Abstract
Dihydroartemisinin-piperaquine, the current first-line drug for uncomplicated malaria caused by Plasmodium falciparum and Plasmodium vivax in Cambodia, was previously shown to be of benefit as malaria chemoprophylaxis when administered as a monthly 3-day regimen. We sought to evaluate the protective efficacy of a compressed monthly 2-day treatment course in the Royal Cambodian Armed Forces. The safety and efficacy of a monthly 2-day dosing regimen of dihydroartemisinin-piperaquine were evaluated in a two-arm, randomized, double-blind, placebo-controlled cohort study with 2:1 treatment allocation. Healthy military volunteers in areas along the Thai-Cambodian border where there is a high risk of malaria were administered two consecutive daily doses of 180 mg dihydroartemisinin and 1,440 mg piperaquine within 30 min to 3 h of a meal once per month for a planned 4-month period with periodic electrocardiographic and pharmacokinetic assessment. The study was halted after only 6 weeks (69 of 231 projected volunteers enrolled) when four volunteers met a prespecified cardiac safety endpoint of QTcF (Fridericia's formula for correct QT interval) prolongation of >500 ms. The pharmacodynamic effect on the surface electrocardiogram (ECG) peaked approximately 4 h after piperaquine dosing and lasted 4 to 8 h. Unblinded review by the data safety monitoring board revealed mean QTcF prolongation of 46 ms over placebo at the maximum concentration of drug in serum (Cmax) on day 2. Given that dihydroartemisinin-piperaquine is one of the few remaining effective antimalarial agents in Cambodia, compressed 2-day treatment courses of dihydroartemisinin-piperaquine are best avoided until the clinical significance of these findings are more thoroughly evaluated. Because ECG monitoring is often unavailable in areas where malaria is endemic, repolarization risk could be mitigated by using conventional 3-day regimens, fasting, and avoidance of repeated dosing or coadministration with other QT-prolonging medications. (This study has been registered at ClinicalTrials.gov under registration no. NCT01624337.)
INTRODUCTION
Worldwide, an estimated 219 million people suffered from malaria infection in 2010 (1). Advancements in malaria prevention could reduce global morbidity and mortality and serve as a means to achieve malaria elimination. This is particularly true in the Greater Mekong Subregion where multidrug-resistant Plasmodium falciparum continues to present challenges to current treatment paradigms. Despite recent containment and control efforts in the region, both multidrug-resistant Plasmodium falciparum and Plasmodium vivax malaria continue to be prevalent in Cambodia, particularly along the Thai border (2).
Dihydroartemisinin-piperaquine is regarded as a well-tolerated medication for malaria treatment with demonstrated efficacy against multidrug-resistant P. falciparum, particularly in Southeast Asia (3, 4). Widely used in Cambodia, dihydroartemisinin-piperaquine is currently recommended as the first-line agent for both P. falciparum and P. vivax malaria (5). Dihydroartemisinin-piperaquine was recently investigated as a chemoprophylaxis agent in neighboring Thailand. A study of 1,000 mobile migrant volunteers on the Thai-Burmese border found that a standard 3-day treatment course consisting of three consecutive daily fixed doses of 120 mg dihydroartemesinin and 960 mg piperaquine given monthly was highly effective compared to placebo (6).
This study aimed to gather further evidence on the protective efficacy and safety of a repeated monthly 2-day treatment course of dihydroartemisinin-piperaquine to prevent malaria. Neither civilian nor military personnel in Cambodia routinely use malaria chemoprophylaxis. While dihydroartemisinin-piperaquine could potentially be used as prophylaxis, carefully controlled studies to determine its cardiac safety profile for long-term and/or repeated use have not been conducted thus far. Corrected QT interval (QTc) prolongation risk has been partially defined for single courses of treatment and incorporated into labeling instructions provided for one formulation known as Eurartesim which recently received regulatory approval from the European Medicines Agency (EMA). Current labeling recommendations include electrocardiogram (ECG) monitoring before treatment and at peak piperaquine concentrations on day 3 of therapy, along with a 3-h fast before and after the piperaquine dose (7, 8). However, electrocardiography is rarely available in areas where malaria is endemic, and a fasting period prior to dihydroartemisinin-piperaquine administration is not routinely observed. The risk for cardiac adverse events in this population is therefore uncertain. At the time of the study, the Cambodian military frequently used 2-day treatment courses of dihydroartemisinin-piperaquine (Artekin) and other piperaquine-containing products such as artemisinin-piperaquine (Artequick) given with food to improve medication compliance (9). Given this compressed dosing period and administration with food, although understandable with respect to compliance, the contradiction of formal dosing recommendations created the need to investigate QTc interval under these modified conditions.
MATERIALS AND METHODS
Study design and oversight.
The study was a two-arm, randomized, double-blind, placebo-controlled study to determine the protective efficacy of a 2-day course of dihydroartemisinin-piperaquine given monthly, with 2:1 allocation of treatment to placebo.
This trial was registered with ClinicalTrials.gov (NCT01624337). The study protocol was approved by institutional review boards at participating institutions, including the Walter Reed Army Institute of Research and the National Ethics Committee for Health Research in Cambodia. All participants provided written informed consent.
Study site and participants.
From May to June 2012, the study team enrolled and monitored otherwise healthy military and civilian dependent personnel aged 18 to 65 years at multiple remote study sites in Oddar Meanchay province in Cambodia near the northern Cambodian-Thai border. Many participants were occupationally required to spend prolonged periods of time outside in forested areas. At the time of the study and at present, there remains no standard of care for chemoprophylaxis or personal protective measures, though treated bed nets have been widely distributed. Study visits were completed at a regional medical treatment facility, or in some cases, by outreach visit to study participants. Entry criteria included volunteers residing in areas where malaria is endemic that were accessible to the study team for the duration of the study, able to provide informed consent, and agreeing to follow the study protocol. Potential volunteers with a history of hypersensitivity or adverse reaction to dihydroartemisinin-piperaquine, positive malaria smear, or history of antimalarial use in the past 30 days, QTcF (Fridericia's formula for corrected QT interval) interval of greater than 450 ms at baseline (470 ms for females), family history of sudden cardiac death, use of QT-prolonging medications, pregnant or lactating females, and/or females unwilling to use an acceptable form of contraception during the study were excluded. Volunteers reporting more than one malaria infection in the past year and/or more than two reported malaria infections in their lifetime if at least one infection occurred in the past 2 years were excluded.
Drug therapy and randomization.
Combination tablets containing 40 mg of dihydroartemisinin and 320 mg of piperaquine phosphate (Duo-cotecxin; Zhejiang Holley Nanhu Pharmaceutical Co., Ltd.) were used, with placebo tablets matched for size, color, and composition provided by the manufacturer. The treatment randomization code was generated prior to the study by a statistician, using the PROC PLAN function in SAS v.9.2 for a sample size of 300 using time-blocked randomization with a block size of three. The test article was received from the manufacturer in identical packaging and prepared by two study pharmacists. All treatment was administered blinded by directly observed therapy by the investigative team to ensure medication compliance. On enrollment, volunteers were randomized to one of two double-blinded treatment arms to receive either 4.5 tablets of dihydroartemisinin-piperaquine daily (180 mg dihydroartemisinin and 1,440 mg piperaquine) for 2 days once monthly (for a total monthly dose of 360 mg dihydroartemisinin and 2,880 mg piperaquine) or placebo.
Allocation was masked by having volunteers select randomization codes in sealed envelopes. Volunteers ate a serving of steamed rice and 40 g fried pork (considered low to moderate fat content of ∼15 g of fat per meal) within 30 min to 3 h of study drug administration. The taste of the test article was concealed by administering the tablets coated with marmalade. Dropouts were replaced in the same group by a pharmacist to ensure a consistent balance of allocation and exposure. Pharmacokinetic analysis was conducted on batched, frozen samples only after the study had been halted.
Procedures.
Volunteers had an initial history and physical examination, baseline 12-lead electrocardiogram (ECG), malaria blood films, and antibody titers to both Plasmodium falciparum merozoite surface protein 1 (PfMSP-1) and Plasmodium vivax merozoite surface protein 1 (PvMSP-1). Safety assessments included a complete blood count (CBC), quantitative and qualitative glucose-6-phosphate dehydrogenase (G6PD) activity, as well as serum levels of creatinine, total protein, aspartate aminotransferase (AST), alanine aminotransferase (ALT), electrolytes, including potassium, magnesium, calcium, and thyroid-stimulating hormone (TSH). Eligible volunteers were enrolled in the study and administered the initial 2-day course of test article; subsequent monthly clinic visits were then scheduled for test article receipt and brief clinical assessment prior to dosing to screen for adverse events and Plasmodium infection. Volunteers were monitored between visits by trained paramedical personnel and referred for blood film examination at any time there was clinical suspicion of malaria.
Giemsa-stained thick and thin smears were examined using a three-reader paradigm where two microscopists, blinded to each other's results and to volunteer treatment status, initially read each slide (2). Parasite densities were calculated based on parasite counts per 200 white blood cells (WBCs) on thick smear or per 5,000 red blood cells (RBCs) on thin smear. A total of 200 oil immersion fields were examined on the thick film before it was considered negative.
A 12-lead ECG study was performed on each volunteer at screening and each month, at 0 h (predose), 4 and 24 h (prior to second dose), and 28 h relative to the first dose (4 h after the second dose) based on prior reports of expected peak maximum concentration of drug in serum (Cmax) in Cambodian adults (10). ECGs were also performed at the time of malaria diagnosis if the volunteer was infected and at study discharge. Volunteers with positive malaria smears during follow-up were considered to have met the primary endpoint and admitted to an inpatient facility for treatment under current national treatment guidelines (artesunate-mefloquine therapy at the time of the study). During treatment, vital signs were evaluated every 8 h until the temperature was less than 38°C for 24 h. Blood film examination was performed daily until 2 consecutive negative smears were obtained. Patients were discharged from the facility once clinical and parasitological resolution of malaria infection (afebrile with 2 negative consecutive smears at least 6 h apart) was achieved, and therapy was completed.
All volunteers were screened for G6PD deficiency using a qualitative fluorescent spot test method (SQMMR500; R&D Diagnostic) and a quantitative Trinity Biotech test kit (product code 345-B; Trinity Biotech, Bray, Ireland), respectively. Volunteers with normal or mild-moderate G6PD deficiency (WHO classes III, IV, and V) were offered presumptive antirelapse therapy with commercially obtained primaquine phosphate upon study discharge and monitored for an additional 3 months to monitor for adverse events and malaria recurrence (11). Primaquine phosphate at a dose of 30 mg per day for 14 days was given to volunteers with normal G6PD levels, while those who were G6PD deficient received 45 mg once weekly for 8 weeks.
To determine test article drug levels, plasma samples were collected from all volunteers at times 0 (predose), 4, 24, and 28 h after the first dose at each monthly visit. Quantification of piperaquine was performed using a previously described method (12), determined by ultraperformance liquid chromatography followed by tandem mass spectrometry (UPLC/MS/MS), and reported as quantities of piperaquine base (molecular weight [MW] of 535.54). If manual QTc (QTcFm) was greater than 450 ms following the second monthly dose of study drug, drug levels were repeated weekly until the QTcFm intervals normalized.
Simulation of three dosing regimens (1,440 mg piperaquine daily for 2 days, 960 mg piperaquine daily for 3 days and for 5 days) based on parameters derived from previously collected data (12) was performed via two-compartment model with first-order absorption and elimination using Phoenix WinNonlin version 1.3, Pharsight, USA.
Safety and electrocardiogram measurement.
All volunteers receiving dihydroartemisinin-piperaquine were monitored for adverse events, including ECG monitoring, from the time of antimalarial treatment administration until study discharge or volunteer's last visit; they were classified using the common terminology criteria for adverse events (CTCAE), v4.03 (13).
Standard 12-lead electrocardiograms were obtained at a paper speed of 25 mm/s and voltage of 10 mm/mV. The RR and QT intervals from each paper tracing were measured manually by one of three trained readers using a standardized approach, and all tracings were digitized and stored using Cardiosoft software (GE Healthcare, v 6.61). Each participant had QT intervals averaged from three consecutive 10-s tracings between 1 and 5 min part. Tracings rendered noninterpretable by patient movement or other artifact were retained but clearly marked and not used for the purposes of calculation.
For each electrocardiogram, the PR, RR, QRS, and QT intervals were manually measured with an analogue micrometer, accurate to 1/1,000 of an inch, for three consecutive beats. Lead II was used unless the T-wave morphology was indistinct, in which case lead V5 or V2 were used. The same lead was used for each subject. The QT interval was defined as the time between QRS onset to the intersection point of the baseline with the tangent line to the steepest down slope of the intervening T wave. U waves were excluded unless they were >50% the height of the T wave and fused without an intervening isoelectric period. The RR interval was measured from the preceding QRS complex to the measured QRS complex (14). QT intervals were corrected based on both Bazett's formula (QTcB = QT/√RR) and Fridericia's formula (QTcF = QT/3√RR).
Initial determinations to halt subjects were made based on the electronic QTcF (QTcFe) as calculated from the QT and RR intervals reported by the MAC 1200 portable ECG (General Electric) based on preprogrammed electronic reading algorithms. If the QTcFe interval at 4 h postdose on day 2 was greater than 450 ms but less than 500 ms, volunteers were asked to return for repeat ECG, drug level, and electrolyte evaluation at 1-week intervals until the QTcFe had returned to its baseline. If the QTcFe interval was greater than 500 ms or prolonged more than 25% compared to the predose value, serial ECGs were obtained every 2 to 4 h until the QTcFe had returned to its baseline. These cases were subsequently referred to the Data Safety Monitoring Board (DSMB) for a safety review for possible individual halting. The DSMB was required to perform a review of all data and suspend the study when more than three volunteers were found to have greater than 500-ms QTcFe prolongation. Final decisions regarding subject disposition and adverse event grading were made based on manual QTcF measurements.
Interrater variability for the three investigators responsible for QT interval measurements in the study (M. Spring, C. Lon, and D. Saunders) was assessed by comparing blinded, independent readings using the same selection of 91 ECGs. One-way analysis of variance (ANOVA) with Tukey's posttest was used to compare the average manual QT, RR, and resultant QTcF intervals measured from three consecutive ECG complexes from a 10-second lead II rhythm strip. A Bland-Altman plot was used to analyze the agreement between readers by plotting the percent difference against mean values (GraphPad Prism 5).
Statistical analysis.
The primary outcome of interest was the protective efficacy of monthly dihydroartemisinin-piperaquine in the modified intention-to-treat population, which included volunteers who received at least one dose of dihydroartemisinin-piperaquine, in a setting of mixed multidrug-resistant P. falciparum and P. vivax malaria. Protective efficacy was defined as the reduction in the incidence of malaria between treatment and placebo groups. Secondary outcomes included the safety and tolerability of repeated monthly dihydroartemisinin-piperaquine treatment with particular attention to cardiac repolarization abnormalities in relation to piperaquine levels.
Based on an a priori estimate using passive and actively collected data, an all-malaria attack rate of at least 30% was expected over the planned 4-month follow-up period. Using a 5% one-sided type 1 error rate, the study had 80% power to detect a protective efficacy (PE) of 95% with 231 projected volunteers enrolled.
The safety analysis population included all volunteers who received at least one dose of study drug. Piperaquine drug levels were compared with changes in QTcFm intervals from baseline in order to determine effect. Drug levels for individuals were correlated with (i) changes in QTcFm intervals from baseline using simple linear regression analysis and (ii) for the entire population using nonparametric correlation analysis to generate Spearman's ρ statistic. Linear regression was used to determine the individual correlation between plasma piperaquine concentration and change of QTcFm over baseline. Subjects who had a significant r2 were considered to be “piperaquine sensitive,” while those who did have a significant r2 were considered “piperaquine insensitive.” Contingency testing (Fisher's exact test) was used to assess the effect of piperaquine on QTc prolongation. The pooled piperaquine concentration-effect data (ΔQTcFm) did not support a maximum-effect (Emax) structure based on visual inspection, so a linear relationship was developed to estimate the slope using observed piperaquine concentrations as the independent variable (GraphPad Prism 5).
For all other descriptive statistics and comparisons, normally distributed continuous data were compared using Student's t test or analysis of variance. Otherwise, Mann-Whitney U test or Kruskal-Wallis test was used. Nominal data, including occurrence of adverse events (AEs), were expressed as percentages and compared using chi-square tests. Ordinal data were compared using rank (nonparametric) methods. With the exception of AEs, nominal significance levels of P < 0.05 were considered statistically significant for preplanned comparisons. For other categorical variables, proportions were examined using χ2 test or Fisher's exact test. Analyses were done using SAS software, version 9.2 (SAS Corporation, Carey, NC), and GraphPad Prism 5 (La Jolla, CA).
RESULTS
Screening and enrollment.
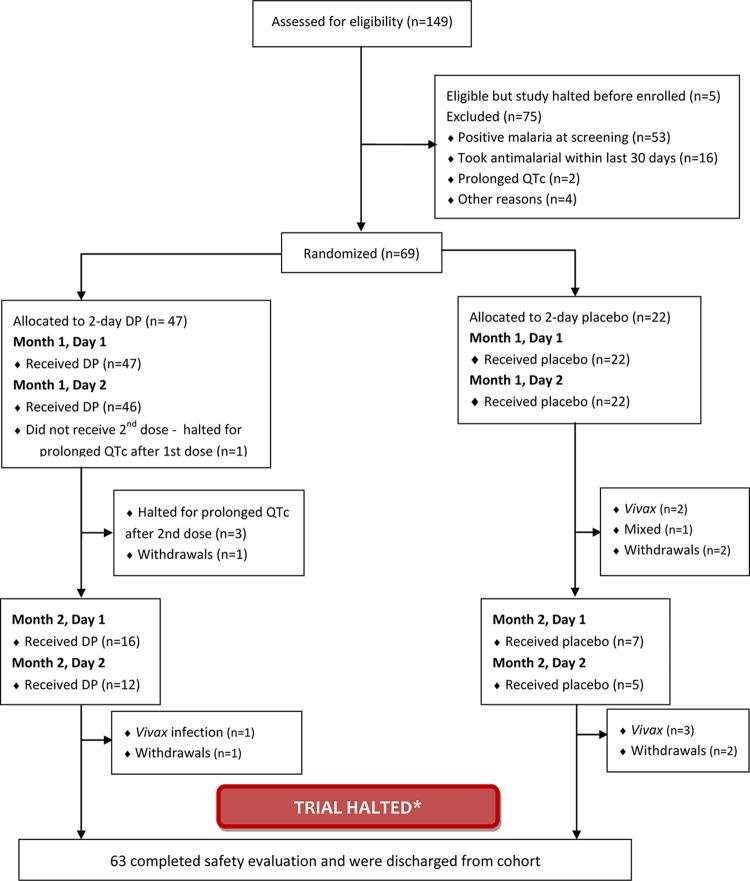
Figure 1 summarizes participant flow before the trial was halted, with 149 volunteers screened, and 69 enrolled and randomized. Of those excluded at screening, only two were found to have prolonged QT intervals. Forty-seven volunteers were randomized to receive dihydroartemisinin-piperaquine and 22 volunteers were randomized to receive placebo. One volunteer in the treatment arm was withdrawn by the investigator for taking antimalarial agents outside of the study. Three volunteers in the placebo arm withdrew from the study because of military duties (n = 2) or moving outside the study area (n = 1). Two volunteers (one in each group) were lost to follow-up, while 63 volunteers completed the scheduled safety follow-up after the study was halted.
FIG 1.
CONSORT (consolidated standards of reporting trials) flow diagram. The patients were given dihydroartemisinin-piperaquine (DP) or placebo. The trial was halted after the prespecified cardiac safety endpoint was met with 4 episodes of QTcF prolongation > 500 ms at 4- to 8-h postdose.
A prespecified cardiac safety endpoint was met, and the study was suspended after only 6 weeks when four volunteers developed a prolonged QTc interval (≥500-ms QTcF interval or ≥25% increase in milliseconds from baseline) (see Table S1 in the supplemental material). Only 69 of the projected 231 volunteers had been enrolled in the study at that point. In the treatment arm, one volunteer met the criteria for prolonged QTc after the first dose of dihydroartemisinin-piperaquine and treatment for this individual was halted (treatment-halted subject) (Fig. 2), while treatment for three additional volunteers was halted for prolonged QTc after completing the second dose. After treatment for the fourth participant was halted for QTc prolongation, the Cardiac Data Safety Monitoring Board (DSMB) reviewed data for all individuals enrolled in the study and determined that the overall study should be halted. The DSMB's recommendations were as follows: (i) to discontinue study enrollment and to not rechallenge previously treatment-halted subjects; (ii) if the study continued, to amend the protocol to intentionally reduce peak systemic exposure to piperaquine by either dosing over 3 days instead of 2 days and/or administering medication after a period of fasting; (iii) consider further targeted research to evaluate any intrinsic genetic/cardiac risk factors of the four treatment-halted volunteers.
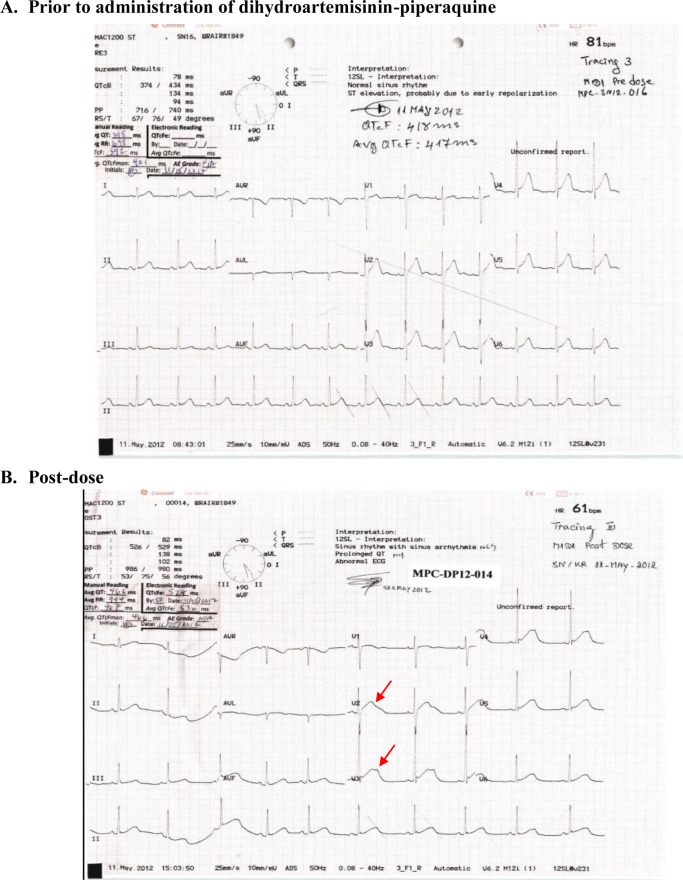
FIG 2.
Pre- and postdosing ECG tracings for the first subject whose treatment was halted. QT-U fusion waves are denoted by red arrows.
While potential cases meeting halting criteria were referred to the DSMB based on electronic readings to avoid bias from manual measurement error, the DSMB's decision to halt the study was made on a global assessment of all data, including the following:
Use of the drug in healthy volunteers with a lack of confounding risk factors
Consistent and reproducible increases in the QTcFm interval resolving on dechallenge, with an average 51-ms increase following the second daily dose of study drug compared to 5 ms with placebo
QTcFm prolongations greater than 60 ms in 3 of the 4 treatment-halted volunteers, and 18 of 46 volunteers overall after the second dose compared to none in the placebo group
Wave forms consistent with drug-induced QT interval prolongation
All volunteers were males of Khmer ethnicity with a median age of 26 years. Baseline demographic, laboratory, and clinical findings for volunteers were not significantly different between the two groups (Table 1). Mild hypokalemia was observed in 29% of participants at baseline, and mild hypomagnesemia was seen in approximately 18%. Baseline electrocardiogram findings by treatment group at enrollment in the study are detailed in Table 2. The dihydroartemisinin-piperaquine group had a higher percentage of electrocardiographic hypertrophy based on voltage criteria (31% in dihydroartemisinin-piperaquine group compared to 27% in the placebo group; P = 0.005). ECGs were not otherwise significantly different at baseline.
TABLE 1.
Baseline characteristics by treatment group
| Characteristica | Value(s) for group treated withb: |
P value | |
|---|---|---|---|
| DHA-PIP (n = 47) | Placebo (n = 22) | ||
| Male gender, no. of subjects (%) | 47 (100) | 22 (100) | |
| Age (yr) | 26.70 (7.51) | 27.27 (7.60) | 0.7704 |
| Military occupation, no. of subjects (%) | 47 (100) | 22 (100) | |
| Tobacco user, no. of subjects (%) | 2 (4.26) | 1 (4.55) | 1.00 |
| Ht (cm) | 165.1 (5.1) | 164.1 (5.5) | 0.4567 |
| Wt (kg) | 61.4 (7.3) | 57.7 (5.4) | 0.0411 |
| Predose vital signs | |||
| Tympanic temp (°C) | 37.1 (0.3) | 37.2 (0.3) | 0.2583 |
| Pulse rate (beats/min) | 73 (10) | 72 (9) | 0.5823 |
| Systolic blood pressure (mm Hg) | 118 (9) | 116 (10) | 0.5030 |
| Diastolic blood pressure (mm Hg) | 71 (9) | 71 (10) | 0.8624 |
| Hemoglobin (g/dl) | 14.1 (3.0) | 14.1 (3.3) | 0.6754 |
| % abnormal (range, 14–18 g/dl) | 28 | 27 | 0.9733 |
| MCV (fl) | 81 (8) | 81 (10) | 0.7900 |
| RDW (%) | 11.8 (2.8) | 11.7 (3.7) | 0.2651 |
| BUN (mg/dl) | 10.52 (4.11) | 9.32 (5.01) | 0.4092 |
| % abnormal (range, 8–21 mg/dl) | 1 | 0 | |
| Creatinine (mg/dl) | 0.932 (0.131) | 0.947 (0.134) | 0.6500 |
| % abnormal (range, 0.7–1.3 mg/dl) | 0 | 0 | |
| Potassium (meq/liter) | 3.84 (0.51) | 3.84 (0.30) | 0.7621 |
| % abnormal (range, 3.5–5.3 meq/liter) | 15 | 14 | |
| Magnesium (mg/dl) | 1.9 (0.3) | 1.7 (0.5) | 0.2148 |
| % abnormal (range, 1.9–2.5 mg/dl) | 12 | 6 | |
| Calcium (mg/dl) | 8.4 (2.0) | 8.1 (2.3) | 0.4404 |
| % abnormal (range, 8.1–10.4 mg/dl) | 0 | 0 | |
| TSH (μIU/ml) | 1.29 (0.54) | 1.54 (0.71) | 0.1053 |
| % abnormal (range, 0.35–4.94 μIU/ml) | 0 | 0 | |
MCV, mean corpuscular volume; RDW, red blood cell distribution width; BUN, blood urea nitrogen; TSH, thyroid-stimulating hormone.
Values for the groups given dihydroartemisinin-piperaquine (DHA-PIP) or placebo. All values are presented as means with the standard deviations (SDs) in parentheses unless otherwise stated.
TABLE 2.
Baseline and postdose ECG findings per treatment group during month 1a
| ECG characteristic | Day 1 predose (0 h) for treatment group |
P value | Day 2 postdose (28 h) for treatment group |
P value | Mean % difference for groups |
P value | |||
|---|---|---|---|---|---|---|---|---|---|
| DHA-PIP (n = 47) | Placebo (n = 22) | DHA-PIP (n = 47) | Placebo (n = 22) | DHA-PIP (n = 47) | Placebo (n = 22) | ||||
| ECG findings, N (%) | |||||||||
| Normal sinus rhythm | 84 (59.6) | 31 (47.0) | 0.089 | 172 (61.0) | 73(55.3) | 0.272 | |||
| Sinus arrhythmia | 9 (6.4) | 6 (9.1) | 0.484 | 7 (2.50) | 6 (4.56) | 0.262 | |||
| Sinus tachycardia | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |||||
| Sinus bradycardia | 41 (29.1) | 26 (39.4) | 0.140 | 16 (27.0) | 44 (33.3) | 0.182 | |||
| Early repolarization | 24 (17.0) | 17 (25.8) | 0.142 | 34 (12.1) | 34 (25.8) | <0.001 | |||
| Hypertrophy by voltage criteria | 31 (22.0) | 27 (41.0) | 0.005 | 67 (23.8) | 43 (32.6) | 0.058 | |||
| ECG intervals,b mean (SD) | |||||||||
| Heart rate | 67 (8.4) | 65 (8.2) | 0.438 | 63 (7) | 66 (10) | 0.181 | −5.5 | 0.6 | 0.005 |
| QT interval (manual) | 375 (25) | 380 (29) | 0.519 | 432 (33) | 382 (26) | <0.001 | 15.1 | 0.9 | <0.001 |
| QTcF interval | |||||||||
| QTcFm | 390 (19) | 390 (19) | 0.685 | 441 (27) | 395 (15) | <0.001 | 13.3 | 1.4 | <0.001 |
| QTcFe | 413 (13) | 414 (15) | 0.762 | 456 (25) | 414 (16) | <0.001 | 10.4 | −0.08 | <0.001 |
| QTcB interval | |||||||||
| QTcBm | 398 (20) | 396 (18) | 0.843 | 445 (27) | 402 (17) | <0.001 | 12.3 | 1.5 | <0.001 |
| QTcBe | 418 (15) | 417 (17) | 0.910 | 457 (27) | 418 (19) | <0.001 | 9.4 | 0.05 | <0.001 |
The values for ECG characteristics are shown for groups given dihydroartemisinin-piperaquine (DHA-PIP) or placebo at day predose (0 h) and day 2 postdose (28 h). The P values for the differences in the values for the two groups at different time points are shown. The values that were significantly different for two groups are shown in boldface type. n is the total number of cases. N is the total number of all tracings because three tracings were done at each time point.
Intervals averaged over three ECG readings per volunteer at each time point.
Tolerability and safety.
Aside from the cardiac safety concerns detailed elsewhere, dihydroartemisinin-piperaquine was well-tolerated. The distribution of other adverse effects between the treatment and placebo group is outlined in Table S2 in the supplemental material. The most common complaints included dizziness, headache, nasopharyngitis, abdominal pain, and nausea and were roughly equally distributed in both groups, suggesting a lack of causal relationship to study drug.
Electrocardiogram findings by treatment group.
Patients treated with dihydroartemisinin-piperaquine experienced significant increases in mean QTcFm interval during the first and second doses (month 1) compared to the placebo group (P < 0.0003; Fig. 3 and Table 3). Overall, the treatment group had a mean QTcFm increase of 25-ms prolongation over baseline at 4 h postdose (421 ± 26 ms in treatment group versus 396 ± 16 ms in the placebo group; P < 0.0001) and 51-ms prolongation at 4 h after the second dose (432 ± 33 ms in treatment group versus 382 ± 26 ms in the placebo group; P < 0.0001; Table 2). In the treatment group, the change in mean QTcFm interval from prior to the first dose to after the last dose was 42.5 ms (standard error, 5). QT interval increases in the treatment-halted volunteers ranged from 19 to 30% by QTcFe and 16 to 19% by QTcFm, with a mean 10% increase overall by QTcFe and 13% by QTcFm for the treated group compared to the placebo group (see Table S2 in the supplemental material). Because the study was halted, month 2 data points were insufficient in number for statistical interpretation, but the data are presented elsewhere (see Fig. S1 in the supplemental material).
FIG 3.
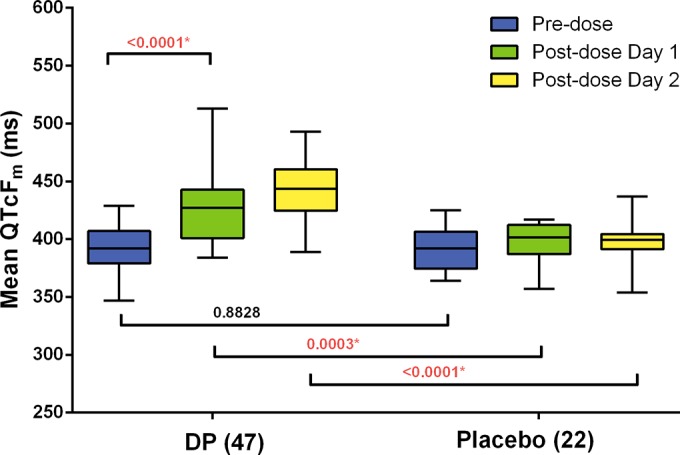
Mean differences between predose QTcF intervals at baseline and at 4-h postdose intervals on days 1 and 2 of dosing during month 1 in the group that received dihydroartemisinin-piperaquine (DP) (n = 47) and the group that received placebo (n = 22). All volunteers who received at least one dose of study drug (n = 69) are shown. One subject whose treatment was halted after the first dose is not included in the postdose day 2 analysis. The center of each box represents the median, the top and bottom represent the 25th and 75th percentiles, the whiskers extend to either the lowest or highest value.
TABLE 3.
QTc interval by category between treatment arms
| QTc interval | No. of subjects with the indicated characteristic after the 1st dose |
P value | No. of subjects with the indicated characteristic after the 2nd dose |
P value | ||
|---|---|---|---|---|---|---|
| DP (n = 47) | Placebo (n = 22) | DP (n = 46) | Placebo (n = 22) | |||
| QTc prolongation | ||||||
| QTcFe | ||||||
| <450 ms | 33 | 22 | 0.003 | 20 | 22 | <0.0001 |
| 450–479 ms | 12 | 0 | 20 | 0 | ||
| 480–499 ms | 1 | 0 | 3 | 0 | ||
| ≥500 ms | 1 | 0 | 3 | 0 | ||
| QTcFm | ||||||
| <450 ms | 38 | 22 | 0.0489 | 28 | 22 | 0.0003 |
| 450–479 ms | 8 | 0 | 14 | |||
| 480–499 ms | 0 | 0 | 4 | |||
| ≥500 ms | 1 | 0 | 0 | |||
| Change of QTc over baseline | ||||||
| QTcFe | ||||||
| <30 ms | 29 | 22 | 0.0003 | 11 | 22 | <0.0001 |
| 30–59 ms | 16 | 0 | 28 | 0 | ||
| ≥60 ms | 2 | 0 | 7 | 0 | ||
| QTcFm | ||||||
| <30 ms | 22 | 19 | 0.0018 | 14 | 19 | <0.0001 |
| 30–59 ms | 13 | 3 | 14 | 3 | ||
| ≥60 ms | 12 | 0 | 18 | 0 | ||
Known confounders of QT interval measurement, including comparison of manual and automated measurements, interreader (between-reader) variability, and heart rate were evaluated. At each time point (predose, day 1, or day 2), there were consistent but moderate differences in QTcF interval measurements between manual and electronic reads (see Fig. S2 in the supplemental material). The QTcFe tended to give a higher estimate of the QTc interval than the QTcFm did. However, there were no significant differences before and after the dose or between the two treatment arms with a range of difference of 15 to 23 ms lower for manual readings performed on the treatment group and 18 to 23 ms lower for the placebo group across the predose and postdose intervals (Fig. S2). Interreader variability in QT interval measurements between the three responsible investigators was compared. While the one-way ANOVA test revealed a statistically significant difference in average QT (P = 0.0223) and QTcFm (P = 0.0317) intervals between readers A and C, the mean percent difference was less than 3% (Fig. S3). Heart rate was also not significantly different for the groups (treatment or placebo) or time (predose or postdose day 1 or 2) (Fig. S4).
Piperaquine pharmacokinetics and QTc prolongation.
The piperaquine concentration in plasma was evaluated at 0, 4, 24 and 28 h in relation to monthly treatment course dosing. The plasma piperaquine levels at 4 and 28 h after dosing were considered to be the estimated Cmax for doses 1 and 2, respectively. Manual ECG reading revealed a significant increase in QTcFm intervals at 4 h postdose, lasting 4 to 8 h (P < 0.0002; Fig. 3). After the first dose at month 1, the mean Cmax was 633.6 ng/ml (standard deviation [SD], 257.3 ng/ml; n = 47) at 4 h and 937.8 ng/ml (SD, 330.1 ng/ml; n = 45) at 28 h. For the second month dosing, Cmax at 4 h and 28 h after the first dose were 573.4 (SD, 314.8; n = 16) and 886.8 (SD, 200.6; n = 13) ng/ml, respectively. None of the four treatment-halted subjects received month 2 treatment. The peak plasma piperaquine concentration of three of the four treatment-halted subjects was higher than the upper bound of the 95% confidence interval (95% CI) of plasma piperaquine concentration during the first dose at month 1 (Fig. 4). Overall, there was no significant correlation between the timing of meals and drug administration at any time point.
FIG 4.
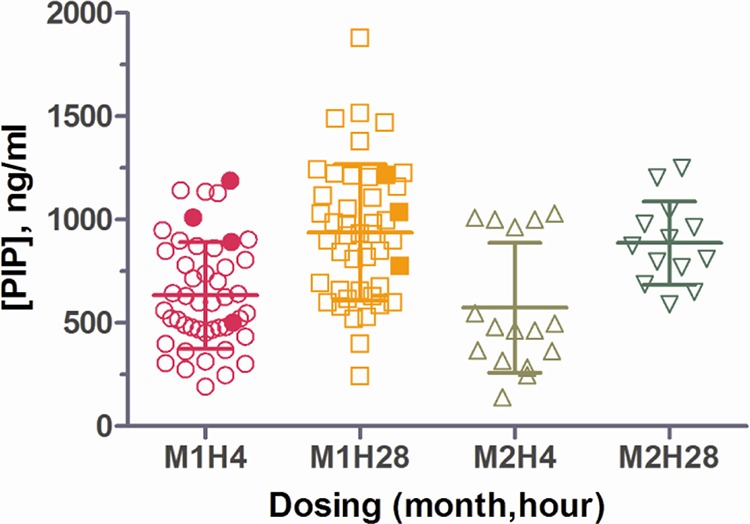
Peak concentrations of piperaquine (PIP) in plasma at 4 and 28 h after the first dose each month. The time of the first dose is given as follows: the month (M) and hours after the first dose (H). Each symbol represents the value for an individual subject. The mean ± standard deviation (error bar) are shown for each group. The values for the treatment-halted subjects are shown by the solid symbols.
The relationship between plasma piperaquine concentration and change in QTcFm from baseline is displayed in Fig. 5 (ΔQTcFm; slope = 0.0462 ms per ng/ml). Spearman correlation analysis of plasma piperaquine level and ΔQTcFm was significant for subjects who received dihydroartemisinin-piperaquine (rho = 0.6706; P < 0.001), with a stronger correlation in the four treatment-halted subjects (rho = 0.8990; P < 0.001) (see Table S2 in the supplemental material). Plasma piperaquine concentration and ΔQTcFm were also significantly correlated in 36 of 47 individuals (76.6%) who received dihydroartemisinin-piperaquine (40 of 47 for QTcFe). In these “piperaquine-sensitive” subjects, piperaquine level had a significant effect on both manual and electronic QTcF prolongation using a contingency test (P < 0.001) (see Fig. S5 in the supplemental material).
FIG 5.
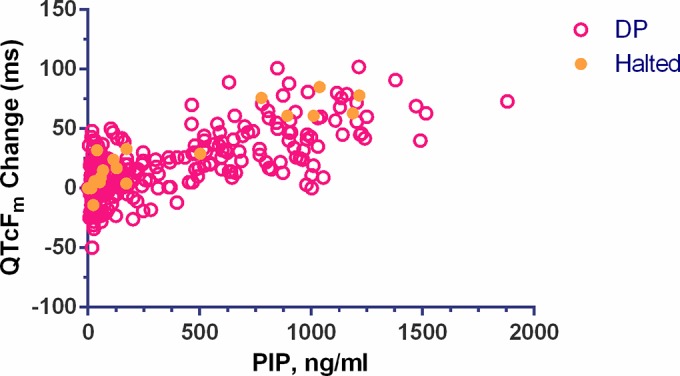
Plot of the change in QTcFm from baseline versus plasma piperaquine concentration for subjects receiving dihydroartemisinin-piperaquine. The values for treatment-halted subjects are indicated by the solid orange circles. Spearman correlation analysis between concentration and QT interval prolongation was significant for subjects treated with dihydroartemisinin-piperaquine (n = 47; rho = 0.6706; P < 0.0001), with stronger correlation in the treatment-halted subjects (n = 4; rho = 0.8990; P < 0.0001).
Simulation based on previously calculated pharmacokinetic parameters from a clinical trial comparing 2- and 3-day dihydroartemisinin-piperaquine regimens (12) was performed to estimate mean drug levels of clinically relevant 2-, 3-, and 5-day dosing regimens. In the simulation, the 2-day regimen had an estimated mean Cmax of 537 ng/ml on the second day of dosing compared to 406 ng/ml on the third day of dosing for the two other regimens. The 5-day regimen reached an estimated mean Cmax of 451 ng/ml on the fifth day, lower than for the 2-day regimen (Fig. 6).
FIG 6.
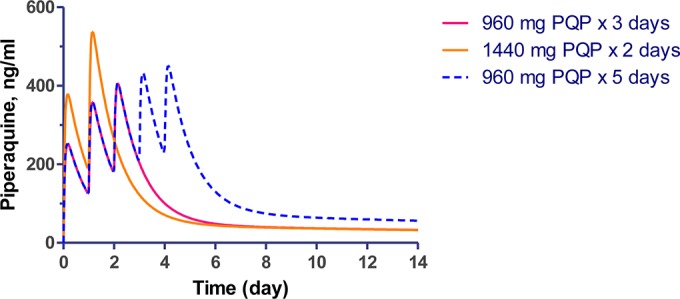
Pharmacokinetic simulation of piperaquine concentration-time profiles when given as 960 mg piperaquine phosphate (PQP) for 3 days, 960 mg PQP for 5 days, and 1,440 mg PQP for 2 days.
Protective efficacy and follow-up.
The primary protective efficacy endpoint could not be evaluated due to the early trial halt. At the time of halting, 69 volunteers had enrolled and received at least one dose of test article. During the 2-month follow-up, a total of seven volunteers developed PCR-confirmed vivax malaria. Six recurrences were in the placebo group (n = 22), with only one recurrence in the treatment arm (n = 47). Upon study termination, 63 volunteers were offered primaquine for radical treatment of P. vivax; of these 63 volunteers, 59 received primaquine for radical cure of vivax malaria and 14 subsequently developed malaria (12 vivax malaria, 1 mixed, and 1 unknown at outside clinic) over the following 3 months.
DISCUSSION
This study aimed to evaluate the safety and efficacy of a compressed 2-day course of dihydroartemisinin-piperaquine for malaria chemoprophylaxis in the Cambodian military. Because there are few safe, cost-effective chemoprevention options available to this population, there is no regular use of chemoprophylaxis currently, nor was there at the time of the study. The de facto standard of care relied on mosquito bite prevention, but the true effectiveness of these measures remains unknown, and given the rates of malaria observed, was relatively ineffective compared to the typical protective efficacy of chemoprophylaxis (80 to 99%). This study attempted to reproduce the previously demonstrated efficacy of monthly and bimonthly 3-day treatment courses of dihydroartemisinin-piperaquine in 2006 to 2008 along the Thai-Myanmar border (6). While the 3-day regimen was found to be effective in western Thailand, electrocardiogram monitoring was not performed (6). This study also followed a recent study by the Armed Forces Research Institute of Medical Science (AFRIMS), Cambodian National Center for Parasitology, Entomology, and Malaria Control (CNM), and Royal Cambodian Armed Forces (RCAF) in 2010 that found similar efficacy between 2- and 3-day treatment courses of dihydroartemisinin-piperaquine for infection of all species (12). Unfortunately, in the present study, the compressed 2-day regimen resulted in unacceptably high levels of QT interval prolongation, and the trial was halted after only 69 participants were enrolled following prespecified cardiac safety rules. Participants receiving dihydroartemisinin-piperaquine had an increase in mean QTcFm by 50 ms compared to placebo, and more than 75% of volunteers receiving dihydroartemisinin-piperaquine had significant correlation between increasing plasma piperaquine concentration and QT interval prolongation, suggesting a concentration-dependent effect. Given the small sample size at the time the study was halted, a conclusion regarding the protective efficacy of dihydroartemisinin-piperaquine cannot be made.
Regulatory agencies such as the U.S. Food and Drug Administration regard QTc interval prolongation over 60 ms as highly concerning for increased risk of torsades de pointes (TdP), though precise risk can be difficult to quantify, even with a known rapidly activating repolarizing potassium ion (IKr) channel blocker (15). Several cardiac safety assessments of dihydroartemisinin-piperaquine have been reported, and while increased risk has been demonstrated, to date, there have not been serious concerns raised regarding life-threatening cardiotoxicity (16–18). One study of 62 adults and children with uncomplicated malaria found a statistically significant prolongation (11-ms mean QTcB increase) at 24 h, but ECGs were not assessed at Cmax. Further, corrections were reported using Bazett's formula which is more susceptible to artifact QTc prolongation at higher heart rates (2, 19). Another study by Mytton et al. of 56 adults performed ECGs at 4 h postdose but found no statistically significant lengthening of the QTc interval regardless of the correction formula used (16). In two large pivotal phase 3 studies conducted in Asia and Africa with Eurartesim, significant QTc prolongation was seen on day 2 after treatment with dihydroartemisinin-piperaquine, although these increases were mild and transient (2, 20). In the predominantly adult Asian study population, there was a statistically significant increase in the proportion of patients with borderline and prolonged QTcB and QTcF values for those receiving dihydroartemisinin-piperaquine compared to artesunate plus mefloquine, but QTc prolongation resolved by day 7 (20). In a pediatric African population, QTcB, but not QTcF, was prolonged in the dihydroartemisinin-piperaquine group compared to the artemether-lumefantrine group, also resolving by day 7 (21). As a result, the current label for the 3-day administration of Eurartesim recommends ECG monitoring “when clinically appropriate,” particularly before treatment and 4 h after the third dose for high-risk patients (8). To date, there have been no published case reports of dihydroartemisinin-piperaquine-induced TdP. However, given limited diagnostics and the difficulty of monitoring outcomes in areas where malaria is endemic, a lack of reported lethal arrhythmias following dihydroartemisinin (DHA)-piperaquine administration may be a function of insufficient surveillance.
Currently, drug-induced QT interval prolongation is attributed to direct blockade of the rapidly activating repolarizing potassium ion channel (denoted as IKr and encoded by the human ether-a-go-go [hERG] gene on chromosome 7). hERG channels are critical in phase 2 of the cardiac action potential, and inhibition of these channels delays ventricular repolarization in a manner that predisposes to life-threatening arrhythmias (15). Even at therapeutic antimalarial levels, halofantrine, now largely abandoned, is known to be a potent inhibitor of IKr, leading to reports of fatal proarrhythmia (19, 22). Chloroquine, on the other hand, predisposes to malignant ventricular rhythms when taken in overdoses but otherwise does not cause cardiotoxicity at antimalarial treatment doses (19, 23). Chloroquine inhibits both the fast-acting IKr and the inwardly rectifying repolarizing potassium channel IK1, which is primarily responsible for phases 3 and 4 of the cardiac action potential. Studies suggest that antagonism of the slower IK1 channel leads to an increase in the U-wave amplitude, which prolongs the QT interval, but not in the classic manner thought to cause potentially lethal arrhythmias (24). It is known that piperaquine weakly inhibits IKr in vitro and requires concentrations 100 times higher than its clinical Cmax to increase torsadogenic risk (25). Dihydroartemisinin has not been shown to significantly inhibit IKr but actually decreased proarrhythmic risk in in vitro rabbit ventricular wedge preparations (25). The effects of dihydroartemisinin or piperaquine on IK1 channels are not currently known, but the structural similarity of piperaquine to chloroquine makes an interaction likely.
The absence of reported malignant ventricular arrhythmias associated with dihydroartemisinin-piperaquine suggests that the degree of QT prolongation seen here may reflect benign changes due to IK1 potassium ion channel behavior. Closer ECG inspection in our study revealed that subjects had increased U-wave amplitude causing QT-U fusion (Fig. 3), particularly subjects whose treatment was halted for prolonged QT. The clinical importance of the U wave remains unclear, but recent work has demonstrated that defective KCNJ2 genes cause decreased IK1 function resulting in dramatic U waves but in the absence of genuine QT prolongation (26, 27). Whether the consequences of pharmacologic IK1 inhibition are clinically deleterious requires further study, but it appears inherently less dangerous than IKr inhibition (24). While manual QTcF readings were less than 500 ms in these treatment-halted volunteers, a cautious approach was taken by the DSMB, given that inspection of QT interval prolongations suggested T-wave changes characteristic of repolarization injury due to piperaquine. Although the QTc prolongations were asymptomatic and transient, halting the study represented a conservative approach to ensure volunteer safety given a large proportion with QTcFm prolonged >60 ms in this austere setting.
Although little effect was seen in this study, food is known to affect piperaquine pharmacokinetics, with fatty meals in particular causing an increase in peak plasma concentrations (Table 4). While studies of piperaquine with modest fat intake in Vietnam (17 g) (28) and Thailand (6.4 g) (6, 29) showed little or no effect on piperaquine Cmax or area under the concentration-time curve (AUC), other studies showed a clear increase. In a randomized crossover study of eight healthy Caucasian adults, Cmax and AUC of piperaquine approximately doubled with a 37-g-fat meal without altering the time to maximum concentration of drug in serum (Tmax) (30). Likewise, a moderate meal with 16.7 g of fat caused a 41% increase in piperaquine exposure compared to the group who fasted in a study of healthy Vietnamese volunteers (31). Food-drug interaction studies by the manufacturer also indicate that piperaquine absorption is increased in the presence of fatty food and may increase its effect on the QTc interval (8). Based on the uncorrected QT data for 208 healthy subjects who took Eurartesim compared to placebo, Eurartesim prolonged the QT interval 39.3 ms in the high-fat/low-calorie group, 38.9 ms in the high-fat/high-calorie group, and 21.2 ms in the group who fasted. There was significant correlation between maximum change from baseline QTcF and piperaquine levels, and both were lower in volunteers who fasted (18). To minimize risks of arrhythmia, the product label recommends taking dihydroartemisinin-piperaquine with water and fasting for 3 h before and after dosing (8).
TABLE 4.
Summary of published findings on food effects of piperaquine dosing
| Reference | No. of subjects | Subject | Piperaquine dose | Mean Cmaxa (ng/ml) | Fasted or meal |
|---|---|---|---|---|---|
| Karunajeewa et al. (17) | 62 | P. falciparum- or P. vivax-infected Cambodian adults and children | 320 mg | 95–124 | Fasted 4 to 6 h |
| Sim et al. (30) | 8 | Healthy Caucasians | 500 mg | 21 for subjects who fasted | Fasted 6 to 8 h |
| 65 for fed subjects | Fed high-fat (37-g) meal | ||||
| Mytton et al. (16) | 56 | P. falciparum-infected Karen or Burmese adults | 55 mg/kg | Not reported | ∼8 hc |
| Liu et al. (36) | 16 | Healthy Chinese | 640 mg | 578–586 | Fed meal 2 h after dosing |
| Nguyen et al. (31) | 12 | Healthy Vietnamese | 500 mg | 69.6 | No food for >10 h |
| 1,000 mg | 195.5 | No food for >10 h | |||
| 500 mg for 3 days | 119.0/198.1d | Fed 16.7 g fat 10 min after breakfast | |||
| Hai et al. (28) | 32 | Healthy Vietnamese | 640 mg | 130b for subjects who fasted | Fasted overnight plus 4 h postdose |
| 212 for fed subjects | Fed meal with 17-g fat | ||||
| Annerberg et al. (29) | 30 | Healthy Thai | 58 mg/kg | 236b for subjects who fasted | Fasted overnight plus 2 h postdose |
| 256b for fed subjects | Fed 200 ml milk and 6.4 g fat | ||||
| This study | 47 | Healthy Cambodians | 2,880 mg over 2 days | 1,183 at 4 h | Within 3 h of fatty meal |
| 1,750 at 28 h |
Cmax 2 h after the first dose.
Median Cmax.
Peak concentration reported to be 8 h by population pharmacokinetic (PK) model. No other data provided; unclear if subjects were fed or fasted.
The first value refers to the mean Cmax for the fasting group, and the second value refers to the mean Cmax for the fed group.
Limitations of this study also included the lack of pharmacokinetic evaluation of dihydroartemisinin and its possible incremental contribution to QT prolongation. This would have required additional ECG studies at 90 min postdose to coincide with the Cmax of dihydroartemisinin (32). Several studies with artemisinin-containing oral compounds have demonstrated excellent cardiac safety profiles despite higher peak plasma concentrations compared to intramuscular formulations (17, 33–35). We cannot determine whether our findings would be similar for the conventional 3-day regimen of dihydroartemisinin-piperaquine. However, our simulation based on previously collected data suggests lower risk given lower mean peak plasma piperaquine levels seen with the 3-day regimen.
Conclusions and recommendations.
Two-day regimens of piperaquine-containing ACTs are widely used in Cambodia despite limited availability of clinical risk assessment and cardiac safety monitoring in areas where malaria is endemic (9). Based on QT prolongation seen in this study, compressed regimens of dihydroartemisinin-piperaquine could be problematic given significant issues with identifying and monitoring high-risk patients. The increased U-wave amplitudes suggest but do not confirm that piperaquine-induced QT prolongation may be less deleterious than other more torsadogenic antimalarials such as halofantrine. Based on the key findings in this study, recommendations include adherence to the warnings in the Eurartesin product label designed to obviate clinical QTc prolongation particularly related to fasting. Other risk-mitigating recommendations like ECG and/or electrolyte monitoring and supplementation will be far more difficult to implement in many settings where malaria is endemic. Given dihydroartemisinin-piperaquine's widespread use and excellent safety record when used in a 3-day dosing regimen, it remains an effective therapeutic tool for malaria treatment, but it may require more pharmacovigilance than previously thought.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to the volunteers who participated in the study and to the staff of Trapang Tao Health center and Anlong Veng Referral Hospital (Pich Sokhla, Nov Sam On, Mok Keng and Kong Phan) for their collaboration and for ensuring volunteer welfare. We thank CNM and AFRIMS colleagues (Thay Kheang Heng, Kong Nareth, You Yom, Va Soch, San Savoeun, Montri Arsanok, Mali Ittiverakul, Piyaporn Saingam, Chaiyawat Mathavarat, Suriya Teopipithaporn, and Somporn Krasaesub) for their technical expertise and research support. We thank collaborators from the Royal Cambodia Armed Forces (Kong Saly, Nhim Vanna, Deth Vantha, Pheap Vannak, So Samen, So Nara, Chou Sophy, Duch Vathna, Mok My, Kul Rong, Leng Chin, He Vireak, Loeung Sopheak, and Mek Buntha) for their collaboration, advice, and liaison. We also thank BIOPHICS (Sarnath Lawpoolsri, Amnat Kamsiriwatchara, Pawinee Jarujareet, Rungrawee Pawarana, Montida Auayporn, and Jesada Hongto) for data management. We are thankful to the study monitors Suchada Chinaworapong and Denise McKinney, sponsor's medical expert Kevin Leary, and others at the United States Army Medical and Material Development Activity (USAMMDA) including Moshe Shmuklarsky, Geoff Dow, William McCarthy, and Bryan Smith.
The views expressed in this manuscript are those of the authors and do not necessarily reflect the official policy or positions of the U.S. Department of the Army, the U.S. Department of Defense, or the U.S. or Cambodian government.
Footnotes
Published ahead of print 4 August 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.02667-14.
REFERENCES
- 1.World Health Organization. 2013. World malaria report 2013. World Health Organization, Geneva, Switzerland [Google Scholar]
- 2.Bethell D, Se Y, Lon C, Tyner S, Saunders D, Sriwichai S, Darapiseth S, Teja-Isavadharm P, Khemawoot P, Schaecher K, Ruttvisutinunt W, Lin J, Kuntawungin W, Gosi P, Timmermans A, Smith B, Socheat D, Fukuda MM. 2011. Artesunate dose escalation for the treatment of uncomplicated malaria in a region of reported artemisinin resistance: a randomized clinical trial. PLoS One 6:e19283. 10.1371/journal.pone.0019283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zwang J, Ashley EA, Karema C, D'Alessandro U, Smithuis F, Dorsey G, Janssens B, Mayxay M, Newton P, Singhasivanon P, Stepniewska K, White NJ, Nosten F. 2009. Safety and efficacy of dihydroartemisinin-piperaquine in falciparum malaria: a prospective multi-centre individual patient data analysis. PLoS One 4:e6358. 10.1371/journal.pone.0006358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Naing C, Mak JW, Aung K, Wong JY. 2013. Efficacy and safety of dihydroartemisinin-piperaquine for treatment of uncomplicated Plasmodium falciparum malaria in endemic countries: meta-analysis of randomised controlled studies. Trans. R. Soc. Trop. Med. Hyg. 107:65–73. 10.1093/trstmh/trs019 [DOI] [PubMed] [Google Scholar]
- 5.Leang R, Barrette A, Bouth DM, Menard D, Abdur R, Duong S, Ringwald P. 2013. Efficacy of dihydroartemisinin-piperaquine for the treatment of uncomplicated Plasmodium falciparum and Plasmodium vivax in Cambodia, 2008 to 2010. Antimicrob. Agents Chemother. 57:818–826. 10.1128/AAC.00686-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lwin KM, Phyo AP, Tarning J, Hanpithakpong W, Ashley EA, Lee SJ, Cheah P, Singhasivanon P, White NJ, Lindegårdh N, Nosten F. 2012. Randomized, double-blind, placebo-controlled trial of monthly versus bimonthly dihydroartemisinin-piperaquine chemoprevention in adults at high risk of malaria. Antimicrob. Agents Chemother. 56:1571–1577. 10.1128/AAC.05877-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Keating GM. 2012. Eurartesim: a guide to its use in the treatment of uncomplicated Plasmodium falciparum malaria. Drugs Ther. Perspect. 28:(10)1–5. 10.1007/BF03262125 [DOI] [Google Scholar]
- 8.Sigma-Tau Industrie Farmaceutiche Riunite s.p.a. Eurartesim. Annex I. Summary of product characteristics. Sigma-Tau Industrie Farmaceutiche Riunite s.p.a., Rome, Italy: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/001199/WC500118113.pdf [Google Scholar]
- 9.Song J, Socheat D, Tan B, Seila S, Xu Y, Ou F, Sokunthea S, Sophorn L, Zhou C, Deng C, Wang Q, Li G. 2011. Randomized trials of artemisinin-piperaquine, dihydroartemisinin-piperaquine phosphate and artemether-lumefantrine for the treatment of multi-drug resistant falciparum malaria in Cambodia-Thailand border area. Malar. J. 10:231. 10.1186/1475-2875-10-231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hung T-Y, Davis TME, Ilett KF, Karunajeewa H, Hewitt S, Denis MB, Lim C, Socheat D. 2004. Population pharmacokinetics of piperaquine in adults and children with uncomplicated falciparum or vivax malaria. Br. J. Clin. Pharmacol. 57:253–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.WHO Working Group. 1989. Glucose-6-phosphate dehydrogenase deficiency. Bull. World Health Organ. 67:601–611 [PMC free article] [PubMed] [Google Scholar]
- 12.Lon C, Manning JE, Vanachayangkul P, So M, Sea D, Se Y, Gosi P, Lanteri C, Chaorattanakawee S, Sriwichai S, Chann S, Kuntawunginn W, Buathong N, Nou S, Walsh DS, Tyner SD, Juliano JJ, Lin J, Spring M, Bethell D, Kaewkungwal J, Tang D, Chuor CM, Satharath P, Saunders D. 2014. Efficacy of two versus three-day regimens of dihydroartemisinin-piperaquine for uncomplicated malaria in military personnel in Northern Cambodia: an open-label randomized trial. PLoS One 9:e93138. 10.1371/journal.pone.0093138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.National Cancer Institute. 2010. Common terminology criteria for adverse events (CCTCAE), version 4.03. National Cancer Institute, National Institutes of Health, US Department of Health and Human Services, Bethesda, MD [Google Scholar]
- 14.Moss AJ. 1993. Measurement of the QT interval and the risk associated with QTc interval prolongation: a review. Am. J. Cardiol. 72:23B–25B. 10.1016/0002-9149(93)90036-C [DOI] [PubMed] [Google Scholar]
- 15.Kannankeril P, Roden DM, Darbar D. 2010. Drug-induced long QT syndrome. Pharmacol. Rev. 62:760–781. 10.1124/pr.110.003723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mytton OT, Ashley EA, Peto L, Price RN, La Y, Hae R, Singhasivanon P, White NJ, Nosten F. 2007. Electrocardiographic safety evaluation of dihydroartemisinin piperaquine in the treatment of uncomplicated falciparum malaria. Am. J. Trop. Med. Hyg. 77:447–450 [PubMed] [Google Scholar]
- 17.Karunajeewa H, Lim C, Hung T-Y, Ilett KF, Denis MB, Socheat D, Davis TME. 2004. Safety evaluation of fixed combination piperaquine plus dihydroartemisinin (Artekin) in Cambodian children and adults with malaria. Br. J. Clin. Pharmacol. 57:93–99. 10.1046/j.1365-2125.2003.01962.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.European Medicines Agency. 2011. Eurartesim assessment report. EMA/739355/2011. Committee for Medicinal Products for Human Use, European Medicines Agency, London, United Kingdom: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/001199/WC500118116.pdf [Google Scholar]
- 19.White NJ. 2007. Cardiotoxicity of antimalarial drugs. Lancet Infect. Dis. 7:549–558. 10.1016/S1473-3099(07)70187-1 [DOI] [PubMed] [Google Scholar]
- 20.Valecha N, Phyo AP, Mayxay M, Newton PN, Krudsood S, Keomany S, Khanthavong M, Pongvongsa T, Ruangveerayuth R, Uthaisil C, Ubben D, Duparc S, Bacchieri A, Corsi M, Rao BHK, Bhattacharya PC, Dubhashi N, Ghosh SK, Dev V, Kumar A, Pukrittayakamee S, Pukittayakamee S. 2010. An open-label, randomised study of dihydroartemisinin-piperaquine versus artesunate-mefloquine for falciparum malaria in Asia. PLoS One 5:e11880. 10.1371/journal.pone.0011880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bassat Q, Mulenga M, Tinto H, Piola P, Borrmann S, Menéndez C, Nambozi M, Valéa I, Nabasumba C, Sasi P, Bacchieri A, Corsi M, Ubben D, Talisuna A, D'Alessandro U. 2009. Dihydroartemisinin-piperaquine and artemether-lumefantrine for treating uncomplicated malaria in African children: a randomised, non-inferiority trial. PLoS One 4:e7871. 10.1371/journal.pone.0007871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Traebert M, Dumotier B, Meister L, Hoffmann P, Dominguez-Estevez M, Suter W. 2004. Inhibition of hERG K+ currents by antimalarial drugs in stably transfected HEK293 cells. Eur. J. Pharmacol. 484:41–48. 10.1016/j.ejphar.2003.11.003 [DOI] [PubMed] [Google Scholar]
- 23.Riou B, Barriot P, Rimailho A, Baud FJ. 1988. Treatment of severe chloroquine poisoning. N. Engl. J. Med. 318:1–6. 10.1056/NEJM198801073180101 [DOI] [PubMed] [Google Scholar]
- 24.Tsuboi M, Antzelevitch C. 2006. Cellular basis for electrocardiographic and arrhythmic manifestations of Andersen-Tawil syndrome (LQT7). Heart Rhythm. 3:328–335. 10.1016/j.hrthm.2005.11.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Borsini F, Crumb W, Pace S, Ubben D, Wible B, Yan G-X, Funck-Brentano C. 2012. In vitro cardiovascular effects of dihydroartemisin-piperaquine combination compared with other antimalarials. Antimicrob. Agents Chemother. 56:3261–3270. 10.1128/AAC.05688-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Postema PG, Ritsema van Eck HJ, Opthof T, van Herpen G, van Dessel PFHM, Priori SG, Wolpert C, Borggrefe M, Kors JA, Wilde AAM. 2009. IK1 modulates the U-wave: insights in a 100-year-old enigma. Heart Rhythm. 6:393–400. 10.1016/j.hrthm.2008.11.024 [DOI] [PubMed] [Google Scholar]
- 27.Zhang H, Garratt C, Zhu J, Holden A. 2005. Role of up-regulation of in action potential shortening associated with atrial fibrillation in humans. Cardiovascular Res. 66:493–502. 10.1016/j.cardiores.2005.01.020 [DOI] [PubMed] [Google Scholar]
- 28.Hai TN, Hietala SF, Van Huong N, Ashton M. 2008. The influence of food on the pharmacokinetics of piperaquine in healthy Vietnamese volunteers. Acta Trop. 107:145–149. 10.1016/j.actatropica.2008.05.013 [DOI] [PubMed] [Google Scholar]
- 29.Annerberg A, Lwin KM, Lindegardh N, Khrutsawadchai S, Ashley E, Day NPJ, Singhasivanon P, Tarning J, White NJ, Nosten F. 2011. A small amount of fat does not affect piperaquine exposure in patients with malaria. Antimicrob. Agents Chemother. 55:3971–3976. 10.1128/AAC.00279-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sim I-K, Davis TME, Ilett KF. 2005. Effects of a high-fat meal on the relative oral bioavailability of piperaquine. Antimicrob. Agents Chemother. 49:2407–2411. 10.1128/AAC.49.6.2407-2411.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nguyen TC, Nguyen NQ, Nguyen XT, Bui D, Travers T, Edstein MD. 2008. Pharmacokinetics of the antimalarial drug piperaquine in healthy Vietnamese subjects. Am. J. Trop. Med. Hyg. 79:620–623 [PubMed] [Google Scholar]
- 32.Na-Bangchang K, Krudsood S, Silachamroon U, Molunto P, Tasanor O, Chalermrut K, Tangpukdee N, Matangkasombut O, Kano S, Looareesuwan S. 2004. The pharmacokinetics of oral dihydroartemisinin and artesunate in healthy Thai volunteers. Southeast Asian J. Trop. Med. Public Health 35:575–582 [PubMed] [Google Scholar]
- 33.Makanga M, Bassat Q, Falade CO, Premji ZG, Krudsood S, Hunt P, Walter V, Beck H-P, Marrast A-C, Cousin M, Rosenthal PJ. 2011. Efficacy and safety of artemether-lumefantrine in the treatment of acute, uncomplicated Plasmodium falciparum malaria: a pooled analysis. Am. J. Trop. Med. Hyg. 85:793–804. 10.4269/ajtmh.2011.11-0069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Price R, van Vugt M, Phaipun L, Luxemburger C, Simpson J, McGready R, ter Kuile F, Kham A, Chongsuphajaisiddhi T, White NJ, Nosten F. 1999. Adverse effects in patients with acute falciparum malaria treated with artemisinin derivatives. Am. J. Trop. Med. Hyg. 60:547–555 [DOI] [PubMed] [Google Scholar]
- 35.Kshirsagar NA, Gogtay NJ, Moorthy NS, Garg MR, Dalvi SS, Chogle AR, Sorabjee JS, Marathe SN, Tilve GH, Bhatt AD, Sane SP, Mull R, Gathmann I. 2000. A randomized, double-blind, parallel-group, comparative safety, and efficacy trial of oral co-artemether versus oral chloroquine in the treatment of acute uncomplicated Plasmodium falciparum malaria in adults in India. Am. J. Trop. Med. Hyg. 62:402–408 [DOI] [PubMed] [Google Scholar]
- 36.Liu C, Zhang R, Hong X, Huang T, Mi S, Wang N. 2007. Pharmacokinetics of piperaquine after single and multiple oral administrations in healthy volunteers. Yakugaku Zacchi 127:1709–1714 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.