-HIV nucleoside analogues for the treatment of human immunodeficiency virus. Biochem. Pharmacol. 51:731–736. 10.1016/0006-2952(96)00056-1 [DOI] [PubMed] [Google Scholar]
- 16.Mulato AS, Cherrington JM. 1997. Anti-HIV activity of adefovir (PMEA) and PMPA in combination with antiretroviral compounds: in vitro analyses. Antiviral Res. 36:91–97. 10.1016/S0166-3542(97)00043-0 [DOI] [PubMed] [Google Scholar]
- 17.Borroto-Esoda K, Vela JE, Myrick F, Ray AS, Miller MD. 2006. In vitro evaluation of the anti-HIV activity and metabolic interactions of tenofovir and emtricitabine. Antivir. Ther. 11:377–384 [PubMed] [Google Scholar]
- 18.Kobayashi M, Yoshinaga T, Seki T, Wakasa-Morimoto C, Brown KW, Ferris R, Foster SA, Hazen RJ, Miki S, Suyama-Kagitani A, Kawauchi-Miki S, Taishi T, Kawasuji T, Johns BA, Underwood MR, Garvey EP, Sato A, Fujiwara T. 2011. In vitro antiretroviral properties of S/GSK1349572, a next-generation HIV integrase inhibitor. Antimicrob. Agents Chemother. 55:813–821. 10.1128/AAC.01209-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ledford RM, Margot NA, Miller MD, McColl DJ. 2007. Elvitegravir (GS-9137/JTK-303), an HIV-1 integrase inhibitor, has additive to synergistic interactions with antiretroviral drugs in vitro, poster MOPEA052. Abstr. 4th International AIDS Society Conference on HIV Pathogenesis, Treatment, and Prevention, Sydney, Australia [Google Scholar]
- 20.Shi C, Mellors JW. 1997. A recombinant retroviral system for rapid in vivo analysis of human immunodeficiency virus type 1 susceptibility to reverse transcriptase inhibitors. Antimicrob. Agents Chemother. 41:2781–2785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weislow OS, Kiser R, Fine DL, Bader J, Shoemaker RH, Boyd MR. 1989. New soluble-formazan assay for HIV-1 cytopathic effects: application to high-flux screening of synthetic and natural products for AIDS-antiviral activity. J. Natl. Cancer Inst. 81:577–586. 10.1093/jnci/81.8.577 [DOI] [PubMed] [Google Scholar]
- 22.Chou T-C, Talalay P. 1984. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv. Enzyme Regul. 22:27–55. 10.1016/0065-2571(84)90007-4 [DOI] [PubMed] [Google Scholar]
- 23.Elion GB, Singer S, Hitchings GH. 1954. Antagonists of nucleic acid derivatives. VIII. Synergism in combinations of biochemically related antimetabolites. J. Biol. Chem. 208:477–488 [PubMed] [Google Scholar]
- 24.Selleseth DW, Talarico CL, Miller T, Lutz MW, Biron KK, Harvey RJ. 2003. Interactions of 1263W94 with other antiviral agents in inhibition of human cytomegalovirus replication. Antimicrob. Agents Chemother. 47:1468–1471. 10.1128/AAC.47.4.1468-1471.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Margot NA, Miller MD. 2005. In vitro combination studies of tenofovir and other nucleoside analogues with ribavirin against HIV-1. Antivir. Ther. 10:343–348 [PubMed] [Google Scholar]
- 26.Baba M, Pauwels R, Balzarini J, Herdewijn P, De Clercq E, Desmyter J. 1987. Ribavirin antagonizes inhibitory effects of pyrimidine 2′,3′-dideoxynucleosides but enhances inhibitory effects of purine 2′,3′-dideoxynucleosides on replication of human immunodeficiency virus in vitro. Antimicrob. Agents Chemother. 31:1613–1617. 10.1128/AAC.31.10.1613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deminie CA, Bechtold CM, Stock D, Alam M, Djang F, Balch AH, Chou TC, Prichard M, Colonno RJ, Lin PF. 1996. Evaluation of reverse transcriptase and protease inhibitors in two-drug combinations against human immunodeficiency virus replication. Antimicrob. Agents Chemother. 40:1346–1351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhu QY, Scarborough A, Polsky B, Chou TC. 1996. Drug combinations and effect parameters of zidovudine, stavudine, and nevirapine in standardized drug-sensitive and resistant HIV type 1 strains. AIDS Res. Hum. Retroviruses 12:507–517. 10.1089/aid.1996.12.507 [DOI] [PubMed] [Google Scholar]
- 29.Hazuda D, Iwamoto M, Wenning L. 2009. Emerging pharmacology: inhibitors of human immunodeficiency virus integration. Annu. Rev. Pharmacol. Toxicol. 49:377–394. 10.1146/annurev.pharmtox.011008.145553 [DOI] [PubMed] [Google Scholar]
- 30.Miller M, Witmer M, Stillmock K, Felock P, Ecto L, Flynn J, Schleif W, Dornadula G, Danovich R, Hazuda D. 2006. Biochemical and antiviral activity of MK-0518, a potent HIV integrase inhibitor, poster THAA0302. Abstr. 16th International AIDS Society Conference, Toronto, Canada [Google Scholar]
- 31.DeJesus E, Berger D, Markowitz M, Cohen C, Hawkins T, Ruane P, Elion R, Farthing C, Zhong L, Cheng AK, McColl D, Kearney BP. 2006. Antiviral activity, pharmacokinetics, and dose response of the HIV-1 integrase inhibitor GS-9137 (JTK-303) in treatment-naive and treatment-experienced patients. J. Acquir. Immune Defic. Syndr. 43:1–5. 10.1097/01.qai.0000233308.82860.2f [DOI] [PubMed] [Google Scholar]
- 32.Grinsztejn B, Nguyen BY, Katlama C, Gatell JM, Lazzarin A, Vittecoq D, Gonzalez CJ, Chen J, Harvey CM, Isaacs RD. 2007. Safety and efficacy of the HIV-1 integrase inhibitor raltegravir (MK-0518) in treatment-experienced patients with multidrug-resistant virus: a phase II randomised controlled trial. Lancet 369:1261–1269. 10.1016/S0140-6736(07)60597-2 [DOI] [PubMed] [Google Scholar]
- 33.Steigbigel RT, Cooper DA, Kumar PN, Eron JE, Schechter M, Markowitz M, Loutfy MR, Lennox JL, Gatell JM, Rockstroh JK, Katlama C, Yeni P, Lazzarin A, Clotet B, Zhao J, Chen J, Ryan DM, Rhodes RR, Killar JA, Gilde LR, Strohmaier KM, Neibohm AR, Miller MD, Hazuda DJ, Nessly ML, DiNubile MJ, Isaacs RD, Nguyen BY, Teppler H. 2008. Raltegravir with optimized background therapy for resistant HIV-1 infection. N. Engl. J. Med. 359:339–353. 10.1056/NEJMoa0708975 [DOI] [PubMed] [Google Scholar]
- 34.Cooper DA, Steigbigel RT, Gatell J, Rockstroh J, Katlama C, Yeni P, Lazzarin A, Clotet B, Kumar P, Eron JE, Schechter M, Markowitz M, Loutfy MR, Lennox JL, Zhao J, Chen J, Ryan DM, Rhodes RR, Killar JA, Gilde LR, Strohmaier KM, Meibohm AR, Miller MD, Hazuda DJ, Nessly ML, DiNubile MJ, Isaacs RD, Teppler H, Nguyen B. 2008. Subgroup and resistance analyses of raltegravir for resistance HIV-1 infection. N. Engl. J. Med. 359:355–365. 10.1056/NEJMoa0708978 [DOI] [PubMed] [Google Scholar]
- 35.Lennox JL, DeJesus E, Lazzarin A, Pollard RB, Madruga JV, Berger DS, Zhao J, Xu X, Williams-Diaz A, Rodgers AJ, Barnard RJ, Miller MD, DiNubile MJ, Nguyen BY, Leavitt R, Sklar P. 2009. Safety and efficacy of raltegravir-based versus efavirenz-based combination therapy in treatment-naive patients with HIV-1 infection: a multicentre, double-blind randomised controlled trial. Lancet 374:796–806. 10.1016/S0140-6736(09)60918-1 [DOI] [PubMed] [Google Scholar]
- 36.Sax PE, DeJesus E, Mills A, Zolopa A, Cohen C, Wohl D, Gallant JE, Liu HC, Zhong L, Yale K, White K, Kearney BP, Szwarcberg J, Quirk E, Cheng AK. 2012. Co-formulated elvitegravir, cobicistat, emtricitabine, and tenofovir versus co-formulated efavirenz, emtricitabine, and tenofovir for initial treatment of HIV-1 infection: a randomised, double-blind, phase 3 trial, analysis of results after 48 weeks. Lancet 379:2439–2448. 10.1016/S0140-6736(12)60917-9 [DOI] [PubMed] [Google Scholar]
- 37.DeJesus E, Rockstroh J, Henry K, Molina J-M, Gathe J, Ramanathan S, Wei X, Yale K, Szwarcberg J, White K, Cheng AK, Kearney BP, GS-236-0103 Study Team 2012. Co-formulated elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate versus ritonavir-boosted atazanavir plus co-formulated emtricitabine and tenofovir disoproxil fumarate for initial treatment of HIV-1 infection: a randomised, double-blind, phase 3, non-inferiority trial. Lancet 379:2429–2438. 10.1016/S0140-6736(12)60918-0 [DOI] [PubMed] [Google Scholar]
- 38.Wohl DA, Cohen C, Gallant JE, Mills A, Sax PE, Dejesus E, Zolopa A, Liu HC, Plummer A, White KL, Cheng AK, Rhee MS, Szwarcberg J. 2014. A randomized, double-blind comparison of single-tablet regimen elvitegravir/cobicistat/emtricitabine/tenofovir DF versus single-tablet regimen efavirenz/emtricitabine/tenofovir DF for initial treatment of HIV-1 infection: analysis of week 144 results. J. Acquir. Immune Defic. Syndr. 65:e118–e121. 10.1097/QAI.0000000000000057 [DOI] [PubMed] [Google Scholar]
- 39.Clumeck N, Molina JM, Henry K, Gathe J, Rockstroh JK, Dejesus E, Wei X, White K, Fordyce MW, Rhee MS, Szwarcberg J. 2014. A randomized, double-blind comparison of single-tablet regimen elvitegravir/cobicistat/emtricitabine/tenofovir DF vs ritonavir-boosted atazanavir plus emtricitabine/tenofovir DF for initial treatment of HIV-1 infection: analysis of week 144 results. J. Acquir. Immune Defic. Syndr. 65:e121–e124. 10.1097/QAI.0000000000000089 [DOI] [PubMed] [Google Scholar]
- 40.Markowitz M, Nguyen BY, Gotuzzo E, Mendo F, Ratanasuwan W, Kovacs C, Prada G, Morales-Ramirez JO, Crumpacker CS, Isaacs RD, Gilde LR, Wan H, Miller MD, Wenning LA, Teppler H. 2007. Rapid and durable antiretroviral effect of the HIV-1 integrase inhibitor raltegravir as part of combination therapy in treatment-naive patients with HIV-1 infection: results of a 48-week controlled study. J. Acquir. Immune Defic. Syndr. 46:125–133. 10.1097/QAI.0b013e318157131c [DOI] [PubMed] [Google Scholar]
- 41.Murray JM, Emery S, Kelleher AD, Law M, Chen J, Hazuda DJ, Nguyen BY, Teppler H, Cooper DA. 2007. Antiretroviral therapy with the integrase inhibitor raltegravir alters decay kinetics of HIV, significantly reducing the second phase. AIDS 21:2315–2321. 10.1097/QAD.0b013e3282f12377 [DOI] [PubMed] [Google Scholar]
- 42.Sedaghat AR, Dinoso JB, Shen L, Wilke CO, Siliciano RF. 2008. Decay dynamics of HIV-1 depend on the inhibited stages of the viral life cycle. Proc. Natl. Acad. Sci. U. S. A. 105:4832–4837. 10.1073/pnas.0711372105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Andrade A, Rosenkranz SL, Cillo AR, Lu D, Daar ES, Jacobson JM, Lederman M, Acosta EP, Campbell T, Feinberg J, Flexner C, Mellors JW, Kuritzkes DR. 2013. Three distinct phases of HIV-1 RNA decay in treatment-naive patients receiving raltegravir-based antiretroviral therapy: ACTG A5248. J. Infect. Dis. 208:884–891. 10.1093/infdis/jit272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miller MD, Farnet CM, Bushman FD. 1997. Human immunodeficiency virus type 1 preintegration complexes: studies of organization and composition. J. Virol. 71:5382–5390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu X, Liu H, Xiao H, Conway JA, Hehl E, Kalpana GV, Prasad V, Kappes JC. 1999. Human immunodeficiency virus type 1 integrase protein promotes reverse transcription through specific interactions with the nucleoprotein reverse transcription complex. J. Virol. 73:2126–2135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhu K, Dobard C, Chow SA. 2004. Requirement for integrase during reverse transcription of human immunodeficiency virus type 1 and the effect of cysteine mutations of integrase on its interactions with reverse transcriptase. J. Virol. 78:5045–5055. 10.1128/JVI.78.10.5045-5055.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Balakrishnan M, Yant SR, Tsai L, O'Sullivan C, Bam RA, Tsai A, Niedziela-Majka A, Stray KM, Sakowicz R, Cihlar T. 2013. Non-catalytic site HIV-1 integrase inhibitors disrupt core maturation and induce a reverse transcription block in target cells. PLoS One 8:e74163. 10.1371/journal.pone.0074163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jurado KA, Wang H, Slaughter A, Feng L, Kessl JJ, Koh Y, Wang W, Ballandras-Colas A, Patel PA, Fuchs JR, Kvaratskhelia M, Engelman A. 2013. Allosteric integrase inhibitor potency is determined through the inhibition of HIV-1 particle maturation. Proc. Natl. Acad. Sci. U. S. A. 110:8690–8695. 10.1073/pnas.1300703110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Department of Health and Human Services (DHHS). 2013. Recommendation on integrase inhibitor use in antiretroviral treatment-naive HIV infected individuals from the HHS Panel on Antiretroviral Guidelines for Adults and Adolescents (October 30, 2013). http://aidsinfo.nih.gov/news/1392/hhs-panel-on-antiretroviral-guidelines-for-adults-and-adolescents-updates-recommendations-on-preferred-insti-based-regimens-for-art-naive-individuals