Abstract
Posaconazole tablets, a new oral formulation of posaconazole, can be effective when given as antifungal prophylaxis to neutropenic patients at high risk for invasive fungal infection (e.g., those with acute myelogenous leukemia or myelodysplastic syndrome). Such effectiveness might be specifically important to patients with poor oral intake because of nausea, vomiting, or chemotherapy-associated mucositis. This was a prospective, global study in high-risk patients to characterize the pharmacokinetics and safety profile of posaconazole tablets and to identify the dose of posaconazole tablets that would provide exposure within a predefined range of exposures (steady-state average concentration [area under the concentration-time curve/24 h] of ≥500 ng/ml and ≤2,500 ng/ml in >90% of patients). The study evaluated two sequential dosing cohorts: 200 mg posaconazole once daily (n = 20) and 300 mg posaconazole once daily (n = 34) (both cohorts had a twice-daily loading dose on day 1) taken without regard to food intake during the neutropenic period for ≤28 days. The exposure target was reached (day 8) in 15 of 19 (79%) pharmacokinetic-evaluable patients taking 200 mg posaconazole once daily and in 31 of 32 (97%) patients taking 300 mg posaconazole once daily; 300 mg posaconazole once daily achieved the desired exposure target. Posaconazole tablets were generally well tolerated in high-risk neutropenic patients. (This study has been registered at ClinicalTrials.gov under registration no. NCT01777763.)
INTRODUCTION
Posaconazole tablets, a new oral formulation of posaconazole, were developed in response to the potential exposure limitations of the current oral suspension in some patients receiving posaconazole antifungal prophylaxis. The clinical development program of the posaconazole tablet included pharmacokinetics (PK) and safety studies in healthy volunteers. This is the first report of use of this new formulation as antifungal prophylaxis in hematology patients at high risk for invasive fungal infection (IFI) (i.e., those with neutropenia as a result of induction chemotherapy for acute myelogenous leukemia [AML] or myelodysplastic syndrome [MDS] with transition to leukemia).
The current commercial formulation, posaconazole oral suspension, has been effective and generally well tolerated as IFI prophylaxis when given to neutropenic patients after chemotherapy for AML or MDS. Previously, in a large, global, phase 3 controlled clinical trial of such patients, treatment with 200 mg posaconazole oral suspension three times daily resulted in fewer proven or probable IFIs (2% versus 8%; P < 0.001), lower incidences of invasive aspergillosis (1% versus 7%; P < 0.001), and prolonged survival (P = 0.04) than treatment with itraconazole or fluconazole (1). In a similar controlled study in immunosuppressed patients with graft-versus-host disease (GVHD) after hematopoietic stem cell transplantation, compared with fluconazole, posaconazole oral suspension effectively prevented invasive mold infections and reduced the related mortality rate (2).
Despite its effectiveness in the registration trials, the posaconazole oral suspension has practical limitations: it must be administered multiple times daily and must be taken with food (high-fat diet preferred) so that adequate systemic exposure can be achieved (3, 4). These limitations are particularly important in hematology patients eligible for antifungal prophylaxis, in whom chemotherapy-induced adverse effects, including severe nausea or vomiting, mucositis, and diarrhea, often make it difficult to ensure adequate food intake to optimize exposure of the posaconazole oral suspension (5–7). Until now, absorption could be enhanced in these patients if posaconazole doses were divided (200 mg four times daily) or if the drug was administered with a liquid nutritional supplement or an acidic drink such as ginger ale (4, 8). Even with these measures, target exposures in many patients could not be achieved; 25% of patients with neutropenia achieved steady-state plasma concentrations of <322 ng/ml when taking the recommended 200-mg dose of the posaconazole oral suspension three times a day for antifungal prophylaxis (9). Despite these limitations, the effectiveness of the posaconazole oral suspension as antifungal prophylaxis was clearly established in two large registration trials (1, 2) in which the steady-state average concentration (Cavg) was 582 ng/ml in neutropenic patients with AML or MDS (10) and 922 ng/ml in patients with GVHD (11). While an exposure-response relationship has been described in salvage treatment for invasive aspergillosis (12), no target plasma level was found in the prophylaxis setting. Suboptimal plasma drug concentrations remain an important area for improvement to maximize the clinical benefits of antifungal prophylaxis with posaconazole, especially in hematology patients at high risk for IFI.
The PK and safety of the new tablet formulation of posaconazole have been evaluated in single-dose and multiple-dose studies (200 mg/d to 400 mg/d) in healthy volunteers and has been reported in the literature (13, 14). The posaconazole tablet showed improved exposure with less variability than the posaconazole oral suspension in fasting healthy volunteers, and tablet-related drug exposure did not seem to be markedly affected by food (13). However, the posaconazole tablet has not been evaluated in the targeted patient population, including patients at risk for IFI. Consequently, the objectives of this study were to fully characterize the PK profile of posaconazole tablets, to evaluate the safety of posaconazole tablets in patients with AML or MDS, and to identify a dose of posaconazole tablets that would attain plasma exposure with a prespecified range. The PK parameter of particular interest was the steady-state Cavg of posaconazole (defined as the average area under the concentration-time curve from 0 to 24 h [AUC0–24 h] [in h · ng/ml]/time interval of 24 h) (9).
(This work was presented at the 52nd Interscience Conference on Antimicrobial Agents and Chemotherapy, San Francisco, CA, 9 to 12 September 2012.)
MATERIALS AND METHODS
Study design.
This was a global, open-label, uncontrolled, dose-escalation, prospective PK and safety study (ClinicalTrials.gov registration no. NCT01777763). The study was conducted in accordance with the principles of good clinical practice, and written informed consent was obtained from each patient before any study-related procedures were performed. The study was conducted at sites in North America and Europe that were experienced in the care and treatment of patients at high risk for IFI and that were capable of performing the extensive serial PK sampling necessary.
Patients were assigned to one of two sequential dosing cohorts (Fig. 1): the first cohort of 20 neutropenic patients received posaconazole tablets at 200 mg once daily (QD); after review of the findings, the second dosing cohort of 34 patients received posaconazole tablets at 300 mg QD. Both cohorts received posaconazole tablets twice daily (BID) (every 12 h) on day 1; thereafter, posaconazole tablet dosing was done QD anytime during the day. The posaconazole tablets were taken without regard to food intake for a maximum of 28 d; 100-mg posaconazole tablets were swallowed whole (two tablets for the 200-mg cohort and three tablets for the 300-mg cohort).
FIG 1.
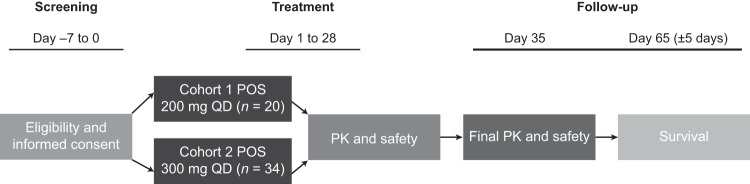
Study design phase 1B. Cohort 1 was completed before cohort 2 patients were administered the study drug. Twice-daily dosing (12 h apart) was given on day 1. For pharmacokinetics and safety, samples were taken on day 1 and day 8 (steady state) at 0 h (predose) and at 2, 4, 6, 8, 12, and 24 h after dose. PK, pharmacokinetics; POS, posaconazole; QD, once daily.
Patients.
Patients were enrolled after undergoing chemotherapy for AML or MDS. Patients had (or, in the opinion of the investigators, were likely to develop within 3 to 5 days) prolonged neutropenia (absolute neutrophil count of <500/mm3 [0.5 × 109/liter]) at baseline that was likely to last ≥7 days. Patients could be of either sex and of any race, were 18 years of age or older, weighed >34 kg, and could tolerate the administration of the oral tablet medication. Patients could not have a known or suspected IFI, a history of type 1 hypersensitivity or idiosyncratic reactions to azoles, moderate or severe liver dysfunction (defined as aspartate aminotransferase or alanine aminotransferase levels greater than three times the upper limit of normal [ULN], and a total bilirubin level greater than two times the ULN), or prolonged corrected QT (QTc) interval (>500 ms). Patients were not allowed to have taken posaconazole within the 10 days preceding study enrollment or undergone systemic antifungal therapy within 30 days of study enrollment for reasons other than antifungal prophylaxis. Patients were not allowed to have taken investigational drugs within 30 days, medications known to interact with azoles and possibly lead to life-threatening effects within 24 h (except astemizole, which could not have been taken within 10 days), or β-hydroxy-β-methylglutaryl-coenzyme A reductase inhibitors metabolized by the cytochrome P450 enzyme 3A4 within 24 h of the start of the study. Concomitant medications were monitored throughout the study.
PK sampling.
Blood samples were collected for PK analysis on day 1 and day 8 (steady state) at 0 h and at 2, 4, 6, 8, 12, and 24 h after dosing. On day 1, the 12-h blood sample was drawn before administration of the next posaconazole dose. Trough samples (minimum plasma concentration [Cmin]) were taken before dosing on days 1, 2, 3, 8, 14, 21, and 28 or at the end of treatment. Approximately 4 ml of blood was collected per time point into tubes containing EDTA, and samples were kept on ice or were refrigerated until centrifugation (within 2 h of collection) for 10 min at 1500 × g at ∼4°C. The plasma samples (two duplicate sets) were immediately frozen to at least −20°C and were maintained in the frozen state until analysis. Plasma samples were assayed for posaconazole at a central laboratory using a validated liquid chromatography coupled to tandem mass spectrometry detection method (15) with a calibration range of 5 to 5,000 ng/ml.
PK evaluations.
The primary PK parameter of interest was steady-state (day 8) Cavg. Other parameters assessed included the AUC during the dosing interval (the dosing interval was 12 h on day 1 [AUC0–12 h] and 24 h on day 8 [AUC0–24 h]), the maximum plasma concentration (Cmax) on days 1 and 8, and the time to Cmax (Tmax) on days 1 and 8.
The posaconazole plasma concentrations and PK parameters were listed and summarized by treatment group using descriptive statistics and graphics. The desired exposure targets were mean steady-state Cavg of ≥500 ng/ml (or mean steady-state AUC0–24 h of ≥12,000 h · ng/ml) in at least 90% of patients per PK-evaluable dosing cohort and a cohort mean steady-state Cavg of 1,200 ng/ml. Similarly, the desired exposure target was a mean steady-state Cavg of ≤2,500 ng/ml (or mean steady-state AUC0–24 h of ≤59,000 h · ng/ml) in at least 90% of patients per PK-evaluable dosing cohort.
Safety.
Safety assessments included adverse event (AE) reports (among these were treatment-related AEs, AEs leading to discontinuation of the study drug, serious AEs [SAEs], treatment-related SAEs, and reports of death), vital signs, clinical laboratory tests (including liver function tests), gastrointestinal (GI) tolerance, and electrocardiography (including incidence of QTc prolongation of >500 ms). Safety was assessed throughout the study until the follow-up visit (7 to 10 days after the last posaconazole dose [through days 35 to 38 of the study]); survival only was assessed at day 65 (±5 days). Safety analyses were descriptive and were summarized by dose level; all safety analyses were performed on the treated population.
RESULTS
Patients.
Fifty-four patients were enrolled in the two cohorts. Patients were treated at nine centers in four countries: United States (four centers), Canada (two centers), Spain (two centers), and Germany (one center). Twenty patients were enrolled in the 200-mg cohort; the mean age was 51 years, 60% were male, and 95% were white. Thirty-four patients were enrolled in the 300-mg cohort; the mean age was 56.5 years, 65% were male, and 97% were white. Patient demographics are shown in Table 1. In the 200-mg cohort, 13 (65%) patients had newly diagnosed AML, 4 (20%) had a first relapse of AML, and 3 (15%) had newly diagnosed MDS. In the 300-mg cohort, 27 (79%) patients had newly diagnosed AML, 5 (15%) had a first relapse of AML, and 2 (6%) had newly diagnosed MDS. All patients received intensive chemotherapy.
TABLE 1.
Patient demographics
| Characteristic | Results for patients receiving POSa tablets at the indicated dosage |
|
|---|---|---|
| 200 mg QD (n = 20)b | 300 mg QD (n = 34)b | |
| Age | ||
| Mean (SD) (yr) | 51.0 (13.2) | 56.5 (13.4) |
| ≥65 yr (no. [%]) | 3 (15) | 12 (35) |
| Sex (no. [%]) | ||
| Male | 12 (60) | 22 (65) |
| Female | 8 (40) | 12 (35) |
| Race (no. [%]) | ||
| White | 19 (95) | 33 (97) |
| Asian | 1 (5) | 0 |
| Native Hawaiian/other Pacific Islander | 0 | 1 (3) |
| Ethnicity (no. [%]) | ||
| Hispanic/Latino | 7 (35) | 1 (3) |
| Not Hispanic/not Latino | 13 (65) | 33 (97) |
| Wt (mean [SD]) (kg) | 76.0 (12.7) | 85.8 (18.7) |
| Ht (mean [SD]) (cm) | 169.37 (10.77) | 170.44 (9.57) |
| Primary diagnosis at study entry (no. [%]) | ||
| AMLc (new diagnosis) | 13 (65) | 27 (79) |
| AML (first relapse) | 4 (20) | 5 (15) |
| MDSd | 3 (15) | 2 (6) |
POS, posaconazole.
All patients received twice-daily dosing on day 1, followed by once-daily (QD) dosing on days 2 to 28 (or until the end of treatment).
AML, acute myelogenous leukemia.
MDS, myelodysplastic syndrome.
PK results.
Patients were considered evaluable for the PK analysis if they adhered to the study drug dosing and PK sampling through the steady-state PK (day 8) visit, had not received posaconazole in the 10 days preceding the study treatment, had a baseline posaconazole plasma concentration of <25 ng/ml, and did not receive concomitant nonstudy posaconazole. For the PK analysis on day 1, all 20 (100%) patients in the 200-mg cohort and 33 (97%) of 34 patients in the 300-mg cohort were considered evaluable; the day 1 PK data were not available for 1 patient in the 300-mg cohort (incomplete sampling). For the PK analysis at steady state (day 8), 19 (95%) of 20 patients in the 200-mg cohort and 32 (94%) of 34 patients in the 300-mg cohort were considered evaluable; the day 8 PK data were not available for 1 patient in the 200-mg cohort (not available) and for 2 patients in the 300-mg cohort (incomplete sampling). The day 1 PK parameters are summarized in Table 2, and the mean (standard deviation [SD]) plasma concentration profiles on day 1 are shown in Fig. 2A. The mean trough concentration before the third dose of posaconazole tablets on day 2 (the day 2 mean Cmin among patients in the 300-mg cohort was already greater than the lower exposure target of >500 ng/ml, indicating that, on average, the therapeutic target was achieved after just two doses of 300 mg of posaconazole tablets) (Fig. 2B). Furthermore, 90% of patients in the 300-mg cohort had a Cmin (24 h after day 1 dose) of >491 ng/ml (n = 32).
TABLE 2.
PK parameter values on day 1 after twice-daily (every 12 h) dosing and on day 8 after multiple once-daily dosing of posaconazole oral tablets to patients at high risk of IFIa
| Day and part | Dose (mg)b | No. of patients | Cmax (ng/ml)c | Tmax (h)d | Cmin (range) (ng/ml)e | AUCtau (h · ng/ml)f | Cavg (ng/ml)g | Accumulation ratioh | 500 ≤ Cavg ≤ 2,500 (no. [%])i |
|---|---|---|---|---|---|---|---|---|---|
| Day 1 | |||||||||
| 1A | 200 BID | 20 | 640 (33) | 3.1 (1.9–4.1) | 163–880 | 4,640 (34) | NAj | NA | NA |
| 1B | 300 BID | 33 | 836 (28) | 4.0 (1.78–8.08) | 440–1,590 | 6,240 (28) | NA | NA | NA |
| Day 8 | |||||||||
| 1A | 200 QD | 19k | 1,270 (49) | 4.9 (2.0–9.2) | 190–1,650 | 22,700 (51) | 951 (50) | 2.17 (60) | 15 (79) |
| 1B | 300 QD | 32l | 1,960 (33) | 2.21 (1.3–8.08) | 343–2,550 | 35,000 (41) | 1,460 (38) | 2.5 (37) | 31 (97) |
PK, pharmacokinetic; IFI, invasive fungal infection.
BID, twice daily; QD, once daily.
Cmax, maximum observed concentration.
Tmax, time of maximum observed concentration. Data are medians (ranges).
Cmin, minimum plasma concentration. Range of Cmin values for day 1 measured at 24 h after day 1 dosing (immediately prior to the third dose); range of Cmin values for day 8 measured at 24 h after day 8 dosing (on day 9).
AUCtau, area under the concentration-time curve during the dosing interval. The dosing intervals were 12 h and 24 h for day 1 and day 8, respectively.
Cavg, average concentration at steady state. Cavg = AUCtau/dosing interval.
The accumulation ratio was calculated as the ratio of Cmax between day 8 and day 1 because the dose was given BID on day 1 and AUC0–24 h was not calculated.
Geometric means of steady-state Cavg observed in healthy subjects were 1,250 and 2,020 ng/ml for 200 mg (QD) and 400 mg (QD), respectively. Based on a linear assumption, the interpolated Cavg in healthy subjects for 300-mg dose was 1,635 ng/ml.
NA, not assessed.
PK data were not available on day 8 for subject 12.
PK data for subjects 53 (days 1 and 8) and 38 (day 8) were excluded because of an incomplete PK profile.
FIG 2.
(A) Mean (SD) plasma concentration profiles (days 1 and 8) of posaconazole after multiple-dose oral administration of tablets to patients at high risk for IFI. (B) Mean (SD) trough plasma concentration profiles of posaconazole after multiple-dose oral administration of tablets to patients at high risk for IFI. BID, twice daily; IFI, invasive fungal infection; QD, once daily.
The day 8 (steady-state) posaconazole tablet PK parameters are summarized in Table 2, and mean (SD) plasma concentration profiles on day 8 are shown in Fig. 2B. The mean posaconazole Cavg values at day 8 (steady state) were 951 and 1,460 ng/ml for the 200-mg and 300-mg cohorts, respectively (Table 2); therefore, the desired exposure target of the mean steady-state Cavg of at least 1,200 ng/ml was achieved in the 300-mg cohort. A trend for increasing mean serum posaconazole levels at day 28 was observed in the 300-mg cohort; however, the variability around this trend was high (up to 58%), and the patient numbers were small (n = 14 to 35 in the 300-mg cohort). Steady state was attained generally between days 6 and 8 after the start of dosing (with BID dosing on day 1 only). In the 200-mg cohort, the desired exposure proportion of ≥90% patients achieving steady-state exposure of ≥500 ng/ml was not met: 4 (21%) of 19 patients had steady-state exposures between ≥350 and 500 ng/ml, and 15 (79%) of 19 patients attained Cavg of between 500 and 2,500 ng/ml at steady state. In this dosing cohort, no patient exceeded Cavg of 2,500 ng/ml.
The desired steady-state exposure target was attained in the 300-mg cohort: all patients treated with 300 mg QD (after BID dosing on day 1) had steady-state Cavg exposure of ≥500 ng/ml. Overall, 31 (97%) of 32 patients attained Cavg of ≥500 ng/ml and remained within the desired upper exposure limit of 2,500 ng/ml. Only one patient exceeded the desired exposure target of 2,500 ng/ml; this patient's steady-state Cavg was 2,680 ng/ml. The Tmax on average was 4 h after daily dosing; this value was similar to that previously reported for the posaconazole oral suspension. For each respective dosing cohort, the mean steady-state Cmax values were 1,270 ng/ml and 1,960 ng/ml for the posaconazole 200-mg and 300-mg cohorts, respectively.
Figure 3 displays the individual AUC0–24 h values at steady state for the posaconazole tablet 200-mg and 300-mg cohorts. These results are consistent with the steady-state Cavg results. In the 200-mg cohort, 15 (79%) of 19 patients attained the desired mean steady-state AUC0–24 h of ≥12,000 h · ng/ml and remained within the desired upper exposure limit of 59,000 h · ng/ml; 4 patients had a mean steady-state AUC0–24 h of <12,000 h · ng/ml. In the 300-mg cohort, 31 (97%) of 32 patients attained AUC0–24 h of ≥12,000 h · ng/ml and ≤59,000 h · ng/ml; the AUC0–24 h in 1 patient was 62,300 h · ng/ml.
FIG 3.

Individual day 8 AUC0–24 h values after multiple doses of posaconazole tablets to patients at high risk for IFI. Dotted lines represent the mean AUC0–24 h steady-state exposure. AUC0–24 h, area under the concentration-time curve from 0 to 24 h; IFI, invasive fungal infection.
Safety results.
As expected for this type of seriously ill patient population (patients with neutropenia after chemotherapy for new-onset AML, AML in first relapse, or MDS with transition to leukemia), all patients (100%) had at least one treatment-emergent AE (TEAE). TEAEs, treatment-related AEs, SAEs, treatment-related SAEs, AEs leading to early study discontinuation, and GI AEs leading to early study discontinuation are summarized in Table 3. GI TEAEs were the most common treatment-related TEAEs reported (25% of patients in the 200-mg cohort and 32% of patients in the 300-mg cohort). However, most patients with GI AEs did not prematurely discontinue the study drug; 1 (5%) patient in the 200-mg cohort and 4 (12%) patients in the 300-mg cohort discontinued the study drug early because of GI AEs. Overall, the early discontinuation of the study therapy because of AEs was reported in 3 (15%) of 20 patients in the 200-mg cohort and in 7 (21%) of 34 patients in the 300-mg cohort. The most commonly reported treatment-related TEAEs were diarrhea and rash (each in 3 [15%] patients) in the 200-mg cohort and diarrhea and vomiting (each in 4 [12%] patients) in the 300-mg cohort. One SAE (renal failure in 1 patient in the 200-mg cohort) was considered possibly related to treatment during the study. No safety concerns with respect to elevated liver function tests and no QTc prolongation longer than the preassigned maximum acceptable level (>500 ms) were reported.
TABLE 3.
Summary of AEs and most commonly reported (≥2 patients in either cohort) treatment-related TEAEsa
| AE | No. (%) of patients receiving POSb tablets |
|
|---|---|---|
| 200 mg QD (n = 20)c | 300 mg QD (n = 34)c | |
| Any TEAE | 20 (100) | 34 (100) |
| Treatment-related AE | 10 (50) | 14 (41) |
| SAEd | 6 (30) | 8 (24) |
| Treatment-related SAE | 1 (5) | 0 |
| Treatment-related GIe AE | 5 (25) | 11 (32) |
| AE leading to study discontinuation | 3 (15) | 7 (21) |
| GI AE leading to study discontinuation | 1 (5) | 4 (12) |
| Most commonly reported treatment-related AE | ||
| Diarrhea | 3 (15) | 4 (12) |
| Rash | 3 (15) | 1 (3) |
| Vomiting | 1 (5) | 4 (12) |
| Abdominal pain | 2 (10) | 3 (9) |
| Decreased appetite | 2 (10) | 0 |
| Hypokalemia | 2 (10) | 3 (9) |
| Nausea | 1 (5) | 3 (9) |
| Dyspepsia | 1 (5) | 2 (6) |
| Headache | 0 | 2 (6) |
| Hypophosphatemia | 1 (5) | 2 (6) |
AE, adverse event; TEAE, treatment-emergent AE.
POS, posaconazole.
All patients received twice-daily dosing on day 1, followed by once-daily (QD) dosing on days 2 to 28 (or until the end of treatment).
SAE, serious AE.
GI, gastrointestinal.
Invasive fungal infection.
Invasive fungal infections were reported in two patients, both in the 200-mg cohort. These two patients were enrolled consecutively at the same study center, and both were distinguishable by their lower posaconazole concentration levels. One patient was diagnosed with a proven Aspergillus infection involving the lungs and blood on day 22. The patient discontinued the study treatment on day 23. Before discontinuation, on day 8, this patient had a trough posaconazole concentration of 668 ng/ml at steady state. On the day of the study drug discontinuation (day 23), the patient had a posaconazole concentration of 261 ng/ml. A second patient in the 200-mg cohort received a diagnosis of invasive fusariosis involving a lung, the sinus, and blood on day 16 of study therapy. The patient was discontinued from the study on day 29. Throughout the course of the study therapy, this patient had a concentration of posaconazole in the blood that was below the target exposure level of 500 ng/ml. On day 8, Cmin was 335 ng/ml, and on day 23, the posaconazole concentration was 162 ng/ml. No further posaconazole concentration levels were available for this patient. No patients in the 300-mg cohort had proven or probable IFI during the study (treatment period or follow-up period).
Survival.
Eighteen (90%) of 20 patients treated in the 200-mg cohort and 32 (94%) of 34 patients treated in the 300-mg cohort were alive at the time of the final survival visit (day 65). In the 200-mg cohort, the two deaths were caused by pneumonia. In the 300-mg cohort, the deaths were caused by septic shock and cardiac arrest (one each). None of the deaths was considered treatment related.
DISCUSSION
In 2006, posaconazole, a broad-spectrum azole, was approved as an oral suspension formulation for the prophylaxis of IFI in patients at high risk for IFI, including leukemia patients with neutropenia after intensive myelosuppressive chemotherapy and patients with GVHD after hematopoietic stem cell transplantation. The efficacy and tolerability of posaconazole oral suspension were compared in two randomized clinical trials, and the control groups were treated with either fluconazole or itraconazole (1, 2). In head-to-head comparisons, posaconazole oral suspension formulation, when given to patients at high risk for IFI, showed a higher IFI protection rate than was seen in the fluconazole/itraconazole group (1, 2). Trial participants took the drug three times a day with food to achieve adequate absorption of posaconazole. Additional reports of therapeutic drug monitoring of posaconazole antifungal prophylaxis in patients with AML or MDS or in hematopoietic stem cell transplant recipients with GVHD have been published (16–18).
This is the first report of the use of the new formulation posaconazole tablet taken by patients at high risk for IFI. The aim of this study was to evaluate the PK, safety, and tolerability of posaconazole tablets in patients at risk for IFI and to identify a dose of posaconazole tablets that would help patients achieve prespecified exposure. The exposure target was selected based on the previous observation of a positive exposure-response relationship in the treatment of IFIs (12). A key fungal pathogen in antifungal prophylaxis studies is Aspergillus species. Based upon in vitro data, the posaconazole MIC for 90% of the isolates (MIC90) for Aspergillus species isolated from clinical infections was 0.5 μg/ml (500 ng/ml). Therefore, the dose selected for this study was required to achieve a minimum exposure target (Cavg) at a steady state of ≥500 ng/ml in the vast majority of subjects. The maximum desired exposure target was related to safety observations from prior clinical and preclinical studies with posaconazole. The clinical development program for the posaconazole tablet sought to maintain exposures within the upper limit of exposures achieved with previous formulations. Within the range of exposures studied with posaconazole oral suspension, no dose-limiting safety events have been identified in either patients or healthy volunteers enrolled in posaconazole oral suspension clinical trials.
This study was a detailed evaluation of the PK of posaconazole tablets at steady state and through a maximum duration of 28 days of dosing. The exposure profile of posaconazole tablets taken by patients with AML or MDS seems to be slightly lower than that of published exposures in healthy volunteers (Table 4). For example, the steady-state Cavg (% coefficient of variation [CV]) for posaconazole tablets at 200 mg QD taken in a fasting state is 1,310 ng/ml (CV, 32%) in healthy subjects, compared with a steady-state Cavg of 981 ng/ml (CV, 48%) when 200 mg of posaconazole tablets is taken without regard to food intake in patients (14). In comparison, in healthy volunteers, the posaconazole oral suspension at 400 mg BID taken with a high-fat meal provided a steady-state Cavg exposure of 2,460 ng/ml, and, in patients, a similar dose of posaconazole oral suspension of 400 mg BID taken with food resulted in a steady-state Cavg of 723 ng/ml (CV, 86%) (19). Patient exposures to posaconazole oral suspension, in contrast to posaconazole tablets, are much reduced compared with those of healthy volunteers. Furthermore, the variability in patient exposure is greater with the posaconazole oral suspension than with the posaconazole tablet (19, 20).
TABLE 4.
Exposure of posaconazole oral suspension at 400 mg BID (with food) and posaconazole tablets at 400 mg QD (without food) in healthy volunteers versus study patientsa
| POSb formulation | POS dose (mg) | Study population | No. of patients | Cmax (ng/ml)c | AUC0–24 h, (h · ng/ml)d | Cavg (ng/ml)e |
|---|---|---|---|---|---|---|
| Oral suspension | 400 BID | Healthy subjects (reference)f | 174 | 2,850 (36) | 59,000 (37) | 2,460 (37) |
| Oral tablet | 200 QD | Healthy subjects (14) | 8 | 1,800 (31) | 31,400 (32) | 1,310 (32) |
| 400 QD | Healthy subjects (14) | 8 | 2,940 (46) | 56,600 (54) | 2,360 (54)g | |
| 200 QD | Patients (this study) | 18 | 1,310 (47) | 23,500 (49) | 981 (48) | |
| 300 QD | Patients (this study) | 33 | 1,930 (32) | 34,300 (36) | 1,440 (36) |
BID, twice daily; QD, once daily.
POS, posaconazole.
Cmax, maximum observed concentration.
AUC0–24 h, area under the concentration-time curve from 0 to 24 h.
Cavg, average concentration at steady state.
Pooled data on file.
Based on linear assumption, the interpolated Cavg in healthy subjects with tablets for a 300-mg dose is 1,835 ng/ml.
Compared with the oral suspension, the tablet formulation might have enhanced benefits. The posaconazole oral suspension is effective and generally well tolerated as antifungal prophylaxis in patients at high risk for IFIs; however, the need for multiple daily dosing and the requirement of taking it with food to enhance absorption are limitations (10, 21). The results of this study suggest that food may not affect the administration of the posaconazole tablet formulation, which may be particularly important for patients undergoing chemotherapy for AML or MDS. This is also consistent with a previous study indicating that administration of posaconazole tablets with a high-fat meal caused a 16% increase in Cmax and a 51% increase in AUC compared with administration in the fasted state (21). The effect of food was therefore much reduced compared with that for the oral suspension, administration of which with a nutritional supplement caused a 3-fold increase in exposure (21). In the present study, the posaconazole tablet was taken without regard for food intake and thus was administered under various food conditions.
A relationship between the plasma concentrations of an antifungal agent and effectiveness can often be determined based on a combination of fungal infection experimental models and assessments of pharmacodynamics and PK in animals and in clinical settings. Although a threshold plasma level for the prevention of breakthrough IFI has not been defined for posaconazole (11, 22), posaconazole plasma levels might be important to maintain the effectiveness of treatment and prophylaxis (9, 12). A steady-state target posaconazole plasma level of 500 ng/ml was used in a trial of patients with compromised GI function who were at high risk for IFI (21). Overall, in the current study of posaconazole tablets, 31 (97%) of 32 patients receiving posaconazole tablets at 300 mg QD (after BID dosing on day 1) attained the prespecified Cavg target of ≥500 ng/ml and remained below the desired upper exposure limit of 2,500 ng/ml. The mean posaconazole concentrations in patients at risk for IFI exceeded the target of 500 ng/ml after only 2 doses of 300 mg of the posaconazole tablets (i.e., within 24 h).
The targeted patient population enrolled in the current study was representative of the type of patient at risk for serious IFIs (who were therefore candidates to receive antifungal prophylaxis) and was demographically similar to the population previously reported for the posaconazole oral suspension. In the 300-mg cohort for posaconazole tablets, all patients reported at least 1 TEAE, and 41% of patients reported a treatment-related TEAE. SAEs were reported in 24% of patients; however, none was considered related to the study drug. The most commonly reported treatment-related TEAEs (reported in ≥9% of patients) in the 300-mg cohort were diarrhea (12%), vomiting (12%), abdominal pain (9%), nausea (9%), and hypokalemia (9%). The AE profile in the present study was generally consistent with that of other studies of the tablet formulation, which reported diarrhea, pyrexia, and nausea as the most common AEs (occurring in >25% of patients) and nausea as the most common AE leading to treatment discontinuation (21). This was also similar to the AE profile of the oral suspension when given as prophylaxis for Aspergillus and Candida infection in which fever, diarrhea, and nausea were reported in >30% of patients, and common AEs leading to treatment discontinuation were nausea, vomiting, and raised hepatic enzymes (1, 2, 21). Overall, the types of adverse reactions reported for posaconazole tablets were considered generally similar to those reported in trials of the posaconazole oral suspension (21).
There were no new safety concerns in the patients in this study. Posaconazole tablets were generally well tolerated, and the safety profile was similar to that previously noted for the posaconazole oral suspension in patients at high risk for IFI (1, 2). Although the experience in the current study is limited, no patient in the 300-mg cohort had a proven or probable IFI during the treatment or follow-up period. In two patients in the 200-mg cohort, IFIs were diagnosed during treatment; both patients had posaconazole concentrations of <500 ng/ml at the time of diagnosis.
Limitations of the current study were that the number of patients was small and the focus was on patients at high risk for IFI (i.e., patients with severe neutropenia after chemotherapy for new-onset AML or MDS with transition to leukemia). Posaconazole tablets continue to be evaluated to obtain further clinical experience and studied in more diverse patient populations who might benefit from the drug.
This new tablet formulation of posaconazole has the potential to predictably and substantially extend the antifungal benefit of this broad-spectrum azole because of its improved absorption characteristics; 300 mg is the dose of choice for further evaluation. The findings of this first-in-patient study will be further evaluated in a larger, more diverse patient population at risk for IFI.
ACKNOWLEDGMENTS
Medical writing and editorial assistance was provided by Sheena Hunt, Ph.D., Susan Quiñones, Ph.D., and Denise Balog, Pharm.D., of ApotheCom, Yardley, PA. This assistance was funded by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Whitehouse Station, NJ. This study was supported by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Whitehouse Station, NJ. Merck provided posaconazole tablets to conduct the study.
We acknowledge the following conflicts of interest: R.F.D. has received a grant from Schering-Plough, is a board member of Genzyme, Merck, Sanofi Oncology, and Schering-Plough, and has received payment for lectures/speaker bureaus from Amgen, Bristol Myers-Squibb, Celgene, Esteve, Genzyme, Gilead Sciences, Merck, Novartis, Pfizer, and Schering-Plough. J.L.-J. reports no conflicts of interest. O.A.C. is a consultant for 3M, Astellas, Basilea, Cubist, F2G, Gilead, GSK, Merck, Optimer, Pfizer, and Sanofi Pasteur, has received research grants from the European Union, German Federal Ministry of Research and Education, 3M, Actelion, Astellas, Basilea, Bayer, Celgene, Cubist, F2G, Genzyme, Gilead, GSK, Merck/MSD, Miltenyi, Optimer, Pfizer, Quintiles, and Viropharma, has received personal fees from Actelion, Astellas, Basilea, F2G, Gilead, Merck/MSD, Optimer, Pfizer, and Sanofi Pasteur, and has served on the speaker bureaus of Astellas, Gilead, Merck/MSD, and Pfizer. M.L. has received honoraria, grant funds, and consultancy fees from Astellas Pharma, Merck Canada Inc., Optimer, and Pfizer. S.H. has served as a consultant for Merck Pharmaceuticals and has served on advisory boards for Merck Canada. P.C. has received payment for speaker bureaus from Astellas and Pfizer and has received research grants from Merck and Pfizer. A.L. has received payment to conduct research for Merck. J.P. has received research grants from Astellas, Merck, and Pfizer and has received payment for consulting with Astellas, F2G, Merck, and Viamet. L.M., M.L.P.S.V.I., N.C., N.K., and H.W. are Merck employees and own stock in Merck.
We all critically reviewed and revised the manuscript and approved the final manuscript. R.F.D., J.L.-J., O.A.C., M.L., D.H., S.H., P.C., A.L., and J.P. were study investigators. L.M., M.L.P.S.V.I., and N.C. were involved with study management and data analysis. N.K. assisted in the analysis and interpretation of the data. H.W. was involved with study management, data analysis, and writing of the manuscript.
Footnotes
Published ahead of print 21 July 2014
REFERENCES
- 1.Cornely OA, Maertens J, Winston DJ, Perfect J, Ullmann AH, Walsh TJ, Helfgott D, Holowiecki J, Stockelberg D, Goh YT, Petrini M, Hardalo C, Suresh R, Angulo-Gonzalez D. 2007. Posaconazole vs. fluconazole or itraconazole prophylaxis in patients with neutropenia. N. Engl. J. Med. 356:348–359. 10.1056/NEJMoa061094 [DOI] [PubMed] [Google Scholar]
- 2.Ullmann AJ, Lipton JH, Vesole DH, Chandrasekar P, Langston A, Tarantolo SR, Greinix H, Morais de Azevedo W, Reddy V, Boparai N, Pedicone L, Patino H, Durrant S. 2007. Posaconazole or fluconazole for prophylaxis in severe graft-versus-host disease. N. Engl. J. Med. 356:335–347. 10.1056/NEJMoa061098 [DOI] [PubMed] [Google Scholar]
- 3.Courtney R, Wexler D, Radwanski E, Lim J, Laughlin M. 2003. Effect of food on the relative bioavailability of two oral formulations of posaconazole in healthy adults. Br. J. Clin. Pharmacol. 57:218–222. 10.1046/j.1365-2125.2003.01977.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krishna G, Moton A, Ma L, Medlock MM, McLeod J. 2009. The pharmacokinetics and absorption of posaconazole oral suspension under various gastric conditions in healthy volunteers. Antimicrob. Agents Chemother. 53:958–966. 10.1128/AAC.01034-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pille S, Bohmer D. 1998. Options for artificial nutrition of cancer patients. Strahlenther. Onkol. 174(suppl 3):52–55 [PubMed] [Google Scholar]
- 6.Sansone-Parsons A, Krishna G, Calzetta A, Wexler D, Kantesaria B, Rosenberg MA, Saltzman MA. 2006. Effect of a nutritional supplement on posaconazole pharmacokinetics following oral administration to healthy volunteers. Antimicrob. Agents Chemother. 50:1881–1883. 10.1128/AAC.50.5.1881-1883.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vehreschild MJ, Meissner AM, Cornely OA, Maschmeyer G, Neumann S, von Lilienfeld-Toal M, Karthaus M, Wattad M, Staib P, Hellmich M, Christ H, Vehreschild JJ. 2011. Clinically defined chemotherapy-associated bowel syndrome predicts severe complications and death in cancer patients. Haematologica 96:1855–1860. 10.3324/haematol.2011.049627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krishna G, Ma L, Vickery D, Yu X, Wu I, Power E, Beresford E, Komjathy S. 2009. Effect of varying amounts of a liquid nutritional supplement on the pharmacokinetics of posaconazole in healthy volunteers. Antimicrob. Agents Chemother. 53:4749–4752. 10.1128/AAC.00889-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jang SH, Colangelo PM, Gobburu JV. 2010. Exposure-response of posaconazole used for prophylaxis against invasive fungal infections: evaluating the need to adjust doses based on drug concentrations in plasma. Clin. Pharmacol. Ther. 88:115–119. 10.1038/clpt.2010.64 [DOI] [PubMed] [Google Scholar]
- 10.Krishna G, Abu Tarif M, Xuan F, Martinho M, Angulo D, Cornely OA. 2008. Pharmacokinetics of oral posaconazole in neutropenic patients receiving chemotherapy for acute myelogenous leukemia or myelodysplastic syndrome. Pharmacotherapy 28:1223–1232. 10.1592/phco.28.10.1223 [DOI] [PubMed] [Google Scholar]
- 11.Krishna G, Martinho M, Chandrasekar P, Ullmann AJ, Patino H. 2007. Pharmacokinetics of oral posaconazole in allogeneic hematopoietic stem cell transplant recipients with graft-versus-host disease. Pharmacotherapy 27:1627–1636. 10.1592/phco.27.12.1627 [DOI] [PubMed] [Google Scholar]
- 12.Walsh TJ, Raad I, Patterson TF, Chandrasekar P, Donowitz GR, Graybill R, Greene RE, Hachem R, Hadley S, Herbrecht R, Langston A, Louie A, Ribaud P, Segal BH, Stevens DA, van Burik JA, White CS, Corcoran G, Gogate J, Krishna G, Pedicone L, Hardalo C, Perfect JR. 2007. Treatment of invasive aspergillosis with posaconazole in patients who are refractory to or intolerant of conventional therapy: an externally controlled trial. Clin. Infect. Dis. 44:2–12. 10.1086/508774 [DOI] [PubMed] [Google Scholar]
- 13.Krishna G, Ma L, Martinho M, O'Mara E. 2012. A single dose phase I study to evaluate the pharmacokinetics of posaconazole new tablet and capsule formulations relative to oral suspension. Antimicrob. Agents Chemother. 56:4196–4201. 10.1128/AAC.00222-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krishna G, Ma L, Martinho M, Preston RA, O'Mara E. 2012. A new solid oral tablet formulation of posaconazole: a randomized clinical trial to investigate rising single- and multiple-dose pharmacokinetics and safety in healthy volunteers. J. Antimicrob. Chemother. 67:2725–2730. 10.1093/jac/dks268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shen JX, Krishna G, Hayes RN. 2007. A sensitive liquid chromatography and mass spectrometry method for the determination of posaconazole in human plasma. J. Pharm. Biomed. Anal. 43:228–236. 10.1016/j.jpba.2006.06.011 [DOI] [PubMed] [Google Scholar]
- 16.Andes D, Pascual A, Marchetti O. 2009. Antifungal therapeutic drug monitoring: established and emerging indications. Antimicrob. Agents Chemother. 53:24–34. 10.1128/AAC.00705-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dolton MJ, Ray JE, Marriott D, McLachlan AJ. 2012. Posaconazole exposure-response relationship: evaluating the utility of therapeutic drug monitoring. Antimicrob. Agents Chemother. 56:2806–2813. 10.1128/AAC.05900-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tonini J, Thiebaut A, Jourdil JF, Berruyer AS, Bulabois CE, Cahn JY, Stanke-Labesque F. 2012. Therapeutic drug monitoring of posaconazole in allogeneic hematopoietic stem cell transplantation patients who develop gastrointestinal graft-versus-host disease. Antimicrob. Agents Chemother. 56:5247–5252. 10.1128/AAC.00815-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Merck & Co. Inc. 2013. Noxafil (posaconazole) oral suspension prescribing information. Merck & Co. Inc., Whitehouse Station, NJ [Google Scholar]
- 20.MSD 2013. Noxafil (posaconazole) oral suspension summary of product characteristics. MSD, Hertfordshire, United Kingdom [Google Scholar]
- 21.Cornely OA, Helfgott D, Langston A, Heinz W, Vehreschild JJ, Vehreschild MJ, Krishna G, Ma L, Huyck S, McCarthy MC. 2012. Pharmacokinetics of different dosing strategies of oral posaconazole in patients with compromised gastrointestinal function and who are at high risk for invasive fungal infection. Antimicrob. Agents Chemother. 56:2652–2658. 10.1128/AAC.05937-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cornely OA, Ullmann AJ. 2011. Lack of evidence for exposure-response relationship of posaconazole used for prophylaxis against invasive fungal infections. Clin. Pharmacol. Ther. 89:351–352. 10.1038/clpt.2010.261 [DOI] [PubMed] [Google Scholar]



