Abstract
Resveratrol is a polyphenol found in black grapes and red wine and has many biological activities. In this study, we evaluated the effect of resveratrol alone and in association with amphotericin B (AMB) against Leishmania amazonensis. Our results demonstrate that resveratrol possesses both antipromastigote and antiamastigote effects, with 50% inhibitory concentrations (IC50s) of 27 and 42 μM, respectively. The association of resveratrol with AMB showed synergy for L. amazonensis amastigotes, as demonstrated by the mean sums of fractional inhibitory index concentration (mean ΣFIC) of 0.483, although for promastigotes, this association was indifferent. Treatment with resveratrol increased the percentage of promastigotes in the sub-G0/G1 phase of the cell cycle, reduced the mitochondrial potential, and showed an elevated choline peak and CH2-to-CH3 ratio in the nuclear magnetic resonance (NMR) spectroscopy analysis; all these features indicate parasite death. Resveratrol also decreased the activity of the enzyme arginase in uninfected and infected macrophages with and without stimulation with interleukin-4 (IL-4), also implicating arginase inhibition in parasite death. The anti-Leishmania effect of resveratrol and its potential synergistic association with AMB indicate that these compounds should be subjected to further studies of drug association therapy in vivo.
INTRODUCTION
Leishmaniasis is a neglected disease that affects 98 countries and 3 territories on 5 continents. Approximately 0.2 to 0.4 million cases of visceral leishmaniasis and 0.9 to 1.2 million cases of cutaneous leishmaniasis occur each year (1). The pentavalent antimonials have been the first-line drugs for treating leishmaniasis for >80 years, but these drugs have high toxicity and adverse side effects. These negative characteristics lead to patient withdrawal and an increased incidence of resistant strains (2, 3). Alternative compounds, such as pentamidine, amphotericin B, and paromomycin, are secondary treatment options for resistant cases, despite their cytotoxicity (4, 5). Miltefosine, the first approved oral treatment for leishmaniasis, has been used since 2002 for visceral leishmaniasis in India with a high cure rate. However, a low efficacy rate has been observed for miltefosine against cutaneous leishmaniasis, and this drug is also teratogenic (6, 7).
Several compounds have been screened against leishmaniasis, especially secondary metabolites of plants. Here, we investigated the antileishmanial activity of resveratrol (3,5,4′-trihydroxystilbene), a natural polyphenol produced by several plants, including Kojo-kon (Polygonum cuspidatum), Kashuwu (Polygonum multiflorum), eucalyptus (Eucalyptus sideroxylon), and black grapes (Vitis vinifera and Vitis labrusca) (8–12). Resveratrol has been described as having anti-inflammatory, anticancer, antioxidant (13–15), antiplatelet aggregation, antifungal, antiviral, antibacterial, and anti-Leishmania major activities (13, 16–20). Importantly, it is well tolerated at high doses, without side effects (21). Moreover, resveratrol has been reported to have a synergistic effect with temozolomide (an antineoplastic agent belonging to a class of alkylating agents, such as one derived from imidazotetrazine), an effect confirmed by isobolographic analysis. This combination was also effective against malignant glioma, occurring by suppressing extracellular signal-regulated kinase (ERK)/reactive oxygen species (ROS)-mediated autophagy and subsequently, inducing apoptosis (22). Resveratrol also acts synergistically with the polyphenols present in black tea to suppress the growth of skin cancer in BALB/c mice, and this suppression occurs by inhibiting the activation of p53 and mitogen-activated protein kinase (MAPK) (23). Recently, resveratrol has received much attention at specific international conferences dedicated to formulating recommendations for research aspects to be addressed and guidelines for human use (24). Although the number of clinical trials addressing the biological properties and activities of resveratrol, pure or mixed with other compounds, against cancer, metabolic syndromes, and cardiovascular diseases has increased, there is still much controversy about the amount needed for its beneficial effects and for a recommendation for use in humans (24).
Here, we describe that resveratrol has anti-Leishmania amazonensis activity demonstrating the induction incidental death markers and that polyamine starvation contributes to parasite death. Furthermore, a synergistic leishmanicidal effect for the association of resveratrol and amphotericin B was demonstrated in vitro using the fractional inhibitory concentration index and isobolographic analysis.
MATERIALS AND METHODS
Ethics statement.
All of the animal experiments were performed in strict accordance with Brazilian animal protection law (Lei Arouca no. 11.794/08) of the National Council for the Control of Animal Experimentation (CONCEA) (Brazil). The protocol was approved by the Committee for Animal Use of the Universidade Federal do Rio de Janeiro (permit IMPPG 001).
Parasite culture.
L. amazonensis (strain WHOM/BR/75/Josefa) promastigotes were cultured at 26°C in Schneider's insect medium (Sigma), 10% fetal calf serum (Gibco-BRL, USA), and 40 μg/ml gentamicin (Schering-Plough, Rio de Janeiro, Brazil).
Antipromastigote activity.
Promastigotes were incubated at 26°C in Schneider's insect medium–10% fetal calf serum (FCS) in the presence of different concentrations of resveratrol (Sigma) and/or amphotericin B (Cristalia, São Paulo, Brazil). Parasite survival was estimated by counting the viable motile forms in a Neubauer chamber at 24, 48, 72, and 96 h after the addition of the drugs. In all tests, medium alone and 0.4% dimethyl sulfoxide (DMSO) were used as controls. All of the cultures were performed in triplicate, and the results were expressed as the percentage of growth compared to that of the controls. Promastigotes were treated with or without 100 μM resveratrol and 1 μM amphotericin B for 48 h at 26°C and then incubated with 4 μM ethidium homodimer 1 (EthD-1) staining solution for 30 min at 37°C, according to the manufacturer's instructions (Molecular Probes). The data regarding dead promastigotes were collected in a BD FACSCalibur and analyzed by CellQuest Pro (BD Biosciences, San Jose, CA). Ten thousand events were harvested from each sample.
Antiamastigote activity.
Peritoneal macrophages from mice obtained after stimulation with 3% thioglycolate for 3 days were harvested in RPMI 1640 medium (Biochrom KG, Germany). The macrophages were plated onto coverslips and allowed to adhere for 2 h at 37°C in 5% CO2. The nonadherent cells were removed, and the macrophages were incubated overnight in RPMI medium and 10% FCS. The adhered macrophages were infected with L. amazonensis promastigotes (stationary growth phase) at a 10:1 parasite-to-macrophage ratio and incubated for 1 h at 34°C in 5% CO2. The free parasites were washed out with 0.01 M phosphate-buffered saline (PBS), and the cultures were maintained for 24 h at 37°C in 5% CO2. The amastigote-infected macrophage cultures were then treated with resveratrol and/or amphotericin B at different concentrations for an additional 24 h. The cells were then washed and stained with Giemsa, and the number of amastigotes and the percentages of infected macrophages were determined by counting ≥200 cells in duplicate cultures. The infectivity indices were obtained by multiplying the percentage of infected macrophages by the mean number of amastigotes per infected macrophage. The results were expressed as the percentage of survival, comparing the infectivity indices of the treated and untreated macrophages (25).
Assessment of the intracellular load of L. amazonensis.
The infected macrophages were treated with 100 μM resveratrol for 24 h or left untreated, were washed and incubated with 0.01% SDS for 10 min, followed by the addition of 1 ml of Schneider's medium and 10% FCS, and were cultured at 26°C for 2 days. The relative intracellular load of viable L. amazonensis amastigotes was measured by a parasite rescue and transformation assay (26, 27).
XTT assay.
Mouse peritoneal macrophages adhered to 96-well plates were treated with resveratrol and amphotericin B. After 24 h of treatment, cell viability was determined by adding 0.5 mg/ml XTT (2,3-bis[2-methoxy-4-nitro-5-sulfophenyl]-2H-tetrazolium-5-carboxinilide inner salt) (Sigma) and 10 μM phenazine methosulfate (PMS). After 3 h of incubation, the reaction product was read at 450 nm. The results were expressed as the percentage of viable cells compared to that in the untreated control (27, 28).
Trypan blue exclusion assay.
BALB/c peritoneal macrophages in a 24-well plate were treated with resveratrol and amphotericin B. After 24 h of treatment, 0.03% trypan blue {tetrasodium 3,3′-[(3,3′-dimethyl[1,1′-biphenyl]-4,4′-diyl)bis(azo)]bis[5-amino-4-hydroxynaphthalene-2,7-disulfonate]} was added, and the number of viable cells was estimated by counting 200 cells in triplicate (29).
Phagocytosis.
BALB/c peritoneal macrophages were treated with resveratrol and amphotericin B and incubated at 37°C in 5% CO2 for 24 h. The macrophages were infected with L. amazonensis promastigotes at a 10:1 parasite-to-cell ratio for 1 h, and the rate of association was determined by randomly counting ≥200 cells in each of the duplicate coverslips (26).
Nitric oxide production.
Thioglycolate-stimulated peritoneal mouse macrophages obtained as described above (106 cells/well in a 24-well plate) were activated with 1 μg/ml recombinant mouse gamma interferon (IFN-γ) (Bioscience, Inc., CA) or left untreated. After the cells were incubated for 24 h at 37°C in 5% CO2, they were treated with 100 μM resveratrol and 0.06 μM amphotericin B. The nitrite concentrations in the culture supernatants were determined by the Griess method. The reaction was read at 540 nm, and concentration of NO2− was determined using a standard curve of sodium nitrite. The results were expressed as micromolar concentrations of nitrite.
Nitric oxide-trapping capacity.
A cell-free system using an NO donor was used to test the capacity of resveratrol and amphotericin B to trap nitric oxide. In solution, SNAP (S-nitroso-N-acetyl dl-penicillamine) (Sigma) liberates nitric oxide, which is then transformed to nitrite in the medium. The addition of an NO scavenger to a SNAP solution results in nitrite decay in the supernatant. Using this protocol, 100 μM resveratrol and 0.06 μM amphotericin B were incubated with 1 mM SNAP. Rutin (1 mM), a known NO scavenger (Sigma), was used as a positive control for the assay. After 6 h of incubation, the nitrite concentration was determined by the Griess method. The results were expressed as the μM concentration of nitrite calculated in comparison with the sodium nitrite standard curve (26).
Cytokine production.
Thioglycolate-stimulated mouse peritoneal macrophages activated with IFN-γ as described above or left untreated were cultured in 24-well plates for 24 h at 37°C in 5% CO2. The macrophages were then treated with resveratrol and amphotericin B, and the production of tumor necrosis factor alpha (TNF-α) and transforming growth factor beta (TGF-β) was evaluated by enzyme-linked immunosorbent assay (ELISA) using capture and detection antibodies obtained from PeproTech (Mexico, DF) and eBioscience (CA), respectively, according to the manufacturer's instructions. Recombinant cytokines were used as standards, and the assays were performed in duplicate. The reactions were detected with streptavidin-alkaline phosphatase (Gibco BRL) and p-nitrophenylphosphate (Sigma) and read at 405 nm. The regression curves were prepared using specific software for microplate assays (Bio-Rad Labs). The assays were performed in triplicate (30).
Promastigote morphology.
The promastigotes (3 × 106/ml) were maintained in the presence or absence of 0.4% DMSO, 100 μM resveratrol, and 0.1 μM amphotericin B for 48 h of treatment, washed 2 times with PBS, fixed, and stained with Giemsa, and ≥200 cells were counted using an Axioplan optical microscope.
Fractional inhibitory concentration determination and isobologram construction.
The fractional inhibitory concentration index (FIC) of resveratrol was calculated using the formula IC50 of resveratrol in association/IC50 of resveratrol alone (IC50, 50% inhibitory concentration). The same formula was applied to amphotericin B (AMB). The sum FICs (ΣFICs) were calculated as FIC of resveratrol + FIC of AMB, as described by Seifert and Croft (31). The mean ΣFIC was calculated for each combination and then compared to the reference values and reported as synergistic (ΣFIC, ≤0.5), indifferent (ΣFIC, >0.5 and ≤4), or antagonistic (ΣFIC, >4) (31). The interaction between resveratrol and AMB was analyzed using FIC values to plot the isobologram, according to the method of Tallarida (32). Antagonistic combinations were defined as points above the line of additivity, and synergistic combinations were defined as points below the line (32).
Cell cycle.
Promastigotes were incubated in Schneider's complete medium with or without 27 μM or 100 μM resveratrol and 0.1 μM amphotericin B for 48 h. The cells were washed with PBS and fixed in 70% (vol/vol) ice-cold methanol-PBS for ≥1 h at 4°C. The fixed cells were washed once with PBS and incubated in cell cycle solution (PBS supplemented with 10 μg/ml propidium iodide and 20 μg/ml RNase) at 37°C for 45 min, as described previously (33). For each sample, 10,000 events were collected on a BD FACSCalibur (Becton, Dickinson) and analyzed using the CellQuest software.
Measurement of ΔΨm.
The change in promastigote ΔΨm (mitochondrial membrane potential) was measured with a mitochondrial staining kit (Sigma-Aldrich). This dye accumulates in the mitochondrial matrix under the influence of ΔΨm, and its monomeric form increases in unhealthy or apoptotic cells. The promastigotes were treated with or without 100 μM resveratrol and 0.1 μM amphotericin B for 48 h. JC-1 (5 μg/ml) staining solution (prepared according to the manufacturer's instructions) was added to the 106 cells for 20 min at 37°C. The ΔΨm was measured at 490 nm excitation and 530 nm emission (for JC-1 monomers) and 525 nm excitation and 590 nm emission wavelengths (for J-aggregates) in a SpectraMax Paradigm (Molecular Devices) using 96-well opaque culture plates (26).
Arginase activity.
Uninfected or infected macrophages stimulated or not with 20 ng/ml of interleukin-4 (IL-4) and treated with or without 100 μM resveratrol, 100 μM spermidine, or 1 μM amphotericin B were lysed to determine the arginase activity. The macrophage lysates were obtained from 106 cells treated with 100 μl of 0.1% Triton X-100 for 30 min, followed by the addition of 100 μl of a buffer containing 25 mM Tris-HCl (pH 7.4) and 10 μl of 10 mM MnSO4. The enzyme was then activated by heating for 10 min at 56°C, and arginine hydrolysis was carried out by incubating 100 μl of the activated lysate with 100 μl of 0.1 M arginine (pH 9.7) at 37°C for 1 h. The reaction was stopped with 800 μl of H2SO4-H3PO4-H2O (1:3:7 [vol/vol/vol]) and 40 μl of 10% α-isonitrosopropiophenone in 100% methanol and heated to 100°C for 30 min. The urea concentration was measured at 540 nm. One unit of enzyme activity was defined as the amount of enzyme that catalyzed the formation of 1 μmol of urea per minute.
NMR experiments.
Promastigotes treated with 100 μM resveratrol were washed 2 times with PBS and then suspended in 600 μl of deuterated PBS. 1H nuclear magnetic resonance (NMR) experiments were recorded at 25°C in an Agilent 500 spectrometer operating at 499.78 MHz, with a 5-mm broadband inverse geometry probe. The following acquisition parameters were used: 90° pulse (zgpr, avance-version 1.7.10.2 1D sequence with f1 presaturation), 64 scans, and spectral width of 8,012.8 ppm. The signal intensities were calculated by performing the appropriate baseline corrections and then integrating the area under each of the resonances using MestReNova version 6.0.2. The integrated regions for CH2 were 1.19 to 1.44 ppm and were 0.77 to 0.95 ppm for CH3.
Statistical analysis.
IC50s were calculated according to a nonlinear regression using a second-order polynomial equation, with 95% confidence intervals, using GraphPad Prism 5 software. The results were expressed as the IC50 ± standard error of the mean (SEM). The data were analyzed by Student's t test when comparing two groups or by one-way analysis of variance (ANOVA) for more than two groups using the GraphPad Prism 5 software. P values of ≤0.05 were considered significant.
RESULTS
Antipromastigote activity.
We first tested the activities of resveratrol and AMB on L. amazonensis promastigote proliferation (Fig. 1A and B). Our results showed dose-dependent antileishmanial activity, with IC50s of 27 ± 0.59 μM and 0.108 ± 0.006 μM for resveratrol and AMB, respectively, after 48 h of treatment. The leishmanicidal activities of both drugs were confirmed by labeling the promastigotes with ethidium homodimer (EthD-1+) after resveratrol and AMB treatment (Fig. 1C). In this assay, resveratrol and AMB increased the percentage of dead EthD-1+ promastigotes in relation to the untreated control by 5.3- and 7.4-fold, respectively.
FIG 1.
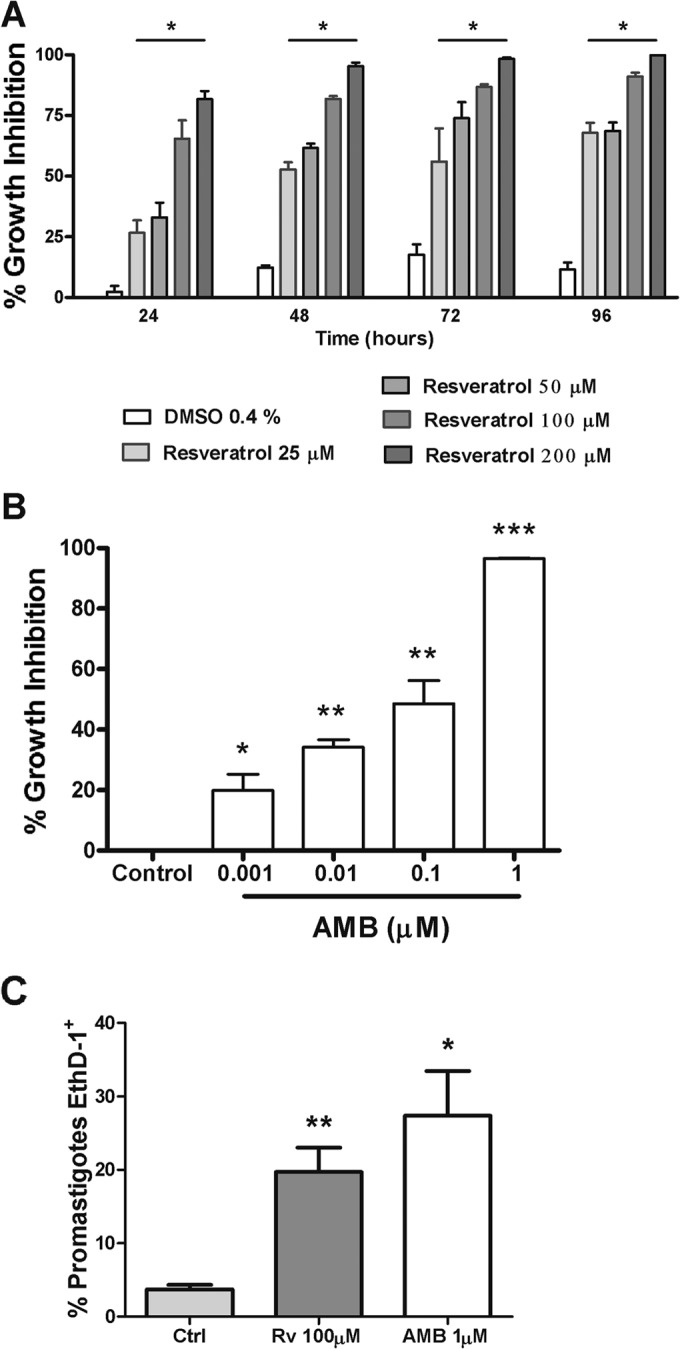
Inhibition of L. amazonensis proliferation by resveratrol and amphotericin B (AMB). Promastigotes (106/ml) were grown in the presence or absence of resveratrol, AMB, or diluent (DMSO), at the indicated concentrations. (A) Percentage of growth inhibition by resveratrol was determined by counting the viable parasites in a Neubauer chamber at the indicated time points. *, P < 0.001 compared to untreated control. (B) Percentage of growth inhibition by AMB was determined as in panel A after 48 h of treatment. The results shown are the mean ± standard error of the mean (SEM) from three independent experiments. *, P < 0.05, **, P < 0.001, and ***, P < 0.0001 compared to the control. (C) Viability analysis of resveratrol (Rv)- and AMB-treated parasites. Promastigotes cultured or not with 100 μM Rv or 1 μM AMB for 48 h were stained with ethidium homodimer 1 (EthD-1+) and analyzed by flow cytometry. The results are shown as the mean ± SEM from 3 independent experiments, with 10,000 events recorded/experimental condition. *, P < 0.05 and **, P < 0.001, compared to the control.
Promastigote morphology.
Next, we examined the morphological changes associated with killing by resveratrol. The promastigotes treated with 100 μM resveratrol for 48 h were stained with Giemsa and compared with the untreated controls (Fig. 2). Typical promastigotes presenting one flagellum, one nucleus, and one kinetoplast were observed in the cultures after 48 h (Fig. 2A). The resveratrol-treated promastigotes showed altered cell division, as demonstrated by the presence of an irregular number of flagella (Fig. 2B), altered cell shape (Fig. 2C), and cells with three nuclei and one kinetoplast (Fig. 2D). No morphological changes were observed in the vehicle (0.4% DMSO)-treated parasites (data not shown).
FIG 2.
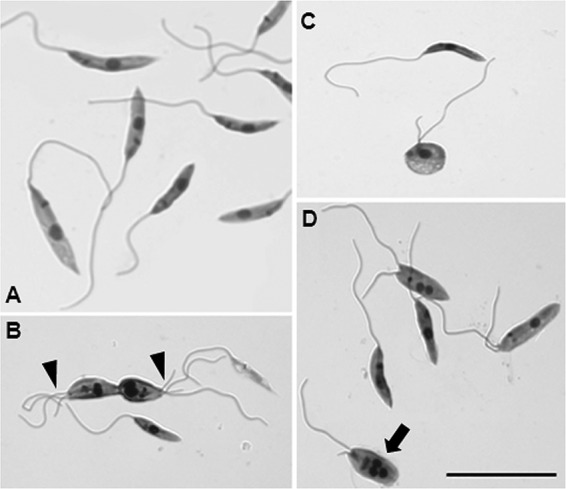
Morphological analysis of resveratrol-treated L. amazonensis. Promastigotes treated with 100 μM resveratrol for 48 h were stained with Giemsa. (A) Untreated control cells. Also shown are resveratrol-treated cells showing an irregular number of flagella (arrowheads in panel B), distorted shape (C), and the presence of 3 nuclei (D, arrow). Scale bar, 20 μm.
The alterations in the number of nuclei (N) and kinetoplasts (K) induced by treating promastigotes with 100 μM resveratrol for 48 h were then quantified. The untreated controls contained 1N/1K and 2N/2K, and 32% of the resveratrol-treated parasites presented 3N/1K or 2N/1K (Fig. 3).
FIG 3.
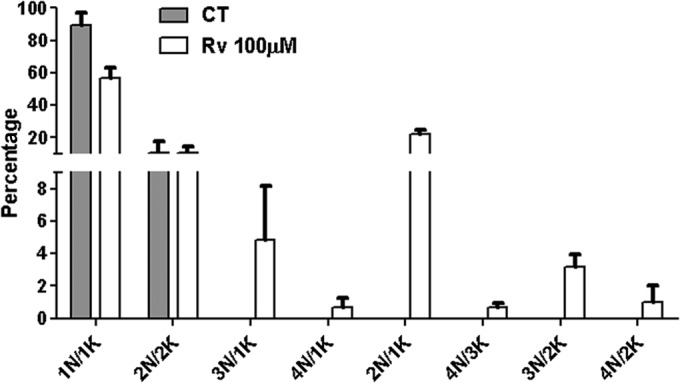
Effects of resveratrol on the cell division of axenic promastigotes. Giemsa-stained promastigotes cultured in the absence or presence of 100 μM resveratrol (Rv) for 48 h were quantified according to the numbers of nuclei (N) and kinetoplasts (K) present in each cell. At least 200 cells were counted in two independent experiments, and the values represent the means ± SEM.
Cell cycle.
The alterations in the nucleus-kinetoplast relationship observed in the resveratrol-treated promastigotes suggested that this drug affects the parasite cell cycle. To test this hypothesis, L. amazonensis promastigotes were treated with resveratrol for 48 h, labeled with propidium iodide in cell cycle solution, and analyzed by flow cytometry (Table 1). Our results confirmed that resveratrol at concentrations of 27 μM and 100 μM affect the division pattern of the promastigotes by increasing the sub-G0/G1 population (5.4- and 11-fold greater than the untreated controls, respectively) and decreasing the G0/G1 population (1.3- and 1.4-fold less than the untreated controls, respectively). Moreover, 100 μM resveratrol decreased the S population by 1.9-fold compared to the DMSO-treated cells and by 1.7-fold compared to the untreated controls. AMB did not alter the cell cycle of the parasite.
TABLE 1.
Analysis of L. amazonensis promastigote cell cycle
| Treatment | % cells in different cell cycle stagesa |
|||
|---|---|---|---|---|
| Sub-G0/G1 | G0/G1 | S | G2 | |
| Control | 1.685 ± 0.170 | 43.867 ± 2.210 | 12.685 ± 0.471 | 18.827 ± 1.308 |
| 0.4% DMSO | 2.117 ± 0.478 | 40.870 ± 0.688 | 13.680 ± 0.841 | 19.932 ± 1.521 |
| 27 μM resveratrol | 9.068 ± 3.065b | 33.628 ± 1.963c | 9.180 ± 0.947 | 17.540 ± 0.270 |
| 100 μM resveratrol | 18.780 ± 5.298c | 31.950 ± 2.000c | 7.285 ± 0.929 | 19.853 ± 1.680 |
| 0.1 μM amphotericin B | 1.360 ± 0.261 | 46.655 ± 0.493 | 13.560 ± 0.556 | 20.700 ± 0.766 |
Results are means ± SEM from four independent experiments.
P < 0.01 in relation to control.
P < 0.001, in relation to control.
Parasite mitochondrial alterations.
Parasites of the genus Leishmania have a single mitochondrion that is involved in apoptotic death, and the increase in the sub-G0/G1 hypodiploid DNA (Table 1) might represent promastigotes that undergo an incidental death. Therefore, we were interested in determining the effect of resveratrol on the parasite mitochondria. We evaluated alterations in the mitochondrial membrane potential (ΔΨm) using the JC-1 assay. Treatment with 100 μM resveratrol reduced by 67% and 76% the ΔΨm compared to the DMSO-treated and untreated control promastigotes, respectively (Fig. 4). Mitochondrial potential was not affected by 0.1 μM AMB (Fig. 4).
FIG 4.
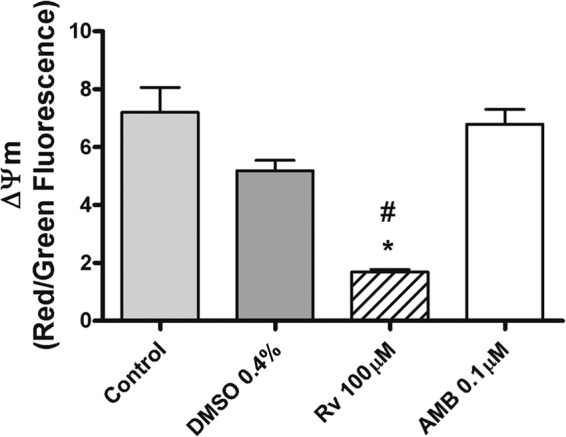
Analysis of the mitochondrial activity of L. amazonensis after resveratrol (Rv) and amphotericin B (AMB) treatments. (A) Promastigotes were cultured in the presence or absence of 100 μM Rv, 0.1 μM AMB, or vehicle (0.4% DMSO) for 4 h. The mitochondrial membrane potential (ΔΨm) was evaluated using a JC-1 assay with 106 parasites/condition. The results are expressed as red/green fluorescence ratios and represent the averages ± SEM from 4 independent experiments. *, P < 0.001 in relation to the untreated control; #, P < 0.05 in relation to DMSO.
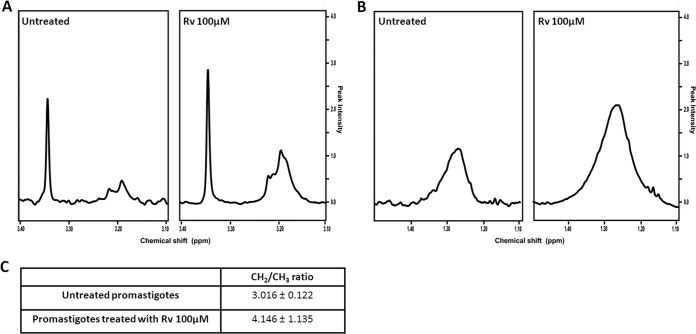
NMR experiments.
To identify possible alterations in the metabolite profile of L. amazonensis, the promastigotes were treated with resveratrol and analyzed by 1H NMR spectroscopy. Our results demonstrated that 100 μM resveratrol increased the CH2-to-CH3 ratio by 1.4-fold after 48 h of treatment. These results from the 1H NMR-visible mobile lipid technique indicated that apoptosis was occurring (Fig. 5A and B). In addition to the changes in the CH2-to-CH3 ratio, a significant modification in the choline region was observed in the resveratrol-treated parasites. Resveratrol increased signals near to 3.18, 3.21, and 3.35 ppm that were assigned to choline, phosphocholine, and phosphatidylcholine, respectively (34). These observations are also characteristic of apoptosis (Fig. 5C).
FIG 5.
1H NMR analysis of promastigotes treated with resveratrol (Rv) for 48 h. (A and B) 1H NMR spectra of promastigotes untreated and treated with 100 μM Rv. (A) choline region; (B) CH2. (C) CH2-to-CH3 ratio.
Antiamastigote activity.
Because resveratrol is active against promastigotes, we were interested in evaluating its activity against amastigotes, the stage that maintains the infection in vertebrate hosts. Thus, parasite killing was assessed in in vitro amastigote-infected macrophages after 24 h of treatment. Our results showed that treatment with 25, 50, 100, and 200 μM resveratrol killed 40%, 60%, 65%, and 78%, respectively, of the amastigotes. The IC50 of resveratrol was calculated as 42 ± 7.18 μM (Fig. 6A). The treatment of amastigote-infected macrophages with 0.001, 0.01, 0.1, and 1 μM AMB resulted in the killing of 40%, 65%, 73%, and 94% of the parasites, respectively, with an IC50 of 0.0088 ± 0.003 μM (Fig. 6B).
FIG 6.
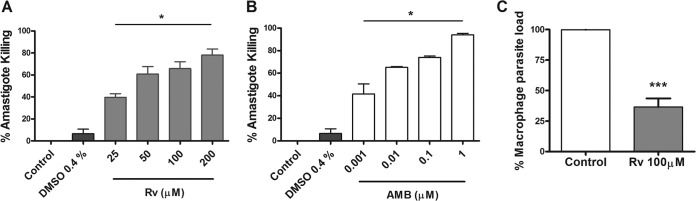
Leishmanicidal effect of resveratrol (Rv) and amphotericin B (AMB) on amastigote-infected macrophages in vitro. Peritoneal macrophages (105) from BALB/c mice were infected with promastigotes at a ratio of 10 parasites to 1 macrophage for 24 h and were either left untreated or were treated with the indicated concentrations of Rv (A) and AMB (B) and 0.4% DMSO. The results represent the mean ± SEM from 3 experiments performed in triplicate. (C) Infected macrophages were left untreated or were treated with 100 μM resveratrol for 24 h, washed, fed with Schneider's complete medium, and cultured at 26°C. The macrophage parasite load was evaluated after 48 h in culture by counting the promastigotes. The results represent the means ± SEM from two experiments performed in triplicate. *, P < 0.01 and ***, P < 0.001 compared to the control.
To further confirm the parasite-killing effect, the amastigote-infected macrophages treated or not with 100 μM resveratrol were cultured under conditions that rescue the amastigotes transformed into promastigotes (28). The viable amastigotes that survived the treatment transformed into motile promastigotes that were counted after 48 h in culture at 26°C (Fig. 6C).
Host cell viability assays.
To test the safety of resveratrol on host cells, we evaluated the mitochondrial activity of the macrophages using the XTT method. Our results showed that treatment of the macrophages with 600 μM resveratrol or 5 μM AMB did not significantly alter the activities of the mitochondrial enzymes (Fig. 7A). However, the cytochrome c oxidase inhibitor sodium azide (1%) inhibited macrophage viability by 63%.
FIG 7.
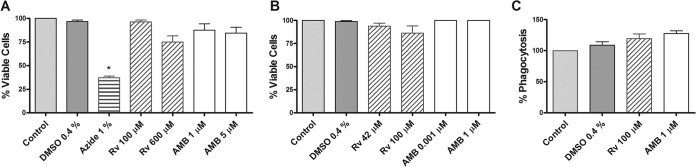
Cytotoxicity of resveratrol (Rv) and amphotericin B (AMB) against murine peritoneal macrophages in vitro. (A) Cells were cultured with 100 and 600 μM Rv and 1 and 5 μM AMB for 24 h at 37°C in 5% CO2. Sodium azide (1%) was used as a positive control. Viability was measured by the XTT method. (B) Macrophages were cultured for 24 h at 37°C in 5% CO2 at the indicated concentrations of Rv and AMB, and viability was measured using a trypan blue exclusion assay. (C) Peritoneal macrophages were treated for 24 h with Rv and AMB at the indicated concentrations and then incubated with promastigotes at a 1:10 ratio. After 1 h, the cells were fixed and stained, and the phagocytic rate was determined by counting ≥200 cells. The results are representative (means) of 3 experiments performed in triplicate ± SEM. *, P < 0.05 compared to the control.
Next, we tested the capacity of the drugs to damage the integrity of the macrophage membranes using a trypan blue dye exclusion assay. Our results showed that 94% and 87% of the cells remained viable following treatment with 42 and 100 μM resveratrol, respectively, compared with that of the untreated control (Fig. 7B). DMSO, the diluent vehicle of resveratrol, as well as 0.001 and 1 μM AMB, did not damage the macrophage membranes.
Finally, we assessed phagocytosis, a functional property of macrophages, as a criterion of cell health. For this assay, the L. amazonensis promastigotes were offered as particles to be phagocytosed by murine macrophages that had been treated or not with resveratrol or AMB. We observed that the concentrations of resveratrol and AMB tested did not affect the phagocytosis of the promastigotes compared to the untreated control cells (Fig. 7C).
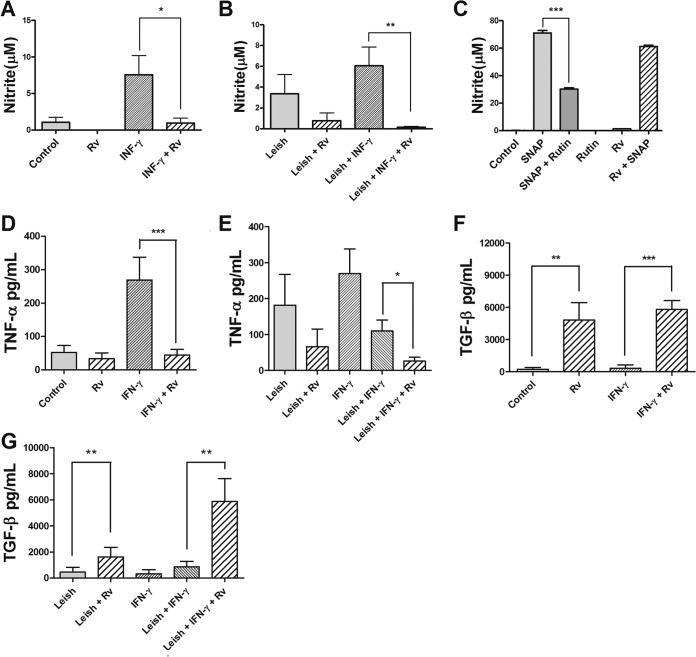
Nitric oxide and cytokine production.
NO is an important mediator of Leishmania death; therefore, we investigated whether resveratrol is capable of modulating NO production by macrophages. Our results showed that resveratrol did not induce NO production by macrophages (Fig. 8A). Instead, resveratrol decreased the NO production by uninfected and infected macrophages stimulated with IFN-γ by 8- and 44-fold, respectively, indicating that the antileishmanial effect of resveratrol was not mediated by the induction of NO (Fig. 8A and B).
FIG 8.
Effects of resveratrol (Rv) on nitric oxide (NO) and cytokine production by murine peritoneal macrophages. Shown are uninfected (105) (A) and L. amazonensis-infected macrophages (Leish) (B) at a 10:1 ratio, stimulated with IFN-γ or left unstimulated; these were incubated in the presence or absence of 100 μM Rv. NO production was evaluated after 48 h of treatment by the Griess method. The results from four independent experiments performed in triplicate are shown as the nitrite concentration means ± SEM. *, P < 0.05 and **, P < 0.001 compared to cells treated with IFN-γ alone. (C) Scavenger effect analysis was performed in a cell-free system by incubating SNAP solution (1 mM) as the NO donor with 100 μM Rv. Rutin (1 mM), an NO scavenger, was used as a positive control, and RPMI medium served as the negative control. The nitrite levels were determined by the Griess method. The data represent the means ± SEM from three independent experiments. ***, P < 0.0001. (D to G) The production of TNF-α (D and E) and TGF-β (F and G) was determined by specific ELISA after 48 h of treatment. The data represent the averages ± SEM from 3 experiments in triplicate. *, P < 0.05; **, P < 0.01; ***, P < 0.0001.
To rule out a possible NO scavenging effect of resveratrol, a cell-free system was used with S-nitroso-N-acetyl-dl-penicillamine (SNAP) as an NO donor in the presence of resveratrol. The addition of rutin, an NO scavenger, to the SNAP solution decreased NO levels by 57%, and the addition of 100 μM resveratrol to the SNAP solution did not reduce the levels of NO, indicating that the decrease in NO production that resulted from treatment with resveratrol was not due to a scavenging effect of the drug (Fig. 8C).
Next, we evaluated if resveratrol could modulate cytokine production by assaying cytokines that promote parasite killing (TNF-α) or survival (TGF-β). According to our data, resveratrol did not stimulate the production of TNF-α by uninfected macrophages or macrophages that were infected with Leishmania (Fig. 8D and E). The uninfected and unstimulated macrophages treated for 48 h with 100 μM resveratrol were unable to produce TNF-α (Fig. 8D). The activation of macrophages with IFN-γ induced a 5.1-fold increase in the production of TNF-α compared to that in unstimulated macrophages, but the addition of 100 μM resveratrol decreased the production of TNF-α by the unstimulated and uninfected macrophages by 6.1-fold (Fig. 8D). In the IFN-γ-activated infected macrophages, resveratrol decreased the production of TNF-α by 4.2-fold (Fig. 8E). However, 100 μM resveratrol increased TGF-β production by the uninfected macrophages that were activated with IFN-γ or left untreated by 21- and 17.5-fold, respectively (Fig. 8F). The infected macrophages treated with resveratrol and activated or not with IFN-γ had 3.6- and 6.8-fold increases in TGF-β production, respectively (Fig. 8G).
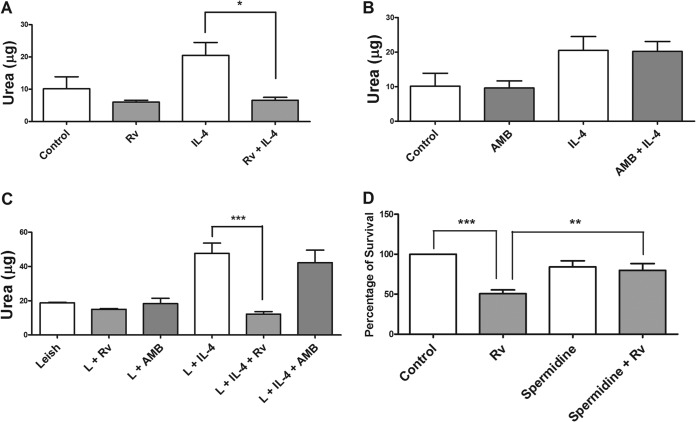
Evaluation of macrophage arginase activity and the effect of spermidine on infection.
The Th2-type immune response induces the expression of arginase by macrophages. This enzyme produces urea and ornithine, with ornithine being the precursor of polyamines that promote Leishmania growth. To assess the effect of resveratrol on the arginine pathway, we determined the arginase activity in the lysates of murine peritoneal macrophages that had been treated or not with resveratrol and AMB for 48 h. Our results showed that in the absence of stimulus, 100 μM resveratrol was unable to significantly reduce the activity of arginase in relation to that of the untreated control. However, the arginase activity after stimulation with 20 ng/ml of IL-4 (an arginase inducer) was decreased by approximately 3-fold with treatment with 100 μM resveratrol (Fig. 9A). In Leishmania-infected macrophages, treatment with resveratrol decreased arginase activity by approximately 4-fold (Fig. 9B). Unlike resveratrol, AMB did not affect arginase activity (Fig. 9C).
FIG 9.
Effect of resveratrol on the arginase activity of murine macrophages. (A) Macrophages (106) were stimulated with 20 ng/ml IL-4 or left unstimulated and then treated with 100 μM resveratrol (Rv) for 48 h. (B) Macrophages (106) were stimulated with 20 ng/ml IL-4 or left unstimulated and then treated with 1 μM AMB. (C) Macrophages (106) were infected with Leishmania (L) promastigotes at a 5:1 ratio for 24 h, stimulated with 20 ng/ml IL-4 or left unstimulated, and then treated with 100 μM Rv or 0.5 μM amphotericin B (AMB) or left untreated for 48 h. (D) Recovery of infectivity by spermidine in Rv-treated macrophages. Macrophages (106) were infected with promastigotes at a 10:1 ratio for 24 h and subsequently treated with 100 μM Rv, 100 μM spermidine, or both Rv and spermidine for 24 h. The results represent the means ± SEM from three (A and B) or two (C) experiments performed in triplicate. *, P < 0.05; **, P < 0.001; ***, P < 0.0001.
To further confirm the effect of resveratrol on arginase modulation, we determined if the addition of exogenous polyamine could restore parasite survival in resveratrol-treated macrophages. We found that the addition of 100 μM spermidine to the Leishmania-infected macrophages treated with 100 μM resveratrol improved parasite survival by 65% compared to the infected macrophages treated with resveratrol only (Fig. 9D).
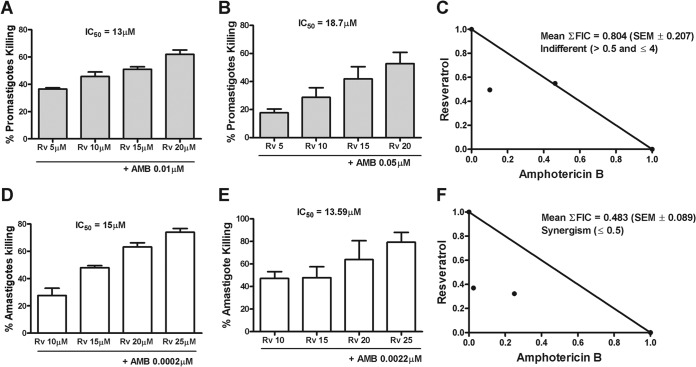
Fractional inhibitory concentration index and isobologram analysis.
An important strategy for the treatment of many diseases is combination therapy. Therefore, we assessed whether resveratrol could synergize with AMB, using the fractional inhibitory concentration index and the isobologram method. Thus, the IC50s for both drugs were determined (Fig. 10A, B, D, and E), the calculated FIC50 value for resveratrol was plotted on the y axis, and the FIC50 values for AMB were plotted on the x axis for promastigotes (Fig. 10C) and amastigotes (Fig. 10F). The two points were connected to form the line of additivity. The points on this line were classified as additive, points above the line indicate antagonistic effects, and points below the line indicate synergistic effects. The combination of resveratrol with AMB showed an indifferent effect against L. amazonensis promastigotes (Fig. 10C) and a synergistic effect against amastigotes (Fig. 10F). The synergism for amastigotes of the resveratrol-AMB association was also indicated by the calculated mean ΣFIC, which was 0.483 ± 0.089.
FIG 10.
Association of resveratrol (RV) and amphotericin B (AMB). (A and B) Promastigotes (106/ml) were grown in the presence or absence of the indicated concentrations of Rv and AMB. Promastigote killing was determined by counting the viable parasites after 48 h. (D and E) Macrophages (105) infected with promastigotes for 24 h were treated with the indicated concentrations of Rv and AMB for 24 h. Amastigote killing was determined as described in Materials and Methods. (C and F) Isobolographic analysis of the Rv-AMB association with L. amazonensis promastigotes and amastigotes. The straight line is the line of additivity and represents all of the additive theoretical combinations that should inhibit survival by 50%. The points below the additivity line represent synergistic combinations. The results represent the means ± SEM from three experiments performed in triplicate. FIC, fractional inhibitory concentration.
DISCUSSION
The polyphenol resveratrol has been widely studied, and several of its biological effects have been described (8, 13–20, 35). Resveratrol is also in clinical trials for the treatment of different diseases (24). It has been demonstrated that resveratrol has antileishmanial activity against L. major and Leishmania donovani, a cutaneous and visceral leishmaniasis agent in the Old World (19, 36). Although it has been shown that resveratrol inhibits L. donovani 3-hydroxy-3-methylglutaryl coenzyme A reductase, an enzyme involved in isoprenoid biosynthesis (36), its leishmanicidal effect is still not clear. Thus, we sought to better characterize the effect of resveratrol by evaluating its leishmanicidal activity against L. amazonensis, the causative agent of cutaneous and diffuse cutaneous forms of leishmaniasis (DCL) in the New World; DCL is more severe and resistant to the conventional therapy. Actually, L. amazonensis produces the whole spectrum of clinical forms and has already been associated with post-kala-azar dermal leishmaniasis and visceral leishmaniasis in humans and dogs (37–40). We determined that the IC50 of resveratrol for L. amazonensis promastigotes was 27 μM; this value is lower than the IC50s of 197 μM and 153 μM that were established for L. major and L. donovani promastigotes, respectively (19, 36). Resveratrol is also effective at killing L. amazonensis amastigotes, with an IC50 of 42 μM. Although the IC50 of resveratrol was not determined for the L. major amastigotes, treatment with 197 μM resveratrol can inhibit macrophage infection by this species by 75% (19). Similarly, we inhibited amastigote intracellular growth by 78% by treating the infected macrophages with 200 μM resveratrol.
Drug association therapy has numerous advantages and can delay the emergence of resistant pathogens and increase the half-lives of the therapeutic agents, as has been shown for HIV-1, malaria, and tuberculosis (41). Specifically, the importance of developing drug association therapy for leishmaniasis has increased over the last several years, especially for the treatment of more-severe forms of this disease (2). Drug association therapy has several important advantages, including reduced dosages and/or treatment duration, both of which result in fewer toxic side effects, increased patient compliance, lower treatment costs, and the prevention or delay of drug resistance development.
Our data show for the first time that the combination of resveratrol and AMB has a synergistic effect against L. amazonensis amastigotes. Studies associating natural products with classical antileishmanials demonstrated synergism when Chenopodium ambrosioides essential oil was used in combination with pentamidine against L. amazonensis; synergism was not observed when this essential oil was used in combination with AMB (42). Recently, it was reported that the association of AMB and allicin, a natural product present in plants of the family Alliaceae, was synergistic for Leishmania infantum and L. donovani (43). It has also been demonstrated that AMB has a synergistic effect when used in association with paromomycin against L. amazonensis, Leishmania braziliensis, and L. infantum in vitro (44). This effect demonstrates the potential for developing drug associations that are effective against microorganisms, particularly when using AMB.
Evaluating the cytotoxicity of natural products to host cells is important, especially considering the strong interest in alternative therapies and the therapeutic use of medicinal plants. To assess the safety of resveratrol, we performed XTT and trypan blue assays. Resveratrol was not toxic to the murine macrophages at the tested concentrations. Neither resveratrol nor AMB decreased the phagocytic capacity of the macrophages at the tested concentrations, demonstrating again the lack of toxicity to macrophages and indicating that the leishmanicidal effect of resveratrol seen in our results was not due to a decrease in the number of viable macrophages. In contrast, Lucas and Kolodziej (20) attributed the anti-Leishmania effect of resveratrol to a dose-dependent toxicity against macrophages. However, we cannot rule out the possibility that the bone marrow-derived macrophages (BMMΦ) and J774-G8 cells tested in that study are more sensitive to resveratrol. This dissimilarity in the sensitivities of different types of macrophages can also be observed by comparing the dehydrogenase activity measured in BMMΦ and J774-G8 cells (20) to that of the peritoneal macrophages used in this study. The 50% cytotoxic concentration (CC50) values of 72 μM and 108 μM were obtained for BMMΦ and J774-G8, respectively, and we demonstrated that the dehydrogenase activity was inhibited only by 25% after treatment with 600 μM resveratrol, a concentration that is 14-fold greater than that required for antiamastigote activity. Importantly, in agreement with Kedzierski and colleagues (19), our results demonstrated that the dehydrogenase activity, membrane integrity, and phagocytosis, all of which are important functions of macrophages, were not altered by treating the peritoneal macrophages with resveratrol.
Trypanosomatids have only one flagellum, one kinetoplast, and one nucleus, and these organelles are duplicated during the cell cycle. It has been observed that the flagellum begins to grow first during the cell division of Leishmania promastigotes, and this is followed by mitosis and the subsequent division of the kinetoplast, which occurs after the initiation of nuclear anaphase (45). Due to the inhibition of parasite cell proliferation by resveratrol, we evaluated their morphology and observed an increase in the number of nuclei and flagella in the promastigotes treated with resveratrol. Changes in the number of nuclei and kinetoplasts were observed in 32.5% of the cells examined, but changes in the G2 phase of the cell cycle were not observed. The changes induced by resveratrol may have occurred due to cell cycle arrest in response to the effect of resveratrol on a component that is essential for completing the process of cytokinesis. Therefore, we decided to investigate the distribution of tubulin in the resveratrol-treated promastigotes. The α-tubulin and β-tubulin components of microtubules play important roles in mitosis, the maintenance of cell shape, cell motility, and the transport of organelles in eukaryotes. Because tubulin is the target of various substances used to treat cancer and infections by fungi and helminthes, it may also be a good target for antileishmanial drugs. Accordingly, it has been shown that paclitaxel, an anticancer drug, affects Leishmania tubulin (46). Thus, we analyzed the distribution pattern of α-tubulin in resveratrol-treated promastigotes, but we did not observe any changes in its distribution (data not shown).
The effects of natural products on the leishmanial cell cycle and the induction of apoptosis have been reported (47). By analyzing the cell cycle of the resveratrol-treated promastigotes, we found an increase in the sub-G0/G1 population, suggesting that the parasites underwent incidental death, according to the suggested nomenclature for protozoan death (48). In agreement with our findings, it was demonstrated that resveratrol induced apoptosis and an accumulation of cells in the G0/G1 phase in LNCaP and PC-3 prostate cancer cells (49). Leishmanial apoptosis and mammalian apoptosis have similar characteristics, such as internucleosomal DNA fragmentation, phosphatidylserine exposure on the external surface of the plasma membrane, and the loss of mitochondrial transmembrane potential (33). Since the maintenance of mitochondrial transmembrane potential is essential for the survival of a single mitochondrion parasite, we evaluated the mitochondrial integrity of promastigotes by testing the mitochondrial membrane potential by JC-1 assay. Our data show that resveratrol induces the depolarization of the mitochondrial membrane potential; this phenomenon may be involved in promastigote incidental death (48), although other mechanisms of death cannot be ruled out. In agreement with our findings, it has been shown that resveratrol reduced the mitochondrial membrane potential in U251 human glioma cells and decreased cell viability, possibly by apoptosis (49, 50).
Nuclear magnetic resonance provides a new means for analyzing biological phenomena in vivo, and this tool can be applied to parasitology with inherent advantages, because it is a nondestructive and noninvasive process (51, 52). The information obtained using this method may provide considerable insight into cellular metabolism, and testing can be performed not only on extracts and media but also on whole cells and tissues under various conditions and across various times (53). The 1H NMR-visible mobile lipid technique had been used to discriminate between different Trypanosoma cruzi death pathways. Apoptotic conditions are characterized by a significantly increased CH2-to-CH3 ratio (34). In agreement, we also observed an increase in the CH2-to-CH3 ratio and an elevation in the choline region after treating L. amazonensis promastigotes with resveratrol. Moreover, we obtained a 2.9-fold increase in the percentage of annexin V-positive promastigotes upon treatment with resveratrol (data not shown).
The production of nitric oxide by macrophages is one of the main microbicidal mechanisms involved in the killing of intracellular forms of these parasites (54). Thus, we evaluated NO production in murine macrophages incubated with resveratrol and AMB. Our results demonstrate that none of the compounds increased NO production, and resveratrol even decreased NO production in both the Leishmania-infected and uninfected macrophages stimulated with IFN-γ. To confirm that the inhibitory effect was not due to NO trapping, resveratrol was incubated with SNAP, an NO donor, in a cell-free system. Our results showed that resveratrol was not capable of scavenging NO. These results indicate that the inhibition of NO production occurred by a direct effect of resveratrol on macrophages and not by a potential scavenger ability. It has been demonstrated that resveratrol inhibits NO production induced by IFN-γ in RAW 264.7 cells by reducing inducible nitric oxide synthase (iNOS) synthesis (55). Our data, together with the literature, indicate that resveratrol inhibits NO, demonstrating that its antiamastigote activity is independent of NO production. Recently, it has been shown that resveratrol inhibits the oxidative activity induced by lipopolysaccharide (LPS) and paraquat by increasing the level of nuclear factor erythroid-like 2 (NFR2) (56, 57). The inhibition of NO and ROS by resveratrol seems to be important for controlling infection by T. cruzi, a trypanosomatid protozoan, as for Leishmania infection. The work of Paiva and colleagues (58) showed that polyphenols, such as resveratrol and pterostilbene, are able to inhibit T. cruzi amastigote growth in infected macrophages by reducing ROS and NO via NFR2 activation, suggesting that an antioxidant drug can be active against intracellular parasites.
Arginase is the main source of l-ornithine in trypanosomatids and is involved in the production of polyamines. Polyamines are important for the synthesis of trypanothione, which plays a crucial role in maintaining the intracellular redox balance and defense against oxidative stress in these parasites (59). Furthermore, the inhibition of macrophage arginase activity leads to the decreased degradation of l-arginine, resulting in a decrease in the availability of ornithine and polyamines and the subsequent reduction of L. major growth in murine macrophages (60). However, the addition of ornithine restores parasite growth. Our results show a decrease in the activity of arginase in macrophages stimulated with IL-4 and treated with resveratrol, and the addition of the polyamine spermidine restores parasite growth. These data suggest that the leishmanicidal activity of resveratrol might involve polyamine starvation, because resveratrol impairs arginase activity, and the addition of spermidine reverses this effect. Additionally, the activity of arginase was not affected by treating the macrophages with AMB.
Our results show that resveratrol decreased the levels of the proinflammatory cytokine TNF-α in the supernatants of uninfected and Leishmania-infected macrophages stimulated with IFN-γ. When we analyzed the levels of the anti-inflammatory cytokine TGF-β in the supernatants of the macrophages treated with resveratrol, we found increased levels of TGF-β in the stimulated and unstimulated macrophages regardless of whether they were infected with L. amazonensis. Corroborating our findings, it has been demonstrated that resveratrol increases the levels of TGF-β in A549 lung epithelial cells (61). In that study, it was found that pretreating the cells with 4-hydroxytamoxifen (tamoxifen), an estrogen receptor antagonist, significantly inhibited the increase in TGF-β levels induced by resveratrol in A549 cells. Considering that the chemical structure of resveratrol is similar to that of estradiol, the authors also showed that 10 pM estradiol stimulated the production of TGF-β in A549 cells, and this production was also blocked by tamoxifen, suggesting that resveratrol induces the formation of TGF-β through estrogen receptors. Macrophages, monocytes, and dendritic cells express both α and β receptors for estrogen (62), and because resveratrol binds to these receptors, our results showing increased levels of TGF-β in macrophages treated with resveratrol may have occurred via its recognition by these receptors.
The results of our study establish the anti-Leishmania effect of resveratrol, and more importantly, our data support the potential synergistic activities of resveratrol and amphotericin B. We show that the leishmanicidal activity of resveratrol is not dependent on proinflammatory cytokines and NO production by murine macrophages; nevertheless, the arginase activity of macrophages seems to be important for killing intracellular amastigotes. Promastigote death was mediated by incidental death (48), with the expression of some apoptotic markers. Further studies are required to determine if the amastigote death induced by resveratrol was due to polyamine starvation or the unavailability of polyamines in the infected macrophages. Overall, this study highlights that resveratrol not only possesses antileishmanial activity but can also synergize with AMB to inhibit the growth of L. amazonensis in murine macrophages.
ACKNOWLEDGMENTS
We thank Norton Heise and Juliany C. Rodrigues (Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil) for their donation of miltefosine.
This work was supported by the Fundação de Apoio a Pesquisa do Estado do Rio de Janeiro (FAPERJ), the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), and the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
Footnotes
Published ahead of print 11 August 2014
REFERENCES
- 1.Alvar J, Vélez ID, Bern C, Herrero M, Desjeux P, Cano J, Jannin J, den Boer M, the WHO Leishmaniasis Control Team 2012. Leishmaniasis worldwide and global estimates of its incidence. PLoS One 7:e35671. 10.1371/journal.pone.0035671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barrett MP, Croft SL. 2012. Management of trypanosomiasis and leishmaniasis. Br. Med. Bull. 104:175–196. 10.1093/bmb/lds031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ouellette M, Drummelsmith J, Papadopoulou B. 2004. Leishmaniasis: drugs in the clinic, resistance and new developments. Drug Resist. Updat. 7:257–266. 10.1016/j.drup.2004.07.002 [DOI] [PubMed] [Google Scholar]
- 4.Berman J. 2003. Current treatment approaches to leishmaniasis. Curr. Opin. Infect. Dis. 16:397–401. 10.1097/00001432-200310000-00005 [DOI] [PubMed] [Google Scholar]
- 5.Santos DO, Coutinho CE, Madeira MF, Bottino CG, Vieira RT, Nascimento SB, Bernardino A, Bourguignon SC, Corte-Real S, Pinho RT, Rodrigues CR, Castro HC. 2008. Leishmaniasis treatment–a challenge that remains: a review. Parasitol. Res. 103:1–10. 10.1007/s00436-008-0943-2 [DOI] [PubMed] [Google Scholar]
- 6.Soto J, Arana BA, Toledo J, Rizzo N, Vega JC, Díaz A, Luz M, Gutiérrez P, Arboleda M, Berman JD, Junge K, Engel J, Sindermann H. 2004. Miltefosine for New World cutaneous leishmaniasis. Clin. Infect. Dis. 38:1266–1272. 10.1086/383321 [DOI] [PubMed] [Google Scholar]
- 7.Sindermann H, Engel J. 2006. Development of miltefosine as an oral treatment for leishmaniasis. Trans. R. Soc. Trop. Med. Hyg. 100(Suppl 1):S17–S20. 10.1016/j.trstmh.2006.02.010 [DOI] [PubMed] [Google Scholar]
- 8.Kubo M, Kimura Y, Shin H, Haneda T, Tani T, Namba K. 1981. Studies on the antifungal substance of crude drug: 2. On the roots of Polygonum cuspidatum (Poligonaceae). Shoyakugaku Zasshe. 35:58–61 [Google Scholar]
- 9.Kimura Y, Ohminam H, Okuda H, Baba K, Kozawa M, Archi S. 1983. Effects of stilbene components of roots of Polygonum ssp. on liver injury in peroxidised oil-fed rats. Planta Med. 49:51–54. 10.1055/s-2007-969810 [DOI] [PubMed] [Google Scholar]
- 10.Hillis WE, Hart JH, Yazaki Y. 1974. Polyphenols of Eucalyptus sideroxylon wood. Phytochemistry 13:1591–1595. 10.1016/0031-9422(74)80334-1 [DOI] [Google Scholar]
- 11.Langcake P, Cornford CA, Pryce RJ. 1979. Identification of pterostilbene as a phytoalexin of Vitis vinifera leaves. Phytochemistry 18:1025–1028. 10.1016/S0031-9422(00)91470-5 [DOI] [Google Scholar]
- 12.Pirola L, Fröjdö S. 2008. Resveratrol: one molecule, many targets. IUBMB Life 60:323–332. 10.1002/iub.47 [DOI] [PubMed] [Google Scholar]
- 13.Baur JA, Sinclair DA. 2006. Therapeutic potential of resveratrol: the in vivo evidence. Nat. Rev. Drug Discov. 5:493–506. 10.1038/nrd2060 [DOI] [PubMed] [Google Scholar]
- 14.Pervaiz S. 2003. Resveratrol: from grapevines to mammalian biology. FASEB J. 17:1975–1985. 10.1096/fj.03-0168rev [DOI] [PubMed] [Google Scholar]
- 15.Jang DS, Kang BS, Ryu SY, Chang IM, Min KR, Kim Y. 1999. Inhibitory effects of resveratrol analogs on unopsonized zymosan-induced oxygen radical production. Biochem. Pharmacol. 57:705–712. 10.1016/S0006-2952(98)00350-5 [DOI] [PubMed] [Google Scholar]
- 16.Chan MM. 2002. Antimicrobial effect of resveratrol on dermatophytes and bacterial pathogens of the skin. Biochem. Pharmacol. 63:99–104. 10.1016/S0006-2952(01)00886-3 [DOI] [PubMed] [Google Scholar]
- 17.Pace-Asciak CR, Hahn S, Diamandis EP, Soleas G, Goldberg DM. 1995. The red wine phenolics trans-resveratrol and quercetin block human platelet aggregation and eicosanoid synthesis: implications for protection against coronary heart disease. Clin. Chim. Acta 235:207–219. 10.1016/0009-8981(95)06045-1 [DOI] [PubMed] [Google Scholar]
- 18.Docherty JJ, Fu MM, Stiffler BS, Limperos RJ, Pokabla CM, DeLucia AL. 1999. Resveratrol inhibition of herpes simplex virus replication. Antiviral Res. 43:145–155. 10.1016/S0166-3542(99)00042-X [DOI] [PubMed] [Google Scholar]
- 19.Kedzierski L, Curtis JM, Kaminska M, Jodynis-Liebert J, Murias M. 2007. In vitro antileishmanial activity of resveratrol and its hydroxylated analogues against Leishmania major promastigotes and amastigotes. Parasitol. Res. 102:91–97. 10.1007/s00436-007-0729-y [DOI] [PubMed] [Google Scholar]
- 20.Lucas IK, Kolodziej H. 2013. In vitro antileishmanial activity of resveratrol originates from its cytotoxic potential against host cells. Planta Med. 79:20–26. 10.1055/s-0032-1328020 [DOI] [PubMed] [Google Scholar]
- 21.Juan ME, Vinardell MP, Planas JM. 2002. The daily oral administration of high doses of trans-resveratrol to rats for 28 days is not harmful. J. Nutr. 132:257–260 [DOI] [PubMed] [Google Scholar]
- 22.Lin CJ, Lee CC, Shih YL, Lin TY, Wang SH, Lin YF, Shih CM. 2012. Resveratrol enhances the therapeutic effect of temozolomide against malignant glioma in vitro and in vivo by inhibiting autophagy. Free Radic. Biol. Med. 52:377–391. 10.1016/j.freeradbiomed.2011.10.487 [DOI] [PubMed] [Google Scholar]
- 23.George J, Singh M, Srivastava AK, Bhui K, Roy P, Chaturvedi PK, Shukla Y. 2011. Resveratrol and black tea polyphenol combination synergistically suppress mouse skin tumors growth by inhibition of activated MAPKs and p53. PLoS One 6:e23395. 10.1371/journal.pone.0023395 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 24.Vang O. 2013. What is new for resveratrol? Is a new set of recommendations necessary? Ann. N. Y. Acad. Sci. 1290:1–11. 10.1111/nyas.12173 [DOI] [PubMed] [Google Scholar]
- 25.Soares DC, Andrade AL, Delorenzi JC, Silva JR, Freire-de-Lima L, Falcão CA, Pinto AC, Rossi-Bergmann B, Saraiva EM. 2010. Leishmanicidal activity of Himatanthus sucuuba latex against Leishmania amazonensis. Parasitol. Int. 59:173–177. 10.1016/j.parint.2010.01.002 [DOI] [PubMed] [Google Scholar]
- 26.Ferreira C, Soares DC, Barreto-Junior CB, Nascimento MT, Freire-de-Lima L, Delorenzi JC, Lima ME, Atella GC, Folly E, Carvalho TM, Saraiva EM, Pinto-da-Silva LH. 2011. Leishmanicidal effects of piperine, its derivatives, and analogues on Leishmania amazonensis. Phytochemistry 72:2155–2164. 10.1016/j.phytochem.2011.08.006 [DOI] [PubMed] [Google Scholar]
- 27.Roehm NW, Rodgers GH, Hatfield SM, Glasebrook AL. 1991. An improved colorimetric assay for cell proliferation and viability utilizing the tetrazolium salt XTT. J. Immunol. Methods 142:257–265. 10.1016/0022-1759(91)90114-U [DOI] [PubMed] [Google Scholar]
- 28.Jain SK, Sahu R, Walker LA, Tekwani BL. 2012. A parasite rescue and transformation assay for antileishmanial screening against intracellular Leishmania donovani amastigotes in THP1 human acute monocytic leukemia cell line. J. Vis. Exp. 70:e4054. 10.3791/4054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Delorenzi JC, Freire-de-Lima L, Gattass CR, de Andrade Costa D, He L, Kuehne ME, Saraiva EM. 2002. In vitro activities of iboga alkaloid congeners coronaridine and 18-methoxycoronaridine against Leishmania amazonensis. Antimicrob. Agents Chemother. 46:2111–2115. 10.1128/AAC.46.7.2111-2115.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Soares DC, Calegari-Silva TC, Lopes UG, Teixeira VL, de Palmer Paixão ICN, Cirne-Santos C, Bou-Habib DC, Saraiva EM. 2012. Dolabelladienetriol, a compound from Dictyota pfaffii algae, inhibits the infection by Leishmania amazonensis. PLoS Negl. Trop. Dis. 6:e1787. 10.1371/journal.pntd.0001787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seifert K, Croft SL. 2006. In vitro and in vivo interactions between miltefosine and other antileishmanial drugs. Antimicrob. Agents Chemother. 50:73–79. 10.1128/AAC.50.1.73-79.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tallarida RJ. 2000. Drug synergism and dose-effect data analysis. Chapman & Hall/CRC, New York, NY [Google Scholar]
- 33.Ambit A, Fasel N, Coombs GH, Mottram JC. 2008. An essential role for the Leishmania major metacaspase in cell cycle progression. Cell Death Differ. 15:113–122. 10.1038/sj.cdd.4402232 [DOI] [PubMed] [Google Scholar]
- 34.Benitez D, Pezaroglo H, Martínez V, Casanova G, Cabrera G, Galanti N, González M, Cerecetto H. 2012. Study of Trypanosoma cruzi epimastigote cell death by NMR-visible mobile lipid analysis. Parasitology 139:506–515. 10.1017/S0031182011002150 [DOI] [PubMed] [Google Scholar]
- 35.Poulsen MM, Jørgensen JO, Jessen N, Richelsen B, Pedersen SB. 2013. Resveratrol in metabolic health: an overview of the current evidence and perspectives. Ann. N. Y. Acad. Sci. 1290:74–82. 10.1111/nyas.12141 [DOI] [PubMed] [Google Scholar]
- 36.Dinesh N, Pallerla DS, Kaur PK, Kishore Babu N, Singh S. 2014. Exploring Leishmania donovani 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMGR) as a potential drug target by biochemical, biophysical and inhibition studies. Microb. Pathog. 6:14–23. 10.1016/j.micpath.2013.11.001 [DOI] [PubMed] [Google Scholar]
- 37.Barral A, Badaró R, Barral-Netto M, Grimaldi G, Jr, Momem H, Carvalho EM. 1986. Isolation of Leishmania mexicana amazonensis from the bone marrow in a case of American visceral leishmaniasis. Am. J. Trop. Med. Hyg. 35:732–734 [DOI] [PubMed] [Google Scholar]
- 38.Barral A, Pedral-Sampaio D, Grimaldi Júnior G, Momen H, McMahon-Pratt D, Ribeiro de Jesus A, Almeida R, Badaro R, Barral-Netto M, Carvalho EM. 1991. Leishmaniasis in Bahia, Brazil: evidence that Leishmania amazonensis produces a wide spectrum of clinical disease. Am. J. Trop. Med. Hyg. 44:536–546 [DOI] [PubMed] [Google Scholar]
- 39.Tolezano JE, Uliana SR, Taniguchi HH, Araújo MF, Barbosa JA, Barbosa JE, Floeter-Winter LM, Shaw JJ. 2007. The first records of Leishmania (Leishmania) amazonensis in dogs (Canis familiaris) diagnosed clinically as having canine visceral leishmaniasis from Araçatuba County, São Paulo State, Brazil. Vet. Parasitol. 149:280–284. 10.1016/j.vetpar.2007.07.008 [DOI] [PubMed] [Google Scholar]
- 40.Aleixo JA, Nascimento ET, Monteiro GR, Fernandes MZ, Ramos AM, Wilson ME, Pearson RD, Jeronimo SM. 2006. Atypical American visceral leishmaniasis caused by disseminated Leishmania amazonensis infection presenting with hepatitis and adenopathy. Trans. R. Soc. Trop. Med. Hyg. 100:79–82. 10.1016/j.trstmh.2005.06.025 [DOI] [PubMed] [Google Scholar]
- 41.Goldberg DE, Siliciano RF, Jacobs WR., Jr 2012. Outwitting evolution: fighting drug-resistant TB, malaria, and HIV. Cell 148:1271–1283. 10.1016/j.cell.2012.02.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Monzote L, Montalvo AM, Scull R, Miranda M, Abreu J. 2007. Combined effect of the essential oil from Chenopodium ambrosioides and antileishmanial drugs on promastigotes of Leishmania amazonensis. Rev. Inst. Med. Trop. Sao Paulo 49:257–260. 10.1590/S0036-46652007000400012 [DOI] [PubMed] [Google Scholar]
- 43.Corral MJ, González-Sánchez E, Cuquerella M, Alunda JM. 2014. In vitro synergistic effect of amphotericin B and allicin on Leishmania donovani and L. infantum. Antimicrob. Agents Chemother. 58:1596–1602. 10.1128/AAC.00710-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.de Morais-Teixeira E, Gallupo MK, Rodrigues LF, Romanha AJ, Rabello A. 2013. In vitro interaction between paromomycin sulphate and four drugs with leishmanicidal activity against three New World Leishmania species. J. Antimicrob. Chemother. 69:150–154. 10.1093/jac/dkt318 [DOI] [PubMed] [Google Scholar]
- 45.Wheeler RJ, Gluenz E, Gull K. 2011. The cell cycle of Leishmania: morphogenetic events and their implications for parasite biology. Mol. Microbiol. 79:647–662. 10.1111/j.1365-2958.2010.07479.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Havens CG, Bryant N, Asher L, Lamoreaux L, Perfetto S, Brendle JJ, Werbovetz KA. 2000. Cellular effects of leishmanial tubulin inhibitors on L. donovani. Mol. Biochem. Parasitol. 110:223–236. 10.1016/S0166-6851(00)00272-3 [DOI] [PubMed] [Google Scholar]
- 47.Sarkar A, Sen R, Saha P, Ganguly S, Mandal G, Chatterjee M. 2008. An ethanolic extract of leaves of Piper betle (Paan) Linn mediates its antileishmanial activity via apoptosis. Parasitol. Res. 102:1249–1255. 10.1007/s00436-008-0902-y [DOI] [PubMed] [Google Scholar]
- 48.Proto WR, Coombs GH, Mottram JC. 2013. Cell death in parasitic protozoa: regulated or incidental? Nat. Rev. Microbiol. 11:58–66. 10.1038/nrmicro2929 [DOI] [PubMed] [Google Scholar]
- 49.Benitez DA, Pozo-Guisado E, Alvarez-Barrientos A, Fernandez-Salguero PM, Castellón EA. 2007. Mechanisms involved in resveratrol-induced apoptosis and cell cycle arrest in prostate cancer-derived cell lines. J. Androl. 28:282–293. 10.2164/jandrol.106.000968 [DOI] [PubMed] [Google Scholar]
- 50.Li J, Qin Z, Liang Z. 2009. The prosurvival role of autophagy in resveratrol-induced cytotoxicity in human U251 glioma cells. BMC Cancer 9:215–224. 10.1186/1471-2407-9-215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thompson SN. 1991. Applications of nuclear magnetic resonance in parasitology. J. Parasitol. 77:1–20. 10.2307/3282548 [DOI] [PubMed] [Google Scholar]
- 52.Adosraku RK, Anderson MM, Anderson GJ, Choi G, Croft SL, Yardley V, Phillipson JD, Gibbons WA. 1993. Proton NMR lipid profile of Leishmania donovani promastigotes. Mol. Biochem. Parasitol. 62:251–262. 10.1016/0166-6851(93)90114-D [DOI] [PubMed] [Google Scholar]
- 53.O'Sullivan WJ, Edwards MR, Norton RS. 1989. The application of nuclear magnetic resonance spectroscopy to parasite metabolism. Parasitol. Today 5:79–82. 10.1016/0169-4758(89)90007-0 [DOI] [PubMed] [Google Scholar]
- 54.Van Assche T, Deschacht M, da Luz RA, Maes L, Cos P. 2011. Leishmania-macrophage interactions: insights into the redox biology. Free Radic. Biol. Med. 51:337–351. 10.1016/j.freeradbiomed.2011.05.011 [DOI] [PubMed] [Google Scholar]
- 55.Chung EY, Kim BH, Hong JT, Lee CK, Ahn B, Nam SY, Han SB, Kim Y. 2010. Resveratrol down-regulates interferon-γ-inducible inflammatory genes in macrophages: molecular mechanism via decreased STAT-1 activation. J. Nutr. Biochem. 22:902–909. 10.1016/j.jnutbio.2010.07.012 [DOI] [PubMed] [Google Scholar]
- 56.Hao E, Lang F, Chen Y, Zhang H, Cong X, Shen X, Su G. 2013. Resveratrol alleviates endotoxin-induced myocardial toxicity via the Nrf2 transcription factor. PLoS One 8:e69452. 10.1371/journal.pone.0069452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.He X, Wang L, Szklarz G, Bi Y, Ma Q. 2012. Resveratrol inhibits paraquat-induced oxidative stress and fibrogenic response by activating the nuclear factor erythroid 2-related factor 2 pathway. J. Pharmacol. Exp. Ther. 342:81–90. 10.1124/jpet.112.194142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Paiva CN, Feijó DF, Dutra FF, Carneiro VC, Freitas GB, Alves LS, Mesquita J, Fortes GB, Figueiredo RT, Souza HS, Fantappié MR, Lannes-Vieira J, Bozza MT. 2012. Oxidative stress fuels Trypanosoma cruzi infection in mice. J. Clin. Invest. 122:2531–2542. 10.1172/JCI58525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Reguera RM, Balaña-Fouce R, Showalter M, Hickerson S, Beverley SM. 2009. Leishmania major lacking arginase are auxotrophic for polyamines but retain infectivity to susceptible BALB/c mice. Mol. Biochem. Parasitol. 165:48–56. 10.1016/j.molbiopara.2009.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kropf P, Fuentes JM, Fähnrich E, Arpa L, Herath S, Weber V, Soler G, Celada A, Modolell M, Müller I. 2005. Arginase and polyamine synthesis are key factors in the regulation of experimental leishmaniasis in vivo. FASEB J. 19:1000–1002. 10.1096/fj.04-3416fje [DOI] [PubMed] [Google Scholar]
- 61.Suenaga F, Hatsushika K, Takano S, Ando T, Ohnuma Y, Ogawa H, Nakao A. 2008. A possible link between resveratrol and TGF-beta: resveratrol induction of TGF-beta expression and signaling. FEBS Lett. 582:586–590. 10.1016/j.febslet.2008.01.024 [DOI] [PubMed] [Google Scholar]
- 62.Cunningham M, Gilkeson G. 2011. Estrogen receptors in immunity and autoimmunity. Clin. Rev. Allergy Immunol. 40:66–73. 10.1007/s12016-010-8203-5 [DOI] [PubMed] [Google Scholar]