Abstract
For the first time, a population approach was used to describe abacavir (ABC) pharmacokinetics in HIV-infected pregnant and nonpregnant women. A total of 266 samples from 150 women were obtained. No covariate effect (from age, body weight, pregnancy, or gestational age) on ABC pharmacokinetics was found. Thus, it seems unnecessary to adapt the ABC dosing regimen during pregnancy.
TEXT
Abacavir (ABC) is a potent nucleoside reverse transcriptase inhibitor administered to treat human immunodeficiency virus (HIV) infection and prevent its transmission. Currently, regimens containing ABC are still recommended as one of the first-line options for adults, including pregnant women (1). ABC pharmacokinetics in adults has been investigated previously (2–9), but only two limited studies (with small numbers of patients, no once-daily administration, and narrow ranges of age and gestational age) were focused on pregnant women (10, 11). During pregnancy, some physiological changes can affect the pharmacokinetics of a drug. Therefore, it is important to characterize these changes in a large population in order to use the drug safely and efficiently during pregnancy. Thus, plasma ABC concentrations in nonpregnant and pregnant women were analyzed using a population approach for the first time. The population pharmacokinetics parameters in HIV-infected nonpregnant and pregnant women were estimated, and the final model was used to determine if the current recommended doses produce efficient drug exposure in pregnancy.
The HIV-infected pregnant (n = 36) and nonpregnant (n = 114) women had a median age of 35.7 years (range, 15 to 67 years) and a median body weight of 62.5 kg (range, 40 to 102 kg). The median gestational age at pharmacokinetics evaluation was 31 weeks (range, 9 to 41 weeks). A total of 266 samples were obtained from HIV-infected women. Among these samples, 16 were collected in pairs (maternal and cord blood samples). Women received orally an ABC-containing regimen: 300 mg twice daily (47.9%) or 600 mg once daily (52.1%). Blood samples for therapeutic-drug monitoring (TDM) were collected during a visit in the pharmacology unit of Hospital Cochin (Paris, France); therefore, the times elapsed between drug administration and sampling were variable. The pregnant women were enrolled at clinical sites of the ANRS-C01-French Perinatal Cohort (EPF). The mother of the child to be born provided signed informed consent. For the nonpregnant women, ethics committee approval and patient consent are not compulsory in France to use TDM data retrospectively. Plasma ABC concentrations were determined by high-performance liquid chromatography, as previously described (12). The limit of quantification (LOQ) was 0.02 mg/liter. The mean interassay precision for the lowest concentration of the quality controls was 10%. The data were analyzed using Nonmem software (version 6.2), and the first-order conditional estimation with interaction (FOCEI) method was applied (13). The 23 concentrations (8.6%) below the LOQ (BLQ) were replaced by the LOQ/2 value (14). The M3 method and the built-in M2 method were also tested for the BLQ data, but they did not improve the results (15). A one-compartment model with first-order absorption and elimination was used to describe the data. The between-subject variability (BSV) could be estimated only for the apparent clearance. The combined model was not significantly better than the proportional model, and the additive model was significantly worse than the combined model. Thus, the proportional error model was the best to describe the residual variability. The absorption constant (Ka) could not be well estimated, but the stability of the model was improved when the value was fixed to 1.8 h−1, a value reported for adults by Jullien et al. (4). No covariate (age, body weight, pregnancy, or gestational age) had a significant effect on pharmacokinetics, so pregnant women and nonpregnant women had the same median population clearance values. This finding is consistent with the previous studies' reporting similar exposures between postpartum or nonpregnant women and pregnant women (10, 11). The final population pharmacokinetics estimates are summarized in Table 1.
TABLE 1.
Population pharmacokinetics parameters of ABC from the final modela
| Pharmacokinetics parameter | Estimate | RSE | 95% CI |
|---|---|---|---|
| Ka (h−1) | 1.8** | ||
| CL/F (liter · h−1) | 41.3 | 4.5 | 34.2–42.4 |
| V/F (liters) | 119 | 5.8 | 91.9–123 |
| ωCL/F | 0.167 | 19.9 | 0.129–0.196 |
| σABC | 0.438 | 13.6 | 0.434–0.574 |
The relative standard error (RSE) is the standard error of the estimate divided by the estimate and multiplied by 100 (95% CI are derived from a bootstrap procedure); **, fixed value; CL/F, apparent clearance; V/F apparent volume of distribution; ωCL/F, square root of between-subject variance for the CL/F ratio; σABC, residual variability for ABC.
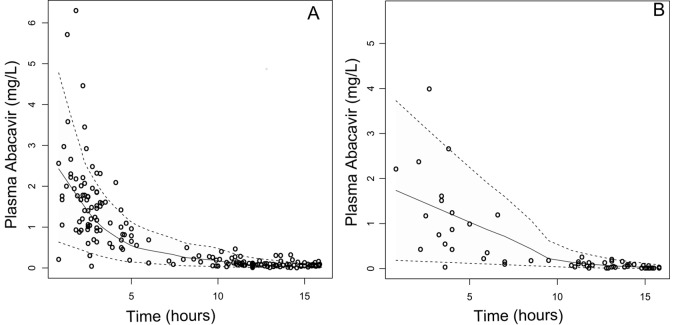
The stability of the model and accuracy of the parameters were assessed by a bootstrap method implemented in Wings for Nonmem (WfN; http://wfn.sourceforge.net/). This method involves repeated random resampling with replacement from the original data set for 1,000 times. The parameters and their associated BSV were accurately estimated, and the confidence intervals (CIs) derived from the bootstrap analysis were reasonably narrow and did not include zero. The model was evaluated by the visual predictive check (VPC) (16) and the normalized prediction distribution errors (NPDE) (17). As confirmed by the VPC in Fig. 1, the average prediction matched the observed concentrations and the variability was reasonably estimated. Moreover, the mean and the variance of the NPDE were not significantly different from 0 (P = 0.10) and 1 (P = 0.66) and the distribution was not different from a normal one (P = 0.53).
FIG 1.
Visual predictive check: comparison between the 5th (lower dashed line), 50th (solid line), and 95th (upper dashed line) percentiles obtained from 1,000 simulations and the observed plasma ABC concentrations (open circles) in pregnant women (A) and nonpregnant women (B). The model predictions and observations have been normalized to the median ABC dose, 600 mg.
The placental transfer during pregnancy was estimated as the median ratio of fetal-to-maternal concentrations at delivery. The median estimated value was 104% (minimum, 62%; maximum, 163%). This value is similar to those reported previously (103% and 106%) (11, 12). We have tried to establish a model to describe fetal concentrations by connecting a peripheral or an effect compartment. However, a successful convergence could not be obtained.
This study presents several limits. Pregnant and nonpregnant women could be included, allowing comparison of ABC pharmacokinetics between groups; however, the number of pregnant women (n = 36) was smaller than the number of nonpregnant women (n = 114). Moreover, the sparse sampling (1 or 2 samples per patient) could have led to a simplified model (a one-compartment model with a fixed Ka value), and these data from TDM resulted in high residual variability. However, the bootstrap, VPC, and NPDE procedures gave a satisfactory evaluation of the model.
To conclude, this is the first time that a population approach has been used to describe ABC pharmacokinetics in a large population of nonpregnant and pregnant women. No covariate was found to influence ABC pharmacokinetics, and therefore pregnant and nonpregnant women had similar pharmacokinetics. Thus, it seems unnecessary to adapt the ABC dosing regimen during pregnancy.
Footnotes
Published ahead of print 28 July 2014
REFERENCES
- 1.World Health Organization. 2013. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. World Health Organization, Geneva, Switzerland: [PubMed] [Google Scholar]
- 2.Zhao W, Cella M, Della Pasqua O, Burger D, Jacqz-Aigrain E, Pediatric European Network for Treatment of AIDS (PENTA) 15 Study Group 2012. Population pharmacokinetics and maximum a posteriori probability Bayesian estimator of abacavir: application of individualized therapy in HIV-infected infants and toddlers. Br. J. Clin. Pharmacol. 73:641–650. 10.1111/j.1365-2125.2011.04121.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Capparelli EV, Englund JA, Connor JD, Spector SA, McKinney RE, Palumbo P, Baker CJ. 2003. Population pharmacokinetics and pharmacodynamics of zidovudine in HIV-infected infants and children. J. Clin. Pharmacol. 43:133–140. 10.1177/0091270002239821 [DOI] [PubMed] [Google Scholar]
- 4.Jullien V, Tréluyer J-M, Chappuy H, Dimet J, Rey E, Dupin N, Salmon D, Pons G, Urien S. 2005. Weight related differences in the pharmacokinetics of abacavir in HIV-infected patients. Br. J. Clin. Pharmacol. 59:183–188. 10.1111/j.1365-2125.2004.02259.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moyle GJ, DeJesus E, Cahn P, Castillo SA, Zhao H, Gordon DN, Craig C, Scott TR, Ziagen Once-Daily in Antiretroviral Combination Therapy (CNA30021) Study Team 2005. Abacavir once or twice daily combined with once-daily lamivudine and efavirenz for the treatment of antiretroviral-naive HIV-infected adults: results of the Ziagen Once Daily in Antiretroviral Combination Study. J. Acquir. Immune Defic. Syndr. 38:417–425. 10.1097/01.qai.0000147521.34369.c9 [DOI] [PubMed] [Google Scholar]
- 6.DiCenzo R, Forrest A, Squires KE, Hammer SM, Fischl MA, Wu H, Cha R, Morse GD, Adult AIDS Clinical Trials Group Protocol 368/886 Study Team 2003. Indinavir, efavirenz, and abacavir pharmacokinetics in human immunodeficiency virus-infected subjects. Antimicrob. Agents Chemother. 47:1929–1935. 10.1128/AAC.47.6.1929-1935.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Staszewski S, Keiser P, Montaner J, Raffi F, Gathe J, Brotas V, Hicks C, Hammer SM, Cooper D, Johnson M, Tortell S, Cutrell A, Thorborn D, Isaacs R, Hetherington S, Steel H, Spreen W, CNAAB3005 International Study Team 2001. Abacavir-lamivudine-zidovudine vs indinavir-lamivudine-zidovudine in antiretroviral-naive HIV-infected adults: a randomized equivalence trial. JAMA 285:1155–1163. 10.1001/jama.285.9.1155 [DOI] [PubMed] [Google Scholar]
- 8.Weller S, Radomski KM, Lou Y, Stein DS. 2000. Population pharmacokinetics and pharmacodynamic modeling of abacavir (1592U89) from a dose-ranging, double-blind, randomized monotherapy trial with human immunodeficiency virus-infected subjects. Antimicrob. Agents Chemother. 44:2052–2060. 10.1128/AAC.44.8.2052-2060.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McDowell JA, Lou Y, Symonds WS, Stein DS. 2000. Multiple-dose pharmacokinetics and pharmacodynamics of abacavir alone and in combination with zidovudine in human immunodeficiency virus-infected adults. Antimicrob. Agents Chemother. 44:2061–2067. 10.1128/AAC.44.8.2061-2067.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mirochnick M, Capparelli E. 2004. Pharmacokinetics of antiretrovirals in pregnant women. Clin. Pharmacokinet. 43:1071–1087. 10.2165/00003088-200443150-00002 [DOI] [PubMed] [Google Scholar]
- 11.Best BM, Mirochnick M, Capparelli EV, Stek A, Burchett SK, Holland DT, Read JS, Smith E, Hu C, Spector SA, Connor JD, PACTG P1026s Study Team 2006. Impact of pregnancy on abacavir pharmacokinetics. AIDS 20:553–560. 10.1097/01.aids.0000210609.52836.d1 [DOI] [PubMed] [Google Scholar]
- 12.Chappuy H, Tréluyer J-M, Jullien V, Dimet J, Rey E, Fouché M, Firtion G, Pons G, Mandelbrot L. 2004. Maternal-fetal transfer and amniotic fluid accumulation of nucleoside analogue reverse transcriptase inhibitors in human immunodeficiency virus-infected pregnant women. Antimicrob. Agents Chemother. 48:4332–4336. 10.1128/AAC.48.11.4332-4336.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beal SL, Sheiner LB. 1998. NONMEM users guide, parts I to VIII. NONMEM Project Group, San Francisco, CA [Google Scholar]
- 14.Beal SL. 2001. Ways to fit a PK model with some data below the quantification limit. J. Pharmacokinet. Pharmacodyn. 28:481–504. 10.1023/A:1012299115260 [DOI] [PubMed] [Google Scholar]
- 15.Ahn JE, Karlsson MO, Dunne A, Ludden TM. 2008. Likelihood based approaches to handling data below the quantification limit using NONMEM VI. J. Pharmacokinet. Pharmacodyn. 35:401–421. 10.1007/s10928-008-9094-4 [DOI] [PubMed] [Google Scholar]
- 16.Post TM, Freijer JI, Ploeger BA, Danhof M. 2008. Extensions to the visual predictive check to facilitate model performance evaluation. J. Pharmacokinet. Pharmacodyn. 35:185–202. 10.1007/s10928-007-9081-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Comets E, Brendel K, Mentré F. 2008. Computing normalised prediction distribution errors to evaluate nonlinear mixed-effect models: the npde add-on package for R. Comput. Methods Programs Biomed. 90:154–166. 10.1016/j.cmpb.2007.12.002 [DOI] [PubMed] [Google Scholar]