Abstract
Nemonoxacin (TG-873870) is a novel nonfluorinated quinolone with potent broad-spectrum activity against Gram-positive, Gram-negative, and atypical pathogens, including vancomycin-nonsusceptible methicillin-resistant Staphylococcus aureus (MRSA), quinolone-resistant MRSA, quinolone-resistant Streptococcus pneumoniae, penicillin-resistant S. pneumoniae, and erythromycin-resistant S. pneumoniae. This first-in-human study was aimed at assessing the safety, tolerability, and pharmacokinetic properties of intravenous nemonoxacin in healthy Chinese volunteers. The study comprised a randomized, double-blind, placebo-controlled, dose escalating safety and tolerability study in 92 subjects and a randomized, single-dose, open-label, 3-period Latin-square crossover pharmacokinetic study in 12 subjects. The study revealed that nemonoxacin infusion was well tolerated up to the maximum dose of 1,250 mg, and the acceptable infusion rates ranged from 0.42 to 5.56 mg/min. Drug-related adverse events (AEs) were mild, transient, and confined to local irritation at the injection site. The pharmacokinetic study revealed that after the administration of 250, 500, and 750 mg of intravenous nemonoxacin, the maximum plasma drug concentration (Cmax) values were 4.826 μg/ml, 7.152 μg/ml, and 11.029 μg/ml, respectively. The corresponding values for the area under the concentration-time curve from 0 to 72 hours (AUC0–72 h) were 17.05 μg · h/ml, 39.30 μg · h/ml, and 61.98 μg · h/ml. The mean elimination half-life (t1/2) was 11 h, and the mean cumulative drug excretion rate within 72 h ranged from 64.93% to 77.17%. Volunteers treated with 250 to 750 mg nemonoxacin exhibited a linear dose-response relationship between the AUC0–72 h and AUC0–∞. These findings provide further support for the safety, tolerability, and pharmacokinetic properties of intravenous nemonoxacin. (This study has been registered at ClinicalTrials.gov under registration no. NCT01944774.)
INTRODUCTION
Nemonoxacin, a nonfluorinated quinolone antibacterial drug, belongs to the class of new-generation selective topoisomerase inhibitors. In vitro studies have demonstrated that nemonoxacin exhibits strong broad-spectrum antibacterial activity against Gram-positive and Gram-negative bacteria, anaerobes, and atypical pathogens, particularly Staphylococcus spp. and Streptococcus spp. Nemonoxacin shows high antimicrobial activity against multidrug-resistant S. pneumoniae and methicillin-resistant Staphylococcus aureus (MRSA) (1–4). In addition, the drug has been proven to significantly reduce viable bacterial counts in the blood and lungs of mice infected with Streptococcus pneumoniae and to protect animals from lethal infection (5, 6).
Completed phase I to III clinical trials of nemonoxacin in China have demonstrated that the drug is rapidly absorbed in healthy subjects after oral administration (at 250-, 500-, and 750-mg doses) and has a long half-life of approximately 11 h. Approximately 70% of the administered drug is excreted by the kidneys. Oral administration of 500 mg or 750 mg nemonoxacin once daily for 10 days is well tolerated in healthy subjects (7). The phase II and III clinical studies also demonstrated that the clinical and microbiological efficacies of oral nemonoxacin (500 mg daily for 7 to 10 days) were comparable to those of oral levofloxacin (500 mg daily) for the treatment of mild to moderate community-acquired pneumonia (CAP). Based on these findings, TaiGen Biotechnology Co., Ltd., developed an intravenous (i.v.) formulation of nemonoxacin for the treatment of moderate to severe CAP infections.
In this study, we investigated the safety and tolerability of i.v. nemonoxacin in healthy Chinese volunteers in a randomized, double-blind, placebo-controlled, single-dose escalation design to determine the safe dose range and appropriate infusion rate. Based on the results for the dose range and acceptable infusion rate, we further performed a single-dose pharmacokinetic (PK) study with three selected doses (low, medium, and high) administered at the suggested i.v. infusion rate. The findings of this study may provide a basis for identifying the dose regimen for future studies on multidose tolerability and PK studies with nemonoxacin infusion.
MATERIALS AND METHODS
Overall design.
This study was composed of two parts, a single-dose escalation study to determine the tolerability of i.v. nemonoxacin and a single-dose PK study. In the single-dose tolerance study, the safe dose range, maximum tolerated dose, and acceptable i.v. infusion rate were determined. The determined i.v. infusion rate was subsequently used in the single-dose PK study. The study was conducted in a university teaching hospital according to the Declaration of Helsinki and all its amendments. The research protocol and informed consent form were reviewed and approved by the ethics committee of the Huashan Hospital, Fudan University. Each subject provided written informed consent before the initiation of screening procedures.
Investigational drug.
Nemonoxacin 0.5 g (volume, 100 ml; containing sodium chloride 0.9 g; lot 08011904;) and placebo (sodium chloride; volume, 100 ml; lot 08060507) were manufactured by Huayu (Wuxi) Pharmaceutical Co., Ltd.
Single-dose tolerability study. (i) Subjects and study design.
The safety and tolerability of i.v. nemonoxacin were assessed in a randomized, double-blind, placebo-controlled, dose escalation study. The maximum recommended starting dose was set at 25 mg based on the results of long-term pharmacotoxicity studies of i.v. nemonoxacin in rats and dogs and the results from the modified Blach well method, the Dollery method, and the modified Fibonacci method (12, 13) and from the maximum level at which no adverse effect was observed (data not shown). According to the results of the phase I clinical trial of oral nemonoxacin in healthy subjects, the highest dose was set at 1,250 mg.
A total of 92 healthy Chinese volunteers (equal numbers of males and females) were to be randomized into 12 groups with different doses and infusion rates. Dosing and infusion rates for each group are shown in Table 1. Safety and tolerability were evaluated by a blinded investigator before subjects were allowed to proceed to the next-higher dose group. Dose escalation was stopped if subjects showed a lack of tolerability in the clinical and/or laboratory examinations or after the highest dose (1,250 mg) was administered. The lack of tolerability was determined according to the investigators' evaluation of dose-limiting toxicity or when ≥3 subjects who received the active drug experienced drug-related adverse events (AEs) of grade 2 or higher toxicity (defined in the U.S. National Cancer Institute Common Terminology Criteria for Adverse Events, version 3.0).
TABLE 1.
Dosing and infusion rates in the single-dose escalation study for assessing the tolerability of i.v. nemonoxacin
| Group | Dose (mg) | Vol of liquid (ml) | No. of subjects (investigational drug + placebo)a | Infusion time (min) | Infusion concn (mg/ml) | Infusion rate (mg/min) |
|---|---|---|---|---|---|---|
| 1 | 25 | 100 | 4 + 2 | 60 | 0.25 | 0.42 |
| 2 | 50 | 100 | 4 + 2 | 60 | 0.5 | 0.83 |
| 3 | 125 | 100 | 6 + 2 | 60 | 1.25 | 2.08 |
| 4 | 250 | 100 | 6 + 2 | 60 | 2.5 | 4.17 |
| 5 | 250 | 50 | 6 + 2 | 45 | 5 | 5.56 |
| 6 | 500 | 100 | 6 + 2 | 60 | 5 | 8.33 |
| 7 | 750 | 150 | 6 + 2 | 180 | 5 | 4.17 |
| 8 | 750 | 150 | 6 + 2 | 135 | 5 | 5.56 |
| 9 | 750 | 150 | 6 + 2 | 90 | 5 | 8.33 |
| 10 | 1,000 | 200 | 6 + 2 | 240 | 5 | 4.17 |
| 11 | 1,000 | 200 | 6 + 2 | 180 | 5 | 5.56 |
| 12 | 1,250 | 250 | 6 + 2 | 300 | 5 | 4.17 |
Total subjects was 92.
(ii) Safety evaluation.
Safety and tolerability were evaluated by AEs, vital signs, physical examinations, laboratory examinations, and 12-lead electrocardiograms (ECGs). AEs were coded according to the Medical Dictionary for Regulatory Activities and classified to assess their association with the investigational drug. Events or results that were possibly, probably, or definitely related to the drug were recorded as adverse drug reactions (ADRs). Higher doses were assessed in volunteers only once the safety evaluation of the preceding dose was completed. Statistical analyses of safety data were performed using SAS software (version 9.1.3).
Single-dose PK study. (i) Investigational drug.
Nemonoxacin, manufactured by Huayu (Wuxi) Pharmaceutical Co., Ltd., was used in the PK study with the same specifications and lot number as in the single-dose tolerability study.
(ii) Subjects and study design.
A randomized, open-label, three-period, 3-dose Latin-square crossover design was used for the single-dose PK study. A washout period of 7 days was included after the follow-up for each experimental period. A total of 12 subjects (equal numbers of males and females) were treated with a single infusion of nemonoxacin 250 mg (50 ml), 500 mg (100 ml), or 750 mg (150 ml). The durations of i.v. infusion were 45, 90, and 135 min, respectively. Linear PK was studied at a 5-mg/ml concentration of nemonoxacin and an infusion rate of 5.56 mg/min.
PK analysis. (i) Blood and urine sample collections.
Blood samples (4 ml) were obtained using a peripheral venous catheter immediately before each dose (0 h), midway through the infusion, immediately after each dose, and at 0.5, 1, 1.5, 2, 3, 4, 6, 8, 12, 16, 24, 36, 48, 60, and 72 h after each dose. The blood samples were centrifuged at 1,207.44 × g for 10 min at 4°C and stored at −40°C for future experiments.
Urine samples were collected −1 to 0 h before each dose and 0 to 2 h, 2 to 4 h, 4 to 8 h, 8 to 12 h, 12 to 24 h, 24 to 36 h, 36 to 48 h, and 48 to 72 h after each dose. Samples were stored at −40°C for future experiments.
(ii) Measurement methods.
The single-dose PK study was conducted according to the method reported by Guo et al. (8), who used liquid chromatography-tandem mass spectrometry (LC-MS/MS) to measure the concentration of nemonoxacin in plasma and urine samples. The LC-MS/MS system included an Alliance 2690 high-performance liquid chromatograph (HPLC) and Finnigan triple-stage quadrupole (TSQ) Quantum mass spectrometer (Waters Co., MA). Chromatography was performed with a symmetry shield RP18 column (50 mm by 2.1 mm; 5-μm pore size; Waters Co., Ltd.), and the mobile phase was 0.1% formic acid–acetonitrile (82:18 [vol/vol]) and 0.1% formic acid–acetonitrile (85:15 [vol/vol]). The flow rate of the mobile phase was set at 0.2 ml/min, and the injection volume used was 5 μl.
Samples were analyzed by MS equipped with an electrospray ionization source operating in a positive ion selected reaction monitoring mode. Nitrogen was used as the sheath gas (40 Arb) and auxiliary gas (10 Arb). Spray voltage was set at 4,000 V; capillary temperature was maintained at 320°C. In order to precipitate proteins, plasma samples were pretreated with 2 volumes of acetonitrile. The resulting supernatant was dried using high-purity nitrogen and reconstructed with the mobile phase before the sample analysis. Urine samples were extracted using 1 ml of ethyl acetate–isopropyl alcohol (75:25 [vol/vol]), and the supernatant was dried with high-purity nitrogen and reconstructed with the mobile phase.
Calibration curves were constructed at concentrations of 0.005 to 1 μg/ml for plasma and 0.005 to 1 μg/ml for urine samples. Intraday precision was expressed by relative standard deviations (RSDs) for the high, medium, and low concentrations, as were the lower limits of quantification (0.8 μg/ml, 0.4 μg/ml, 0.015 μg/ml, and 0.005 μg/ml, respectively). The intraday RSDs were ≤6.94% for plasma samples and ≤13.12% for urine samples. The corresponding RSDs from interday measurements were ≤10.90% and ≤6.58%. The intraday recovery rates were 98.73% to 118.03% and 100.95% to 118.46%, respectively, and interday recovery rates were 96.02% to 107.89% and 93.83% to 114.20%, respectively.
(iii) Calculation of PK parameters.
The PK parameters of single-dose i.v. nemonoxacin were determined from concentrations of the unchanged drug in plasma and urine samples by using noncompartmental analysis with WinNonlin 5.2.1 software (Pharsight Corp., CA).
PK parameters included determination of the peak plasma drug concentration (Cmax), 24-h and 72-h plasma drug concentrations (C24 h and C72 h), areas under the concentration-time curve from 0 to 24 h, 0 to 72 h, and 0 h to infinity after administration (AUC0–24 h, AUC0–72 h, and AUC0–∞, respectively), total plasma clearance (CLZ), apparent volume of distribution (Vz), mean residence time (MRT0–∞, the ratio of area under the moment of the concentration-time curve [integration of C × t versus time from 0 to infinity] to AUC0–∞), and terminal elimination half-life (t1/2). Urinary excretion of nemonoxacin from 0 to 24 h and from 0 to 72 h (UA0–24 h and UA0–72 h), urinary excretion rate (the ratio of urinary excretion to dose) from 0 to 24 h and from 0 to 72 h (Ae0–24 h and Ae0–72 h), and renal clearance between 0 and 72 h (CLR) were also calculated.
Statistical analysis.
Continuous data are expressed as means ± standard deviations (SDs). Descriptive statistical analysis was performed on PK parameters, including Cmax, Cmin, time to reach Cmax (Tmax), AUC0–24 h, AUC0–72 h, AUC0–∞, λz (first-order rate for terminal elimination), t1/2, Vz, MRT0-∞, CLZ, CLR, Ae%0–24 h (cumulative percentage of urinary excretion from 0 to 24 h), and Ae%0–72 h for each single-dose PK group. The calculated statistical measures included the number of subjects, mean, standard deviation, coefficient of variation, 95% confidence interval, and minimum and maximum values. Data were categorized by gender to determine statistical differences in PK parameters between males and females.
The proportionality between the doses and the PK parameters (AUC0–t, AUC0–∞, and Cmax) was also analyzed using ordinary least-squares regression (primary method) and the mixed-effect (supplementary method) models. Since the AUC usually conforms to a log-normal distribution, the relationships between natural log-transformed AUC0–72 h and AUC0–∞ of the 3 doses and factors such as dosage, period, drug-administration sequence, and individual subjects were analyzed by ordinary least-squares regression. A mixed-effect model was used when natural log-transformed doses were used as the independent variables, cycle and drug administration sequences were used as fixed-effect covariates, individual subjects were used as a random-effect covariate, and AUC0–72 h and AUC0–∞ were used as dependent variables. P values of <0.05 were considered statistically significant (bilateral).
RESULTS
Subject characteristics of the single-dose tolerability study.
A total of 92 subjects (equal numbers of male and female) were enrolled in the 12 dosing groups in the single-dose tolerability study. Of the subjects, 91 belonged to the Chinese Han ethnic group, and the remaining subject belonged to a different ethnic group. The subjects were assigned to the study drug group (n = 68) or the placebo group (n = 24), and all of them completed the trial and were included in the safety analysis set. The age distributions of the subjects were similar in each dose group (20 to 29 years of age in the active drug group and 22 to 28 years of age in the placebo group). The mean body weights in the active drug and placebo groups were 58.9 ± 7.1 kg and 61.3 ± 7.7 kg, respectively, and the mean body mass index (BMI) values were 21.2 ± 1.5 kg/m2 and 21.4 ± 1.6 kg/m2, respectively. All the groups contained even numbers of male and female subjects, except for the 50-mg-dose group (1 male, 3 females), the 750-mg-dose group with an infusion rate of 4.17 mg/min (2 males, 4 females), the 1,000-mg-dose group with an infusion rate of 5.56 mg/min (2 males, 4 females), and the placebo group (15 males, 9 females).
Subject characteristics of the single-dose PK study.
A total of 12 Chinese Han subjects (6 male, 6 female) were enrolled in the single-dose PK study. One subject who received single-dose i.v. infusions of 500 mg nemonoxacin in the first period and 250 mg nemonoxacin in the second period discontinued use due to AEs. Therefore, there were 12 subjects in the 250-mg and 500-mg groups and 11 subjects in the 750-mg group. The study subjects were 21 to 25 years old and had a mean body weight of 58.9 ± 5.1 kg and a mean BMI of 21.0 ± 1.0 kg/m2.
Safety and tolerability of i.v. nemonoxacin.
The maximum tolerated dose of i.v. nemonoxacin was 1,250 mg, and the most appropriate infusion rate was 5.56 mg/min. All AEs were mild and transient, and no serious or severe AEs were reported. The most common AE was an injection site reaction (Tables 2 and 3). There were no apparent correlations between the frequency of injection site reactions and doses or infusion rates. The next most common AE was erythematous rash with or without pruritus that was observed during drug administration and occurred on the skin of the face, neck, or trunk (Tables 2 and 3). All AEs described above spontaneously resolved during drug administration or within 121 min after administration, except in two subjects in the 750-mg-dose group with an infusion rate of 4.17 mg/min and one subject in the 750-mg-dose group with an infusion rate of 8.33 mg/min who were treated with 10 mg cetirizine for rash on the head, face, or chest. No clinically significant abnormalities were observed in the vital signs or on physical examination of the subjects.
TABLE 2.
Common drug-related adverse events in the single-dose tolerability study of i.v. nemonoxacin
| Adverse event | Placebo (pooled n = 24) | No. (%) of subjects with indicated adverse event at a dosage ofa: |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 25 mg (0.42) (n = 4) | 50 mg (0.83) (n = 4) | 125 mg (2.08) (n = 6) | 250 mg (4.17) (n = 6) | 250 mg (5.56) (n = 6) | 500 mg (8.33) (n = 6) | 750 mg (4.17) (n = 6) | 750 mg (5.56) (n = 6) | 750 mg (8.33) (n = 6) | 1,000 mg (4.17) (n = 6) | 1,000 mg (5.56) (n = 6) | 1,250 mg (4.17) (n = 6) | Total (n = 68) | ||
| Injection site reaction | 1 (4) | 0 (0) | 1 (25) | 0 (0) | 4 (67) | 4 (67) | 5 (83) | 1 (17) | 1 (17) | 4 (67) | 3 (50) | 1 (17) | 3 (50) | 27 (40) |
| Erythematous rash with or without pruritus | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 4 (67) | 3 (50) | 1 (17) | 3 (50) | 0 (0) | 1 (17) | 1 (17) | 13 (19) |
| Abnormal electrocardiogram T-wave | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 3 (50) | 0 (0) | 3 (4) |
Values in parentheses immediately after the dosages are the infusion rates (in mg/min).
TABLE 3.
Common drug-related adverse events in the single-dose PK study of i.v. nemonoxacin
| Adverse event | No. (%) of subjects with indicated adverse event at a nemonoxacin dosage ofa: |
||
|---|---|---|---|
| 250 mg (n = 12) | 500 mg (n = 12) | 750 mg (n = 11) | |
| Injection site reaction | 3 (25) | 4 (33) | 7 (64) |
| Erythematous rash | 0 (0) | 1 (8) | 2 (18) |
| Total | 3 (25) | 5 (42) | 9 (82) |
The infusion rate for each group was 5.56 mg/min.
Some laboratory AEs had low incidences, and all were mild in severity, except in the case of one subject in the nemonoxacin 750-mg group who showed elevated blood creatine phosphokinase levels and one subject in the placebo group who showed a >3-fold increase in transaminase levels above normal. These two events were considered to be of moderate severity. The former subject recovered spontaneously, and the latter received treatment and recovered in the end. There were no clinically significant changes in corrected QT (QTc). None of the male and female subjects had QTc values of >450 and >470 ms, respectively. No subjects had a QTc difference of >60 ms except one subject each in the 750-mg-dose group with an infusion rate of 8.33 mg/min and the 1,000-mg-dose groups with infusion rates of 4.17 and 5.56 mg/min (single-dose tolerability study) and one subject in the 500-mg group (single-dose PK study). Categorical analysis revealed that there were no increases in QTc values with the administration dose or in QTc difference from baseline compared with placebo for the single-dose safety study.
PK parameters of single-dose i.v. nemonoxacin.
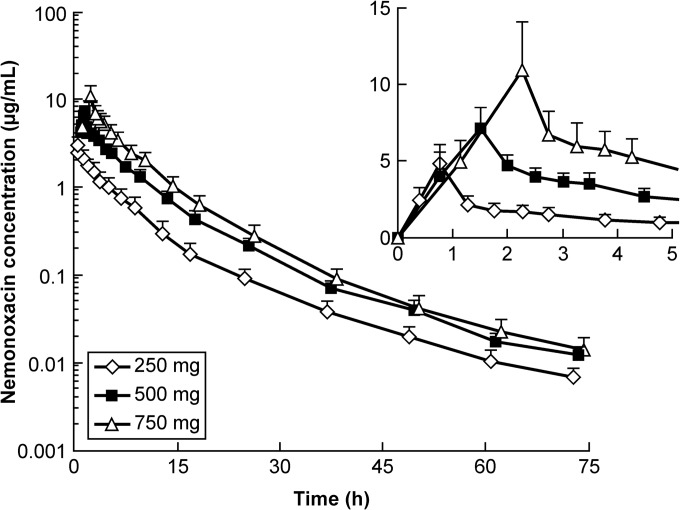
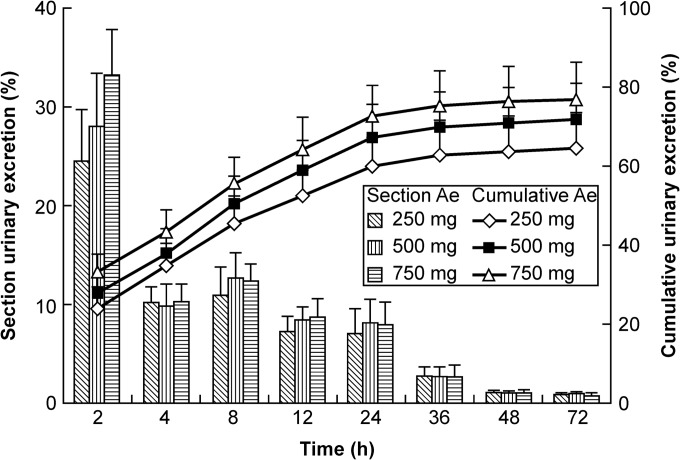
The drug concentration-time curves of the 250-, 500-, and 750-mg groups (infusion time, 45, 90, and 135 min, respectively; infusion rate, 5.56 mg/min) are shown in Fig. 1, and the mean PK parameters are shown in Table 4. The Cmax values for the 250-, 500-, and 750-mg groups were 4.826 μg/ml, 7.152 μg/ml, and 11.029 μg/ml, respectively. The AUC0–72 h values were 17.05 μg · h/ml, 39.30 μg · h/ml, and 61.98 μg · h/ml, respectively. The t1/2 values were 11.10 h, 10.92 h, and 10.52 h, respectively. The rates of drug excreted 72 h after administration of the three doses were 64.93%, 71.88%, and 77.17%, respectively (Fig. 2).
FIG 1.
Drug concentration-time curves following single administration of 250 mg, 500 mg, or 750 mg i.v. nemonoxacin in healthy Chinese volunteers (mean ± SD; n = 12). The infusion rate of nemonoxacin in each group was 5.56 mg/min. (Inset) Time profiles within 5 h of drug administration.
TABLE 4.
Mean PK parameters in healthy subjects following i.v. administration of a single dose of nemonoxacin
| Dose (mg) | n | Statistical measurea |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cmax (μg/ml) | C24 h (μg/ml) | C72 h (μg/ml) | t1/2 (h) | Vz (liters) | CLZ (liter/h) | CLR (liter/h) | AUC0–24 h (μg · h/ml) | AUC0–72 h (μg · h/ml) | AUC0–∞ (μg · h/ml) | MRT0–∞ (h) | Ae0–72 h (%) | ||
| 250 | 12 | 4.826 (1.320) | 0.093 (0.026) | 0.007 (0.002) | 11.10 (2.24) | 242.03 (66.00) | 15.30 (3.68) | 10.03 (2.79) | 15.65 (3.78) | 17.05 (4.09) | 17.21 (4.10) | 8.81 (0.98) | 64.93 (8.76) |
| 500 | 12 | 7.152 (1.333) | 0.216 (0.050) | 0.012 (0.003) | 10.92 (2.96) | 201.10 (50.62) | 12.87 (1.75) | 9.38 (2.04) | 36.37 (5.10) | 39.30 (5.33) | 39.51 (5.36) | 8.47 (0.98) | 71.88 (9.09) |
| 750 | 11 | 11.029 (3.134) | 0.287 (0.082) | 0.014 (0.005) | 10.52 (3.19) | 184.50 (47.40) | 12.46 (2.48) | 9.68 (2.29) | 58.31 (10.89) | 61.98 (11.40) | 62.21 (11.47) | 7.80 (0.92) | 77.17 (9.25) |
Values are means (SD). Abbreviations: Cmax, peak plasma drug concentration; C24 h, 24-h plasma drug concentration; C72 h, 72-h plasma drug concentration; t1/2, terminal half-life; Vz, apparent volume of distribution; CLZ, total plasma clearance; CLR, renal clearance; AUC0–24 h, area under the concentration-time curve from 0 to 24 h; AUC0–72 h, area under the concentration-time curve from 0 to 72 h; AUC0–∞, area under the concentration-time curve from 0 h to infinity; MRT0–∞, mean residence time; Ae0–72 h, urinary excretion rate from 0 to 72 h.
FIG 2.
Urinary excretion of nemonoxacin following single administration of 250 mg, 500 mg, or 750 mg i.v. nemonoxacin in healthy Chinese volunteers (mean ± SD; n = 12). Histogram and lines represent the section excretion rate (left y axis) and cumulative excretion rate (right y axis), respectively, of nemonoxacin.
PK parameters were analyzed by gender. No significant differences in Cmax, C24 h, C72 h, t1/2, CLZ, Vz, AUC0–72 h, AUC0–∞, or Ae were detected between male and female subjects who received 250 mg nemonoxacin. In subjects who received 500 mg nemonoxacin, significant differences in Vz (P = 0.018) and MRT0-∞ (P = 0.016) were observed between the two genders. Of the subjects who received the highest dose of nemonoxacin (750 mg), significant differences between the genders were observed only in MRT0-∞ (P = 0.016). In male volunteers, the mean drug excretion rates 72 h after administration were 64.75%, 71.55%, and 75.78% in the 250-mg, 500-mg, and 750-mg groups, respectively. In female subjects, the proportions of drug excreted after 72 h were 65.11%, 72.22%, and 78.84%, respectively. No significant differences in Ae0–72 h (P > 0.05) were observed between the male and female subjects.
For the relationship between noncompartmental parameters and nemonoxacin dose, analysis results indicated that the increase in AUC0–72 h and AUC0–∞ had a linear correlation in the dose range of 250 to 750 mg. The regression coefficients of AUC0–72 h and AUC0–∞ were 1.192 (1.122 to 1.261) and 1.186 (1.117 to 1.255), respectively.
DISCUSSION
No serious or severe AEs were reported in the single-dose safety and tolerability studies of i.v. nemonoxacin. No AEs led to study discontinuation, and no clinically significant AEs related to ECG changes were observed. The predominant drug-related clinical AEs were injection site reactions such as mild rash and pain or pruritus at the injection site or along blood vessels. Erythematous rash also appeared during drug infusion and occurred on the skin of the face, neck, or trunk, with or without pruritus. The observed AEs were mild and transient histamine-releasing reactions characterized by rapid onset and disappearance. These differed from general allergic reactions. Most events did not require treatment and resolved during drug administration or within 121 min. Intravenous infusion rates in the range 0.42 to 5.56 mg/min were well tolerated by the study subjects. Drug-related laboratory abnormalities were mainly mild and transient.
The findings of the single-dose tolerability study provided dosage and infusion rate information for the single-dose PK study. The dose range of 25 to 1,250 mg and infusion rate of 5.56 mg/min were selected on the basis of tolerability and safety. Since the target dose was 500 mg, a dose higher (750 mg) and a dose lower (250 mg) than the target dose were selected for the single-dose PK study of i.v. nemonoxacin.
Subjects in the single-dose PK study received 3 single i.v. doses of nemonoxacin. The t1/2 values ranged from 10 to 12 h, and this range was similar to the single- and multidose PK parameters reported by Guo et al. (7), in which oral administration of nemonoxacin capsules was assessed in healthy Chinese subjects. In contrast, t1/2 values between 8 and 16 h were reported by Lin et al. (9) following single doses of oral nemonoxacin capsules and by Chung et al. (10) after multiple doses of oral nemonoxacin capsules in healthy North American study subjects. These differences may be explained by variations in the final PK blood-sampling time points in the various studies. In the present study, the percentage of drug excreted within 72 h ranged between 64.93% and 77.17%, which was similar to the values reported by Guo et al. (Ae0–72 h after administration of 250 to 750 mg reported as 66.00% to 70.28% [7]) and higher than values obtained from a North American study in which subjects received a single dose of between 25 mg and 1,500 mg of nemonoxacin (Ae0–72 h, 34.15% to 56.24%). Cmax values following i.v. administration of 3 doses in the present study were higher than those obtained following oral administration of the capsule formulation of nemonoxacin (8). This difference may be explained by differences in the figures and body surface areas between Chinese and North American study subjects. It may also be related to the differences in drug absorption characteristics and in the levels between oral and i.v. administrations. By comparing additional PK parameters such as CLZ and Vz with published findings, we determined that the elimination rate of nemonoxacin is similar between Chinese and Western populations (8, 9).
Our previous work reported the PK of nemonoxacin oral capsules in Chinese subjects (7). The difference in the Cmax of nemonoxacin between Chinese and Western populations was 1.46 mg/ml. Moreover, the AUC0–24 h was 21.6% higher and the urinary excretion rate was 12.1% lower in the Chinese population following once-daily oral administration of 500 mg nemonoxacin for 10 days (10). We also investigated the absolute bioavailability (BA) of nemonoxacin oral capsules in healthy Chinese subjects and found that it was nearly 100% (unpublished data). Thus, the PK of intravenous nemonoxacin is expected to be similar to that of oral nemonoxacin in Chinese and Western populations.
Following i.v. administration of nemonoxacin at doses of 250, 500, and 750 mg, the volume of distribution was high, as Vz values were >3 liters/kg. This indicated that nemonoxacin is widely distributed in tissue fluids. Similar to other quinolones, higher concentrations of nemonoxacin are expected to have greater penetration in the tissue fluids of the bronchi and lungs (11). The expected clinical applications of nemonoxacin injection include the treatment of moderate or severe CAP or use in patients in whom oral administration is not possible. Such patients could potentially receive sequential therapy, in which intravenous therapy is used first and followed by oral therapy.
In conclusion, the present study confirmed that nemonoxacin has a good safety and tolerability profile at dose ranges of 25 to 1,250 mg and infusion rates of ≤5.56 mg/min in healthy Chinese subjects. In the 250- to 750-mg-dose range, drug exposure correlated linearly with the dose administration. Nemonoxacin is widely distributed in the human body, with a long elimination half-life, and is mainly excreted by the kidneys.
ACKNOWLEDGMENTS
We thank TaiGen Biotechnology Company, Ltd., Taipei, Taiwan, for support of the study and for reviewing the manuscript.
This work was also supported by the major research and development project of innovative drugs initiative of the Ministry of Science and Technology, China (2012ZX09303004-001).
Footnotes
Published ahead of print 4 August 2014
REFERENCES
- 1.Wang F, Zhang Y. 2012. Chapter 12. In Practical anti-infective therapeutics, 2nd ed. People's Medical Publishing House, Beijing, China [Google Scholar]
- 2.Roychoudhury S, Twinem T, Makin KM, McIntosh EJ, Ledoussal B, Catrenich CE. 2001. Activity of non-fluorinated quinolones (NFQs) against quinolone-resistant Escherichia coli and Streptococcus pneumoniae. J. Antimicrob. Chemother. 48:29–36. 10.1093/jac/48.1.29 [DOI] [PubMed] [Google Scholar]
- 3.Chen YH, Liu CY, Lu JJ, King CH, Hsueh PR. 2009. In vitro activity of nemonoxacin (TG-873870), a novel non-fluorinated quinolone, against clinical isolates of Staphylococcus aureus, enterococci and Streptococcus pneumoniae with various resistance phenotypes in Taiwan. J. Antimicrob. Chemother. 64:1226–1229. 10.1093/jac/dkp370 [DOI] [PubMed] [Google Scholar]
- 4.Adam HJ, Laing NM, King CR, Lulashnyk B, Hoban DJ, Zhanel GG. 2009. In vitro activity of nemonoxacin, a novel nonfluorinated quinolone, against 2,440 clinical isolates. Antimicrob. Agents Chemother. 53:4915–4920. 10.1128/AAC.00078-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andersson MI, MacGowan AP. 2003. Development of the quinolones. J. Antimicrob. Chemother. 51(Suppl 1):1–11. 10.1093/jac/dkg212 [DOI] [PubMed] [Google Scholar]
- 6.Arjona A. 2009. Nemonoxacin. Drugs Future 34:196–203. 10.1358/dof.2009.034.03.1350294 [DOI] [Google Scholar]
- 7.Guo B, Wu X, Zhang Y, Shi Y, Yu J, Cao G, Zhang J. 2012. Safety and clinical pharmacokinetics of nemonoxacin, a novel non-fluorinated quinolone, in healthy Chinese volunteers following single and multiple oral doses. Clin. Drug Invest. 32:475–486. 10.2165/11632780-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 8.Guo B, Zhang J, Yu J, Wu X, Shi Y, Tsai CY. 2012. A liquid chromatography–tandem mass spectrometry assay for the determination of nemonoxacin (TG-873870), a novel nonfluorinated quinolone, in human plasma and urine and its application to a single-dose pharmacokinetic study in healthy Chinese volunteers. Biomed. Chromatogr. 26:1333–1340. 10.1002/bmc.2699 [DOI] [PubMed] [Google Scholar]
- 9.Lin L, Chang LW, Tsai CY, Hsu CH, Chung DT, Aronstein WS, Ajayi F, Kuzmak B, Lyon RA. 2010. Dose escalation study of the safety, tolerability, and pharmacokinetics of nemonoxacin (TG-873870), a novel potent broad-spectrum nonfluorinated quinolone, in healthy volunteers. Antimicrob. Agents Chemother. 54:405–410. 10.1128/AAC.00682-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chung DT, Tsai CY, Chen SJ, Chang LW, King CH, Hsu CH, Chiu KM, Tan HC, Chang YT, Hsu MC. 2010. Multiple-dose safety, tolerability, and pharmacokinetics of oral nemonoxacin (TG-873870) in healthy volunteers. Antimicrob. Agents Chemother. 54:411–417. 10.1128/AAC.00683-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rodvold KA, Danziger LH, Gotfried MH. 2003. Steady-state plasma and bronchopulmonary concentrations of intravenous levofloxacin and azithromycin in healthy adults. Antimicrob. Agents Chemother. 47:2450–2457. 10.1128/AAC.47.8.2450-2457.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao M, Yang JB, Xie SM. 2008. Discussion on basic requirement and concerned questions in human tolerance test of new anti-bacterial drugs. Chin. J. Clin. Pharmacol. 24:373–375 (In Chinese) [Google Scholar]
- 13.Food and Drug Administration. 2005. Estimating the maximum safe starting dose in initial clinical trials for therapeutics in adult healthy volunteers. Food and Drug Administration, Washington, DC: http://www.fda.gov/cder/guidance/ [Google Scholar]