Abstract
The intestinal reservoir is central to the epidemiology of Pseudomonas aeruginosa, but the dynamics of intestinal colonization by different phenotypes have been poorly described. To determine the impact of antimicrobial exposure on intestinal colonization by multidrug-resistant (MDR) and extensively drug-resistant (XDR) P. aeruginosa, we screened intensive care unit (ICU) patients for rectal colonization on admission and at weekly intervals. During an 18-month study period, 414 ICU patients were enrolled, of whom 179 (43%) were colonized; 112 (63%) of these were identified at ICU admission and 67 (37%) during their ICU stay. At 10 days after ICU admission, the probabilities of carriage were 44%, 24%, and 24% for non-MDR, MDR-non-XDR, and XDR P. aeruginosa strains, respectively (log rank, 0.02). Pulsed-field gel electrophoresis showed 10 pairs of non-MDR P. aeruginosa and subsequent MDR-non-XDR strains isolated from the same patients to be clonally identical and another 13 pairs (8 MDR-non-XDR and 5 XDR) to be unrelated. There was one specific clone between the 8 MDR-non-XDR strains and an identical genotype in the 5 XDR isolates. The Cox regression analysis identified MDR P. aeruginosa acquisition as associated with the underlying disease severity (adjusted hazard ratio [aHR], 1.97; 95% confidence interval [CI], 1.22 to 3.18; P = 0.006) and prior use of fluoroquinolones (aHR, 1.02; 95% CI, 1.00 to 1.04; P = 0.039), group 2 carbapenems (aHR, 1.03; 95% CI, 1.00 to 1.07; P = 0.041), and ertapenem (aHR, 1.08; 95% CI, 1.02 to 1.14; P = 0.004). The epidemiology of MDR P. aeruginosa is complex, and different clusters may coexist. Interestingly, ertapenem was found to be associated with the emergence of MDR isolates.
INTRODUCTION
Pseudomonas aeruginosa is one of the most common nosocomial pathogens worldwide, and continuously evolving resistance to multiple antimicrobial agents has become a significant health problem. The control of multidrug-resistant (MDR) P. aeruginosa in intensive care units (ICUs) is an important method for preserving the limited number of drugs available for treating P. aeruginosa infections. However, this control is difficult to achieve because the path of emergence and dissemination of MDR P. aeruginosa is not fully understood. As an endogenous source of endemic or epidemic infection with Gram-negative bacilli (GNB), the intestinal reservoir is central to the epidemiology of P. aeruginosa because prior rectal colonization is typically present in most patients developing GNB infections (1).
Several studies have demonstrated that prior antimicrobial drug exposure is also a strong risk factor for colonization with a drug-resistant pathogen (2, 3). In fact, we have previously reported an association between carbapenem and fluoroquinolone consumption with the probability of colonization by carbapenem-resistant P. aeruginosa in ICU patients (4).
In recent years, important changes have occurred in the epidemiology of MDR P. aeruginosa. First, in addition to the characteristic polyclonal pattern of endogenous P. aeruginosa, a newly identified epidemic clonal pattern has been described during MDR P. aeruginosa outbreaks (5). Second, several reports have provided strong evidence for the existence of MDR P. aeruginosa high-risk clones (6), with biological parameters that may explain the success of these specific clones (7). Finally, a recent consensus document (8) proposed a set of MDR definitions in pathogenic bacteria that may improve the comparability of surveillance data. To our knowledge, no study has applied these new definitions to the epidemiological behavior of MDR P. aeruginosa and extensively drug-resistant (XDR) P. aeruginosa. In addition, the impact of ertapenem use over time is an issue of recent controversy (9).
For all these reasons, we decided to analyze the influence of antimicrobial use on intestinal P. aeruginosa colonization in ICU patients. Additionally, we assessed the temporal changes and the dynamic characteristics of intestinal colonization by different PA phenotypes in ICU patients after the start of antibiotic therapy. This study aimed to compare the probability of intestinal colonization by non-MDR P. aeruginosa and MDR P. aeruginosa strains and to assess the relationship between the duration of antibiotic therapy exposure and intestinal colonization with P. aeruginosa.
MATERIALS AND METHODS
We conducted a prospective cohort study of patients admitted to a medical-surgical ICU at the Hospital Universitari de Bellvitge between January 2012 and June 2013. All samples and examinations were performed as part of the standard care procedures for epidemiological control in this population. The local ethics committee of our hospital approved the study, and the patients or family provided informed consent.
Study design.
We conducted an active surveillance program with ICU patients. Weekly rectal swab samples to detect digestive tract carriage of P. aeruginosa were obtained immediately on ICU admission and between ICU admission and discharge. To study patients at risk for digestive tract carriage during their ICU stay, all patients admitted to the unit for more than 48 h were included. We collected the following information for each patient: demographic data, including sex, age, hospital admission date, ICU admission date, and ICU discharge date; and severity of the acute illness on ICU admission using the simplified acute physiologic score (SAPS) (10) and the Charlson comorbidity index (11).
Definitions.
We defined ICU admission carriers as patients with P. aeruginosa-positive culture samples at ICU admission. We defined ICU-acquired carriers as patients with P. aeruginosa-negative culture samples at ICU admission, who developed a P. aeruginosa-positive culture either during ICU admission or at ICU discharge. In addition, we defined new ICU-acquired carriers as patients in whom the P. aeruginosa phenotype isolated in a rectal sample differed from that of a previous isolate.
The phenotype stratification of P. aeruginosa isolates was made in accordance with recent standard definitions (8). MDR P. aeruginosa was defined as a strain nonsusceptible to ≥1 agent in ≥3 antipseudomonal antimicrobial categories. XDR P. aeruginosa was defined as a strain nonsusceptible to ≥1 agent in all but ≤2 antipseudomonal antimicrobial categories; thus, XDR P. aeruginosa isolates were included as MDR P. aeruginosa. To study the specific epidemiology of XDR P. aeruginosa, MDR P. aeruginosa isolates were distributed as follows: XDR P. aeruginosa as defined above and MDR-non-XDR P. aeruginosa defined as P. aeruginosa strains nonsusceptible to ≥1 agent in ≥3 antipseudomonal antimicrobial categories, but susceptible in at least >2 antipseudomonal antimicrobial classes. All other P. aeruginosa isolates, including those nonsusceptible to ≥1 agent in <3 antimicrobial categories, were considered non-MDR P. aeruginosa. Thus, three phenotypes of P. aeruginosa isolates were considered: non-MDR, MDR-non-XDR, and XDR. Only the first P. aeruginosa isolate of a specific phenotype was taken into account for each patient. Isolates of different phenotypes in the same patient were considered to be differentiated colonization episodes.
The length of exposure to antibiotic therapy for patients colonized by P. aeruginosa was defined by the number of days of therapy with different groups of antibiotics that a patient had received in the 3 months prior to hospital admission and until P. aeruginosa rectal colonization. The antibiotics analyzed were non-antipseudomonal penicillins (penicillin G, ampicillin, amoxicillin-clavulanic acid, and cloxacillin), antipseudomonal penicillin (piperacillin-tazobactam), non-antipseudomonal cephalosporins, antipseudomonal cephalosporins (ceftazidime and cefepime), aztreonam, aminoglycosides, glycopeptides, fluoroquinolones, group 2 antipseudomonal carbapenems (meropenem and imipenem), and ertapenem (group 1 carbapenem). For new ICU-acquired carrier status, the length of exposure to antibiotic therapy was calculated to the date of the new positive sample. For patients not colonized by P. aeruginosa, the length of exposure was defined as the number of days of prior antibiotic consumption to the date of withdrawal or ICU discharge.
Microbiological studies.
We identified P. aeruginosa strains and tested for their antimicrobial susceptibility using a MicroScan automated microdilution system with CN1S and CO1S panels (Dade International, West Sacramento, CA). The Clinical and Laboratory Standards Institute criteria (12) were used to define susceptibility or resistance to these antimicrobial agents. Pulsed-field gel electrophoresis (PFGE), as described previously (13), was used to determine the relatedness for patients who acquired intestinal colonization of P. aeruginosa and who subsequently acquired different phenotypes of P. aeruginosa. The DNA restriction patterns generated by the PFGE, using SpeI (New England BioLabs, Izasa, Spain), were interpreted according to the criteria established by Tenover et al. (14). We selected 23 pairs of susceptible and multiresistant strains from 23 patients, with each isolated pair originating from the same patient. This resulted in 18 paired samples of non-MDR P. aeruginosa and MDR-non-XDR P. aeruginosa strains and 5 paired samples of non-MDR P. aeruginosa and XDR P. aeruginosa strains. In addition, a PFGE analysis was performed in all MDR P. aeruginosa isolates (37 MDR-non-XDR and 46 XDR phenotype strains). Multilocus sequence typing (MLST) as previously described (15) for the P. aeruginosa MLST database (http://pubmlst.org/paeruginosa) was used to assign sequence types (16) in one representative isolate from each of the two major clones (5, 17).
Statistical analysis.
Quantitative variables were tested for normal distribution and compared by the two-tailed t test or Mann-Whitney U test. The chi-square test or Fisher's exact test was used to compare categorical variables. Variables with a P value of <0.05 were considered statistically significant. Carriers were defined as those patients with at least one P. aeruginosa-positive rectal test result. Only the first P. aeruginosa isolate of a specific phenotype in each patient was included in the analysis (non-MDR, MDR-non-XDR, and XDR), and they were considered different colonization episodes in the same patient. The time at risk (in days) for ICU acquisition carriers and new ICU acquisition carriers was defined as the time elapsed from ICU admission to P. aeruginosa isolation for colonized patients; for noncolonized patients, we used the length of the ICU stay.
The probability of P. aeruginosa carriage in the digestive tract was calculated using the Kaplan-Meier method: the outcome evaluated was P. aeruginosa colonization, using the date of ICU admission as time zero. Patients were monitored to ICU discharge. Only patients with a negative baseline sample at ICU admission were included in this analysis.
To assess the risk factors for MDR P. aeruginosa colonization, we included all first episodes of P. aeruginosa colonization (according to phenotype stratification) and all noncolonized patients. To control for confounding, multivariate analyses were performed by Cox regression, using time to colonization as the dependent variable and MDR P. aeruginosa (MDR-non-XDR and XDR) colonization as the explanatory variable of interest. In the crude analysis, variables associated with exposure were candidates for multivariate analysis at a P value of <0.20. Data were analyzed using the SPSS statistical software package (version 15.0; SPSS Institute Inc., Chicago, IL).
RESULTS
Epidemiological characteristics.
Over a period of 18 months, a cohort of 414 patients had culture samples obtained on ICU admission, with 936 rectal swabs being taken in total. Of these, 206 (50%) were screened once, while 208 (50%) had at least 2 swabs (range, 2 to 12 swabs). During their ICU stay, 179 (43%) patients had a P. aeruginosa-positive rectal sample (112 [63%] at ICU admission and 67 [37%] during ICU admission), while 235 (57%) patients had no evidence of colonization. Among the 179 carriers, 143 (80%) were colonized by a single resistance phenotype at ICU discharge, and 34 (20%) were colonized by different P. aeruginosa resistance phenotypes. The key epidemiological characteristics of patients included in the study are illustrated in Table 1.
TABLE 1.
Epidemiological characteristics of the 414 ICU patients included in the study
| Characteristic | Results at ICU admission for patients with: |
P | |
|---|---|---|---|
| P. aeruginosa intestinal colonization (n = 112) | No P. aeruginosa intestinal colonization (n = 302) | ||
| Age (mean ± SD) (yr) | 65.3 ± 13.3 | 62.2 ± 14.3 | 0.049 |
| Male sex (no. [%]) | 78 (69) | 186 (61) | 0.13 |
| Prior hospital stay | |||
| No. (%) | 74 (66) | 136 (45) | <0.001 |
| Days (median [IQRa]) | 5 (2–12) | 2 (0–7) | <0.001 |
| Charlson index (mean ± SD) | 2.7 ± 1.9 | 2.5 ± 2.1 | 0.40 |
| SAPS II score (mean ± SD) | 45.8 ± 14.5 | 43.0 ± 13.0 | 0.059 |
| Prior antibiotic exposure (no. [%]) | |||
| Fluoroquinolones | 18 (16) | 61 (20) | 0.34 |
| Group 2 carbapenems | 20 (18) | 73 (24) | 0.17 |
| Group 1 carbapenems | 7 (6) | 20 (7) | 0.89 |
| Aminoglycosides | 4 (4) | 20 (7) | 0.24 |
| Colistin | 2 (2) | 10 (3) | 0.41 |
| Monobactam/antipseudomonal cephalosporins | 12 (11) | 32 (11) | 0.97 |
| Non-antipseudomonal cephalosporins | 7 (6) | 46 (15) | 0.015 |
| Antipseudomonal penicillins | 17 (15) | 104 (34) | <0.001 |
| Non-antipseudomonal penicillins | 33 (29.5) | 89 (29.5) | 0.99 |
| Glycopeptides | 11 (10) | 48 (16) | 0.11 |
| Total days prior antibiotics (median [IQR]) | 9 (2–22) | 10.5 (4–21) | 0.22 |
IQR, interquartile range.
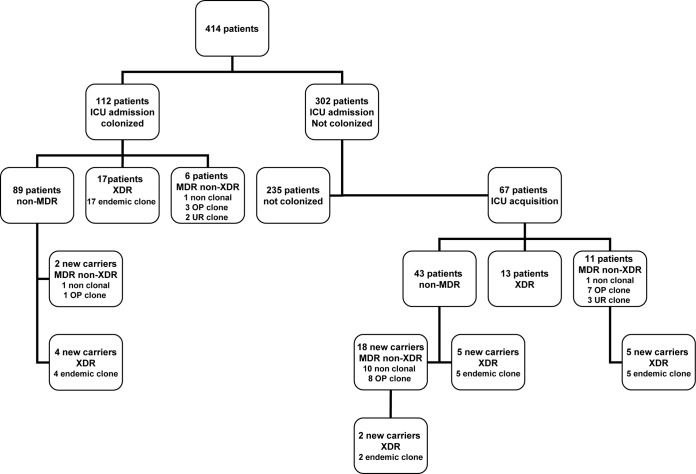
The prevalence of P. aeruginosa colonization at ICU admission was 27% (112/414 patients): 89 (22%) for non-MDR P. aeruginosa, 6 (1%) for MDR-non-XDR P. aeruginosa, and 17 (4%) for XDR P. aeruginosa. In addition, the prevalence of ICU-acquired P. aeruginosa colonization was 22% (67/302 patients): 43 (14%) for non-MDR, 11 (4%) for MDR-non-XDR, and 13 (4%) for XDR. Finally, among the P. aeruginosa-colonized patients, 36 new carrier episodes were observed in 34 patients (8%) during their ICU stay (Fig. 1). Thus, the total number of P. aeruginosa episodes was 215: 132 non-MDR and 83 MDR (37 MDR-non-XDR and 46 XDR) episodes.
FIG 1.
Study schematic diagram of the number of patients: phenotype and genotype of Pseudomonas aeruginosa colonization episodes during the 18-month study period. “10 non clonal” refers to the genotype relatedness of 10 MDR-non-XDR isolates and non-MDR isolates. For an explanation of “endemic clone,” see reference 5. UR clone, unrelated clone; OP clone, clone overexpressing MexXY-OprM (17).
Microbiological and genotypic analysis.
Among the 37 MDR-non-XDR isolates analyzed, 13 were polyclonal and 19 had genotypes identical to previously described isolates (17) and were identified as the ST111 clone; the remaining 5 had clonal relatedness by PFGE, suggesting the occurrence of another cluster. In addition, XDR P. aeruginosa isolates were comparable with those of the predominant endemic clone in our hospital (5) and belonged to the ST175 strain. Identical susceptibility patterns were observed in all XDR P. aeruginosa strains; only amikacin and colistin retained activity in the XDR phenotype.
PFGE analysis of 18 pairs of non-MDR isolated from the same patients were clonally identical in 10 pairs (56%) of subsequent MDR-non-XDR strains. The remaining 8 pairs (44%) were unrelated to the non-MDR P. aeruginosa strain by PFGE; additionally, all these 8 MDR-non-XDR strains showed clonal relatedness. In the remaining pairs, 5 non-MDR and subsequent XDR had isolates with PFGE patterns that were unrelated to prior P. aeruginosa isolates but similar to those obtained from other patients (Table 2). These data confirmed clonal dissemination of both MDR-non-XDR and XDR-P. aeruginosa isolates in the ICU (5, 17).
TABLE 2.
Descriptive analysis of the phenotype and genotype of the 23 patients with non-MDR and MDR P. aeruginosa isolates
| Patient no. | Days from ICU admission to colonization 1 | Prior antibiotic 1 (days)a | Phenotype/genotype 1 | Days of colonization 2 (days) | Prior antibiotic 2 (days)b | Phenotype/genotype 2 |
|---|---|---|---|---|---|---|
| 1 | 5 | None | Non-MDR/1 | 50 | APCEF (17), TZP (17) | MDR-non-XDR/1 |
| 2 | 6 | NAPEN (2) | Non-MDR/2 | 29 | FQ (2), AG (2), CARB-2 (2), TZP (8) | MDR-non-XDR/2 |
| 3 | 28 | NAPEN (13) | Non-MDR/3 | 28 | NAPEN (13) | MDR-non-XDR/3 |
| 4 | 8 | NACEF (6) | Non-MDR/4 | 14 | TZP (18) | MDR-non-XDR/4 |
| 5 | 11 | None | Non-MDR/5 | 9 | CARB-2 (5), TZP (7), NAPEN (3), GLYC (5) | MDR-non-XDR/5 |
| 6 | 10 | CARB-2 (6), TZP (3) | Non-MDR/6 | 20 | CARB-2 (15), CARB-1 (8), CST (9), TZP (6), GLYC (12) | MDR-non-XDR/6 |
| 7 | 12 | CARB-2 (10), NAPEN (9) | Non-MDR/7 | 28 | TZP (28), GLYC (5) | MDR-non-XDR/7 |
| 8 | 7 | CARB-1 (5) | Non-MDR/8 | 16 | FQ (4), CARB-2 (9), GLYC (9) | MDR-non-XDR/8 |
| 9 | 7 | NACEF (5), TZP (2) | Non-MDR/9 | 5 | CARB-2 (5) | MDR-non-XDR/9 |
| 10 | 12 | APCEF | Non-MDR/10 | 6 | TZP (6) | MDR-non-XDR/10 |
| 11 | 49 | NAPEN (5), TZP (3), GLYC (14) | Non-MDR/11 | 11 | FQ (6), CST (10) | MDR-non-XDR/OP clone |
| 12 | 8 | CARB-2 (1), NACEF (10) | Non-MDR/12 | 0c | CARB-2 (1), NACEF (10) | MDR-non-XDR/OP clone |
| 13 | 7 | None | Non-MDR/13 | 8 | TZP (10) | MDR-non-XDR/OP clone |
| 14 | 8 | NAPEN (3) | Non-MDR/14 | 9 | AG (4), TZP (8), GLYC (3) | MDR-non-XDR/OP clone |
| 15 | 6 | None | Non-MDR/15 | 7 | FQ (7) | MDR-non-XDR/OP clone |
| 16 | 9 | TZP (2), NAPEN (6) | Non-MDR/16 | 14 | TZP (8), GLYC (14) | MDR-non-XDR/OP clone |
| 17 | 8 | NAPEN (5) | Non-MDR/17 | 14 | FQ (7), APCEF (9) | MDR-non-XDR/OP clone |
| 18 | 9 | CARB-2 (9), GLYC (3) | Non-MDR/18 | 15 | CARB-1 (12), TZP (4) | MDR-non-XDR/OP clone |
| 19 | 39 | NACEF (2), TZP (18), NAPEN (7) | Non-MDR/19 | 0c | NACEF (2), TZP (18), NAPEN (7) | XDR/endemic clone |
| 20 | 18 | CST (15), TZP (8) | Non-MDR/20 | 0c | CST (15), TZP (8) | XDR/endemic clone |
| 21 | 10 | CARB-2 (6), TZP (3) | Non-MDR/21 | 36 | AG (15), TZP (16) | XDR/endemic clone |
| 22 | 8 | CARB-1 (5) | Non-MDR/22 | 16 | None | XDR/endemic clone |
| 23 | 12 | FQ (10), TZP (3), NAPEN (8) | Non-MDR/23 | 5 | None | XDR/endemic clone |
NAPEN, non-antipseudomonal penicillin; NACEF, non-antipseudomonal cephalosporin; CARB-2, group 2 carbapenem; TZP, piperacillin-tazobactam; CARB-1, group 1 carbapenem; APCEF, antipseudomonal cephalosporin; GLYC, glycopeptide; CST, colistin.
FQ, fluoroquinolone; AG, aminoglycoside.
Non-MDR and MDR isolate in the same sample.
Dynamics of intestinal P. aeruginosa colonization.
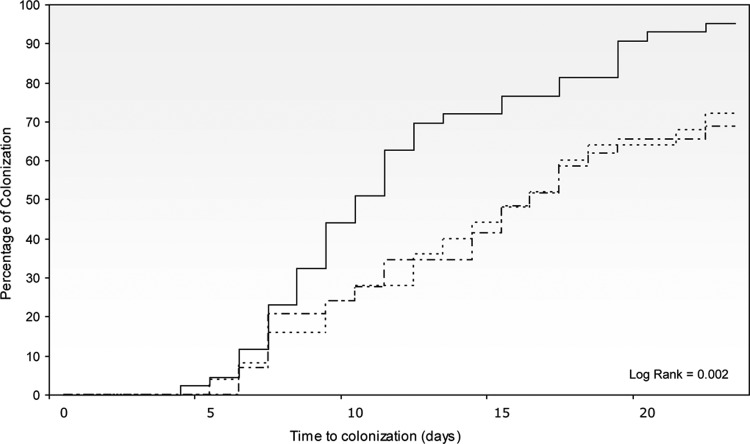
The unadjusted probabilities of P. aeruginosa carriage were analyzed among the 302 noncarriers identified at ICU admission. The mean ICU stay for 235 noncolonized patients was 10.8 ± 9.2 days versus 15.6 ± 10.5 days for 67 colonized patients (P = 0.001). Of these colonized patients, we identified 97 episodes: 67 initial episodes of ICU-acquired colonization and 30 subsequent new carrier episodes (Fig. 1). At 10 days after ICU admission, the probabilities of P. aeruginosa carriage were 44%, 24%, and 24% for non-MDR, MDR-non-XDR, and XDR-P. aeruginosa strains, respectively (log rank, P = 0.02) (Fig. 2). Although the epidemic nature of an MDR-P. aeruginosa strain might promote spread and facilitate more rapid exogenous P. aeruginosa colonization, nonstatistically significant differences existed in digestive tract carriage of polyclonal and clonal MDR-non-XDR P. aeruginosa strains (log rank, P = 0.37).
FIG 2.
Probability of Pseudomonas aeruginosa digestive tract colonization. Solid line, non-MDR P. aeruginosa colonization; dotted-dashed line, MDR non-XDR P. aeruginosa colonization; dotted line, XDR P. aeruginosa colonization.
Of note, among the 235 noncolonized patients, 30 (13%) with a median ICU stay of 25 days (interquartile range [IQR], 21.75 to 37.25) and a median antibiotic duration of 23.5 days (IQR, 16.75 to 33.25) remained noncolonized during their ICU stay.
Risk factors for MDR-P. aeruginosa carriage.
In total, 450 patient episodes were included (Fig. 1): 235 noncolonized, 132 colonized with non-MDR P. aeruginosa, and 83 colonized with MDR P. aeruginosa (37 MDR-non-XDR and 46 XDR). We tried to define the patients at risk of acquiring intestinal colonization with MDR P. aeruginosa (MDR-non-XDR and XDR). The demographic characteristics and variables examined as possible risk factors are displayed in Table 3. A Cox regression model was adjusted for underlying condition severity, acute illness severity at the ICU admission, and duration of prior fluoroquinolone, group 2 carbapenem, ertapenem, colistin, and piperacillin-tazobactam consumption as input variables. The model identified underlying condition severity (adjusted hazard ratio [aHR], 1.97; 95% confidence interval [CI], 1.22 to 3.18; P = 0.006) and prior fluoroquinolone (aHR, 1.02; 95% CI, 1.00 to 1.04; P = 0.039), group 2 carbapenem (aHR, 1.03; 95% CI, 1.00 to 1.07; P = 0.041), and ertapenem (aHR, 1.08; 95% CI, 1.02 to 1.14; P = 0.004) consumption as independently associated with MDR P. aeruginosa intestinal colonization.
TABLE 3.
Cox regression analysis of risk factors for MDR P. aeruginosa intestinal colonization in ICU patients: impact of exposure to antibiotic therapy
| Characteristic | Results for crude analysis: |
Results for adjusted analysis: |
||
|---|---|---|---|---|
| cHRa (95% CI) | P | aHR (95% CI) | P | |
| Age > 65 yr | 1.11 (0.72–1.73) | 0.63 | 1.38 (0.86–2.19) | 0.17 |
| Male gender | 1.20 (0.94–1.52) | 0.13 | 1.20 (0.94–1.54) | 0.14 |
| SAPS score at ICU admission (>40) | 1.53 (0.79–2.37) | 0.054 | 1.26 (0.79–2.02) | 0.32 |
| Charlson index of ≥3 | 1.96 (1.26–3.04) | 0.003 | 1.97 (1.22–3.18) | 0.006 |
| Prior hospital stay | 1.15 (0.75–1.78) | 0.52 | ||
| Prior fluoroquinolonesb | 1.02 (1.00–1.04) | 0.013 | 1.02 (1.00–1.04) | 0.039 |
| Prior aminoglycosidesb | 1.02 (0.94–1.12) | 0.59 | ||
| Prior carbapenemsb | 1.04 (1.01–1.07) | 0.007 | 1.03 (1.00–1.07) | 0.041 |
| Prior ertapenemb | 1.08 (1.03–1.14) | 0.002 | 1.08 (1.02–1.14) | 0.004 |
| Prior colistinb | 1.03 (0.99–1.07) | 0.11 | 1.03 (0.99–1.08) | 0.12 |
| Prior antipseudomonal cephalosporinsb | 1.01 (0.98–1.05) | 0.37 | ||
| Prior other cephalosporinsb | 1.00 (0.98–1.03) | 0.58 | ||
| Prior piperacillin-tazobactamb | 0.98 (0.94–1.00) | 0.13 | 0.98 (0.95–1.01) | 0.29 |
| Prior penicillinb | 1.00 (0.99–1.02) | 0.65 | ||
| Prior glycopeptidesb | 0.99 (0.97–1.01) | 0.53 | ||
cHR, crude hazard ratio.
Antibiotics per day.
Analysis was performed in 37 episodes of MDR-non-XDR P. aeruginosa colonization (13 polyclonal and 24 clonal isolates) based on the different molecular epidemiology and behavior of polyclonal and clonal strains. Cox regression identified prior ertapenem use (aHR, 1.10; 95% CI, 1.01 to 1.19; P = 0.026) as the only independent variable associated with the risk of clonal versus polyclonal MDR-non-XDR intestinal colonization (Table 4).
TABLE 4.
Cox regression analysis of risk factors for clonal MDR-non-XDR P. aeruginosa intestinal colonization in ICU patients: impact of exposure to antibiotic therapy
| Characteristic | Results for crude analysis |
Results for adjusted analysis |
||
|---|---|---|---|---|
| cHRa (95% CI) | P | aHR (95% CI) | P | |
| Age > 65 yr | 0.77 (0.33–1.80) | 0.55 | 1.20 (0.47–3.04) | 0.69 |
| Male gender | 1.90 (0.74–4.87) | 0.18 | 0.43 (0.12–1.53) | 0.19 |
| SAPS score at ICU admission (>40) | 1.23 (0.53–2.87) | 0.62 | ||
| Charlson index of ≥3 | 2.48 (1.01–6.11) | 0.047 | 2.05 (0.76–5.52) | 0.15 |
| Prior hospital stay | 0.75 (0.33–1.68) | 0.48 | ||
| Prior non-MDR colonization | 0.43 (0.17–1.09) | 0.076 | 0.61 (0.21–1.75) | 0.36 |
| Prior fluoroquinolonesb | 1.05 (0.960–1.16) | 0.23 | ||
| Prior aminoglycosidesb | 114 (0.79–1.64) | 0.46 | ||
| Prior carbapenemsb | 0.94 (0.84–1.04) | 0.23 | ||
| Prior ertapenemb | 1.07 (1.00–1.15) | 0.048 | 1.10 (1.01–1.19) | 0.026 |
| Prior colistinb | 0.96 (0.88–1.03) | 0.24 | ||
| Prior antipseudomonal cephalosporinsb | 0.96 (0.88–1.05) | 0.38 | ||
| Prior other cephalosporinsb | 0.99 (0.97–1.02) | 0.95 | ||
| Prior piperacillin-tazobactamb | 0.92 (0.86–0.98) | 0.024 | 0.95 (0.88–1.02) | 0.19 |
| Prior penicillinsb | 1.27 (0.57–2.84) | 0.56 | ||
| Prior glycopeptidesb | 0.96 (0.89–1.03) | 0.23 | ||
cHR, crude hazard ratio.
Antibiotics per day.
DISCUSSION
In this study, we undertook active surveillance of intestinal P. aeruginosa colonization in ICU patients over an 18-month period. Overall, 936 rectal swab samples were obtained from 414 patients, of whom 8% were newly colonized by different P. aeruginosa phenotypes. There were significant differences in age, prior hospitalization, and acute illness severity but not prior antibiotic use between colonized and noncolonized patients at ICU admission. Molecular epidemiology research revealed that XDR P. aeruginosa phenotypes had identical genotypes, whereas 65% of MDR-non-XDR isolates presented two concomitant clusters. Furthermore, the MLST study showed that the two major MDR clusters belonged to the ST175 and ST111 high-risk clones (5, 17).
We conducted an extensive evaluation of how drug resistance levels differed at the time of intestinal colonization. We found differences in colonization dynamics, with intestinal colonization occurring more prematurely for non-MDR P. aeruginosa isolates. Consequently, strains exhibiting high resistance levels for antimicrobials have delayed intestinal colonization due to the time required for selective pressure to facilitate the emergence of new resistant mutants or for preexisting subpopulations of resistant organisms to emerge. In fact, molecular analysis of paired non-MDR and MDR-non-XDR P. aeruginosa isolates showed that genetically identical isolates occurred in about 56% of the strains studied, due to the development of resistance in patients exposed to antibiotics. In parallel, antibiotic pressure appeared to provide a selective growth advantage for MDR organisms in the remaining paired isolates (44%), which had different genotypic patterns.
In addition, the observation that similar P. aeruginosa genotypes colonized several ICU patients strongly suggests cross-colonization. The presumed route of colonization in MDR-non-XDR and XDR epidemic strains of P. aeruginosa might initially be exogenous and may occur earlier than intestinal colonization. Unfortunately, the only surveillance samples in this study were by rectal swab; no other patient or environmental cultures were performed. Thus, rectal cultures may not be adequate to quantify the initial colonization of patients with clonal strains, despite epidemiological findings, suggesting that patients provide a reservoir for further environmental contamination and cross-transmission (5).
Interestingly, 13% of the patients were not colonized during prolonged ICU stays despite high antimicrobial selection pressures. It is possible that our detection methods for P. aeruginosa were insufficiently sensitive, allowing cases of rectal colonization to be missed. However, it is equally plausible that some unknown non-antimicrobial-related host factors may have increased the level of colonization resistance in some patients (18).
We studied antimicrobial use and the extent of exposure before study inclusion. It is important to know the duration of exposure to understand the relationship between antibiotic resistance and microorganisms. The present study provides data concerning the impact of the duration of use of antimicrobials on their ability to promote digestive tract colonization with MDR P. aeruginosa (2, 3). After multivariate analysis, the only agents that remained significantly associated with the MDR P. aeruginosa isolation were the fluoroquinolones and the carbapenems (both ertapenem and group 2 carbapenems).
As expected, the underlying disease severity and prior fluoroquinolone and carbapenem consumption were associated with MDR P. aeruginosa acquisition. Exposure to group 2 carbapenems and the fluoroquinolones is known to be associated with the development of P. aeruginosa infection with wider resistance profiles. Previous studies on digestive colonization in ICU patients have shown that carbapenem exposure was associated with the acquisition of carbapenem-resistant P. aeruginosa strains, with odds ratios ranging from 3.4 to 7.8 (4, 19). In addition, the ability of fluoroquinolones to promote P. aeruginosa-resistant strains was also comparable with that in previous reports (3, 4, 19–21), although a recent study has shown a clear divergence in the role of fluoroquinolones in P. aeruginosa resistance (22).
Our study also demonstrated that ertapenem might increase the likelihood of development of polyclonal and clonal MDR P. aeruginosa intestinal colonization. There are lingering concerns that extensive ertapenem use may compromise the susceptibility of P. aeruginosa to group 2 carbapenems. P. aeruginosa is considered to have inherent resistance to ertapenem, and its clinical use was expected to delay the emergence of carbapenem resistance. Previous studies (23, 24) have concluded that, although ertapenem can select in vitro carbapenem resistance in P. aeruginosa, this phenomenon only occurs briefly in vivo. In fact, several studies suggest that ertapenem is not associated with increased carbapenem resistance in P. aeruginosa (25, 26); however, these reports drew their conclusions from ecological analyses. Other studies have examined the effect of introducing ertapenem into a hospital on the susceptibility of P. aeruginosa to group 2 carbapenems (27, 28); in these studies, the authors suspected that the improved susceptibility of P. aeruginosa to group 2 carbapenems was related to decreased fluoroquinolone use. However, in line with our finding, a recent patient-centered analysis provided a possible association of ertapenem with the appearance of P. aeruginosa resistance patterns (9). This may be explained through collateral damage on the indigenous microflora, with the high capacity of ertapenem to kill significant numbers of normal gastrointestinal flora, promoting an ecological pressure for the spread of MDR P. aeruginosa; another possibility is that substantial and prolonged ertapenem use in the past might selectively promote the development of resistance mechanisms in P. aeruginosa.
We found a trend toward reduced risks of clonal MDR-non-XDR P. aeruginosa versus polyclonal MDR-non-XDR intestinal P. aeruginosa colonization with piperacillin-tazobactam. These data suggest that piperacillin-tazobactam was more selection neutral than other agents and may result in the selection of fewer resistant P. aeruginosa strains.
There are several limitations in our study. The data set on which we performed our analysis was limited by the small number of patients colonized by different P. aeruginosa phenotypes. Although the conclusions are statistically significant, we recommend confirmation of our findings in larger epidemiological studies. In addition, restricting screening to stool samples might have resulted in an underestimation of early colonization with clonal MDR P. aeruginosa strains. For example, the possibility that clonal MDR P. aeruginosa strains were already present at other gut sites cannot be excluded. Future studies that include multiple screening sites might help. Finally, the results may have been influenced by local epidemiological variables not applicable in other settings, including a relatively high rate of horizontal transmission and almost universal antibiotic exposure. On balance, we believe that this was a useful approach for the epidemiological evaluation of MDR P. aeruginosa and even XDR P. aeruginosa strains.
In conclusion, our study contributes to a better understanding of the dynamics of endogenous P. aeruginosa colonization. The molecular data alerted us to the fact that different clusters of MDR P. aeruginosa coexist in our ICU, although few of those evolve in the same patient. Additionally, the risk of patients acquiring MDR P. aeruginosa colonization varied between antibiotic classes; group 2 carbapenems and fluoroquinolones had established associations, while ertapenem may also have contributed to promoting MDR P. aeruginosa strains. On the other hand, piperacillin-tazobactam appeared to be less selective for the development of MDR P. aeruginosa strains. It is possible that use of such a very broad-spectrum antibiotic might offer a valuable strategy to minimize the spread of MDR P. aeruginosa strains.
ACKNOWLEDGMENTS
This study was supported by National Health Service grant FIS 11/00164 from the Fondo de Investigación Sanitarias Instituto de Salud Carlos III and by the Ciber de Enfermedades Respiratorias (CB06/06/0037).
Footnotes
Published ahead of print 21 July 2014
REFERENCES
- 1.Bonten MJ, Bergmans DC, Speijer H, Stobberingh EE. 1999. Characteristics of polyclonal endemicity of Pseudomonas aeruginosa colonization in intensive care units. Am. J. Respir. Crit. Care Med. 160:1212–1219. 10.1164/ajrccm.160.4.9809031 [DOI] [PubMed] [Google Scholar]
- 2.Philippe E, Weiss M, Schultz JM, Yeomans F, Ehrenkraz NJ. 1999. Emergence on highly antibiotic resistant Pseudomonas aeruginosa in relation to duration of empirical antipseudomonal antibiotic treatment. Clin. Perform. Qual. Health Care 7:83–87 [PubMed] [Google Scholar]
- 3.Paramythiotou E, Lucet JC, Timsit JF, Vanjak D, Paugam-Burtz C, Trouillet JL, Belloc S, Kassis N, Karabinis A, Andremont A. 2004. Acquisition of multidrug-resistant Pseudomonas aeruginosa in patients in intensive care units: role of antibiotics with antipseudomonal activity. Clin. Infect. Dis. 38:670–677. 10.1086/381550 [DOI] [PubMed] [Google Scholar]
- 4.Peña C, Guzmán A, Suarez C, Dominguez MA, Tubau F, Pujol M, Gudiol F, Ariza J. 2007. Effects of carbapenem exposure on the risk for digestive tract carriage of intensive care unit endemic carbapenem-resistant Pseudomonas aeruginosa strain in critically ill patients. Antimicrob. Agents Chemother. 51:1967–1971. 10.1128/AAC.01483-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Suarez C, Peña C, Arch O, Dominguez MA, Tubau F, Juan C, Gavaldá L, Sora M, Oliver A, Pujol M, Ariza J. 2011. A large sustained endemic outbreak of multiresistant Pseudomonas aeruginosa: a new epidemiological scenario for nosocomial acquisition. BMC Infect. Dis. 11:272–279. 10.1186/1471-2334-11-272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cabot G, Ocampo-Sosa AA, Dominguez MA, Gago JF, Juan C, Tubau F, Rodriguez C, Moyà B, Peña C, Martínez-Martínez L, Oliver A, Spanish Network for Research in Infectious Diseases (REIPI) 2012. Genetic markers of widespread extensively drug-resistant Pseudomonas aeruginosa high-risk clones. Antimicrob. Agents Chemother. 56:6349–6357. 10.1128/AAC.01388-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mulet X, Cabot G, Ocampo-Sosa AA, Dominguez MA, Zamorano L, Juan C, Tubau F, Rodriguez C, Moyà B, Peña C, Martínez-Martínez L, Oliver A, Spanish Network for Research in Infectious Diseases (REIPI) 2013. Biological markers of Pseudomonas aeruginosa epidemic high-risk clones. Antimicrob. Agents Chemother. 57:5527–5535. 10.1128/AAC.01481-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, Harbarth S, Hindler JF, Kahimeter G, Olsson-Liljequist B, Paterson DL, Rice LB, Stelling J, Struelens MJ, Vatopoulos A, Weber JT, Monnet DL. 2012. Multidrug resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 18:268–281. 10.1111/j.1469-0691.2011.03570.x [DOI] [PubMed] [Google Scholar]
- 9.Cohen MJ, Block CS, Moses AE, Nir-Paz R. 2014. Exposure to ertapenem is possibly associated with Pseudomonas aeruginosa antibiotic resistance. Clin. Microbiol. Infect. 20:O188–O196. 10.1111/1469-0691.12362 [DOI] [PubMed] [Google Scholar]
- 10.Le Gall JR, Loirat P, Alperovitch A, Glaser P, Granthil C, Mathieu D, Mercier P, Thomas R, Villers D. 1984. A simplified acute physiologic score for ICU patients. Crit. Care Med. 12:975–977. 10.1097/00003246-198411000-00012 [DOI] [PubMed] [Google Scholar]
- 11.Charlson ME, Pompei P, Ales KL, MacKenzie CR. 1987. A new method of classifying prognostic co-morbidity in longitudinal studies: development and validation. J. Chron. Dis. 40:373–383. 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 12.Clinical and Laboratory Standards Institute. 2012. Performance standards for antimicrobial susceptibility testing; 20th informational supplement. M100-S22 Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 13.Deplano A, Denis O, Poirel L, Hocquet D, Nonhoff C, Byl B, Nordmann P, Vincent JL, Struelens MJ. 2005. Molecular characterization of an epidemic clone of panantibiotic-resistant Pseudomonas aeruginosa. J. Clin. Microbiol. 43:1198–1204. 10.1128/JCM.43.3.1198-1204.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tenover FC, Arbeit RD, Goering RV, Mickelsen PA, Murray BE, Persing DH, Swaminathan B. 1995. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J. Clin. Microbiol. 33:2233–2239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Curran B, Jonas D, Grudmann H, Pitt T, Dowson CG. 2004. Development of a multilocus sequence typing scheme for the opportunistic pathogen Pseudomonas aeruginosa. J. Clin. Microbiol. 42:5644–5649. 10.1128/JCM.42.12.5644-5649.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jolley KA, Chan MS, Maiden MC. 2004. mlstdbNet-distributed multilocus sequence typing (MLST) databases. BMC Bioinformatics 5:86. 10.1186/1471-2105-5-86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peña C, Suarez C, Tubau F, Juan C, Moya B, Dominguez MMA, Oliver A, Pujol M, Ariza J. 2009. Nosocomial outbreak of a non-cefepime-susceptible ceftazidime susceptible Pseudomonas aeruginosa strain overexpressing MexXY-OprM and producing an integron-borne PSE-1 β-lactamase. J. Clin. Microbiol. 47:2381–2387. 10.1128/JCM.00094-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vollaard EJ, Clasener HAL. 1994. Colonization resistance. Antimicrob. Agents Chemother. 38:409–414. 10.1128/AAC.38.3.409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lepelletier D, Cady A, Caroff N, Marraillac J, Reynaud A, Lucet JC, Corvec S. 2010. Imipenem-resistant Pseudomonas aeruginosa gastrointestinal carriage among hospitalized patients: risk factors and resistance mechanisms. Diagn. Microbiol. Infect. Dis. 66:1–6. 10.1016/j.diagmicrobio.2009.08.014 [DOI] [PubMed] [Google Scholar]
- 20.Cao B, Wang H, Sun H, Zhu Y, Chen M. 2004. Risk factors and clinical outcomes of nosocomial multi-drug resistant Pseudomonas aeruginosa. J. Hosp. Infect. 57:112–118. 10.1016/j.jhin.2004.03.021 [DOI] [PubMed] [Google Scholar]
- 21.Defez C, Fabbro-Peray P, Bouziges N, Gouby A, Mahamat A, Daurès JP, Sotto A. 2004. Risk factors for multidrug-resistant Pseudomonas aeruginosa nosocomial infection. J. Hosp. Infect. 57:209–216. 10.1016/j.jhin.2004.03.022 [DOI] [PubMed] [Google Scholar]
- 22.Martínez JA, Delgado E, Martí S, Marco F, Vila J, Mensa J, Torres A, Codina C, Trilla A, Soriano A, Alquezar A, Castro P, Nicolás JM. 2009. Influence of antipseudomonal agents on Pseudomonas aeruginosa colonization and acquisition of resistance in critically ill medical patients. Intensive Care Med. 35:439–447. 10.1007/s00134-008-1326-y [DOI] [PubMed] [Google Scholar]
- 23.Livermore DM, Mushtaq S, Warner M. 2005. Selectivity of ertapenem for Pseudomonas aeruginosa mutants cross-resistant to other carbapenems. J. Antimicrob. Chemother. 55:306–311. 10.1093/jac/dki009 [DOI] [PubMed] [Google Scholar]
- 24.Vainio S, Wilhelm A, Vandenbroucke-Grauls C, Murk JL, Debets-Ossenkopp Y. 2013. Rapid selection of carbapenem-resistant by clinical concentrations of ertapenem. Int. J. Antimicrob. Agents 41:492–494. 10.1016/j.ijantimicag.2013.01.010 [DOI] [PubMed] [Google Scholar]
- 25.Carmeli Y, Lidji SK, Shabtai E, Navon-Venezia S, Schwaber MJ. 2011. The effects of group 1 versus group 2 carbapenems on imipenem-resistant Pseudomonas aeruginosa: an ecological study. Diagn. Microbiol. Infect. Dis. 70:367–372. 10.1016/j.diagmicrobio.2011.03.009 [DOI] [PubMed] [Google Scholar]
- 26.Nicolau DP, Carmeli Y, Crank CW, Goff DA, Graber CJ, Lima AL, Goldstein EJC. 2012. Carbapenem stewardship: does ertapenem affect Pseudomonas susceptibility to other carbapenems? A review of the evidence. Int. J. Antimicrob. Agents 39:11–15. 10.1016/j.ijantimicag.2011.08.018 [DOI] [PubMed] [Google Scholar]
- 27.Cook PP, Gooch M, Rizzo S. 2011. Reduction in fluoroquinolones use following introduction of ertapenem into a hospital formulary is associated with improvement in susceptibility of Pseudomonas aeruginosa to group 2 carbapenems: a 10-year study. Antimicrob. Agents Chemother. 55:5597–5601. 10.1128/AAC.00742-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sousa D, Castelo-Corral L, Gutierrez-Urbón JM, Molina F, López-Calviño B, Bou G, Llinares P. 2013. Impact of ertapenem use on Pseudomonas aeruginosa and Acinetobacter baumannii imipenem susceptibility rates: collateral damage or positive effect on hospital ecology? J. Antimicrob. Chemother. 68:1917–1925. 10.1093/jac/dkt091 [DOI] [PubMed] [Google Scholar]