Abstract
Poly(amidoamine)s (PAAs) are multifunctional tert-amine polymers endowed with high structural versatility. Here we report on the screening of a minilibrary of PAAs against a panel of viruses. The PAA AGMA1 showed antiviral activity against herpes simplex virus, human cytomegalovirus, human papillomavirus 16, and respiratory syncytial virus but not against human rotavirus and vesicular stomatitis virus. The results suggest the contribution of both a polycationic nature and side guanidine groups in imparting antiviral activity.
TEXT
The development of antiviral molecules usually focuses on either preventing virus entry into the host cell or inhibiting virus replication following host infection. The first strategy may be based on antiviral polyanionic polymers capable of competitively blocking the interaction between viral proteins and cell surface heparan sulfate proteoglycans (HSPGs), which are exploited as attachment receptors by many viruses (1–4). Notwithstanding the large number of studies demonstrating their efficacy in preclinical models, polyanionic polymers somehow failed in clinical trials (5). Unlike polyanions, polycationic polymers have been less investigated as antiviral compounds. In principle, polycations may act as antivirals by electrostatically interacting either with the negatively charged cell membrane or with the envelope of lipid-enveloped viruses, thus preventing virus adsorption onto cell surfaces, or by directly inactivating the virus particle. In this context, it was shown that the cationic poly(acrylic ester) Eudragit E 100, endowed with a membrane-destabilizing activity, exerts antiviral activity against a panel of lipid-enveloped viruses (6, 7). Another study demonstrated that polyethylenimine, a cationic polymer able to condense DNA and mediate gene transfer into mammalian cells, inhibits infection by human cytomegalovirus (HCMV) and human papillomavirus (HPV), a lipid-enveloped virus and a nonenveloped virus, respectively (8).
Poly(amidoamine)s (PAAs) are multifunctional tert-amine polymers endowed with high structural versatility, obtained by Michael polyaddition of amines and bis-acrylamides (9). The repeating units of PAAs can be designed to be reminiscent of peptides. For instance, an amphoteric, prevailingly cationic PAA named AGMA1 is a polymer mimic of the arg-gly-glu peptide (RGD) (10, 11).
In the search for new antiviral compounds, a minilibrary of PAAs was screened against a panel of seven viruses, namely, herpes simplex virus type 1 and 2 (HSV-1, HSV-2), HCMV, HPV-16, human respiratory syncytial virus (RSV), human rotavirus (HRV), and vesicular stomatitis virus (VSV), chosen as representative of different virus characteristics, such as the presence or absence of lipid envelope, a DNA or RNA genome, and HSPG dependency for virus attachment (12–16).
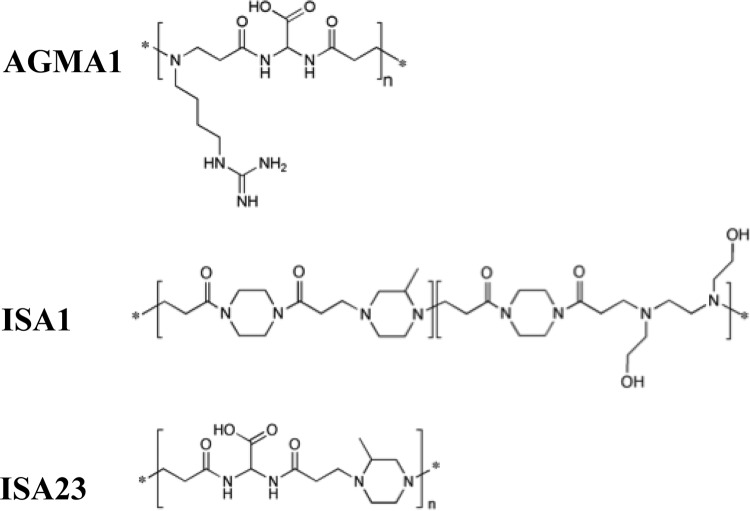
The minilibrary included three water-soluble PAAs, ISA1, ISA23, and AGMA1, whose structures are reported in Fig. 1. The copolymeric PAA ISA1 (17), containing two randomly distributed repeating units present in equal amounts, and the homopolymeric PAAs ISA23 (17, 18) and AGMA1 (10) were prepared as previously reported. AGMA1 fractions with different average molecular weights were obtained by ultrafiltration against water using membranes with different nominal molecular weight cutoffs, as previously described (11). ISA1 is weakly cationic, but ISA23 and AGMA1 are amphoteric, with isoelectric points of ∼5.2 and ∼10.3. As reported in Table 1, at pH 7.4, these PAAs have, respectively, +0.55, −0.55, and +0.55 average charges per unit. For ISA1, the reported value corresponds to the ionization degree of its tert-amine groups, with no other ionizable groups being present. For ISA23 and AGMA1, the reported figures correspond to the excess negative-over-positive charges and vice versa, that is, respectively, −1 plus 0.45 and −1 plus 1.55 per unit. Thus, at pH 7.4, the overall cationic charges of ISA1 and AGMA1 are superficially similar, but a deeper investigation reveals that their real charge distributions are different.
FIG 1.
Chemical structures of the AGMA1, ISA1, and ISA23 repeating units.
TABLE 1.
Physicochemical characteristics of PAAs
| Polymer | M̄na | Net avg charge per unit at pH 7.4 | Avg negative charge per unit at pH 7.4 | Avg positive charge per unit at pH 7.4 |
|---|---|---|---|---|
| Polydisperse | 10,100 | +0.55 | −1.00 | +1.55 |
| AGMA1 | ||||
| AGMA14 | 4,500 | +0.55 | −1.00 | +1.55 |
| AGMA17 | 7,800 | +0.55 | −1.00 | +1.55 |
| AGMA120 | 20,500 | +0.55 | −1.00 | +1.55 |
| ISA1 | 13,600 | +0.55 | 0.0 | +0.55 |
| ISA23 | 16,500 | −0.55 | −1.00 | +0.45 |
, number average molecular weight.
where Ni is the number of macromolecules containing i repeating units and Mi is the weight of macromolecules containing i repeating units.
Antiviral assays were performed by infecting cell monolayers in the presence of serial dilutions of compounds for 2 h at 37°C to generate dose-response curves and a selectivity profile of the PAAs' antiviral spectra. The inocula were subsequently washed out and replaced with culture medium containing the same concentration of compounds. The effect on HSV and VSV infections was evaluated by a standard plaque reduction assay on preseeded Vero cells in 24-well plates (10 × 104 cells) infected with 300 PFU/well of clinical isolates of HSV-1 and HSV-2 (19) and VSV serotype Indiana; after incubation for 24 h (HSV-2 and VSV) or 48 h (HSV-1) at 37°C in 5% CO2, cells were fixed and stained with 0.1% crystal violet in 20% ethanol and viral plaques were counted. The mean plaque count for each drug concentration was expressed as a percentage of the mean plaque count of the control.
In HCMV and RSV inhibition assays, infected cells were fixed and subjected to virus-specific immunostaining as described previously (20, 21). In these assays, cells were preseeded at a density of 6 × 103/well in 96-well plates. Hep-2 cells were infected with RSV strain A2 (60 PFU/well), whereas HELF cells were infected with HCMV strain AD169 (24 PFU/well). Three days (RSV) or 5 days (HCMV) postinfection, immunostained viral plaques were microscopically counted.
HPV inhibition assays were performed on preplated 293TT cells (2 × 104/well in 96-well plates) using HPV-16 secreted alkaline phosphatase (SEAP) pseudoviruses (PsV) at the final concentration of 1 ng/ml liter−1; 3 days postinfection, the SEAP content in the clarified supernatant was determined as previously described (22). Plasmids used for PsV production were kindly provided by J. Schiller (NCI, Bethesda, MD, USA). Antiviral assays for rotavirus were carried out on preplated MA104 cells (1 × 104/well in 96-well plates) using human rotavirus strain Wa (200 PFU/well). After 16 h, viral foci were determined by indirect immunostaining (22).
The endpoints of the assays were the effective compound concentration that reduced the viral plaque/focus formation or SEAP activity by 50% (EC50) in comparison to that in the untreated control. The F test was used to compare logEC50s, and two-way analysis of variance was used to analyze the significance between percentages of infection at the same doses of different compounds not able to generate EC50s. P values of <0.05 were considered statistically significant. The EC50s were calculated and all statistical analyses were performed by using the program PRISM 4 (GraphPad Software, San Diego, CA, USA). The viability of cells preseeded in 96-well plates was determined under identical culture conditions in antiviral assays (i.e., cell density and time of incubation with compounds) using a CellTiter 96 proliferation assay kit (Promega, Madison, WI, USA). The 50% cytotoxic concentrations (CC50) were determined using Prism software, and the selectivity index (SI) was calculated by dividing the CC50 by the EC50 (19). All data were generated from duplicate wells in at least three independent experiments. Heparin was included in the study as a positive control, being a known inhibitor of HSPG-dependent viruses (e.g., HSV-1, HSV-2, HCMV, RSV, and HPV-16) (23–26). As expected, heparin blocked infection by HSPG-dependent viruses but not that by VSV and HRV, which are not dependent on HSPG (Table 2).
TABLE 2.
Antiviral activities of PAAs and heparina
| Compound | Virus | EC50 (μg/ml) (95% CI) | CC50 (μg/ml) | SI |
|---|---|---|---|---|
| AGMA1 | HSV-1 | 3.04 (1.75–5.28) | >300 | >98.7 |
| HSV-2 | 5.34 (1.85–15.4) | >300 | >56.2 | |
| HCMV | 0.76 (0.40–1.47) | >300 | >395 | |
| HPV-16 | 0.54 (0.53–0.55) | >300 | >556 | |
| RSV | >100 | >300 | NA | |
| HRV | >100 | >300 | NA | |
| VSV | >100 | >300 | NA | |
| AGMA14 | HSV-1 | 1.93 (1.43–2.61) | >300 | >155 |
| HSV-2 | 1.35 (0.57–3.17) | >300 | >222 | |
| HCMV | 0.39 (0.11–1.30) | >300 | >769 | |
| HPV-16 | 0.92 (0.53–1.58) | >300 | >326 | |
| RSV | 8.87 (6.51–12.1) | >300 | >33.8 | |
| HRV | >100 | >300 | NA | |
| VSV | >100 | >300 | NA | |
| AGMA17 | HSV-1 | 17.0 (11.4–25.4) | >300 | >17.6 |
| HSV-2 | 4.80 (3.13–7.35) | >300 | >62.5 | |
| HCMV | 4.45 (3.28–5.90) | >300 | >67.4 | |
| HPV-16 | 0.79 (0.44–1.44) | >300 | >380 | |
| RSV | 7.44 (3.11–17.8) | >300 | >40.3 | |
| HRV | >100 | >300 | NA | |
| VSV | >100 | >300 | NA | |
| AGMA120 | HSV-1 | 5.10 (3.21–8.10) | >300 | >58.8 |
| HSV-2 | 2.82 (1.72–4.64) | >300 | >106 | |
| HCMV | 4.14 (2.50–6.86) | >300 | >72.5 | |
| HPV-16 | 0.72 (0.50–1.06) | >300 | >417 | |
| RSV | 1.37 (1.11–1.68) | >300 | >219 | |
| HRV | >100 | >300 | NA | |
| VSV | >100 | >300 | NA | |
| ISA1 | HSV-1 | >100 | >300 | NA |
| HSV-2 | >100 | >300 | NA | |
| HCMV | 1.26 (0.79–2.00) | >300 | >238 | |
| HPV-16 | 3.55 (1.97–6.40) | >300 | >84.5 | |
| RSV | 9.54 (5.51–16.5) | >300 | >31.4 | |
| HRV | >100 | >300 | NA | |
| VSV | >100 | >300 | NA | |
| ISA23 | HSV-1 | >100 | >300 | NA |
| HSV-2 | >100 | >300 | NA | |
| HCMV | >100 | >300 | NA | |
| HPV-16 | >100 | >300 | NA | |
| RSV | >100 | >300 | NA | |
| HRV | >100 | >300 | NA | |
| VSV | >100 | >300 | NA | |
| Heparin | HSV-1 | 5.22 (4.22–6.45) | >300 | >57.5 |
| HSV-2 | 0.67 (0.39–1.18) | >300 | >448 | |
| HCMV | 0.38 (0.24–0.64) | >300 | >789 | |
| HPV-16 | 2.88 (1.81–4.57) | >300 | >104 | |
| RSV | 0.05 (0.04–0.06) | >300 | >6,000 | |
| HRV | >100 | >300 | NA | |
| VSV | >100 | >300 | NA |
EC50, 50% effective concentration; 95% CI, 95% confidence interval; CC50, 50% cytotoxic concentration; SI, selectivity index; NA, not assessable.
Data reported in Table 2 prompt the following observations. The PAA antiviral effect was not a consequence of cytotoxicity, since none of the screened compounds significantly reduced cell viability at any concentration tested (i.e., up to 300 μg/ml); therefore, their CC50 values may be considered to be higher than 300 μg/ml in all the cell lines tested.
Polydisperse AGMA1 strongly inhibited infections by HSV-1, HSV-2, HCMV, and HPV-16, generating dose-response curves with EC50s of 3.04, 5.34, 0.76, and 0.54 μg/ml, respectively. Interestingly, AGMA1 was significantly more active than heparin against HSV-1 and HPV-16 infections, whereas it was as active as heparin against HCMV infection (P < 0.05). In contrast, polydisperse AGMA1 was inactive against RSV, HRV, and VSV.
To evaluate the influence of molecular weight on antiviral potency, three additional linear AGMA1 fractions were prepared, namely, AGMA14 (M̄n, 4,500), AGMA17 (M̄n, 7,800), and AGMA120 (M̄n, 20,500) (Table 1). As depicted in Table 2, fractions with lower and higher molecular weights than that of polydisperse AGMA1 (M̄n, 10,100) maintained inhibitory activity against HSV-1, HSV-2, HCMV, and HPV-16 although to different extents. AGMA14 showed a stronger anti-HSV-1 activity than that of heparin, and all fractions were more active than heparin against HPV-16 infection (P < 0.05). No statistically significant differences were observed between the EC50 of heparin and the EC50s of AGMA14 against HSV-2 and HCMV infections and between the EC50 of heparin and the EC50 of AGMA120 against HSV-1 infection.
Unlike polydisperse AGMA1, AGMA14, AGMA17, and AGMA120 were also active against RSV, with EC50 values of 8.87, 7.44, and 1.37 μg/ml, respectively. Both of the polydisperse AGMA1 and AGMA1 fractions failed to display any significant inhibitory effect against HRV and VSV. The antiviral activity of AGMA1 seems not to be dependent on its molecular weight for HSV-1, HSV-2, HCMV, and HPV-16; instead, there is a clear relationship between the AGMA1 fractions' sizes and their anti-RSV potency. Explaining why polydisperse AGMA1 did not exert a detectable anti-RSV activity while all of the size fractions did demands further investigation.
Polymers do not consist of a single molecular species but rather of families of homologous species differing in their numbers of repeating units. Therefore, it is considered inappropriate to adopt the molar concept describing their properties. Nevertheless, to compare activities across compounds, Table 3 shows the EC50s of AGMA1 fractions and heparin expressed in terms of molarity instead of μg/ml, considering the average molecular weight reported in Table 1. It was not possible to convert the average molecular mass of polydisperse AGMA1 in terms of molar equivalents, since its molecular mass is not univocally defined. Interestingly, the relationship between the AGMA1 fractions' sizes and their anti-RSV potency, reported in the text where data are expressed in terms of μg/ml, is preserved. Furthermore, AGMA17 and AGMA120 preserved a higher anti-HPV-16 activity than that of heparin (P < 0.05). In contrast, the antiviral activity of AGMA14 in terms of molarity is lower than that in terms of μg/ml; its activity is similar to that of heparin against HSV-1 and HPV-16 infections and is lower than that of heparin against HSV-2 and HCMV (P < 0.05). This behavior might be ascribed to a greater rigidity of the polymer with the lowest molecular weight. Because all of the polymers are polyelectrolytes, it is necessary to take into account that the charge density markedly affects the dynamic rheological properties, the flexibility, and the chain entanglements. Increased polymer charge density results in intermolecular electrostatic repulsion and increased polymer solubility.
TABLE 3.
Antiviral activities of poly(amidoamine)s expressed in terms of approximate molar valuesa
| Compound | Virus | EC50 (μM) (95% CI) | CC50 (μM) |
|---|---|---|---|
| AGMA14 | HSV-1 | 0.43 (0.30–0.61) | >66.67 |
| HSV-2 | 0.30 (0.11–0.80) | >66.67 | |
| HCMV | 0.33 (0.09–1.27) | >66.67 | |
| HPV-16 | 0.20 (0.12–0.33) | >66.67 | |
| RSV | 1.97 (1.44–2.69) | >66.67 | |
| HRV | >22.22 | >66.67 | |
| VSV | >22.22 | >66.67 | |
| AGMA17 | HSV-1 | 2.18 (0.65–7.33) | >38.46 |
| HSV-2 | 0.61 (0.38–1.00) | >38.46 | |
| HCMV | 0.56 (0.41–0.76) | >38.46 | |
| HPV-16 | 0.10 (0.06–0.18) | >38.46 | |
| RSV | 0.95 (0.40–2.28) | >38.46 | |
| HRV | >12.82 | >38.46 | |
| VSV | >12.82 | >38.46 | |
| AGMA120 | HSV-1 | 0.25 (0.16–0.40) | >14.63 |
| HSV-2 | 0.14 (0.08–0.23) | >14.63 | |
| HCMV | 0.20 (0.12–0.33) | >14.63 | |
| HPV-16 | 0.04 (0.02–0.51) | >14.63 | |
| RSV | 0.07 (0.05–0.08) | >14.63 | |
| HRV | >4.87 | >14.63 | |
| VSV | >4.87 | >14.63 | |
| Heparin | HSV-1 | 0.38 (0.30–0.49) | >21.90 |
| HSV-2 | 0.04 (0.03–0.07) | >21.90 | |
| HCMV | 0.03 (0.02–0.05) | >21.90 | |
| HPV-16 | 0.21 (0.13–0.36) | >21.90 | |
| RSV | 0.01 (0.00–0.01) | >21.90 | |
| HRV | >7.30 | >21.90 |
EC50, 50% effective concentration; 95% CI, 95% confidence interval; CC50, 50% cytotoxic concentration.
Next, to investigate whether the activity of AGMA1 was specifically due to the structure of its repeating unit, the antiviral activities of ISA1 and ISA23 were assessed. Overall, while AGMA1 was active against HSV-1, HSV-2, HCMV, RSV, and HPV-16 infection, ISA1 was active only against HCMV and RSV, with a lower activity than that of heparin, and was as active as heparin against HPV-16 (P < 0.05). ISA23 was inactive in all cases. At pH 7.4, both AGMA1 and ISA1 are positively charged, whereas ISA23 is negatively charged. It is known that polycationic polymers establish ionic interactions with the cell surface HSPG (27, 28), a feature that may impart antiviral activity to these compounds. This feature, along with the finding that the active PAAs have the same antiviral activity spectrum as heparin, supports the hypothesis that PAAs may exert their antiviral action, at least in part, by interacting with HSPG, thus preventing virus attachment. However, notwithstanding the fact that AGMA1 and ISA1 carry the same density of positive charges, i.e., +0.55, AGMA1 showed a greater activity for HSV-1, HSV-2, and HPV-16. This may be due to the different real charge distributions on the macromolecules and to their side guanidine groups reinforcing membrane interactions, according to their well-known chaotropic properties (29). In contrast, the guanidine side group does not seem to be necessary for the anti-HCMV activity. Furthermore, a different chain entanglement might explain the different activity of AGMA1 with respect to that of RSV. Overall, these results provide a starting point to tailor a macromolecule with enhanced antiviral activity against a selected virus. Future work will be focused on narrowing the molecular mass distribution of PAA samples to assist in preclinical development.
Studies are ongoing to elucidate the mechanisms of action of the active PAAs and their antiviral potential and biocompatibility profile in preclinical models.
ACKNOWLEDGMENT
This work was supported by a grant from Ricerca Finanziata dall'Università degli Studi di Torino (ex 60%) 2012 to D.L.
Footnotes
Published ahead of print 4 August 2014
REFERENCES
- 1.Spillmann D. 2001. Heparan sulfate: anchor for viral intruders? Biochimie 83:811–817. 10.1016/S0300-9084(01)01290-1 [DOI] [PubMed] [Google Scholar]
- 2.Liu J, Thorp SC. 2002. Cell surface heparan sulfate and its roles in assisting viral infections. Med. Res. Rev. 22:1–25. 10.1002/med.1026 [DOI] [PubMed] [Google Scholar]
- 3.Tiwari V, Maus E, Sigar IM, Ramsey KH, Shukla D. 2012. Role of heparan sulfate in sexually transmitted infections. Glycobiology 22:1402–1412. 10.1093/glycob/cws106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rusnati M, Vicenzi E, Donalisio M, Oreste P, Landolfo S, Lembo D. 2009. Sulfated K5 Escherichia coli polysaccharide derivatives: a novel class of candidate antiviral microbicides. Pharmacol. Ther. 123:310–322. 10.1016/j.pharmthera.2009.05.001 [DOI] [PubMed] [Google Scholar]
- 5.Pirrone V, Wigdahl B, Krebs FC. 2011. The rise and fall of polyanionic inhibitors of the human immunodeficiency virus type 1. Antiviral Res. 90:168–182. 10.1016/j.antiviral.2011.03.176 [DOI] [PubMed] [Google Scholar]
- 6.Alasino RV, Ausar SF, Bianco ID, Castagna LF, Contigiani M, Beltramo DM. 2005. Amphipathic and membrane-destabilizing properties of the cationic acrylate polymer Eudragit E100. Macromol. Biosci. 5:207–213. 10.1002/mabi.200400168 [DOI] [PubMed] [Google Scholar]
- 7.Alasino RV, Bianco ID, Vitali MS, Zarzur JA, Beltramo DM. 2007. Characterization of the inhibition of enveloped virus infectivity by the cationic acrylate polymer eudragit E100. Macromol. Biosci. 7(9-10):1132–1138. 10.1002/mabi.200700102 [DOI] [PubMed] [Google Scholar]
- 8.Spoden GA, Besold K, Krauter S, Plachter B, Hanik N, Kilbinger AF, Lambert C, Florin L. 2012. Polyethylenimine is a strong inhibitor of human papillomavirus and cytomegalovirus infection. Antimicrob. Agents Chemother. 56:75–82. 10.1128/AAC.05147-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferruti P. 2013. Poly(amidoamine)s: past, present, and perspectives. J. Polymer Sci. A Polymer Chem. 51:2319–2353. 10.1002/pola.26632 [DOI] [Google Scholar]
- 10.Ferruti P, Franchini J, Bencini M, Ranucci E, Zara GP, Serpe L, Primo L, Cavalli R. 2007. Prevailingly cationic agmatine-based amphoteric polyamidoamine as a nontoxic, nonhemolytic, and “stealthlike” DNA complexing agent and transfection promoter. Biomacromolecules 8(5):1498–1504. 10.1021/bm061126c [DOI] [PubMed] [Google Scholar]
- 11.Cavalli R, Bisazza A, Sessa R, Primo L, Fenili F, Manfredi A, Ranucci E, Ferruti P. 2010. Amphoteric agmatine containing polyamidoamines as carriers for plasmid DNA in vitro and in vivo delivery. Biomacromolecules 11(10):2667–2674. 10.1021/bm100685t [DOI] [PubMed] [Google Scholar]
- 12.Roizman B, Knipe MM, Whitley RJ. 2007. Herpes simplex viruses, p 2501–2601 In Fields BN, Knipe DM, Howley PM. (ed), Fields virology. Lippincott-Raven, Philadelphia, PA [Google Scholar]
- 13.Lowy DR, Howley PM. 2001. Papillomaviruses, p 2231–2264 In Fields BN, Knipe DM, Howley PM. (ed), Fields virology. Lippincott-Raven, Philadelphia, PA [Google Scholar]
- 14.Collins PL, Crowe JE., Jr 2007. Respiratory syncytial virus and metapneumovirus, p 1601–1646 In Knipe DM, Howley PM. (ed) Fields virology, 5th ed. Lippincott Williams and Wilkins, Philadelphia, PA [Google Scholar]
- 15.Landolfo S, Gariglio M, Gribaudo G, Lembo D. 2003. The human cytomegalovirus. Pharmacol. Ther. 98:269–297. 10.1016/S0163-7258(03)00034-2 [DOI] [PubMed] [Google Scholar]
- 16.Cox E, Christenson JC. 2012. Rotavirus. Pediatr. Rev. 33:439–445. 10.1542/pir.33-10-439 [DOI] [PubMed] [Google Scholar]
- 17.Richardson S, Ferruti P, Duncan R. 1999. Poly(amidoamine)s as potential endosomolytic polymers: evaluation in vitro and body distribution in normal and tumour-bearing animals. J. Drug Target. 6:391–404. 10.3109/10611869908996846 [DOI] [PubMed] [Google Scholar]
- 18.Ferruti P, Manzoni S, Richardson SCW, Duncan R, Patrick NG, Mendichi R, Casolaro M. 2000. Amphoteric linear poly(amido-amine)s as endosomolytic polymers: correlation between physicochemical and biological properties. Macromolecules 33:7793–7800. 10.1021/ma000378h [DOI] [Google Scholar]
- 19.Donalisio M, Nana HM, Ngono Ngane RA, Gatsing D, Tiabou Tchinda A, Rovito R, Cagno V, Cagliero C, Boyom FF, Rubiolo P, Bicchi C, Lembo D. 2013. In vitro anti-herpes simplex virus activity of crude extract of the roots of Nauclea latifolia Smith (Rubiaceae). BMC Compl. Altern. Med. 13:266. 10.1186/1472-6882-13-266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Donalisio M, Rusnati M, Cagno V, Civra A, Bugatti A, Giuliani A, Pirri G, Volante M, Papotti M, Landolfo S, Lembo D. 2012. Inhibition of human respiratory syncytial virus infectivity by a dendrimeric heparan sulfate-binding peptide. Antimicrob. Agents Chemother. 56:5278–5288. 10.1128/AAC.00771-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Funaro A, Gribaudo G, Luganini A, Ortolan E, Lo Buono N, Vicenzi E, Cassetta L, Landolfo S, Buick R, Falciola L, Murphy M, Garotta G, Malavasi F. 2008. Generation of potent neutralizing human monoclonal antibodies against cytomegalovirus infection from immune B cells. BMC Biotechnol. 8:85. 10.1186/1472-6750-8-85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Donalisio M, Rusnati M, Civra A, Bugatti A, Allemand D, Pirri G, Giuliani A, Landolfo S, Lembo D. 2010. Identification of a dendrimeric heparan sulfate-binding peptide that inhibits infectivity of genital types of human papillomaviruses. Antimicrob. Agents Chemother. 54:4290–4299. 10.1128/AAC.00471-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.WuDunn D, Spear PG. 1989. Initial interaction of herpes simplex virus with cells is binding to heparan sulphate. J. Virol. 63:52–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kari B, Gehrz R. 1992. A human cytomegalovirus glycoprotein complex designated gC-II is a major heparin-binding component of the envelope. J. Virol. 66:1761–1764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hallak LK, Spillmann D, Collins PL, Peeples ME. 2000. Glycosaminoglycan sulfation requirements for respiratory syncytial virus infection. J. Virol. 74:10508–10513. 10.1128/JVI.74.22.10508-10513.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Joyce JG, Tung J-S, Przysiecji CT, Cook JC, Lehman ED, Sands JA, Jansen KU, Keller PM. 1999. The L1 major capsid protein of human papillomavirus type 11 recombinant virus-like particles interacts with heparin and cell-surface glycosaminoglycans on human keratinocytes. J. Biol. Chem. 274:5810–5822. 10.1074/jbc.274.9.5810 [DOI] [PubMed] [Google Scholar]
- 27.Poon GM, Gariépy J. 2007. Cell-surface proteoglycans as molecular portals for cationic peptide and polymer entry into cells. Biochem. Soc. Trans. 35:788–793. 10.1042/BST0350788 [DOI] [PubMed] [Google Scholar]
- 28.Mislick KA, Baldeschwieler JD. 1996. Evidence for the role of proteoglycans in cation-mediated gene transfer. Proc. Natl. Acad. Sci. U. S. A. 93:12349–12354. 10.1073/pnas.93.22.12349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Myers JK, Pace CN, Scholtz JM. 1995. Denaturant m values and heat capacity changes: relation to changes in accessible surface areas of protein unfolding. Protein Sci. 4:2138–2148. 10.1002/pro.5560041020 [DOI] [PMC free article] [PubMed] [Google Scholar]