Abstract
Artesun-Plus is a fixed-dose combination antimalarial agent containing artesunate and amodiaquine. The current study was conducted to compare the pharmacokinetic and safety profiles of Artesun-Plus and the WHO-designated comparator product Artesunate Amodiaquine Winthrop. To overcome the high intrasubject variability of artesunate, the study applied a two-sequence and four-period crossover (2 by 4), replicate study design to assess bioequivalence between the two products in 31 healthy male Chinese volunteers under fasting conditions. The results showed that the values of the geometric mean ratios of maximum concentration of drug in plasma (Cmax) and area under the concentration-time curve from time zero to the last blood sample collection (AUC0-last) for the artesunate component in the test and reference products were 95.9% and 93.9%, respectively, and that the corresponding 90% confidence intervals were 84.5% to 108.7% and 87.2% to 101.1%, while the geometric mean ratios for the amodiaquine component in the test and reference products were 95.0% and 100.0%, respectively, and the corresponding 90% confidence intervals were 86.7% to 104.1% and 93.5% to 107.0%. In conclusion, bioequivalence between the two artesunate and amodiaquine fixed-dose combination products was demonstrated for both components. The study also confirmed high intrasubject variability, especially for artesunate: the coefficients of variation (CV) of Cmax values for the test and reference products were 39.2% and 43.7%, respectively, while those for amodiaquine were 30.6% and 30.2%, respectively.
INTRODUCTION
Artemisinin-based combination therapies (ACTs) are the first-line treatments for uncomplicated falciparum malaria recommended by the World Health Organization (WHO) (1, 2). Treatment using the combination of artesunate and amodiaquine (ASAQ) is such an ACT regimen and consists of a quick-acting antimalarial, artesunate (AS), and a slow-acting “partner medicine,” amodiaquine (AQ). After oral administration, AS is rapidly converted into its active metabolite, dihydroartimisinin (DHA); both are short-lived, with an elimination half-life of less than an hour (1, 3). The “partner medicine”—AQ—is also extensively metabolized into its active metabolite, desethylamodiaquine (DEAQ), and both work in infected red blood cells via a mechanism of action that is different from that of artemisinin derivatives (1, 4). The elimination half-life of AQ was reported to be close to 30 h in whole blood, while that of DEAQ is longer than 70 h (1, 4).
Two previous studies have compared the pharmacokinetics (PK) of a loose-dose combination of AS and AQ to that of fixed-dose combination (FDC) tablets in healthy volunteers (5, 6). However, bioequivalence between the non-FDC and FDC regimens has not been demonstrated with regard to AS for unknown reasons and, in part, due to its high inter- and intrasubject variability, particularly with respect to Cmax values, with analysis of variance (ANOVA) coefficients of variation (CV) reported to be as high as 56% (6).
To improve malaria treatment adherence and delay the development of artemisinin resistance, FDC products containing an artemisinin derivative are strongly preferred by WHO (1). Artesun-Plus is an FDC formulation of ASAQ developed by Guilin Pharmaceutical Co., Ltd. In an effort to participate in the WHO program for prequalification of medicines, the current study was conducted to compare the pharmacokinetic and safety profiles of Artesun-Plus to those of the WHO-designated comparator product ASAQ Winthrop, which has demonstrated therapeutic efficacy against uncomplicated falciparum malaria in several large clinical trials (4). In light of previous data on the high variability of AS, we applied a two-sequence and four-period crossover (2 by 4), replicate study design, such that the true intrasubject variability for the test and reference products can be established independently and the bioequivalence acceptance limit could be adjusted based on the variability for the reference product, using the scaled-average-bioequivalence method, according to European Medicines Agency (EMA) Guidelines on the Investigation of Bioequivalence for highly variable drugs (7).
MATERIALS AND METHODS
Materials.
The test product Artesun-Plus (ASAQ; 100/270-mg tablets) was provided by Guilin Pharmaceutical Co., Ltd., and the reference product ASAQ Winthrop (100/270-mg tablets, Sanofi-Aventis) was purchased from Kama Health Services ACC, Morocco. Both products were shipped and stored below 30°C until use in the study and were tested by Guilin Pharmaceutical Co., Ltd., within a year of the study, with demonstrated quality and strength, according to the certificate of analyses. Bioanalytical standards AS and DHA were obtained from the European Directorate for Quality Medicines (EDQM), while AQ (amodiaquine hydrochloride) was purchased from US Pharmacopeial Convention Reference Standard. Analytical internal standards (IS) [2H4]AS and [2H10]AQ were purchased from Toronto Research Chemicals Inc., Ontario, Canada, while [13C, 2H4]DHA was purchased from Comipso, Bordeaux, France.
Subjects.
Thirty-six healthy Chinese male volunteers aged between 18 and 45 years with a body mass index (BMI) of between 18 and 25 kg/m2 were enrolled in the study. Subjects were in good health, as determined by a detailed medical history, full physical examination, vital signs (oral body temperature, heart rate, respiratory rate, and sitting blood pressure), 12-lead electrocardiogram (ECG) tests, and clinical laboratory tests (urinalysis, hematology, blood chemistry, and serologic tests, including tests for hepatitis B virus surface antigen, hepatitis C virus antibody, HIV antibody, and falciparum malaria), with values within the reference range or deemed normal by the clinical investigator. Subjects were excluded if they had history of clinically significant cardiovascular, endocrine, gastrointestinal, hematological, hepatic, neurological, psychiatric, pulmonary, and renal diseases or a history of drug abuse or alcohol abuse (more than 14 drinks per week per subject [1 drink = 360 ml of beer or 150 ml of wine or 45 ml of hard liquor]) or heavy smoking (more than 10 cigarettes per day) or if they received any prescription or over-the-counter medication, including traditional Chinese medicines, within 14 days prior to or during the study period. Anyone who had participated in an investigational drug study within 30 days, or who was allergic to the study medications or any other similar compounds, was excluded.
Study design and conduct.
This was a randomized, open label, two-sequence, four-period, single-dose bioequivalence study, conducted in 36 healthy male Chinese volunteers under fasting conditions, to compare the pharmacokinetic and safety profiles of Artesun-Plus (ASAQ 100/270-mg tablets), manufactured by Guilin Pharmaceutical Co., Ltd., to those of ASAQ Winthrop (100/270-mg tablets), manufactured by Sanofi-Aventis. Study subjects were randomized into two treatment sequence groups, sequence 1 (TRTR, where T represents the test product and R represents the reference product) and sequence 2 (RTRT), and the study periods were separated by 7-day washout periods. The study protocol was reviewed by the WHO Technical Assessment team of the Prequalification of Medicines Programme and was approved by the Ethic Committee at Shanghai Xuhui Central Hospital. The study was conducted in accordance with the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) Guidelines for Good Clinical Practices (GCP) and the EMA/Committee for Medicinal Products for Human Use (CHMP) Guidelines on the Investigation of Bioequivalence and Bioanalytical Method Validation, as well as WHO Guidance on In Vivo Bioequivalence Studies and the Chinese FDA Guideline for Human Bioavailability and Bioequivalence Studies for Pharmaceuticals (7–10).
In the evening before the study, study subjects were assigned randomization numbers into two treatment sequence groups (TRTR and RTRT) and underwent an overnight (12-h) fast. The next morning, each subject received either an oral dose of two tablets of the Artesun-Plus test product (ASAQ 100/270-mg tablets × 2, batch no. SH100701) or two tablets of the ASAQ Winthrop reference product (100/270-mg tablets × 2, batch no. 5355) according to the randomly assigned treatment sequence. Blood samples (4 ml each) were drawn at time 0 (predose) and at 10, 20, 30, 45 min and 1, 1.5, 2, 3, 4, 5, 6, 8, 10, 12, 24, 48, and 72 h postdose. No water was consumed between 1 h before and 2 h after drug administration, except the 240 ml of water provided to enable subjects to swallow the study medications. Lunch and dinner, prepared as standardized Chinese low-fat meals (approximately 890 kcal containing 61% carbohydrate, 20% protein, and 19% fat) and noncaffeinated drinks (except grapefruit juice) were provided approximately 4 and 9 h postdose, respectively. Subjects were confined to the phase I Clinical Research Center under conditions of clinical monitoring 12 h prior to and 24 h after drug administration and were required to abstain from engaging in strenuous exercise. Subjects were discharged after the 24-h blood sample was drawn and tolerability assessments were completed and were asked to return to the hospital at 48 and 72 h after dosing for blood sampling and safety assessments. Throughout the study, subjects were required to abstain from smoking, taking concomitant medications (including traditional Chinese medicines), and consuming food or beverages containing alcohol or caffeine or grapefruit juice and to take contraceptive measures.
Safety was assessed by physical examinations, vital signs (sitting blood pressure, heart rate, breathing rate, and oral body temperature), clinical laboratory tests (hematology, blood biochemistry, and urinalysis), 12-lead ECGs, and subject interviews on adverse events (AEs) or serious AEs (SAEs) at baseline and at completion of the study. All AEs or/and SAEs were assessed by clinical investigators for their severity and their relationship to the study medications.
Study periods 2, 3, and 4 followed the prespecified treatment sequence, and the above-described procedures were repeated. The four study periods were separated by 7-day washout periods.
Blood sampling and storage.
Blood samples were collected in polypropylene tubes containing sodium heparin as an anticoagulant and were temporarily stored on ice before storage to minimize further enzymatic or chemical hydrolysis of AS and were divided into two parts: the part one samples were centrifuged at 4°C within 30 min of collection to obtain plasma specimens, which were stored at below −70°C pending analysis of AS and DHA; and the part two samples were stored directly as whole blood at below −70°C pending analysis of AQ.
Drug analysis.
The bioanalytical method validation and clinical sample analyses were conducted in accordance with the EMA Guideline on Bioanalytical Method Validation and WHO Good Laboratory Practice Guidelines (7, 11). Throughout this study, the analysts were blinded from the randomized treatment sequence and clinical sample analyses included all subjects who completed two or more study periods.
Concentrations of AS and its metabolite DHA in plasma were analyzed using a validated liquid chromatography tandem mass spectrometry (LC-MS/MS) method, while concentrations of AQ were analyzed in whole blood using another validated LC-MS/MS method, according to published methods (12, 13).
Briefly, AS and DHA were extracted from human plasma samples using a protein precipitation method: an aliquot (100 μl) of plasma was spiked with 10 μl of the internal standard working solution ([2H4]AS–[13C, 2H4]DHA–80% methanol-water solution) and added with 10 μl of an 80% methanol–water solution and mixed well, and then 200 μl of acetonitrile was added and the mixture was subjected to a vortex procedure for ∼10 s to precipitate plasma proteins. The resulting mixture was then centrifuged at 15,000 rpm (25,650 × g) for 3 min at 4°C. Upon centrifugation, the supernatant was transferred to autosampler vials and an aliquot (15 μl) was injected into the LC-MS/MS system for the analysis of AS and DHA, in which the LC system employed a reverse-phase column (C18; 4.6 by 50 mm) and isocratic elution (63% acetonitrile/37% water, both containing 0.1% formic acid), and the analytes were detected by a triple-quadruple mass detector (API5500; AB Sciex) in positive-ion mode with electrospray ionization (ESI) in multiple-reaction-monitoring (MRM) mode (m/z 402.2/267.2 for AS and m/z 302.2/267.2 for DHA). Peak area ratios of AS and DHA over those of the corresponding deuterium-labeled internal standard (m/z 406.2/267.2 for [2H4]AS and m/z 307.2/272.2 for [13C, 2H4]DHA) were used to quantify AS and DHA plasma concentrations. Calibration curves were constructed by the LC-MS/MS software Analyst 1.5.1 using quadratic-regression curve fitting for both AS and DHA. Calibration curve fitting processes for both analytes included a weighting factor of 1/x. Quantitation of quality control (QC) and clinical samples was also performed by use of the Analyst software using the same mathematical algorithm as that used for the calibration standard curves.
For the analysis of AS, the lower limit of quantitation (LLOQ) was 1 ng/ml and the assay dynamic range was 1 to 1,000 ng/ml. Intrabatch accuracy and precision were 96.3% to 104.0% and ≤5.5%, while interbatch accuracy and precision were 98.2% to 101.0% and ≤5.4%, respectively. For DHA, the LLOQ was 3 ng/ml and the assay dynamic range was 3 to 3,000 ng/ml. Intrabatch accuracy and precision were 97.4% to 105.0% and ≤6.8%, while interbatch accuracy and precision were 98.4% to 101.0% and ≤4.9%, respectively. Short-term stability at room temperature (bench top) showed that both AS and DHA were unstable (>±15% of initial values) beyond 6 h. However, both analytes were stable (within ±10%) for at least 24 h when stored at 4°C. Long-term storage stability after 35 days at −70°C indicated that changes from day 0 were within ±13.1% for AS and within ±13.6% for DHA.
For the analysis of AQ, an aliquot (50 μl) of whole blood was spiked with 5 μl of the internal standard working solution ([2H10]AQ), 5 μl of standard working solution, and 50 μl of water and mixed well, and then 200 μl of acetonitrile was added and the mixture was subjected to a vortex procedure for ∼30 s to precipitate proteins. The resulting mixture was then centrifuged at 15,000 rpm (25,650 × g) for 3 min at 4°C. Upon centrifugation, an aliquot (50 μl) of the supernatant was transferred to autosampler vials, followed by addition of 20 μl of water, and mixed well, and 5 μl was injected into the LC-MS/MS system for the analysis of AQ. AQ was analyzed on an LC-MS/MS system, in which the LC system employed a reverse-phase column (C18; 2.0 by 100 mm) and gradient elution (water and acetonitrile, both containing 0.2% formic acid, with the acetonitrile portion increasing from 2% to 90% in 3 min), and the analyte and internal standard (IS) were detected by a triple-quadruple mass detector (API4000; AB Sciex) in positive-ion mode with electrospray ionization (ESI) in multiple-reaction-monitoring (MRM) mode (m/z 358.2/285.2 for AQ). Peak area ratios of AQ over those of the corresponding deuterium-labeled internal standard (m/z 368.2/285.2 for [2H10]AQ) were used to quantify AQ blood concentrations. Calibration curves were constructed by the LC-MS/MS software Analyst 1.5.1 using linear-regression curve fitting. Curve-fitting processes for AQ included a weighting factor of 1/x2. Quantitation of QC and study samples was also performed by the use of Analyst software using the same mathematical algorithm for AQ as that used for the calibration curves.
For AQ, the lower limit of quantitation (LLOQ) was 1 ng/ml and the assay dynamic range was 1 to 100 ng/ml. Intrabatch accuracy and precision were 92.2% to 101.0% and ≤4.9%, while interbatch accuracy and precision were 94.1% to 97.9% and ≤4.9%, respectively. Short-term-stability determinations at room temperature (bench top) showed that AQ was stable for at least 24 h (±15% of initial values). Long-term-storage stability for AQ could be demonstrated for only 21 days when AQ was stored at −70°C (±15% of initial values); as a result, clinical samples had to be analyzed within 3 weeks after blood collection.
Pharmacokinetic and statistical analyses.
Pharmacokinetic and statistical analyses were performed using SAS software (version 9.2; SAS Institute, Cary, NC). Pharmacokinetic (PK) calculations followed the standard noncompartmental analysis and linear trapezoidal rule for estimations of area under the concentration-time curve (AUC) and included the area under the concentration-time curve from time zero to the last blood sample collection (AUC0-last), the area under the concentration-time curve from time zero to infinity (AUC0-inf), the maximum observed plasma concentration (Cmax), the elimination rate constant (λz), and the elimination half-life (t1/2). All PK parameters underwent natural log transformation before statistical analyses were performed, and then the geometric least square mean ratios (test/reference) and corresponding 90% confidence intervals were calculated using the linear-mixed-effects-model procedure (PROC MIXED in SAS) recommended by the U.S. FDA (14). Bioequivalence assessment was made based on statistical comparisons of the Cmax and AUC0-last values from the test and reference products for AS and AQ for subjects who completed all four periods of the study per protocol (PP), while comparisons of artesunate metabolite DHA and all other PK parameters were considered secondary endpoints or supportive data. Artesunate has been reported to be a highly variable drug; therefore, the scaled-average-bioequivalence method was to be applied to the acceptance limit of Cmax, based on the intrasubject variability of the reference product obtained from this study, while the conventional acceptance limit range of 80% to 125% was applied to AUC0-last. For tmax, median (range) values of test and reference products were compared.
RESULTS
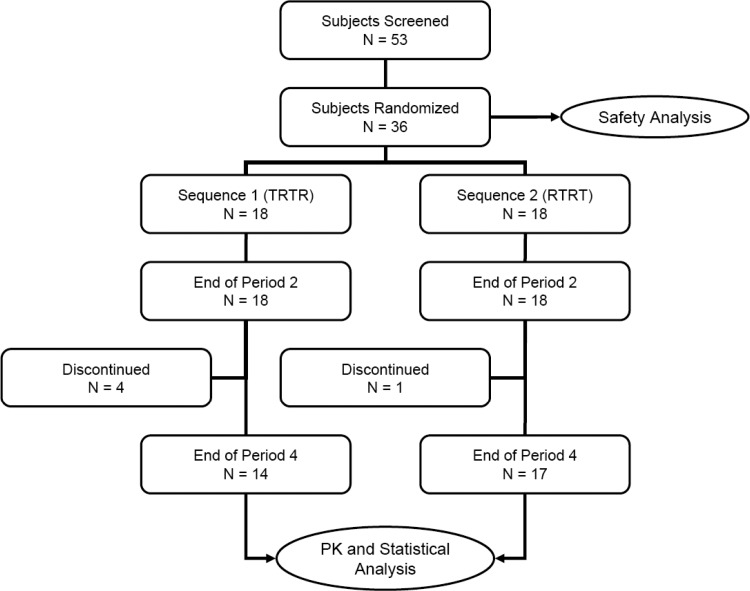
Figure 1 shows the disposition of study subjects. A total of 53 volunteers were screened within 2 weeks prior to the study, and 36 were enrolled and randomized into the treatment sequence of TRTR or RTRT according to the randomization sequence table. Five subjects discontinued the study at the end of period 2; as a result, 31 subjects completed all four periods of the study (86%) and were included in pharmacokinetic and statistical analysis for per protocol analyses. The mean ages (±standard deviations [SD]) of all subjects who completed the study (PP) were 24.8 (±3.4) years (range, 20 to 32), and the mean body weights (±SD) were 63.4 (±5.4) kilograms (range, 54.2 to 72.5 kg).
FIG 1.
Study design and subject disposition (T = test, R = reference).
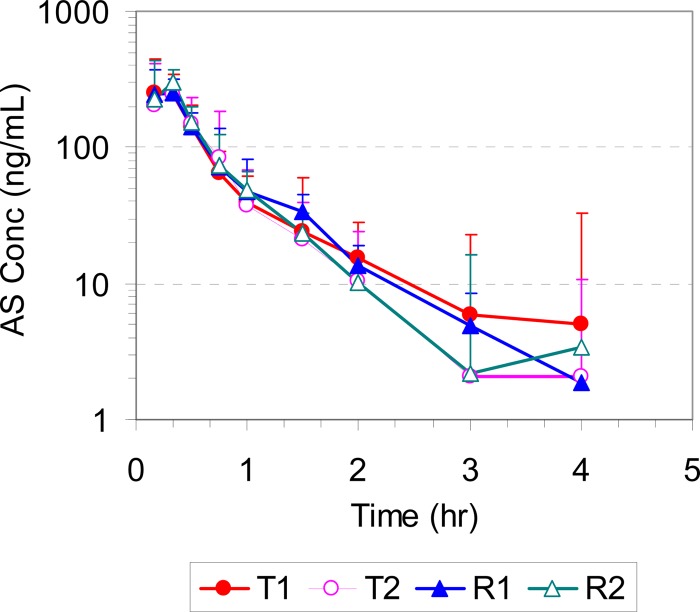
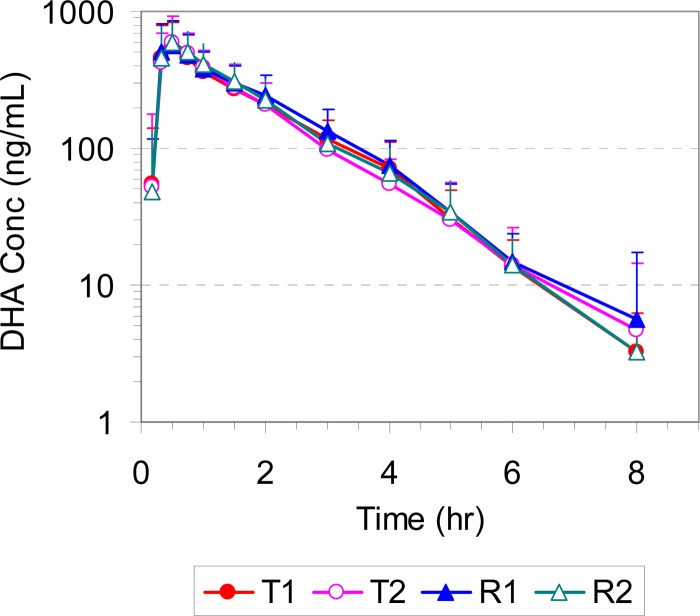
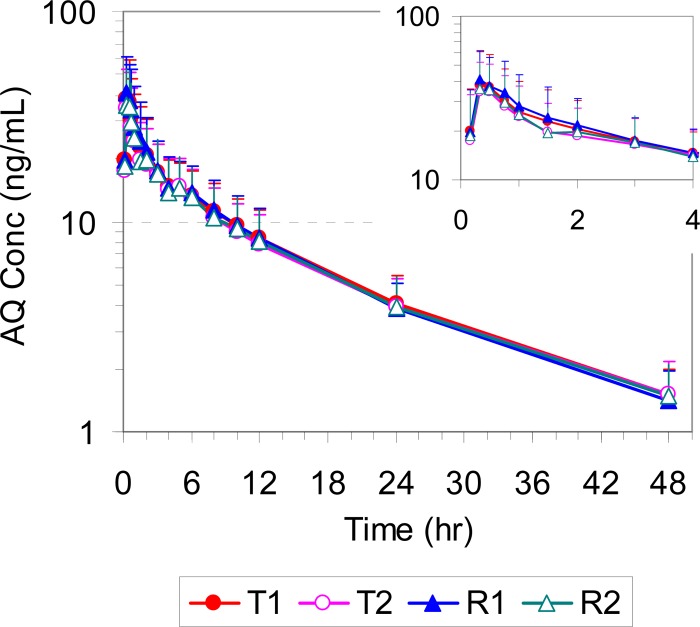
The mean plasma/blood concentrations of AS, DHA, and AQ for all four study periods (T1, T2, R1, and R2) are presented in Fig. 2, 3, and 4, respectively. The corresponding pharmacokinetic parameters are summarized in Table 1. All pharmacokinetic data and statistical calculations included 31 subjects who completed the entire study per study protocol (4 periods).
FIG 2.
Mean plasma concentration-time profiles of AS in healthy volunteers following administration of a single oral dose of the test (T1 and T2) and reference (R1 and R2) products (2× 100/270 mg ASAQ).
FIG 3.
Mean plasma concentration-time profiles of DHA in healthy volunteers following administration of a single oral dose of the test (T1 and T2) and reference (R1 and R2) products (2× 100/270 mg ASAQ).
FIG 4.
Mean plasma concentration-time profiles of AQ in healthy volunteers following administration of a single oral dose of the test (T1 and T2) and reference (R1 and R2) products (2× 100/270 mg ASAQ). The inset shows the plasma concentration-time profile from 0 to 4 h.
TABLE 1.
Summary of pharmacokinetic parameters of AS, DHA, and AQ following administration of a single oral dose of the test and reference products in Chinese healthy volunteersa
| Drug and metabolite parameter | Values for indicated treatment |
|||
|---|---|---|---|---|
| T1 | T2 | R1 | R2 | |
| Artesunate | ||||
| Cmax (ng/ml) | 312 ± 161 (51.6) | 307 ± 197 (64.1) | 330 ± 189 (57.2) | 342 ± 214 (62.5) |
| Median Tmax in h (range) | 0.33 (0.17–1.5) | 0.33 (0.17–1.0) | 0.33 (0.17–1.0) | 0.33 (0.17–1.0) |
| AUC0-last (ng · h/ml) | 174 ± 54.3 (31.3) | 162 ± 65.5 (40.6) | 180 ± 59.7 (33.1) | 180 ± 73.0 (40.6) |
| AUC0-inf (ng · h/ml) | 186 ± 75.4 (40.5) | 168 ± 65.4 (39.1) | 188 ± 61.2 (32.5) | 187 ± 71.5 (38.1) |
| t1/2 (h) | 0.56 ± 0.37 (66) | 0.54 ± 0.43 (79) | 0.57 ± 0.39 (68) | 0.53 ± 0.45 (85) |
| λz (1/h) | 1.8 ± 1.1 (61) | 1.9 ± 1.2 (62) | 1.7 ± 1.1 (63) | 2.0 ± 1.2 (58) |
| Dihydroartemisinin | ||||
| Cmax (ng/ml) | 606 ± 319 (52.6) | 661 ± 304 (46.0) | 648 ± 288 (44.5) | 644 ± 265 (41.2) |
| Median Tmax in h (range) | 0.50 (0.33–1.5) | 0.50 (0.33–1.5) | 0.50 (0.33–2.0) | 0.50 (0.33–2.0) |
| AUC0-last (ng · h/ml) | 983 ± 338 (34.4) | 980 ± 272 (28.1) | 1,081 ± 275 (25.4) | 1,040 ± 277 (26.6) |
| AUC0-inf (ng · h/ml) | 993 ± 338 (34.1) | 991 ± 338 (27.5) | 1,090 ± 274 (25.1) | 1,052 ± 274 (26.0) |
| t1/2 (h) | 1.1 ± 0.3 (23) | 1.1 ± 0.5 (45) | 1.1 ± 0.3 (24) | 1.1 ± 0.3 (30) |
| λz (1/h) | 0.7 ± 0.1 (21) | 0.7 ± 0.1 (24) | 0.7 ± 0.1 (20) | 0.7 ± 0.1 (22) |
| Amodiaquine | ||||
| Cmax (ng/ml) | 41.2 ± 22.2 (53.7) | 39.1 ± 19.8 (50.6) | 45.1 ± 20.5 (45.4) | 39.7 ± 19.3 (48.6) |
| Median Tmax in h (range) | 0.50 (0.33–1.0) | 0.50 (0.17–2.0) | 0.50 (0.33–1.0) | 0.50 (0.33–0.75) |
| AUC0-last (ng · h/ml) | 331 ± 125 (37.9) | 311 ± 137 (44.1) | 327 ± 134 (40.8) | 317 ± 140 (44.1) |
| AUC0-inf (ng · h/ml) | 355 ± 126 (35.4) | 337 ± 139 (41.2) | 352 ± 134 (38.0) | 341 ± 142 (41.7) |
| t1/2 (h) | 12.2 ± 2.3 (19.0) | 12.2 ± 2.2 (17.9) | 11.6 ± 2.4 (21.0) | 12.1 ± 2.4 (20.0) |
| λz (1/h) | 0.06 ± 0.01 (21.8) | 0.06 ± 0.01 (21.1) | 0.06 ± 0.02 (24.2) | 0.06 ± 0.01 (23.1) |
The test and reference products were administered as 2× 100/270 mg ASAQ. Results are presented as means ± standard deviations (SD) (interindividual percent coefficient of variation [CV]) for all subjects who completed the study per protocol (n = 31) except where otherwise indicated. T1, the first time a subject received the test product; T2, the second time a subject received the test product; R1, the first time a subject received the reference product; R2, the second time a subject received the reference product.
Following oral administration of both test and reference products, AS was rapidly absorbed, reaching the peak concentration in 20 min, and formed its main active metabolite DHA, whose concentration peaked at 30 min. Both AS and DHA were cleared rapidly from the systemic circulation, with plasma concentrations falling below the limit of quantitation by 4 and 8 h postdose and with mean elimination half-lives of approximately 0.55 and 1.1 h, respectively. AQ was also rapidly absorbed, reaching its peak concentration in 30 min, but cleared much more slowly, with a mean elimination half-life of 11.6 to 12.2 h.
Statistical comparisons of the AS component of the test and reference products showed that the geometric least square mean ratios (T/R) of Cmax and AUC0-last values were 95.9% and 93.9%, respectively, and the corresponding 90% confidence intervals were 84.5% to 108.7% and 87.2% to 101.1%; all fell within the conventional 80% to 125% acceptance limits for bioequivalence (Table 2). The study did demonstrate high intrasubject variability for Cmax values for AS at 39.2% and 43.7% for the test and reference products, respectively (Table 2). Nevertheless, it was not necessary to widen the bioequivalence limit using the scaled-average-bioequivalence approach in this particular study. For the metabolite DHA, geometric least square mean ratios (T/R) of Cmax and AUC0-last were 96.2% and 91.9%, respectively, and the corresponding 90% confidence intervals were 86.8% to 106.6% and 87.4% to 96.6%, respectively. Both were within the conventional 80% to 125% acceptance limits and provided supportive evidence for bioequivalence. For AQ, the second component of two FDC products, geometric least square mean ratios (T/R) of Cmax and AUC0-last were 95.0% and 100.0%, respectively, and the corresponding 90% confidence intervals were 86.7% to 104.1% and 93.5% to 107.0, respectively. Again, all were within the 80% to 125% acceptance limits for bioequivalence (Table 2). Intrasubject variability for AQ was slightly higher than the 30% defined by the EMA guideline for highly variable drug products.
TABLE 2.
Statistical comparison of pharmacokinetic parameters for AS, DHA, and AQ following administration of a single oral dose of the test and reference products in Chinese healthy volunteersa
| Drug and metabolite parameters | GLSM ratio (test/reference) | 90% CI | Intrasubject CV% |
|
|---|---|---|---|---|
| Test | Reference | |||
| Artesunate | ||||
| Cmax (ng/ml) | 95.9 | 84.5–108.7 | 39.2 | 43.7 |
| AUC0-last (ng · h/ml) | 93.9 | 87.2–101.1 | 24.2 | 25.1 |
| AUC0-inf (ng · h/ml) | 94.2 | 87.2–101.8 | 24.9 | 25.8 |
| t1/2 (h) | 99.9 | 86.2–115.8 | ||
| Dihydroartemisinin | ||||
| Cmax (ng/ml) | 96.2 | 86.8–106.6 | 36.5 | 31.9 |
| AUC0-last (ng · h/ml) | 91.9 | 87.4–96.6 | 14.3 | 18.6 |
| AUC0-inf (ng · h/ml) | 91.9 | 87.5–96.5 | 18.2 | 13.7 |
| t1/2 (h) | 98.5 | 92.7–104.6 | ||
| Amodiaquine | ||||
| Cmax (ng/ml) | 95.0 | 86.7–104.1 | 30.6 | 30.2 |
| AUC0-last (ng · h/ml) | 100.0 | 93.5–107.0 | 20.8 | 21.8 |
| AUC0-inf (ng · h/ml) | 100.1 | 94.2–106.4 | 18.7 | 19.6 |
| t1/2 (h) | 102.7 | 97.8–107.9 | ||
The test and reference products were administered as 2× 100/270 mg ASAQ. GLSM ratio, geometric least square mean ratio of natural log transformed PK parameters; 90% CI, 90% confidence interval; intrasubject CV%, percent coefficient of variation of intrasubject variability. Results were calculated using SAS software for all subjects who completed the study per protocol (n = 31).
Safety data were collected for all subjects who enrolled the study (n = 36). A total of 172 adverse events (AEs) were reported in this study, 154 of which were deemed by the clinical investigator to be probably treatment related. Table 3 lists all AEs reported from this study. Overall, the incidences of AEs between the test and reference products were similar (n = 78 for the test product and n = 76 for the reference product). In general, these adverse events are consistent with those reported for ASAQ Winthrop (Coarsucam) or amodiaquine, with the most frequent adverse events including gastrointestinal and nervous system disorders, both of which are listed in the Summary of Product Characteristics (SmPC) as common (1% to 10% frequency) adverse reactions (4). It was noted that the frequency of skin reactions (flush) was greater than that reported in the SmPC, possibly due to sensitivity in the current study population. Most of the adverse events were mild to moderate in severity, and most subjects fully recovered within a few hours to a few days after onset, without requiring medical treatment. Three subjects were asked by the principal investigator (PI) to withdraw from the study due to AE-related tolerability issues after they completed the first half of the study (end of period 2), and two other subjects voluntarily withdrew from the study without reported AEs. Since this was a 4-period study, all five discontinued subjects had received both test and reference products after completing 2 periods of the study.
TABLE 3.
Incidence of all adverse eventsa
| Adverse event | No. (%) of subjects with adverse events by treatment group |
|||
|---|---|---|---|---|
| Test drug (n = 36) |
Reference drug (n = 36) |
|||
| Treatment related | Non-treatment related | Treatment related | Non-treatment related | |
| Body as a whole | ||||
| Fatigue | 3 (1.7) | 2 (1.2) | ||
| Cardiovascular | ||||
| Atrioventricular block, first degree | 1 (0.6) | 1 (0.6) | ||
| Palpitation | 2 (1.2) | |||
| Postural hypotension | 2 (1.2) | 1 (0.6) | ||
| Gastrointestinal | ||||
| Abdominal pain | 5 (2.9) | 9 (5.2) | ||
| Bloating | 5 (2.9) | 3 (1.7) | ||
| Burning stomach | 1 (0.6) | 1 (0.6) | ||
| Nausea | 6 (3.5) | 11 (6.4) | ||
| Vomiting | 1 (0.6) | 1 (0.6) | ||
| Diarrhea | 6 (3.5) | 6 (3.5) | ||
| Respiratory | ||||
| Cough | 2 (1.2) | 1 (0.6) | 1 (0.6) | |
| Flu symptoms | 1 (0.6) | 1 (0.6) | 1 (0.6) | |
| Nasal congestion | 3 (1.7) | 3 (1.7) | ||
| Upper respiratory infection | 1 (0.6) | 1 (0.6) | ||
| Psychiatric and nervous system | ||||
| Loss of appetite | 2 (1.2) | 3 (1.7) | ||
| Dizziness | 19 (11.0) | 15 (8.7) | ||
| Drowsiness | 2 (1.2) | 1 (0.6) | ||
| Skin | ||||
| Flushed | 12 (7.0) | 14 (8.1) | ||
| Laboratory test | ||||
| Decrease in neutrophiles | 1 (0.6) | 2 (1.2) | ||
| Decrease in WBC | 1 (0.6) | 2 (1.2) | 1 (0.6) | |
| Increase in ALT | 2 (1.2) | |||
| Increase in AST | 1 (0.6) | |||
| Increase in bilirubin | 1 (0.6) | |||
| Miscellaneous | ||||
| Amaurosis | 1 (0.6) | 2 (1.2) | ||
| Dental ulcer | 1 (0.6) | 1 (0.6) | ||
| Dyspepsia | 1 (0.6) | |||
| Headache | 1 (0.6) | |||
| External ear inflammation | 1 (0.6) | 1 (0.6) | 1 (0.6) | |
| Night sweat | 1 (0.6) | |||
| Pharyngalgia | 1 (0.6) | |||
| Total (R or NR) | 78 (45) | 8 (4.7) | 76 (44) | 10 (5.8) |
| Total (R + NR) | 86 (50) | 86 (50) | ||
Data represent adverse events of mild to moderate severity. WBC, white blood cells; ALT, alanine transaminase; AST, aspartate transaminase; R, treatment related; NR, non-treatment related.
DISCUSSION
The current study was conducted to participate in the WHO Prequalification of Medicines Programme, which aims to make high-quality and affordable medicines available for the benefit of those in need. Artesun-Plus is a fixed-dose combination antimalarial agent containing artesunate and amodiaquine formulated to be an alternative to the brand-name product ASAQ Winthrop. To establish the interchangeability of Artesun-Plus with ASAQ Winthrop, this study compared the in vivo pharmacokinetic and safety profiles of the two products and adopted a full replicate study design to overcome the difficulty presented by the high variability of AS in which the subjects took both the test and reference products twice. This study design enabled us to determine the true intrasubject variability for the test and reference products independently and enabled us to apply the scaled-average-bioequivalence approach, which offers more-flexible bioequivalence acceptance criteria for highly variable drug products, according to the current EMA/CHMP Guidelines on the Investigation of Bioequivalence (7).
This report also emphasizes the evaluation of parent drugs, as bioequivalence studies aim to compare the absorption profiles of the test and reference formulations. As such, the PK and statistical results determined for DHA, the major and active metabolite of AS, were used only as supportive evidence for bioequivalence assessment. Furthermore, desethylamodiaquine (DEAQ), the major and active metabolite of AQ, was not even included in the bioanalytical assay. DEAQ was previously reported to have a half-live of 3 to 8 days in whole blood (5, 6) and was expected to accumulate using the 7-day washout periods between the four study periods. Such an accumulation would result in a carryover effect on DEAQ and confound the bioequivalence evaluation of this metabolite. However, as long as the accumulation of the metabolite did not impact the clearance of the parent drug AQ, it would not undermine the bioequivalence assessment for AQ. Indeed, results from bioanalysis of predose samples for study periods 2, 3, and 4 did not show any detectable AQ levels and no carryover effect was observed by statistical calculations (P value ≥0.11 for either sequence or period effects). This study design shortened the duration of this four-period study and possibly reduced the number of dropouts. However, the potential for accumulation of DEAQ may have contributed to the large number of adverse events reported from this study.
This 2-by-4 crossover study demonstrated, for the first time, high intrasubject variability for Cmax values of the AS component in FDC products (39.2% and 43.7% for the test and reference products, respectively). Previous studies have compared FDC and non-FDC products in 2-by-2 crossover studies, which estimated that the intraindividual variability for Cmax values of AS was as high as 56% (6). However, those results were confounded by the study design, averaging ANOVA results from the FDC and non-FDC products, as it is possible that the non-FDC product of AS is much more variable.
Although the intrasubject variability data from this study qualify AS in this FDC product as a “highly variable” drug and the bioequivalence acceptance criteria could be widened using the scaled-average-bioequivalence approach (7), the actual results of statistical comparisons between the test and reference products for AS (Cmax and AUC0-last) fell within the conventional acceptance limits for bioequivalence of 80% to 125%. Similar results were obtained for the DHA metabolite, which supported the bioequivalence conclusion, despite high intrasubject variability values at 36.5% and 31.9% for the test and reference products, respectively. It is noteworthy that the variability for the metabolite was considerably lower than that of the parent drug, and these findings lend more support for the study design on the basis of the understanding that it is more appropriate to assess bioequivalence using the parent drug, as opposed to its metabolite, as the parent drug is more sensitive to subtle changes, even within a given subject.
In conclusion, the current study demonstrated that Artesun-Plus, a FDC product composed of artesunate and amodiaquine (100/270 mg), is bioequivalent to the reference product Artesunate Amodiaquine Winthrop and that the two products showed similar pharmacokinetic and safety profiles.
ACKNOWLEDGMENTS
This study was supported by Guilin Pharmaceutical Co., Ltd. (Guilin, China), the sponsor of the Artesun-Plus for WHO Prequalification of Medicines Programme. We thank the study volunteers, clinical investigators, study coordinators, clinical research associates (CRAs), and the administrative staff at the Shanghai Xuhui Central Hospital who made this study possible.
We have no other conflicts of interest regarding the sponsor or the content of this article.
Footnotes
Published ahead of print 28 July 2014
REFERENCES
- 1.World Health Organization. 2010. Guidelines for treatment of malaria, 2nd ed. World Health Organization, Geneva, Switzerland [Google Scholar]
- 2.World Health Organization. 2010. World malaria report. World Health Organization, Geneva, Switzerland [Google Scholar]
- 3.German PI, Aweeka FT. 2008. Clinical pharmacology of artemisinin-based combination therapies. Clin. Pharmacokinet. 47:91–102. 10.2165/00003088-200847020-00002 [DOI] [PubMed] [Google Scholar]
- 4.Sanofi-Aventis. 2010. Summary of product characteristics: ASAQ Winthrop (artesunate, amodiaquine). Sanofi-Aventis, Reston, VA [Google Scholar]
- 5.Navaratnam V, Ramanathan S, Wahab MS, Siew Hua G, Mansor SM, Kiechel JR, Vaillant M, Taylor WR, Olliaro P. 2009. Tolerability and pharmacokinetics of non-fixed and fixed combinations of artesunate and amodiaquine in Malaysian healthy normal volunteers. Eur. J. Clin. Pharmacol. 65:809–821. 10.1007/s00228-009-0656-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fortin A, Verbeeck RK, Jansen FH. 2011. Comparative oral bioavailability of non-fixed and fixed combinations of artesunate and amodiaquine in healthy Indian male volunteers. Eur. J. Clin. Pharmacol. 67:267–275. 10.1007/s00228-010-0911-5 [DOI] [PubMed] [Google Scholar]
- 7.European Medicines Agency (EMA)/Committee for Medicinal Products for Human Use (CHMP). 2010. Guideline on the investigation of bioequivalence. EMA/CHMP, London, United Kingdom [Google Scholar]
- 8.European Medicines Agency (EMA)/Committee for Medicinal Products for Human Use (CHMP). 2011. Guideline on bioanalytical method validation. EMA/CHMP, London, United Kingdom [Google Scholar]
- 9.World Health Organization. 2006. Guidelines on registration requirements to establish interchangeability and for in vivo bioequivalence studies, annex 7 and 9, WHO Technical Report Series 937, 4th report World Health Organization, Geneva, Switzerland [Google Scholar]
- 10.Chinese FDA. 2005. Guideline for human bioavailability and bioequivalence studies for pharmaceuticals. China Food and Drug Administration, Beijing, China [Google Scholar]
- 11.WHO. 2000. Good laboratory practice, special programme for research and training in tropical diseases. World Health Organization, Geneva, Switzerland [Google Scholar]
- 12.Hanpithakpong W, Kamanikom B, Dondorp AM, Singhasivanon P, White NJ, Day NP, Lindegardh N. 2008. A liquid chromatographic-tandem mass spectrometric method for determination of artesunate and its metabolite dihydroartemisinin in human plasma. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 876:61–68. 10.1016/j.jchromb.2008.10.018 [DOI] [PubMed] [Google Scholar]
- 13.Hodel EM, Zanolari B, Mercier T, Biollaz J, Keiser J, Olliaro P, Genton B, Decosterd LA. 2009. A single LC-tandem mass spectrometry method for the simultaneous determination of 14 antimalarial drugs and their metabolites in human plasma. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 877:867–886. 10.1016/j.jchromb.2009.02.006 [DOI] [PubMed] [Google Scholar]
- 14.U.S. FDA. 2001. Statistical approaches to establishing bioequivalence, guidance to industry. Center for Drug Evaluation and Research (CDER). U.S. FDA, Rockville, MD [Google Scholar]