LETTER
The recent paper by Abdelraouf et al. (1) aimed to demonstrate polymyxin B (PMB) uptake by renal tubular cells using a discontinued commercial product, BODIPY-PMB (Life Technologies). We agree that polymyxins are taken up by renal tubular cells, as evidenced by very extensive tubular reabsorption following glomerular filtration in animals and humans (2, 3) and confirmed by our synchrotron X-ray fluorescence microscopy study in tubular cells using a polymyxin probe that maintains the cationic and hydrophobic domains of PMB (4). As polymyxin-induced nephrotoxicity occurs in up to ∼60% of patients (5–7), it is essential to understand the mechanism of polymyxin uptake by renal tubular cells. However, it is important to note that the results of Abdelraouf et al. (1) may not reflect the intracellular disposition of PMB, because they used a fluorescent BODIPY-PMB probe in which key domains of the PMB core structure are altered.
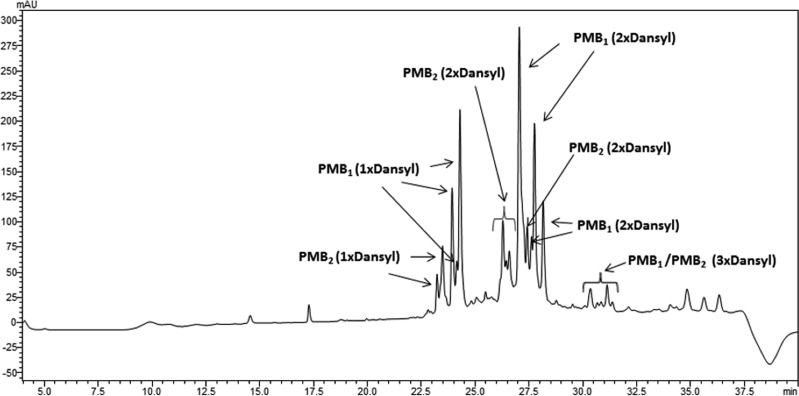
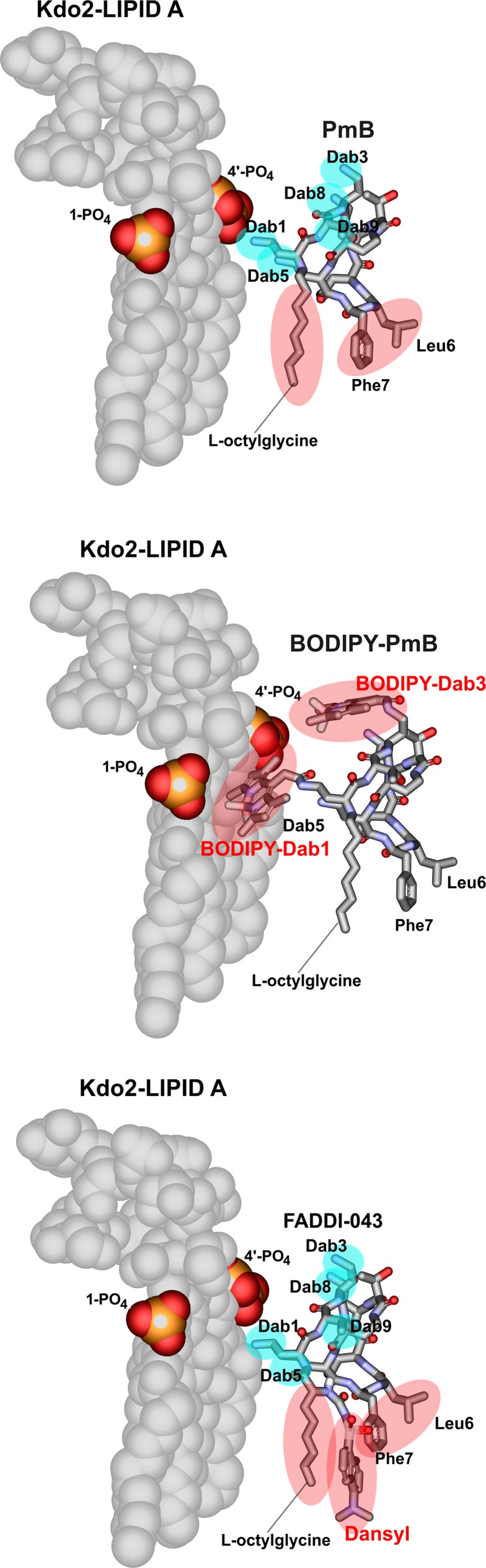
Lipopolysaccharide (LPS) is the initial bacterial target of polymyxins. Binding of polymyxins to LPS involves an initial electrostatic interaction of the positively charged diaminobutyric acid (Dab) residues with the negatively charged phosphate groups of lipid A, displacing divalent cations (Ca2+ and Mg2+) that bridge adjacent LPS molecules (8–10). We have previously highlighted the deficiencies of directly amine coupling dansyl groups onto the Dab side chains in semisynthetic preparations of dansyl-PMB (11). Analysis of these semisynthetic dansyl-PMB preparations revealed a mixture of mono-, di-, and tri-dansyl-substituted species (Fig. 1). Furthermore, as PMB is comprised of two major components, the potential for each component to be replaced at any of the five Dab residues with multiple dansyl molecules results in an extremely variable mixture of dansylated derivatives (11). Like the commercial dansyl-PMB (also discontinued; Life Technologies), the commercial BODIPY-PMB employed by Abdelraouf et al. (1) is prepared by nonspecifically labeling the Dab residues of PMB with hydrophobic BODIPY. Based upon the structure-activity relationship (SAR) model (10), modifications of the Dab residues with BODIPY substantially affect the electrostatic interaction between polymyxin and lipid A (Fig. 2). Considering the loss of native antibacterial activity universally seen across polymyxin analogs modified at the Dab residues (10), results of cell uptake studies with BODIPY-PMB (1) or dansyl-PMB as imaging probes for polymyxins may be very misleading (11) and must be interpreted with caution. The binding of such probes with mammalian cells may differ substantially from that of PMB; confirmation of the intracellular localization of BODIPY-PMB is essential using techniques such as confocal microscopy, rather than ultraperformance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS) quantitation of whole-cell lysate (1). We reported the first fluorescent polymyxin probe which maintains PMB pharmacological properties (12). The probe was designed based upon polymyxin SAR (10, 12) and regio-selectively modified so as to incorporate a single fluorescent dansyl moiety without significantly disturbing the pharmacological properties of the native PMB scaffold (Fig. 2).
FIG 1.
LC-MS profile of commercial dansyl-PMB. mAU, milliabsorbance units.
FIG 2.
Molecular models of the complex between lipid A and polymyxin B (PMB) (top), BODIPY-PMB (middle), and FADDI-043 (bottom). The cyan and red areas represent electrostatic and hydrophobic contacts with Kdo2-lipid A, respectively.
In conclusion, the structural requirements for fluorescent probes that truly represent the native polymyxins are crucial for understanding the mechanisms of polymyxin uptake and nephrotoxicity. Such mechanistic information is essential for developing novel, safer polymyxins.
ACKNOWLEDGMENTS
J.L., T.V., R.L.N., P.E.T., and K.D.R. are supported by a research grant from the National Institute of Allergy and Infectious Diseases of the National Institutes of Health (R01AI098771). J.L., T.V., R.L.N., and P.E.T. are also supported by the Australian National Health and Medical Research Council (NHMRC grant APP1026109). J.L. is an Australian NHMRC Senior Research Fellow. T.V. is an Australian NHMRC Industry Career Development Research Fellow.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases or the National Institutes of Health.
Footnotes
For the author reply, see doi:10.1128/AAC.03784-14.
REFERENCES
- 1.Abdelraouf K, Chang KT, Yin T, Hu M, Tam VH. 2014. Uptake of polymyxin B into renal cells. Antimicrob. Agents Chemother. 58:4200–4202. 10.1128/AAC.02557-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sandri AM, Landersdorfer CB, Jacob J, Boniatti MM, Dalarosa MG, Falci DR, Behle TF, Bordinhao RC, Wang J, Forrest A, Nation RL, Li J, Zavascki AP. 2013. Population pharmacokinetics of intravenous polymyxin B in critically ill patients: implications for selection of dosage regimens. Clin. Infect. Dis. 57:524–531. 10.1093/cid/cit334 [DOI] [PubMed] [Google Scholar]
- 3.Li J, Milne RW, Nation RL, Turnidge JD, Smeaton TC, Coulthard K. 2003. Use of high-performance liquid chromatography to study the pharmacokinetics of colistin sulfate in rats following intravenous administration. Antimicrob. Agents Chemother. 47:1766–1770. 10.1128/AAC.47.5.1766-1770.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li J. 2013. Mechanism of nephrotoxicity: how understanding may be used advantageously. 1st Int. Conf. Polymyxins, 2 to 4 May 2013, Prato, Italy: http://monash.edu/pharm/about/events/polymyxins/ [Google Scholar]
- 5.Hartzell JD, Neff R, Ake J, Howard R, Olson S, Paolino K, Vishnepolsky M, Weintrob A, Wortmann G. 2009. Nephrotoxicity associated with intravenous colistin (colistimethate sodium) treatment at a tertiary care medical center. Clin. Infect. Dis. 48:1724–1728. 10.1086/599225 [DOI] [PubMed] [Google Scholar]
- 6.Akajagbor DS, Wilson SL, Shere-Wolfe KD, Dakum P, Charurat ME, Gilliam BL. 2013. Higher incidence of acute kidney injury with intravenous colistimethate sodium compared with polymyxin B in critically ill patients at a tertiary care medical center. Clin. Infect. Dis. 57:1300–1303. 10.1093/cid/cit453 [DOI] [PubMed] [Google Scholar]
- 7.Kubin CJ, Ellman TM, Phadke V, Haynes LJ, Calfee DP, Yin MT. 2012. Incidence and predictors of acute kidney injury associated with intravenous polymyxin B therapy. J. Infect. 65:80–87. 10.1016/j.jinf.2012.01.015 [DOI] [PubMed] [Google Scholar]
- 8.Pristovsek P, Kidric J. 2004. The search for molecular determinants of LPS inhibition by proteins and peptides. Curr. Top. Med. Chem. 4:1185–1201. 10.2174/1568026043388105 [DOI] [PubMed] [Google Scholar]
- 9.Velkov T, Roberts KD, Nation RL, Thompson PE, Li J. 2013. Pharmacology of polymyxins: new insights into an ‘old' class of antibiotics. Future Microbiol. 8:711–724. 10.2217/fmb.13.39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Velkov T, Thompson PE, Nation RL, Li J. 2010. Structure-activity relationships of polymyxin antibiotics. J. Med. Chem. 53:1898–1916. 10.1021/jm900999h [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Soon RL, Velkov T, Chiu F, Thompson PE, Kancharla R, Roberts K, Larson I, Nation RL, Li J. 2011. Design, synthesis, and evaluation of a new fluorescent probe for measuring polymyxin-lipopolysaccharide binding interactions. Anal. Biochem. 409:273–283. 10.1016/j.ab.2010.10.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deris ZZ, Swarbrick JD, Roberts KD, Azad MA, Akter J, Horne AS, Nation RL, Rogers KL, Thompson PE, Velkov T, Li J. 2014. Probing the penetration of antimicrobial polymyxin lipopeptides into gram-negative bacteria. Bioconjug. Chem. 25:750–760. 10.1021/bc500094d [DOI] [PMC free article] [PubMed] [Google Scholar]