Abstract
We studied the development of antimony-resistant Leishmania infantum in natural vectors Lutzomyia longipalpis and Phlebotomus perniciosus to ascertain the risk of parasite transmission by sand flies. All three resistant strains produced fully mature late-stage infections in sand flies; moreover, the resistant phenotype was maintained after the passage through the vector. These results highlight the risk of circulation of resistant Leishmania strains and question the use of human drugs for treatment of dogs as Leishmania reservoirs.
TEXT
Drug-resistant microorganisms are currently a major global health threat. They are the main cause of nonresponse to treatment, prolonged illness, and higher risk of death. Furthermore, they seriously reduce treatment options. For visceral leishmaniasis, a vector-borne and widespread disease with potentially fatal outcomes, treatment alternatives are limited and largely dictated by economic conditions (1). The worldwide emergence of Leishmania resistance to the most widely used antileishmanial agent, antimonials, represents a threatening problem (2, 3, 4, 5). In leishmaniasis, various environmental factors can contribute to the selection of antimony (Sb)-resistant strains (6, 7, 8). Several field studies suggest a probability of natural cyclical transmission of resistant strains to explain the observed epidemiological patterns in different regions of the world (9, 10). In Algeria, where antimony drug pressure is sought to be low, antimony-resistant Leishmania infantum strains have been repeatedly isolated from dogs (11), which serve as a main reservoir of visceral leishmaniasis. Previously, it was shown in in vitro experiments that selection for drug resistance affects the amastigote-promastigote differentiation process of Leishmania (12) and is associated with competitive cost (13). In addition, a higher fitness of Sb-resistant Leishmania donovani strains corresponding to an increased number of metacyclic stages has been described (14). However, many Leishmania strains developed efficiently in the culture but did not produce late-stage infection in the sand fly vector (15). During passage through the sand fly gut, Leishmania must overcome several barriers which are critical for successful completion of the parasite life cycle (16).
Strains of L. infantum isolated from dogs that have undergone several courses of antimonial therapy were shown to grow in Phlebotomus perniciosus sand flies used for xenodiagnosis in these dogs (17), and this finding raised questions about circulation of these strains in nature and their transmissibility to humans (18). While these studies dealt with resistant strains under strong drug pressure, there is no study performed on antimony-resistant strains isolated in areas where antimony is not the primary pressure source, like the ones that have been isolated in Algeria and South America (11, 19).
Thus, we investigated in detail the development of antimony-resistant L. infantum parasites in Lutzomyia longipalpis and Phlebotomus perniciosus, two natural sand fly vectors of visceral leishmaniasis (20). Three antimony-resistant (LEM5285-R, LEM5696-R, and LEM5695-R) and three antimony-susceptible (LEM5680-S, LEM5684-S, and LEM5688-S) L. infantum strains, isolated from naturally infected dogs originating from various localities in the Algiers region (Algeria), where leishmaniasis is endemic (11), were used; two of them belong to the MON-1 zymodeme and four to the MON-281 zymodeme (Table 1) (21). We classified these strains as resistant ones because they express 50% inhibitory concentrations (IC50s) toward trivalent derivatives of Sb (SbIII) and pentavalent derivatives of Sb (SbV) that are 2- to 3-fold higher than the IC50s determined for 50 L. infantum strains isolated in France and Algeria (11, 22). Before passage through the sand fly, susceptibilities of promastigotes and amastigotes were measured twice within a 6-month interval to confirm that the resistant phenotype is stable.
TABLE 1.
Antimony susceptibility of L. infantum before and after experimental infections of P. perniciosusa
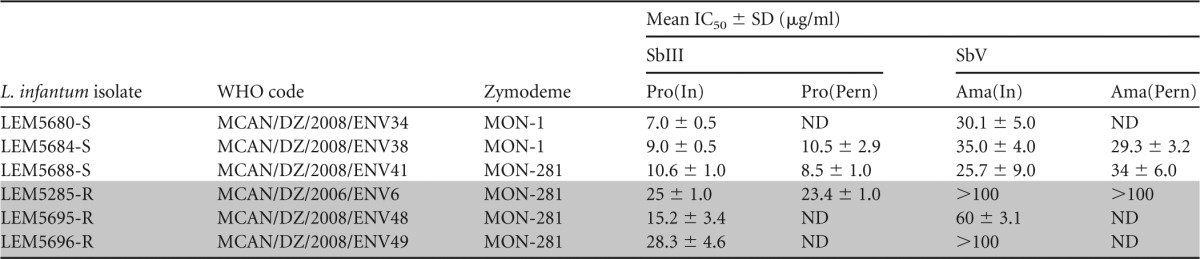
The susceptibility of promastigotes to SbIII before [Pro(In)] and after [Pro(Pern)] development in P. perniciosus and susceptibility of intramacrophagic amastigotes to SbV before P. perniciosus infection [Ama(In)] and amastigotes derived from promastigotes after P. perniciosus infection [Ama(Pern)]. Results are mean values from three independent experiments. Shading indicates results obtained in experiments with the three Sb-resistant L. infantum strains. ND, not determined.
The susceptibility of Leishmania promastigote forms to SbIII was determined by cell counting using a flow cytometer as previously described (11). Briefly, parasites were incubated with increasing concentrations of SbIII, ranging from 3.12 to 50 μg/ml, for 3 days. Dead cells were discriminated from the whole population by propidium iodide staining and counted on a flow cytometer (FACSCalibur; Becton Dickinson). As summarized in Table 1, the IC50s of promastigotes to SbIII vary between 7.0 and 10.6 μg SbIII/ml for susceptible strains, compared to IC50s of 15.2 to 28.3 μg SbIII/ml for resistant strains.
To confirm these results, we also ascertained the susceptibility of intracellular amastigotes (the clinically relevant stage of Leishmania) to SbV. Briefly, THP-1 monocytes were differentiated into macrophages in a 16-well chamber (LABTECKII; Nunc) by adding 20 ng/ml of phorbol myristate acetate (Sigma) for 48 h. Macrophages were washed two times with RPMI medium (Life Technology) and infected for 4 h at a parasites/host cell ratio of 10:1. Then, 12.5, 25.0, 50.0, or 100.0 μg SbV/ml was added. Drugs were renewed after 48 h, and after 120 h macrophages were fixed with methanol and stained with Giemsa to evaluate the susceptibility to SbV antimony. As shown in Table 1, three isolates belonging to the MON-281 zymodeme were characterized as antimony (SbV) resistant, with IC50s greater than 100 μg SbV/ml for LEM5285-R and LEM5696-R and an IC50 of 60.0 ± 3.1 μg SbV/ml for LEM5695-R. Susceptible isolates expressed IC50s for SbV between 25.7 and 35.0 μg SbV/ml (Table 1).
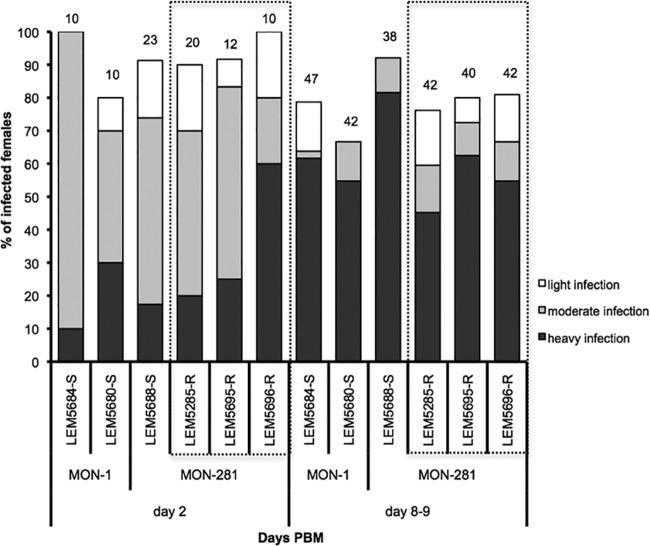
Then, we compared the intravectorial development of resistant and susceptible strains. Three Sb-resistant and three Sb-susceptible L. infantum strains were used for experimental infection of Lutzomyia longipalpis females. In each experiment, approximately 150 sand fly females (5 to 7 days old) were fed through a chick skin membrane on heat-inactivated rabbit blood containing 106 promastigotes/ml. Engorged females were separated, maintained at 26°C under conditions described previously (23), and dissected at days 2, 8, and 9 after blood meal (pbm). Localization and intensity of Leishmania infection in sand fly midguts were estimated in situ under a light microscope, by scoring the proportions of sand flies with weak (<100 parasites/gut), moderate (100 to 1,000 to parasites/gut), and heavy (>1,000 parasites/gut) infections (24). All experiments were repeated twice. The χ2 test was used for comparison of infection rates and intensities using the STATISTICA 6.0 program (StatSoft, Inc.). On day 2, no differences were observed in the development of Sb-resistant and Sb-susceptible L. infantum strains (P = 0.46), with both phenotypes showing high infection rates (>80%). Most sand fly females produced heavy or moderate infection in their abdominal midgut, with parasites enclosed within the peritrophic matrix. In late-stage infection, days 8 and 9 pbm, parasites migrated anteriorly to the thoracic midgut (infection rates above 70%) and colonized the stomodeal valve in 70 to 90% of infected females. All tested strains produced mature late-stage infection with metacyclic promastigotes, and no significant differences in infection intensities were observed between Sb-resistant and Sb-susceptible strains (P = 0.27) (Fig. 1).
FIG 1.
Development of antimony-resistant (box with dashed lines) and antimony-susceptible L. infantum strains in Lutzomyia longipalpis. Infection rates and intensities of infection by Sb-susceptible (LEM5684-S, LEM5680-S, LEM5688-S) and Sb-resistant (LEM5285-R, LEM5695-R, LEM5696-R) L. infantum strains in Lutzomyia longipalpis determined at day 2 and days 8 and 9 post blood meal (PBM). Numbers above the bars indicate the number of dissected females.
To test the stability of the Sb resistance phenotype after development in sand flies, we isolated one resistant strain (LEM5285-R) and two susceptible strains (LEM5688-S and LEM5684-S) from the guts of P. pernicious females with mature infections (on day 9 pbm). P. perniciosus females were infected using the same methods described above for Lutzomyia longipalpis. Sand fly-derived promastigotes were transferred into SDM79 medium supplemented with 10% fetal calf serum (FCS) and antibiotics (amikacin, penicillin, and fluorocytosine) in order to prevent bacterial and fungal growth (25). Seven to 10 days after isolation from the sand fly gut, their susceptibilities to SbIII and SbV were tested as described above. We also checked the SbV resistance of intramacrophagic amastigotes derived from gut-isolated promastigotes. As shown in Table 1, the cyclical passage of the LEM5384-R Leishmania strain through sand fly did not alter its SbIII or SbV antimony resistance profiles. As suspected, the cyclical passages of susceptible strains (LEM5688-S and LEM5684-S) did not change their antimony susceptibility.
The worldwide increase in drug resistance frequency among the Leishmania population, including areas where antimonial pressure is low (6), and the description of fitness advantages in Nepalese Sb-resistant L. donovani strains (26) raise concerns about the risk of spreading of such strains. Here, we confirmed previous finding by Gradoni et al. (17) that Sb-resistant L. infantum strains grow in sand flies. Importantly, we studied in detail the development of L. infantum originating from an area with a low drug pressure, and for the first time we showed that resistant strains are comparable to the resistant ones in all studied aspects of the vectorial part of the life cycle. For the first time, we demonstrated that the Sb resistance phenotype was maintained after passage through the natural sand fly under conditions without any drug pressure. The most important prerequisite for successful transmission, a high number of parasites colonizing the stomodeal valve and the thoracic part of the sand fly midgut, was achieved in all the strains tested. However, this aspect should be confirmed in the future using additional field-isolated antimony-resistant strains.
Nevertheless, our results highlight the risk of circulation of resistant Leishmania strains and also question the use of human drugs for treatment of dogs as Leishmania reservoirs. Treatment of canine and human leishmaniasis, both based on antimonial compounds, provide favorable conditions for selection and long-lasting circulation of resistant strains, particularly in areas where leishmaniasis is endemic and where no cost-effective therapeutic alternatives are available. As mentioned by Aït-Oudhia et al. (27), further work should be done in order to verify the epidemiological consequences of the natural occurrence of L. infantum antimony resistance in field conditions and their impact on the emergence and spreading of chemoresistance and consequently on control of the disease.
ACKNOWLEDGMENTS
We are grateful to P. Lami (National Reference Center for Leishmaniasis) for Leishmania isoenzyme typing, D. Garcia (IRD-MIVEGEC) for technical assistance, and J. Votypka for help with statistical analysis. We also thank anonymous reviewers for helpful suggestions, corrections, and comments.
V.S. and P.V. were partially funded by grants GACR 13-07 500S. D.S. and B.O. are supported by IRD. N.E. is supported in part by grants from IRD (BEST 2012) and from the Agence Nationale du Développement de la Recherche en Santé (ANDRS) Algeria (PNR no. 08/ANDRS/2011). C.M. holds a fellowship from Fundação para a Ciência e a Tecnologia, Ministério da Ciência, Tecnologia e Ensino Superior, Portugal (SFRH/BPD/44082/2008). This study was partially funded by EU grant FP7-261504 EDENext and is cataloged by the EDENext Steering Committee as EDENext 0192 (http://www.edenext.eu).
Footnotes
Published ahead of print 21 July 2014
REFERENCES
- 1.Vanlerberghe V, Diap G, Guerin JP, Meheus F, Gerstl S, Van der Stuyft P, Boelaert M. 2007. Drug policy for visceral leishmaniasis: a cost-effectiveness analysis. Trop. Med. Int. Health 12:274–283. 10.1111/j.1365-3156.2006.01782.x [DOI] [PubMed] [Google Scholar]
- 2.Abdo MG, Elamin WM, Khalil EAG, Mukhtar MM. 2003. Antimony-resistant Leishmania donovani in eastern Sudan: incidence and in vitro correlation. East Mediterr. Health J. 9:837–843 [PubMed] [Google Scholar]
- 3.Hadighi R, Mohebali M, Boucher P, Hajjaran H, Khamesipour A, Ouellette M. 2006. Unresponsiveness to glucantime treatment in Iranian cutaneous leishmaniasis due to drug-resistant Leishmania tropica parasites. PLoS Med. 3:e162. 10.1371/journal.pmed.0030162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mittal MK, Rai S, Ashutosh Ravinder Gupta S, Sundar S, Goyal NN. 2007. Characterization of natural antimony resistance in Leishmania donovani isolates. Am. J. Trop. Med. Hyg. 76:681–688 [PubMed] [Google Scholar]
- 5.Rojas R, Valderrama L, Valderrama M, Varona MX, Ouellette M, Saravia NG. 2006. Resistance to antimony and treatment failure in human Leishmania (Viannia) infection. J. Infect. Dis. 193:1375–1383. 10.1086/503371 [DOI] [PubMed] [Google Scholar]
- 6.Sereno D, Maia C, Aït-Oudhia K. 2012. Leishmania antimony resistance and environment: elusive links to explore. Int. J. Parasitol. 2:200–203. 10.1016/j.ijpddr.2012.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aït-Oudhia K, Gazanion E, Vergnes B, Oury B, Sereno D. 2011. Leishmania antimony resistance: what we know what we can learn from the field. Parasitol. Res. 109:1225–1232. 10.1007/s00436-011-2555-5 [DOI] [PubMed] [Google Scholar]
- 8.Perry MR, Wyllie S, Raab A, Feldmann J, Fairlamb AH. 2013. Chronic exposure to arsenic in drinking water can lead to resistance to antimonial drugs in a mouse model of visceral leishmaniasis. Proc. Natl. Acad. Sci. U. S. A. 110:19932–19937. 10.1073/pnas.1311535110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adaui V, Maes I, Huyse T, Van der Broeck F, Talledo M, Kuhls K, De Doncker S, Maes L, Llanos-Cuentas A, Schönian G, Arevalo J, Dujardin JC. 2011. Multilocus genotyping reveals a polyphyletic pattern among naturally antimony-resistant Leishmania braziliensis isolates from Peru. Infect. Genet. Evol. 11:1873–1880. 10.1016/j.meegid.2011.08.008 [DOI] [PubMed] [Google Scholar]
- 10.Singh S, Kumar DV, Ramesh Negi NS, Singh S, Salotra P. 2006. Visceral leishmaniasis, or kala azar (KA): high incidence of refractoriness to antimony is contributed by anthroponotic transmission via post-KA dermal leishmaniasis. J. Infect. Dis. 194:302–306. 10.1086/505079 [DOI] [PubMed] [Google Scholar]
- 11.Aït-Oudhia K, Gazanion E, Sereno D, Oury B, Dedet JP, Pratlong F, Lachaud L. 2012. In vitro susceptibility to antimonials and amphotericin B of Leishmania infantum strains isolated from dogs in a region lacking drug selection pressure. Vet. Parasitol. 187:386–393. 10.1016/j.vetpar.2012.01.034 [DOI] [PubMed] [Google Scholar]
- 12.Sereno D, Guilvard E, Maquaire S, Cavaleyra M, Holzmuller P, Ouaissi A, Lemesre JL. 2001. Experimental studies on the evolution of antimony-resistant phenotype during the in vitro life cycle of Leishmania infantum: implications for the spread of chemoresistance in endemic areas. Acta Trop. 80:195–205. 10.1016/S0001-706X(01)00154-1 [DOI] [PubMed] [Google Scholar]
- 13.Agnew P, Holzmuller P, Michalakis Y, Sereno D, Lemesre JL, Renaud F. 2001. In vitro growth of Leishmania amazonensis promastigotes resistant to pentamidine is dependent on interactions among strains. Antimicrob. Agents Chemother. 45:1928–1929. 10.1128/AAC.45.6.1928-1929.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ouakad M, Vanaerschot M, Rijal S, Sundar S, Speybroeck N, Kestens L, Boel L, De Doncker S, Maes I, Decuypere S, Dujardin JC. 2011. Increased metacyclogenesis of antimony-resistant Leishmania donovani clinical lines. Parasitology 138:1392–1399. 10.1017/S0031182011001120 [DOI] [PubMed] [Google Scholar]
- 15.Cihakova J, Volf P. 1997. Development of different Leishmania major strains in the vector sandflies Phlebotomus papatasi and Phlebotomus duboscqi. Ann. Trop. Med. Parasitol. 91:267–279. 10.1080/00034989761120 [DOI] [PubMed] [Google Scholar]
- 16.Dostalova A, Volf P. 2013. Leishmania development in sand flies: parasite-vector interactions overview. Parasit. Vectors 5:276. 10.1186/1756-3305-5-276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gradoni L, Maroli M, Gramiccia M, Macianti F. 1987. Leishmania infantum infection rates in Phlebotomus perniciosus fed on naturally infected dogs under antimonial treatment. Med. Vet. Entomol. 4:339–342 [DOI] [PubMed] [Google Scholar]
- 18.Gramiccia M, Gradoni L, Orsini S. 1992. Decreased sensitivity to meglumine antimoniate (Glucantime) of Leishmania infantum isolated from dogs after several courses of drug treatment. Ann. Trop. Med. Parasitol. 86:613–620 [DOI] [PubMed] [Google Scholar]
- 19.Robledo SM, Valencia AZ, Saravia NG. 1999. Sensitivity to Glucantime of Leishmania viannia isolated from patients prior to treatment. J. Parasitol. 85:360–366. 10.2307/3285647 [DOI] [PubMed] [Google Scholar]
- 20.Maroli M, Feliciangeli M, Bichaud L, Charrel R, Gradoni L. 2013. Phlebotomine sand flies and the spreading of leishmaniasis and other diseases of public health concern. Med. Vet. Entomol. 27:123–147. 10.1111/j.1365-2915.2012.01034.x [DOI] [PubMed] [Google Scholar]
- 21.Ait-Oudhia K, Lami P, Lesceu S, Harrat Z, Hamrioui B, Dedet JP, Pratlong F. 2009. Increase in the prevalence of canine leishmaniasis in urban Algiers (Algeria) following the 2003 earthquake. Ann. Trop. Med. Parasitol. 103:679–692. 10.1179/000349809X12554106963591 [DOI] [PubMed] [Google Scholar]
- 22.Oury B, Diabate M, Gazanion E, Barnabe C, Vergnes B, Garcia D, Pratlong F, Bastien P, Dedet JP, Sereno D. 2008. Chemosensitivity to antimony of Leishmania infantum in the region of Montpellier (South of France), p 37–42 In Dupoy-Camet J, Dei-Cas E. (ed), X European Multicolloquium of Parasitology. Medimond, Paris, France [Google Scholar]
- 23.Volf P, Volfova V. 2011. Establishment and maintenance of sand fly colonies. J. Vector Ecol. 36:S1–S9. 10.1111/j.1948-7134.2011.00106.x [DOI] [PubMed] [Google Scholar]
- 24.Myskova J, Votypka J, Volf P. 2008. Leishmania in sand flies: comparison of quantitative polymerase chain reaction with other techniques to determine the intensity of infection. J. Med. Entomol. 45:133–138. 10.1603/0022-2585(2008)45[133:LISFCO]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- 25.Sadlova J, Yeo M, Seblova V, Lewis MD, Mauricio I, Volf P, Miles MA. 2011. Visualisation of Leishmania donovani fluorescent hybrids during early stage development in the sand fly vector. PLoS One 6:e19851. 10.1371/journal.pone.0019851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vanaerschot M, Decuypere S, Berg M, Roy S, Dujardin JC. 2013. Drug-resistant microorganisms with a higher fitness—can medicines boost pathogens? Crit. Rev. Microbiol. 39:384–394. 10.3109/1040841X.2012.716818 [DOI] [PubMed] [Google Scholar]
- 27.Aït-Oudhia K, Gazanion E, Vergnes B, Oury B, Sereno D. 2011. The fitness of antimony-resistant Leishmania parasites: lessons from the field. Trends Parasitol. 27:141–142. 10.1016/j.pt.2010.12.003 [DOI] [PubMed] [Google Scholar]