Abstract
In tuberculosis treatment, susceptibility is defined by a critical concentration of 1.0 mg/liter for rifampin and 0.2 or 1.0 mg/liter for low- and high-level isoniazid resistance on the basis of an epidemiologic cutoff method that uses the distribution of the MICs for isolates. However, pharmacokinetics-pharmacodynamics-based clinical trial simulations suggested that the breakpoints should be 0.0625 mg/liter for rifampin and 0.0312 or 0.125 mg/liter for isoniazid. We examined the outcomes of 36 patients with drug-susceptible tuberculosis whose rifampin and isoniazid MICs were determined, whose plasma drug concentrations were also measured, and who were part of a prospective cohort study in Western Cape, South Africa. We performed classification and regression tree analysis to identify clinical and laboratory factors that predicted 2-month sputum conversion rates and long-term clinical outcomes. Poor long-term clinical outcomes were defined as microbiological failure, relapse, or death within a 2-year follow-up period. Peak drug concentrations and areas under the concentration-time curve were most predictive of outcomes and constituted the primary node, similar to our findings on the larger cohort. However, rifampin and isoniazid MICs improved the predictive capacity of the primary decision node by 20 and 17%, respectively, for these 36 patients. The rifampin MIC cutoff above which there was therapy failure was 0.125 mg/liter, while that of isoniazid was 0.0312 mg/liter; these are similar to those derived in clinical trial simulations. The critical concentrations used to define multidrug resistance for clinical decision making should take clinical outcomes into account.
INTRODUCTION
Patients with drug-resistant tuberculosis, especially multidrug-resistant tuberculosis (MDR-TB; defined as tuberculosis resistant to both isoniazid and rifampin) and extremely drug-resistant tuberculosis (XDR-TB; MDR-TB with additional resistance to fluoroquinolones and injectable compounds) need to be accurately categorized from the outset, as they need specific management with second-line regimens (1–5). Hence, it is crucial that resistance testing identify infections with a susceptibility threshold beyond which patients receiving the first-line regimen with standard doses of rifampin and isoniazid will respond poorly. Resistance to isoniazid and rifampin is said to be present when ≥1% of a Mycobacterium tuberculosis culture grows in medium supplemented with the critical concentration of each drug. The critical concentrations of rifampin and isoniazid were derived from the MICs for ≥95% of wild-type isolates of M. tuberculosis, termed epidemiologic cutoff values (6–8). While they are extensively used, as evidenced by the regular release of MDR-TB worldwide figures by the World Health Organization (3), it is nevertheless unclear if the epidemiologic cutoff values are predictive of clinical responses and if they are the most accurate. Here, we investigated this possibility on the basis of data from a prospective cohort study.
Another approach that has been used to identify susceptibility breakpoints is a pharmacokinetics (PK)-pharmacodynamics (PD)-based derivation that takes into account the PK variability of each drug (9). PK-PD studies in preclinical models such as the hollow-fiber model, as well as in clinical studies of tuberculosis patients, have identified peak-to-MIC and area under the concentration-time curve (AUC)-to-MIC ratios below which there is a poor outcome (10–14). In addition, PK variability has been shown to be one of the important determinants of therapy failure in patients and has delineated optimal AUCs and peak concentrations below which therapy fails (15, 16). Therefore, we have proposed that the critical concentration be defined as the MIC above which maximum tolerated doses fail to effectively kill M. tuberculosis at the site of infection (17). If the recommended doses in the treatment regimen are unable to effectively kill bacilli in patients because of the MIC, then that MIC defines clinically meaningful drug resistance. Monte Carlo simulations that utilized PK-PD exposures associated with optimal outcomes and antibiotic PK variability encountered in patients led to the proposal that critical rifampin concentrations be lowered from the epidemiologic cutoff-derived value of 1.0 to 0.0625 mg/liter and isoniazid low- and high-level resistance concentrations be lowered from 0.2 and 1.0 to 0.0312 and 0.125 mg/liter in Middlebrook medium (17). Here, we used an agnostic machine learning method to identify the rifampin and isoniazid MICs above which treatment with short-course chemotherapy in the clinic fails.
MATERIALS AND METHODS
One hundred forty-two patients with sputum culture-positive tuberculosis were enrolled in a PK-PD study at Brewelskloof Hospital in Worcester, Western Cape, South Africa as described in prior reports (15, 18). Patients were recruited between August 1999 and February 2002. The isoniazid and rifampin MICs for the isolates from 36 of these 142 patients, randomly chosen, were also identified at the time of diagnosis. Patients were treated with the following daily doses during the intensive phase of therapy: 300 mg of isoniazid, 20 to 35 mg/kg pyrazinamide, 15 mg/kg ethambutol, and 600 mg of rifampin daily if they weighed >50 kg or 450 mg if they weighed less. Patients with prior tuberculosis also received intramuscular streptomycin (1 g if they weighed more than 55 kg, 0.75 g if they weighed 38 to 54 kg, and 0.5 g if they weighed ≤37 kg). In the continuation phase, all of the patients received the same isoniazid and rifampin doses but for 5 in 7 days and patients with prior tuberculosis were continued on ethambutol. All patients were hospitalized during the first 2 months of therapy to ensure directly observed therapy, for reasons outlined in the primary PK study publication (18). PK studies were performed over 24 h during the 8th week of therapy. The compartmental PK analyses have been published elsewhere (15). Sputum microscopy and culture (using liquid cultures in the Bactec 460 instrument) were performed after 8 weeks of treatment. Patients were then followed up prospectively for sputum conversion and long-term outcomes for 2 years.
All of the patients' isolates were directly tested for sensitivity with the Bactec 460 instrument. The isoniazid and rifampin MICs for the initial isolates from 36 patients were determined at diagnosis. Isoniazid susceptibility was determined in the Bactec system by using the following concentrations: 0.0125, 0.025, 0.05, 0.1, and 0.2 mg/liter. For rifampin, the following concentrations were examined: 0.06, 0.125, 0.25, 0.5, 1.0, and 2.0 mg/liter. MICs of pyrazinamide or ethambutol were not identified.
Classification and regression tree (CART) analysis is a nonparametric method that uses recursive partitioning to classify data (19–21), which we and others have used for clinical decision making to examine outcomes in several infectious-disease studies (15, 22–25). We were interested in the classification of the 36 patients into two categories, therapy failure versus success. Two clinical outcome measures were used, sputum microbiology status at 2 months and long-term outcome. A poor long-term outcome was defined as a relapse, microbiologic failure, or death within the 2-year follow-up period since the start of therapy (15). Several outcome predictors were examined, including the AUC, peak drug concentrations in serum, trough concentrations, isoniazid MIC, rifampin MIC, age, gender, weight, HIV status, and treatment with streptomycin. CART analysis examines all of these potential outcome predictors and examines all of the possible threshold values of the predictors to create a tree. A step-by-step description of this method of analyzing tuberculosis therapy was previously published (15). Briefly, the upside tree created starts with a root node, which is the most important potential predictor or decision node. Daughter nodes are added to the tree, and a score of how much they improve the primary decision node (as a percent score of the primary node) is computed. For each outcome, maximal trees were generated by splitting each daughter node so that each class was homogeneous in the outcome examined until further splits were not possible. Maximal trees are important in identifying data structure, as well as clinically meaningful interactions between covariates, particularly among fewer patients (small daughter nodes). We then pruned the maximal trees based on relative misclassification costs, complexity, and maximization of parsimony. Next, we performed a 10-fold validation. In this process, the data set is randomly split 10 times in order to construct optimal trees, to identify how predictive the primary analysis was with these randomly created “test” data sets. This process obviates the need for a validation data set.
RESULTS
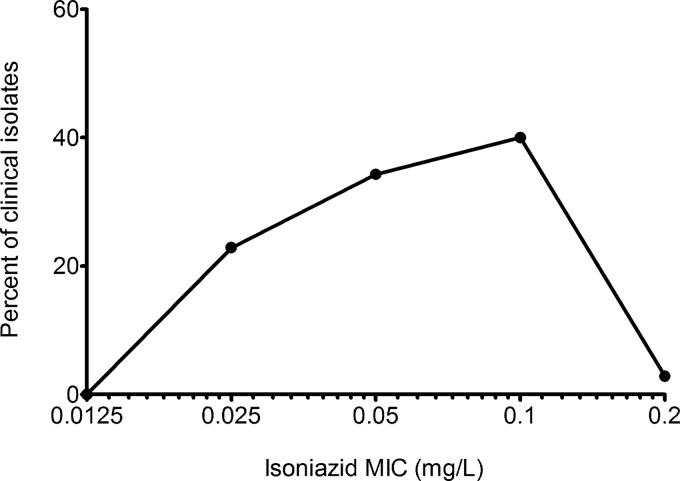
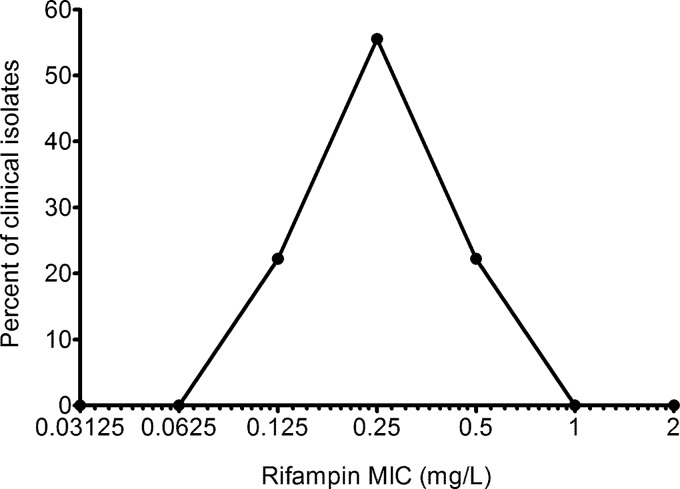
The full clinical characteristics of the 36 patients whose isoniazid and rifampin MICs were determined are shown in Table 1. The characteristics are similar to those of the entire set of 142 patients (15), confirming that this subset of patients was randomly chosen from the larger data set. The distribution of isoniazid MICs is shown in Fig. 1. The mean isoniazid MIC was 0.07 ± 0.04 mg/liter. The epidemiologic cutoff value was 0.2 mg/liter. The distribution of rifampin MICs is shown in Fig. 2. The mean rifampin MIC was 0.28 ± 0.13 mg/liter. The rifampin epidemiologic cutoff value was 0.5 mg/liter.
TABLE 1.
Clinical and demographic factors of 35 patients treated for tuberculosis in the Western Cape, South Africa
| Characteristic | Value |
|---|---|
| No. (%) of females | 23 (65.71) |
| Median age, yr (range) | 35 (20–71) |
| No. (%) self-identified as: | |
| Mixed-race South African | 31 (88.57) |
| Black South African | 4 (11.43) |
| Median body wt, kg (range) | 47.3 (30–68) |
| No. (%) HIV infected | 3 (8.57) |
| Median dose, mg/kg (range) | |
| Rifampin | 10.71 (8.38–15.00) |
| Isoniazid | 6.39 (4.41–10.00) |
| Pyrazinamide | 35.14 (19.69–50.00) |
| Ethambutol | 23.87 (12.88–30.77) |
| No. (%) with prior tuberculosis | 23 (65.71) |
| Median hemoglobin level, g/dl (range) | 12.00 (7.40–14.00) |
| Median white blood cell count, 109/ml (range) | 8.60 (3.80–25.70) |
| Median platelet count, 109 cells/ml (range) | 410.0 (66.0–813.0) |
| Median ESR,a mm/h (range) | 58 (12–136) |
| Median total protein level, g/liter (range) | 77 (62–92) |
| Median albumin level, g/liter (range) | 34.00 (21–43) |
ESR, erythrocyte sedimentation rate.
FIG 1.
Distribution of isoniazid MICs for isolates from 36 patients. The Gaussian distribution is skewed towards the left and shows a slightly higher epidemiologic cutoff value than in the literature (6).
FIG 2.
Distribution of rifampin MICs for isolates from 36 patients. The Gaussian distribution of the rifampin MICs shows a range of MICs of the drug for clinical isolates. Thus, the standard notion of stating that the rifampin MIC for clinical isolates is, say, 0.125 mg/liter is meaningless.
With regard to the 2-month sputum conversion rates, 18/36 patients (50%) had a positive culture or smear at 8 weeks. The factors most predictive of 2-month sputum conversion included peak pyrazinamide, rifampin, and isoniazid concentrations, as identified in the larger 142-patient study (15). However, in the 36 patients, isoniazid and rifampin MICs were also predictive of 2-month sputum conversion; MIC thresholds are shown in Table 2. While 2-month sputum conversion is an important surrogate, the gold standard of antituberculosis therapy efficacy is still the long-term outcome. With regard to the long-term outcomes, the pyrazinamide AUC was the primary root node, followed by the rifampin AUC and the isoniazid AUC, consistent with findings from the entire data set of 142 patients. However, the next root node was the rifampin MIC, followed by the isoniazid MIC. Rifampin and isoniazid MICs improved the primary decision node by 20 and 17%, respectively. The rifampin and isoniazid cutoff points associated with outcomes are shown in Table 2. However, in standard assays, MICs are determined on the basis of 2-fold dilution steps, so that the nearest observed MIC that fulfills the nonstrict inequality values shown in Table 2 was chosen as the breakpoint MIC (Table 2). The results held in the 10-fold validation process. Since predictive power is defined as the performance of the training set-derived tree with the test data set, our results suggest a good role for these rifampin and isoniazid MICs in predicting therapy failure. As shown in Table 2, the isoniazid MIC breakpoint for patients whose therapy failed was 0.0312 mg/liter, while that of rifampin was 0.125 mg/liter.
TABLE 2.
CART analysis-derived MIC breakpoints for isolates from 36 patients
| Parameter | MIC (mg/liter) cutoff (2-log scale) |
|
|---|---|---|
| Isoniazid | Rifampin | |
| 2-mo sputum conversion | ≤0.150 (0.125) | ≤0.188 (0.125) |
| Long-term outcome | ≤0.038 (0.0312) | ≤0.38 (0.25) |
DISCUSSION
Isoniazid and rifampin MICs were predictive of clinical outcomes, both at the 2-month time point and in the long term. These data are consistent with classic antimicrobial PK-PD theory, which is that the MIC for a bacterial species is an important outcome determinant (26–29). Therefore, as with many other pathogens, such as standard Gram-negative and Gram-positive bacteria, the MICs of antituberculosis drugs affect clinical outcome. The MIC's effect on microbial killing is considered relative to the drug concentration achieved at the site of infection. The drug concentrations achieved in patients have a ceiling at the maximum tolerated dose and are driven mainly by PK variability between patients. As the MIC rises in the face of that ceiling concentration, the AUC/MIC or peak/MIC ratio falls, leading to less and less killing. Eventually, an MIC is reached above which microbial killing is not effective in most patients and which defines clinical resistance. This MIC above which therapeutic failure occurs is not necessarily linked to the 95% epidemiologic cutoff value derived from the MIC distribution. Indeed, there should be no mathematical or physiological reason to expect the patient response to be linked to Gaussian parameters of drug MICs for M. tuberculosis isolates. On the other hand, a shift in the 95% epidemiologic cutoff value in different time periods indicates that the population of isolates from the locale is moving more and more toward drug resistance, making it an excellent index for epidemiologic and public health work tracking of MDR-TB and XDR-TB. Our present study suggests that for clinical decision making during combination therapy, however, susceptibility breakpoint values should be lowered to 0.0312 mg/liter for isoniazid and 0.125 mg/liter for rifampin and do not coincide with the 95% epidemiologic cutoff value. The implication is that MDR-TB rates in the world are likely multiple-fold higher than currently assumed.
Our results redefine MDR-TB and, by extension, XDR-TB, given that these definitions are dependent on critical concentrations of isoniazid and rifampin. In the case of rifampin, in four sets of case reports from New Zealand, Australia, and The Netherlands, a total of 11 clinical isolates from these three centers had mutations in the β subunit of RNA polymerase (rpoB), were flagged as rifampin resistant by Gene-Xpert technology, and were found to have rifampin MICs of 0.125 to 1.0 mg/liter, rifampin-containing multiple-drug therapy failed (30–32). The authors termed this “phenotypically occult” resistance. We propose, instead, that the rifampin breakpoint should, in fact, be lower than the current standard, at ≤0.125 mg/liter. Moreover, our proposed breakpoint is based on the failure of patients to respond to therapy and is therefore not defined by chromosomal mutations, as is the case with Gene-Xpert. That is because not all drug resistance is due to mutations; the MICs for some M. tuberculosis isolates are naturally high, while other mechanisms of drug resistance such as efflux pump induction could also lead to drug resistance (33–36). There have not been any case reports on isoniazid similar to the 11 case reports on rifampin since there is no rapid molecular test with which to identify isoniazid-resistant mutants. Nevertheless, mechanisms such as efflux pumps are also known to play a role in isoniazid resistance, which means that the final definition of isoniazid resistance will ultimately have to be based on phenotypic tests such as MIC determinations (11, 13, 37, 38). Our present findings suggest that the critical concentration of isoniazid for clinical decision making should also be lowered. In essence, there is now clinical support for a change in the critical concentrations that define MDR-TB. These new concentrations should be considered for clinical decision making by the several bodies important in the clinical care of tuberculosis around the world, such as the WHO and STOP TB, as well as by groups designing assays for the diagnosis of MDR-TB.
The approach using hollow-fiber PK-PD and PK variability in Monte Carlo simulations to identify provisional and definitive susceptibility breakpoints of antituberculosis agents (17) identified breakpoints virtually the same as those identified in our present clinical study. Similarly, the breakpoint of pyrazinamide was correctly identified by this method and was also recently identified by CART as identical (25). This is noteworthy because CART analyses did not prespecify the susceptibility breakpoints that should be selected but were “agnostic” in identifying the MIC as a clinical outcome predictor from among several clinical factors and also calculated threshold MICs that classify patients as those whose therapy fails and those whose therapy succeeds without relying on a prior choice. Therefore, the pharmacometric pathway consisting of (i) preclinical PK-PD studies of monotherapy to identify optimal drug exposures in the hollow-fiber or other preclinical model, followed by (ii) the use of population PK in Monte Carlo experiments and (iii) the choice of a susceptibility breakpoint based on >10% of patients failing to achieve optimal exposures, is validated for setting anti-TB drug susceptibility breakpoints. We propose its use for the new and experimental antituberculosis therapies currently being studied.
Our study has several limitations. First is the size of the clinical study, which could limit the generalizability of the findings. However, CART has been able to correctly identify thresholds with similarly small populations in the past (24, 25, 39). Second, several other clinical factors also determine clinical outcomes, including drug concentrations, cavitary disease, and bacterial burdens. However, these factors need not exclude a role for MICs in outcome prediction. Indeed, our CART analysis also examined some of these as possible predictors, and with the exception of drug concentrations, they were outranked by MICs. Third, one potential limitation of CART is fitting and biasing toward covariates with many possible splits. Thus, our findings should be taken with these factors in context. Nevertheless, cross-validation identified the same MIC thresholds, which were virtually identical to Monte Carlo simulation results published 5 years earlier (17). This means that the same breakpoints have now been identified by two completely independent methods. In the case of rifampin, retrospective case reports led to the same conclusion, adding a third independent method. Thus, critical concentrations of 0.125 mg/liter for rifampin and 0.0312 mg/liter for isoniazid should be used to define MDR-TB. Such PK-PD evaluations should form the basis of the accurate determination of susceptibility breakpoints. The continued use of breakpoints that disregard the drug exposures of patients administered recommended doses will lead to the incorrect categorization of patients and treatment with inappropriate regimens (40).
ACKNOWLEDGMENTS
We acknowledge the patients who participated in this study; the nursing staff of Brewelskloof Hospital, Western Cape, for taking care of patients; and Andrew Whitelaw for MIC determination. We also acknowledge the University of Cape Town Department of Medicine and the University of Texas Southwestern Medical Center Office of Global Health for making this collaboration possible.
T.G. founded Jacaranda Biomed. J.G.P. and H.M. have no conflicts of interest.
This work was supported by the National Institutes of Health via the NIH Director New Innovator Award (National Institutes of General Medical Sciences DP2 OD001886 to J.G.P. and T.G. and National Institute of Allergy and Infectious Diseases R01AI079497 to J.G.P. and T.G.). Funding for the recruitment of patients and for Helen McIlleron was provided by the Division of Pharmacology of the University of Cape Town and the Medical Research Council of South Africa.
Footnotes
Published ahead of print 4 August 2014
REFERENCES
- 1.Migliori GB, Sotgiu G, Gandhi NR, Falzon D, DeRiemer K, Centis R, Hollm-Delgado MG, Palmero D, Pérez-Guzmán C, Vargas MH, D'Ambrosio L, Spanevello A, Bauer M, Chan ED, Schaaf HS, Keshavjee S, Holtz TH, Menzies D, Collaborative Group for Meta-Analysis of Individual Patient Data in MDR-TB 2013. Drug resistance beyond extensively drug-resistant tuberculosis: individual patient data meta-analysis. Eur. Respir. J. 42:169–179. 10.1183/09031936.00136312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention (CDC). 2006. Emergence of Mycobacterium tuberculosis with extensive resistance to second-line drugs–worldwide, 2000-2004. MMWR Morb. Mortal. Wkly. Rep. 55:301–305 [PubMed] [Google Scholar]
- 3.World Health Organization. 2010. Multidrug and extensively drug-resistant TB (M/XDR-TB): 2010 global report on surveillance and response. World Health Organization, Geneva, Switzerland: http://whqlibdoc.who.int/publications/2010/9789241599191_eng.pdf?ua=1 [Google Scholar]
- 4.Gandhi NR, Moll A, Sturm AW, Pawinski R, Govender T, Lalloo U, Zeller K, Andrews J, Friedland G. 2006. Extensively drug-resistant tuberculosis as a cause of death in patients co-infected with tuberculosis and HIV in a rural area of South Africa. Lancet 368:1575–1580. 10.1016/S0140-6736(06)69573-1 [DOI] [PubMed] [Google Scholar]
- 5.Dheda K, Gumbo T, Gandhi NR, Murray M, Theron G, Udwadia Z, Migliori GB, Warren R. 2014. Global control of tuberculosis: from extensively-drug resistant to untreatable tuberculosis. Lancet Respir. Med. 2:321–338. 10.1016/S2213-2600(14)70031-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Canetti G, Froman S, Grosset J, Hauduroy P, Langerova M, Mahler HT, Meissner G, Mitchison DA, Sula L. 1963. Mycobacteria: laboratory methods for testing drug sensitivity and resistance. Bull. World Health Organ. 29:565–578 [PMC free article] [PubMed] [Google Scholar]
- 7.Ambrose PG. 2006. Monte Carlo simulation in the evaluation of susceptibility breakpoints: predicting the future: insights from the society of infectious diseases pharmacists. Pharmacotherapy 26:129–134. 10.1592/phco.2006.26.1.129 [DOI] [PubMed] [Google Scholar]
- 8.Clinical and Laboratory Standards Institute. 2003. Susceptibility testing of mycobacteria, nocardiae, and other aerobic actinomycetes; approved standard. Clinical and Laboratory Standards Institute, Wayne, PA: [PubMed] [Google Scholar]
- 9.Drusano GL, Preston SL, Hardalo C, Hare R, Banfield C, Andes D, Vesga O, Craig WA. 2001. Use of preclinical data for selection of a phase II/III dose for evernimicin and identification of a preclinical MIC breakpoint. Antimicrob. Agents Chemother. 45:13–22. 10.1128/AAC.45.1.13-22.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gumbo T, Louie A, Deziel MR, Liu W, Parsons LM, Salfinger M, Drusano GL. 2007. Concentration-dependent Mycobacterium tuberculosis killing and prevention of resistance by rifampin. Antimicrob. Agents Chemother. 51:3781–3788. 10.1128/AAC.01533-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gumbo T, Louie A, Liu W, Brown D, Ambrose PG, Bhavnani SM, Drusano GL. 2007. Isoniazid bactericidal activity and resistance emergence: integrating pharmacodynamics and pharmacogenomics to predict efficacy in different ethnic populations. Antimicrob. Agents Chemother. 51:2329–2336. 10.1128/AAC.00185-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gumbo T, Siyambalapitiyage Dona CS, Meek C, Leff R. 2009. Pharmacokinetics-pharmacodynamics of pyrazinamide in a novel in vitro model of tuberculosis for sterilizing effect: a paradigm for faster assessment of new antituberculosis drugs. Antimicrob. Agents Chemother. 53:3197–3204. 10.1128/AAC.01681-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Srivastava S, Musuka S, Sherman C, Meek C, Leff R, Gumbo T. 2010. Efflux-pump-derived multiple drug resistance to ethambutol monotherapy in Mycobacterium tuberculosis and the pharmacokinetics and pharmacodynamics of ethambutol. J. Infect. Dis. 201:1225–1231. 10.1086/651377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chigutsa E, Pasipanodya JG, Sirgel FA, Gumbo T, McIlleron H. 2013. Multivariate adaptive regression splines analysis of the effect of drug concentration and MIC on sterilizing activity in patients on multidrug therapy, abstr. 14, p 15 Abstr. 6th Int. Workshop Clin. Pharmacol. Tuberc. Drugs, Denver, CO [Google Scholar]
- 15.Pasipanodya JG, McIlleron H, Burger A, Wash PA, Smith P, Gumbo T. 2013. Serum drug concentrations predictive of pulmonary tuberculosis outcomes. J. Infect. Dis. 208:1464–1473. 10.1093/infdis/jit352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pasipanodya JG, Srivastava S, Gumbo T. 2012. Meta-analysis of clinical studies supports the pharmacokinetic variability hypothesis for acquired drug resistance and failure of antituberculosis therapy. Clin. Infect. Dis. 55:169–177. 10.1093/cid/cis353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gumbo T. 2010. New susceptibility breakpoints for first-line antituberculosis drugs based on antimicrobial pharmacokinetic/pharmacodynamic science and population pharmacokinetic variability. Antimicrob. Agents Chemother. 54:1484–1491. 10.1128/AAC.01474-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McIlleron H, Wash P, Burger A, Norman J, Folb PI, Smith P. 2006. Determinants of rifampin, isoniazid, pyrazinamide, and ethambutol pharmacokinetics in a cohort of tuberculosis patients. Antimicrob. Agents Chemother. 50:1170–1177. 10.1128/AAC.50.4.1170-1177.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Breiman L, Friedman J, Stone CJ, Olshen RA. 1984. Classification and regression trees. Chapman & Hall/CRC, Boca Raton, FL [Google Scholar]
- 20.Kim H, Loh W-Y. 2001. Classification trees with unbiased multiway splits. J. Am. Stat. Assoc. 88:457–467. 10.1198/016214501753168271 [DOI] [Google Scholar]
- 21.Steinberg D, Colla P. 1995. CART: tree-structured non-parametric data analysis. Salford Systems, San Diego, CA [Google Scholar]
- 22.Lewis RJ. 2000. An introduction to classification and regression tree (CART) analysis. Annu. Meet. Soc. Acad. Emerg. Med. 2000. Society for Academic Emergency Medicine, Des Plaines, IL: http://www.freewebs.com/achi/lewis1.pdf [Google Scholar]
- 23.Andes D, Ambrose PG, Hammel JP, Van Wart SA, Iyer V, Reynolds DK, Buell DN, Kovanda LL, Bhavnani SM. 2011. Use of pharmacokinetic-pharmacodynamic analyses to optimize therapy with the systemic antifungal micafungin for invasive candidiasis or candidemia. Antimicrob. Agents Chemother. 55:2113–2121. 10.1128/AAC.01430-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jain MK, Pasipanodya JG, Alder L, Lee WM, Gumbo T. 2013. Pegylated interferon fractal pharmacokinetics: individualized dosing for hepatitis C virus infection. Antimicrob. Agents Chemother. 57:1115–1120. 10.1128/AAC.02208-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gumbo T, Chigutsa E, Pasipanodya JG, Visser M, McIlleron H, Sirgel FA. 2014. The pyrazinamide susceptibility breakpoint above which combination therapy fails. J. Antimicrob. Chemother. 69:2420–2425. 10.1093/jac/dkt524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Drusano GL, Johnson DE, Rosen M, Standiford HC. 1993. Pharmacodynamics of a fluoroquinolone antimicrobial agent in a neutropenic rat model of Pseudomonas sepsis. Antimicrob. Agents Chemother. 37:483–490. 10.1128/AAC.37.3.483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Craig WA. 1998. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin. Infect. Dis. 26:1–10. 10.1086/516284 [DOI] [PubMed] [Google Scholar]
- 28.Craig WA. 2007. Pharmacodynamics of antimicrobials: general concepts and applications, p 1–19 In Nightangle CH, Ambrose PG, Drusano GL, Murakawa T. (ed), Antimicrobial pharmacodynamics in theory and practice, 2nd ed, vol. 44 Informa Healthcare USA, Inc., New York, NY [Google Scholar]
- 29.Ambrose PG, Bhavnani SM, Rubino CM, Louie A, Gumbo T, Forrest A, Drusano GL. 2007. Pharmacokinetics-pharmacodynamics of antimicrobial therapy: it's not just for mice anymore. Clin. Infect. Dis. 44:79–86. 10.1086/510079 [DOI] [PubMed] [Google Scholar]
- 30.Williamson DA, Roberts SA, Bower JE, Vaughan R, Newton S, Lowe O, Lewis CA, Freeman JT. 2012. Clinical failures associated with rpoB mutations in phenotypically occult multidrug-resistant Mycobacterium tuberculosis. Int. J. Tuberc. Lung Dis. 16:216–220. 10.5588/ijtld.11.0178 [DOI] [PubMed] [Google Scholar]
- 31.van Ingen J, Aarnoutse R, de Vries G, Boeree MJ, van Soolingen D. 2011. Low-level rifampicin-resistant Mycobacterium tuberculosis strains raise a new therapeutic challenge. Int. J. Tuberc. Lung Dis. 15:990–992. 10.5588/ijtld.10.0127 [DOI] [PubMed] [Google Scholar]
- 32.Ho J, Jelfs P, Sintchencko V. 2013. Phenotypically occult multidrug-resistant Mycobacterium tuberculosis: dilemmas in diagnosis and treatment. J. Antimicrob. Chemother. 68:2915–2920. 10.1093/jac/dkt284 [DOI] [PubMed] [Google Scholar]
- 33.Pasipanodya JG, Gumbo T. 2011. A new evolutionary and pharmacokinetic-pharmacodynamic scenario for rapid emergence of resistance to single and multiple anti-tuberculosis drugs. Curr. Opin. Pharmacol. 11:457–463. 10.1016/j.coph.2011.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schmalstieg AM, Srivastava S, Belkaya S, Deshpande D, Meek C, Leff R, van Oers NS, Gumbo T. 2012. The antibiotic resistance arrow of time: efflux pump induction is a general first step in the evolution of mycobacterial drug resistance. Antimicrob. Agents Chemother. 56:4806–4815. 10.1128/AAC.05546-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zimic M, Fuentes P, Gilman RH, Gutierrez AH, Kirwan D, Sheen P. 2012. Pyrazinoic acid efflux rate in Mycobacterium tuberculosis is a better proxy of pyrazinamide resistance. Tuberculosis (Edinb.) 92:84–91. 10.1016/j.tube.2011.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Louw GE, Warren RM, Gey van Pittius NC, Leon R, Jimenez A, Pando RH, McEvoy CR, Grobbelaar M, Murray M, van Helden PD, Victor TC. 2011. Rifampicin reduces susceptibility to ofloxacin in rifampicin resistant Mycobacterium tuberculosis through efflux. Am. J. Respir. Crit. Care Med. 184:269–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gumbo T, Louie A, Liu W, Ambrose PG, Bhavnani SM, Brown D, Drusano GL. 2007. Isoniazid's bactericidal activity ceases because of the emergence of resistance, not depletion of Mycobacterium tuberculosis in the log phase of growth. J. Infect. Dis. 195:194–201. 10.1086/510247 [DOI] [PubMed] [Google Scholar]
- 38.Machado D, Couto I, Perdigao J, Rodrigues L, Portugal I, Baptista P, Veigas B, Amaral L, Viveiros M. 2012. Contribution of efflux to the emergence of isoniazid and multidrug resistance in Mycobacterium tuberculosis. PLoS One 7(4):e34538. 10.1371/journal.pone.0034538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gumbo T, Hiemenz J, Ma L, Keirns JJ, Buell DN, Drusano GL. 2008. Population pharmacokinetics of micafungin in adult patients. Diagn. Microbiol. Infect. Dis. 60:329–331. 10.1016/j.diagmicrobio.2007.09.018 [DOI] [PubMed] [Google Scholar]
- 40.Pasipanodya JG, Srivastava S, Gumbo T. 2012. Scientific and patient care evidence to change susceptibility breakpoints for first-line anti-tuberculosis drugs. Int. J. Tuberc. Lung Dis. 16:706–707. 10.5588/ijtld.11.0850 [DOI] [PubMed] [Google Scholar]