Abstract
The whole-genome sequence of a carbapenem-resistant Klebsiella pneumoniae strain, PittNDM01, which coproduces NDM-1 and OXA-232 carbapenemases, was determined in this study. The use of single-molecule, real-time (SMRT) sequencing provided a closed genome in a single sequencing run. K. pneumoniae PittNDM01 has a single chromosome of 5,348,284 bp and four plasmids: pPKPN1 (283,371 bp), pPKPN2 (103,694 bp), pPKPN3 (70,814 bp), and pPKPN4 (6,141 bp). The contents of the chromosome were similar to that of the K. pneumoniae reference genome strain MGH 78578, with the exception of a large inversion spanning 23.3% of the chromosome. In contrast, three of the four plasmids are unique. The plasmid pPKPN1, an IncHI1B-like plasmid, carries the blaNDM-1, armA, and qnrB1 genes, along with tellurium and mercury resistance operons. blaNDM-1 is carried on a unique structure in which Tn125 is further bracketed by IS26 downstream of a class 1 integron. The IncFIA-like plasmid pPKPN3 also carries an array of resistance elements, including blaCTX-M-15 and a mercury resistance operon. The ColE-type plasmid pPKPN4 carrying blaOXA-232 is identical to a plasmid previously reported from France. SMRT sequencing was useful in resolving the complex bacterial genomic structures in the de novo assemblies.
INTRODUCTION
Carbapenemase-producing Enterobacteriaceae have been a major global public health problem since the late 2000s. Among the various carbapenemases that have been identified to date, the three enzyme groups underpinning the expansion are Klebsiella pneumoniae carbapenemases (KPCs), the metallo-β-lactamase NDM-1, and the oxacillinase OXA-48, as well as their variants (1). Among them, NDM-1 was initially reported in 2009 in a K. pneumoniae strain from a Swedish patient who had traveled from India (2), and then it rapidly spread worldwide in the following years (3). It is now apparent that NDM-1-producing Enterobacteriaceae are endemic in the Indian subcontinent. The blaNDM-1 gene has been identified on multidrug resistance plasmids belonging to various incompatibility groups, often accompanied by extended-spectrum β-lactamases (ESBL) or plasmid-mediated class C β-lactamase genes (e.g., blaCTX-M-15 and blaCMY-2) and aminoglycoside resistance 16S rRNA methyltransferase genes (e.g., armA and rmtC) (3). OXA-48 was initially identified in 2004 from a K. pneumoniae strain in Turkey (4). Besides Turkey, blaOXA-48 appears to have spread primarily in K. pneumoniae strains in the Middle East and North Africa (5). On the Indian subcontinent, where NDM-1 is endemic, variants of OXA-48, including OXA-181 and OXA-232, also seem to be spreading among Enterobacteriaceae (6, 7). As a consequence of the rapid spread of these two groups of carbapenemases, the coproduction of NDM-1 and OXA-48 group carbapenemases is also increasingly reported in Enterobacteriaceae (8–16). However, the genetics supporting the coacquisition of multiple carbapenemase genes have not been elucidated.
We recently described a K. pneumoniae strain coproducing NDM-1 and OXA-232 that was identified from an inpatient at our hospital (17). The aim of this study was to conduct a whole-genome assembly using a single-molecule, real-time (SMRT) sequencing technology to characterize the genomic structure of this strain, with a focus on the antimicrobial resistance determinants, including these carbapenemase genes.
MATERIALS AND METHODS
Bacterial strain.
K. pneumoniae strain PittNDM01 coproducing NDM-1 and OXA-232 was isolated from an inpatient at a hospital in Pittsburgh, PA, in March 2013 (17). In brief, this patient had been transferred from a hospital in India a month earlier for continuing care for a subarachnoid hemorrhage. PittNDM01 was isolated from a urine sample of the patient when she was hospitalized for fever. OXA-232 is a variant of the OXA-48 carbapenemase, which was recently identified from clinical isolates in India (6). The strain belongs to sequence type 14 (ST14) and was found to be resistant to all β-lactams, including carbapenems, aminoglycosides, and fluoroquinolones, but it remained susceptible to fosfomycin and colistin.
Library preparation and sequencing.
Genomic DNA from K. pneumoniae PittNDM01 was isolated from 3 ml of lysogenic broth culture using the DNeasy blood and tissue kit (Qiagen, Valencia, CA). Genomic DNA was then sheared to fragments of approximately 10 to 15 kb using ag-TUBE (Covaris, Woburn, MA). SMRTbell sequencing libraries were made as suggested by the manufacturer (Pacific Biosciences, Menlo Park, CA). Briefly, fragmented DNA underwent DNA damage repair, end repair, and ligation to hairpin adapters. Following exonuclease treatment, the SMRTbell library was size selected by BluePippin (Sage Science, Beverly, MA) to retain SMRTbell templates of <5 kb. The SMRTbell libraries were sequenced on the PacBio RS II instrument (Pacific Biosciences) using the recommended protocol for large-insert libraries. The sequence was obtained from a total of 4 SMRT cells using P4-C2 sequencing chemistry, which resulted in an average depth of sequencing coverage of approximately 250-fold. At this coverage, a base-calling accuracy of >99.999% is expected (18).
Genome assembly and annotation.
The genome was assembled de novo using the hierarchical genome assembly process (HGAP) (19). The assembled sequence was polished using Quiver to obtain a high consensus accuracy (19). One sequence gap was closed manually using the Bridge Mapper tool that is part of the SMRT Analysis software package (version 2.1). With the input of the raw sequence reads, HGAP directly assembled and closed the chromosome and the two largest plasmids (pPKPN1 and pPKPN2). The third plasmid (pPKPN3) did not initially assemble as a closed circular DNA with HGAP but was subsequently closed into a circular plasmid by using the initially unmapped reads and resequencing in silico for consensus building by Bridge Mapper. The fourth plasmid (pPKPN4) was initially not detected by HGAP, likely because the pipeline primarily utilizes long reads (>10 kb) to build the consensus sequences. However, pPKPN4 was identified and circularized with the input of the blaOXA-232 sequence, which was known from our earlier work to exist on the PittNDM01 genome (17). No gap-filling procedures were required.
The following custom primers were designed and used to confirm the chromosomal inversion by PCR and sequencing: KPN-NDM-end3-F (5′-CACCACCAGAGACGCTACAA-3′), KPN-NDM-end3-R (5′-TCCGGTGCAGATCTGTTATG-3′), KPN-NDM-end5-F (5′-CTCGCCTGGCAGACAAGT-3′), KPN-NDM-end5-R (5′-GTTCTTCATCCATGGCCTGT-3′), KPN-NDM-endpre3-F (5′-CAGATGGCTCCGGATATGAT-3′), and KPN-NDM-endpre3-R (5′-AGAACCAGATCCGCTCACAC-3′).
Annotation was performed with RAST (http://rast.nmpdr.org) and the NCBI Prokaryotic Genome Annotation Pipeline. The sequences were deposited in GenBank under accession no. CP006798 to CP006802. The sequencing project was assigned BioProject no. PRJNA221868 and ID 221868. The DNA methylation data of the K. pneumoniae PittNDM01 strain were captured in the process of SMRT sequencing, as previously described (20), and were deposited to the restriction enzyme database (REBASE) (21).
Phylogenetic analysis.
Phylogenomic analysis of PittNDM01 was performed as previously described (22) by aligning the whole genome of PittNDM01 with the whole genomes of other representative Klebsiella and Enterobacter strains available in the public domain. The genomic data were aligned using Mugsy (23), and the homologous blocks from each genome that aligned were concatenated with the bx-python tool kit (https://bitbucket.org/james_taylor/bx-python). A maximum-likelihood phylogeny with 100 bootstrap replicates was generated for the concatenated blocks of the aligned sequence using RAxML version 7.2.8 (24) and was visualized using FigTree version 1.3.1 (http://tree.bio.ed.ac.uk/software/figtree/).
RESULTS
Overall features of the genome.
K. pneumoniae PittNDM01 has a single chromosome of 5,348,284 bp and four plasmids: pPKPN1 (283,371 bp), pPKPN2 (103,694 bp), pPKPN3 (70,814 bp), and pPKPN4 (6,141 bp). The chromosome has a G+C content of 57.5%, with a total of 4,940 predicted open reading frames identified by the NCBI Prokaryotic Genome Annotation Pipeline. It shares 99% identity with the chromosome of the reference genome K. pneumoniae strain MGH 78578 (GenBank accession no. CP000647), with a large inverted region spanning 23.3% of the entire chromosome (Fig. 1). This inversion was confirmed with PCR and sequencing using custom primers designed to cover the positions at which it occurred.
FIG 1.
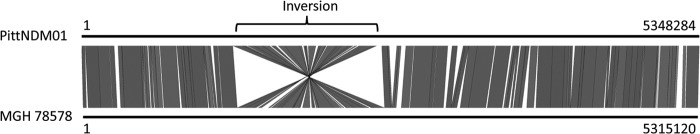
BLAST comparison results for the chromosomes of K. pneumoniae strains PittNDM01 and MGH 78578. A large inversion was identified and verified by PCR in PittNDM01 in comparison with MGH 78578, covering 23.3% of the chromosome.
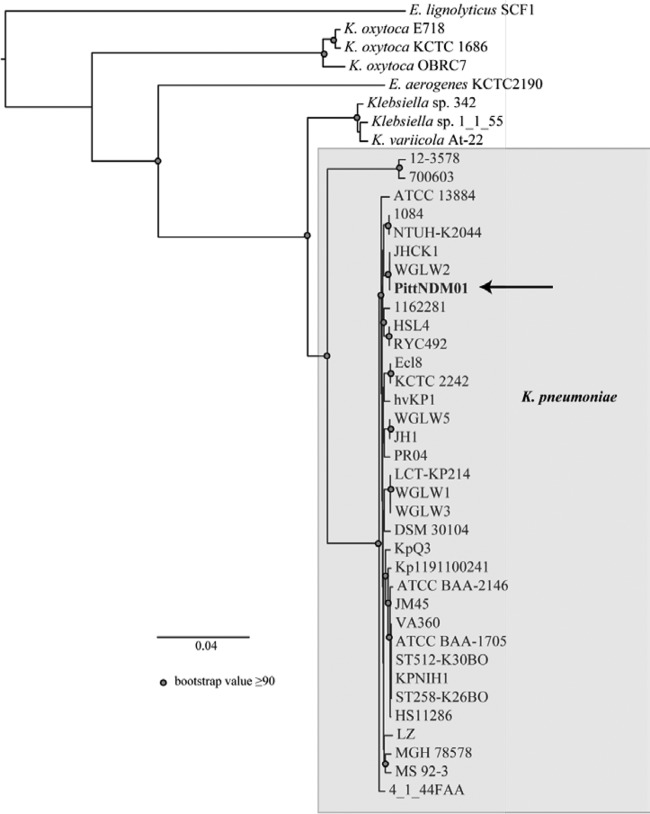
A phylogenetic comparison of the PittNDM01 strain with other sequenced K. pneumoniae strains and closely related species identified that this strain is highly similar to other K. pneumoniae strains (Fig. 2). This measure of genome similarity did not take into account the inversion identified above.
FIG 2.
Whole-genome phylogeny of K. pneumoniae PittNDM01 relative to other Klebsiella strains. Bootstrap values of ≥50 are shown. The scale bar indicates the distance of 0.04 nucleotide substitutions per site. The tree demonstrates that the core genome of K. pneumoniae PittNDM01 is similar to that of other K. pneumoniae isolates that have been sequenced.
The antimicrobial resistance genes identified on the chromosome include the β-lactamase genes blaSHV-28 and blaOXA-1, the aminoglycoside and fluoroquinolone acetyltransferase gene aac(6′)-Ib-cr, the chloramphenicol acetyltransferase gene catB4, the macrolide efflux genes macA and macB, and the fosfomycin resistance gene fosA. blaSHV, catB4, macA, macB, and fosA are frequently present on the K. pneumoniae chromosomes (GenBank accession no. CP000647) (25, 26).
IncHI1B-like plasmid pKPN1 carrying blaNDM-1.
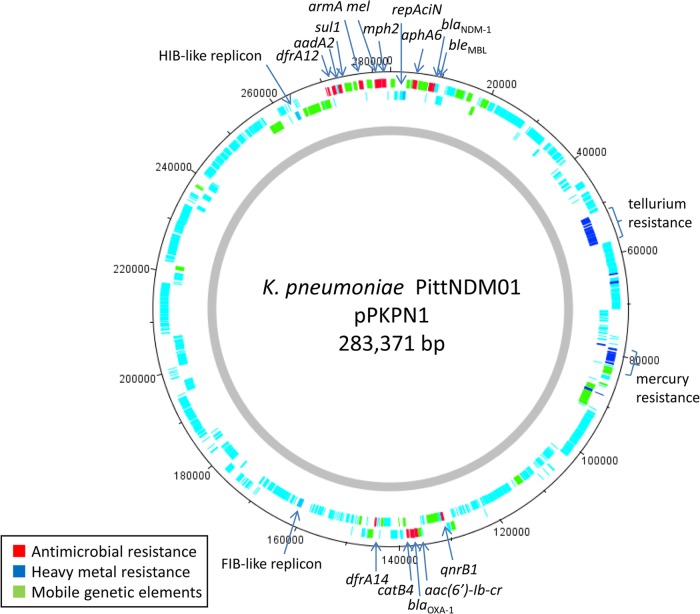
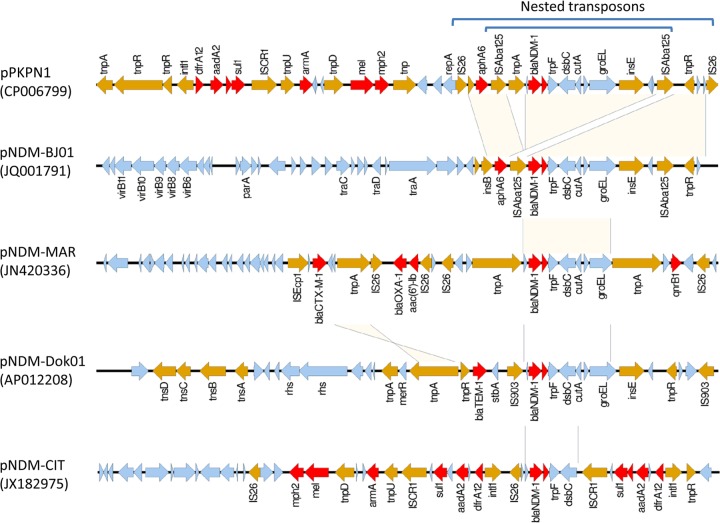
pPKPN1 was found to be the largest of the plasmids, carries blaNDM-1, and contains 291 predicted open reading frames (Fig. 3). It possesses three replicon genes: repAciN, recently identified in plasmid pNDM-CIT carrying blaNDM-1 and speculated to have originated from a plasmid in Acinetobacter spp. (27), and repA encoding an FIB-like replicon (predicted) and repA encoding an HI1B-like replicon, both recently identified in pNDM-MAR, a blaNDM-1-carrying plasmid in a K. pneumoniae strain from Morocco (28). Overall, 99% identity was observed in >87% of the pPKPN1 sequence with the pNDM-MAR sequence. However, the region surrounding blaNDM-1 differed substantially between these two plasmids (Fig. 4). The genetic context of the blaNDM-1 gene in pPKPN1 resembled that of pNDM-BJ01 from Acinetobacter lwoffii (29), in which the blaNDM-1 region is bracketed by two copies of insertion sequence ISAba125 and is located downstream of aphA6 (encoding aminoglycoside phosphotransferase for amikacin and neomycin resistance). However, this entire ISAba125-bracketed structure is further bracketed by two tandem copies of insertion sequence IS26, commonly found in Enterobacteriaceae (Fig. 4). Therefore, it may be inferred that the region was first mobilized onto a plasmid in Acinetobacter spp. by an ISAba125-mediated event, followed by a transfer to K. pneumoniae and capture of this element onto this IncHI1B-like plasmid by an IS26-mediated transpositional event.
FIG 3.
Overview of pPKPN1. The plasmid carries 12 resistance genes, including blaNDM-1, armA, and qnrB1, which are clustered in two regions of the plasmid containing class 1 integrons. pPKPN1 therefore confers resistance to the three key antimicrobial classes (carbapenems, aminoglycosides, and fluoroquinolones). The numbers indicate the positions on the plasmid.
FIG 4.
Alignment of the blaNDM-1 region of pPKPN1 and representative blaNDM-1-carrying plasmids. The antimicrobial resistance genes and mobile genetic elements are colored in red and brown, respectively. The region surrounding blaNDM-1 differed substantially among the plasmids, with pPKPN1 being especially rich in antimicrobial resistance genes, along with pNDM-CIT.
pPKPN1 also carries aac(6′)-Ib-cr, blaOXA-1, and catB4 (encoding chloramphenicol acetyltransferase) on a class 1 integron, dfrA12 (encoding dihydrofolate reductase for trimethoprim resistance), aadA2 (encoding aminoglycoside adenylyltransferase for streptomycin resistance), and sul1 (encoding dihydropteroate synthase for sulfonamide resistance) on another class 1 integron, armA (encoding 16S rRNA methyltransferase for high-level aminoglycoside resistance), mel (encoding macrolide efflux), mph2 (encoding macrolide phosphotransferase), bleMBL (encoding bleomycin resistance), and qnrB1 (encoding ciprofloxacin resistance). As such, the acquisition of this plasmid would compromise the activities of all β-lactams (with the exception of aztreonam), aminoglycosides, fluoroquinolones, and sulfonamides.
IncFIB-like plasmid pPKPN2.
pPKPN2, with 101 predicted open reading frames, possesses an IncFIB-like replicon previously reported in pKPN-IT from a carbapenem-resistant KPC-3-producing K. pneumoniae ST258 strain in Italy (30), and it contains loci for plasmid partition, conjugal transfer, and a type I restriction/modification system. Approximately 78% of the pPKPN2 sequence aligned at 99% identity with that of pKPN-IT. However, pPKPN2 (103.7 kb) is much smaller than pKPN-IT (208.2 kb) and lacks all of the heavy metal resistance loci that characterize pKPN-IT-type plasmids. In addition, antimicrobial resistance determinants were completely absent on pPKPN2.
IncFIA-like plasmid pPKPN3.
pPKPN3, with 80 predicted open reading frames, has an IncFIA-like replicon reported in pEK499, which is a multidrug resistance blaCTX-M-15-carrying plasmid found in an Escherichia coli ST131 strain from the United Kingdom (31). It also possesses a repB gene that is commonly observed in blaKPC-carrying plasmids, including pKPN101-IT from Italy (32). pPKPN3 possesses a large array of antimicrobial resistance genes (Table 1). A class 1 integron contains the gene cassettes arr-2 (encoding rifampin ADP-ribosyl transferase for rifampin resistance), ereC (encoding erythromycin esterase for erythromycin resistance), aadA1 (encoding aminoglycoside adenylyltransferase for streptomycin resistance), cmlA5 (encoding an efflux pump for chloramphenicol resistance), and sul1 (encoding sulfonamide resistance). Other resistance genes are scattered across the plasmid and include blaCTX-M-15 (encoding ESBL), blaSHV-11 and blaTEM-1 (encoding non-ESBL), aacC2 (encoding aminoglycoside acetyltransferase for gentamicin resistance), strB (encoding aminoglycoside phosphotransferase for streptomycin resistance), and sul2 (encoding dihydropteroate synthase for sulfonamide resistance).
TABLE 1.
Overall features of the K. pneumoniae PittNDM01 genome
| Parameter | Chromosome | pPKPN01 | pPKPN02 | pPKPN03 | pPKPN04 |
|---|---|---|---|---|---|
| Size (bp) | 5,348,284 | 283,371 | 103,694 | 70,814 | 6,141 |
| No. of reads | 391,218 | 19,936 | 1,505 | 1,315 | 99 |
| G+C % | 57.50 | 46.41 | 51.00 | 52.92 | 52.19 |
| No. of predicted open reading frames | 4,940 | 291 | 101 | 80 | 8 |
| Resistance gene(s) | blaSHV-28, blaOXA-1, aac(6′)-Ib-cr, cat, macA/macB, fosA | blaNDM-1, blaOXA-1, armA, aac(6′)-Ib-cr, aadA2, aphA6, qnrB1, catB4, mel, mph2, dfrA12, sul1 | None | blaCTX-M-15, blaSHV-11, blaTEM-1, aadA1, aacC2, strB, ereC, cmlA5, sul1, sul2, arr-2 | blaOXA-232 |
blaOXA-232-carrying ColE-like plasmid pPKPN4.
pPKPN4 is nearly identical to the fully sequenced plasmid carrying blaOXA-232 with eight predicted open reading frames, including the carbapenemase gene, which was identified in K. pneumoniae and E. coli isolates recovered from patients who were transferred from India to France (6). Other than the antimicrobial resistance gene, the remainder of the plasmid appears to be cryptic in nature.
Methylome of PittNDM01.
There were five active methyltransferases (MTases) that were detected, as shown in Table 2. In addition to the dam methyltransferase, which modifies 5′-GATC-3′ sequences (with the base in bold type as the site of methylation), there are 3 different active type I MTases that also modify their recognition sequences with N6-methyladenine (6mA). 5-Methylcytosine (5mC) modifications at the 5′-CCWGG-3′ sequences imparted by a homolog of the dcm gene were also detected. As described previously (33), the direct detection of 5mC during SMRT sequencing without enhancement techniques is limited, because the signal is more subtle and dispersed than in other modifications. This explains why only 27.5% of the sites were detected, even though the expectation is that the majority of the 5′-CCWGG-3′ motifs are modified.
TABLE 2.
Detection of methylation in K. pneumoniae PittNDM01
| Motifa | Modification type | No. of motifs detected | No. of motifs in genome | % motifs detected |
|---|---|---|---|---|
| 5′-GATC-3′ | 6mA | 63,453 | 63,698 | 99.6 |
| 3′-CTAG-5′ | ||||
| 5′-CAACNNNNNCGT-3′ | 6mA | 756 | 758 | 99.7 |
| 3′-GTTGNNNNNGCA-5′ | 756 | 758 | 99.7 | |
| 5′-GATGNNNNNNTTG-3′ | 6mA | 1,040 | 1,043 | 99.7 |
| 3′-CTACNNNNNNAAC-5′ | 1,037 | 1,043 | 99.4 | |
| 5′-GTCANNNNNNNNTGT-3′ | 6mA | 322 | 325 | 99.1 |
| 3′-CAGTNNNNNNNNACA-5′ | 324 | 325 | 99.7 | |
| 5′-CCWGG-3′ | 5mC | 7,296 | 26,524 | 27.5 |
| 3′-GGWCC-5′ |
The methylated positions in the methyltransferase recognition sequence motifs are in bold type.
DISCUSSION
NDM-1 and OXA-48 group carbapenemases are rapidly emerging worldwide in Enterobacteriaceae, in particular in K. pneumoniae. In the United States, however, KPC remains by far the most common carbapenemase in Enterobacteriaceae, and clinical strains producing NDM-1 or OXA-48 group carbapenemases are still rare. The finding of NDM-1 and OXA-232 coproduction in K. pneumoniae PittNDM01 was therefore surprising. This strain was also highly drug resistant, only sparing fosfomycin and colistin. We therefore set out to characterize the genomic features of this unique clinical strain in order to obtain insights into the evolution of multidrug resistance.
While K. pneumoniae PittNDM01 originated in India, its chromosome shares 99% identity with that of the reference genome K. pneumoniae MGH 78578, a strain that was isolated in the United States in 1994. In contrast, three of the four plasmids are unique to Klebsiella isolates in strain PittNDM01. pPKPN1, a multidrug resistance plasmid, has a hybrid structure of two blaNDM-1-carrying plasmids, pNDM-MAR and pNDM-CIT. Its blaNDM-1 region revealed a distinctive arrangement in which the original ISAba125-bracketed blaNDM-1 seems to have been further mobilized by an IS26-mediated event downstream of a class 1 integron, generating an extended multidrug resistance scaffold. Also, among the genes carried by pPKPN1 are armA and qnrB1, which compromise the activity of aminoglycosides and fluoroquinolones, respectively. pPKPN2 is somewhat unusual for its lack of striking functional features, but it might be considered the progenitor of the series of heavy metal resistance plasmids represented by pKPN-IT. Finally, pPKPN3 is the second multidrug resistance plasmid after pPKPN1 and carries a variety of genes conferring resistance to expanded-spectrum cephalosporins, gentamicin, sulfonamides, and chloramphenicol. Together, these plasmids provide an extraordinary array of antimicrobial and heavy metal resistance determinants.
Given that this was an isolated imported case from India, we were not able to identify an adequate reference genome prior to this study. Another consideration was the high variability of blaNDM-1-carrying plasmids reported to date, which may confound the assembly of the blaNDM-1-carrying plasmid in K. pneumoniae PittNDM01. For these reasons, we conducted a de novo assembly of the genome of this strain using the SMRT sequencing technology, whose reads of >10 kb facilitated scaffold building. The assembly was conducted on the Celera-based HGAP pipeline (7). This allowed for closure of the chromosome and the two largest plasmids directly from the raw sequence read input. The third plasmid (pPKPN3) was initially assembled as an approximately 67-kb linear element. The identification of the missing 3 kb and closure was achieved by remapping the SMRT sequencing data to the assembled contigs and identifying long sequencing reads that connected the 5′ and 3′ ends of the linear element. The linear element sequence was manually extended using the identified reads and polished with the Quiver consensus tool (19). Finally, the smallest plasmid carrying blaOXA-232 (pPKPN4) was initially not recognized by the SMRT Analysis software as the BluePippin size selection of fragments of >7 kb was carried out. Therefore, with the presence of very small plasmids, a lower size selection cutoff or the alternative use of the AMPure purification method would likely facilitate the automated assembly of these smaller plasmids from the SMRT sequencing data. A whole-genome mapping-assisted de novo assembly method was recently proposed and was used in the full-genome assembly of an NDM-1-producing Providencia stuartii strain (34). The SMRT sequencing used in our study has the advantage of achieving closure of the genome using a single platform. The cost of SMRT sequencing per bacterial strain is slightly higher than those of the other popular sequencing platforms that yield shorter reads, but the total cost required to finish a bacterial genome is substantially lower with SMRT sequencing (18). Therefore, the most cost-effective use of this technology likely lies in the de novo sequencing of highly plastic bacterial genomes.
In conclusion, we characterized the genomic features of a double carbapenemase-producing K. pneumoniae clinical isolate through the use of a long-read sequencing platform and semiautomated de novo assembly process. The approach provided fully assembled genome sequences without the need for gap-filling procedures, and it was particularly useful in elucidating the complex structures of the large multidrug resistance plasmids.
ACKNOWLEDGMENTS
We thank K. Luong for assistance with data analysis and Rich Roberts (New England BioLabs) for entering the methylome information into REBASE.
The whole-genome sequencing was supported by Pacific Biosciences. The effort of Y.D. was supported in part by research grants from the National Institutes of Health (grants R21AI107302 and R01AI104895).
M.B., Y.-C.T., T.A.C., and J.K. are employees of Pacific Biosciences.
Footnotes
Published ahead of print 28 July 2014
REFERENCES
- 1.Nordmann P, Naas T, Poirel L. 2011. Global spread of carbapenemase-producing Enterobacteriaceae. Emerg. Infect. Dis. 17:1791–1798. 10.3201/eid1710.110655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yong D, Toleman MA, Giske CG, Cho HS, Sundman K, Lee K, Walsh TR. 2009. Characterization of a new metallo-β-lactamase gene, blaNDM-1, and a novel erythromycin esterase gene carried on a unique genetic structure in Klebsiella pneumoniae sequence type 14 from India. Antimicrob. Agents Chemother. 53:5046–5054. 10.1128/AAC.00774-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nordmann P, Poirel L, Walsh TR, Livermore DM. 2011. The emerging NDM carbapenemases. Trends Microbiol. 19:588–595. 10.1016/j.tim.2011.09.005 [DOI] [PubMed] [Google Scholar]
- 4.Poirel L, Héritier C, Tolün V, Nordmann P. 2004. Emergence of oxacillinase-mediated resistance to imipenem in Klebsiella pneumoniae. Antimicrob. Agents Chemother. 48:15–22. 10.1128/AAC.48.1.15-22.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Poirel L, Potron A, Nordmann P. 2012. OXA-48-like carbapenemases: the phantom menace. J. Antimicrob. Chemother. 67:1597–1606. 10.1093/jac/dks121 [DOI] [PubMed] [Google Scholar]
- 6.Potron A, Rondinaud E, Poirel L, Belmonte O, Boyer S, Camiade S, Nordmann P. 2013. Genetic and biochemical characterisation of OXA-232, a carbapenem-hydrolysing class D β-lactamase from Enterobacteriaceae. Int. J. Antimicrob. Agents 41:325–329. 10.1016/j.ijantimicag.2012.11.007 [DOI] [PubMed] [Google Scholar]
- 7.Castanheira M, Deshpande LM, Mathai D, Bell JM, Jones RN, Mendes RE. 2011. Early dissemination of NDM-1- and OXA-181-producing Enterobacteriaceae in Indian hospitals: report from the SENTRY Antimicrobial Surveillance Program, 2006–2007. Antimicrob. Agents Chemother. 55:1274–1278. 10.1128/AAC.01497-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poirel L, Ros A, Carricajo A, Berthelot P, Pozzetto B, Bernabeu S, Nordmann P. 2011. Extremely drug-resistant Citrobacter freundii isolate producing NDM-1 and other carbapenemases identified in a patient returning from India. Antimicrob. Agents Chemother. 55:447–448. 10.1128/AAC.01305-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dash N, Panigrahi D, Zarouni MA, Darwish D, Ghazawi A, Sonnevend A, Pal T, Yasin F, Hadi SA. 2014. High incidence of New Delhi metallo-β-lactamase-producing Klebsiella pneumoniae isolates in Sharjah, United Arab Emirates. Microb. Drug Resist. 20:52–56. 10.1089/mdr.2013.0040 [DOI] [PubMed] [Google Scholar]
- 10.Khajuria A, Praharaj AK, Grover N, Kumar M. 2013. First report of an Enterobacter ludwigii isolate coharboring NDM-1 and OXA-48 carbapenemases. Antimicrob. Agents Chemother. 57:5189–5190. 10.1128/AAC.00789-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ben Nasr A, Decré D, Compain F, Genel N, Barguellil F, Arlet G. 2013. Emergence of NDM-1 in association with OXA-48 in Klebsiella pneumoniae from Tunisia. Antimicrob. Agents Chemother. 57:4089–4090. 10.1128/AAC.00536-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alp E, Perçin D, Colakoğlu S, Durmaz S, Kürkcü CA, Ekincioğlu P, Günes T. 2013. Molecular characterization of carbapenem-resistant Klebsiella pneumoniae in a tertiary university hospital in Turkey. J. Hosp. Infect. 84:178–180. 10.1016/j.jhin.2013.03.002 [DOI] [PubMed] [Google Scholar]
- 13.Samuelsen Ø, Naseer U, Karah N, Lindemann PC, Kanestrøm A, Leegaard TM, Sundsfjord A. 2013. Identification of Enterobacteriaceae isolates with OXA-48 and coproduction of OXA-181 and NDM-1 in Norway. J. Antimicrob. Chemother. 68:1682–1685. 10.1093/jac/dkt058 [DOI] [PubMed] [Google Scholar]
- 14.Barguigua A, El Otmani F, Lakbakbi El Yaagoubi F, Talmi M, Zerouali K, Timinouni M. 2013. First report of a Klebsiella pneumoniae strain coproducing NDM-1, VIM-1 and OXA-48 carbapenemases isolated in Morocco. APMIS 121:675–677. 10.1111/apm.12034 [DOI] [PubMed] [Google Scholar]
- 15.Balm MND, Ngan G, Jureen R, Lin RTP, Teo JWP. 2013. OXA-181-producing Klebsiella pneumoniae establishing in Singapore. BMC Infect. Dis. 13:58. 10.1186/1471-2334-13-58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Székely E, Damjanova I, Jánvári L, Vas KE, Molnár S, Bilca DV, Lõrinczi LK, Tóth A. 2013. First description of blaNDM-1, blaOXA-48, blaOXA-181 producing Enterobacteriaceae strains in Romania. Int. J. Med. Microbiol. 303:697–700. 10.1016/j.ijmm.2013.10.001 [DOI] [PubMed] [Google Scholar]
- 17.Doi Y, O'Hara JA, Lando JF, Querry AM, Townsend BM, Pasculle AW, Muto CA. 2014. Co-production of NDM-1 and OXA-232 by Klebsiella pneumoniae. Emerg. Infect. Dis. 20:163-165. 10.3201/eid2001.130904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koren S, Harhay GP, Smith TP, Bono JL, Harhay DM, McVey SD, Radune D, Bergman NH, Phillippy AM. 2013. Reducing assembly complexity of microbial genomes with single-molecule sequencing. Genome Biol. 14:R101. 10.1186/gb-2013-14-9-r101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chin CS, Alexander DH, Marks P, Klammer AA, Drake J, Heiner C, Clum A, Copeland A, Huddleston J, Eichler EE, Turner SW, Korlach J. 2013. Nonhybrid, finished microbial genome assemblies from long-read SMRT sequencing data. Nat. Methods 10:563–569. 10.1038/nmeth.2474 [DOI] [PubMed] [Google Scholar]
- 20.Murray IA, Clark TA, Morgan RD, Boitano M, Anton BP, Luong K, Fomenkov A, Turner SW, Korlach J, Roberts RJ. 2012. The methylomes of six bacteria. Nucleic Acids Res. 40:11450–11462. 10.1093/nar/gks891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roberts RJ, Macelis D. 2000. REBASE—restriction enzymes and methylases. Nucleic Acids Res. 28:306–307. 10.1093/nar/28.1.306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sahl JW, Steinsland H, Redman JC, Angiuoli SV, Nataro JP, Sommerfelt H, Rasko DA. 2011. A comparative genomic analysis of diverse clonal types of enterotoxigenic Escherichia coli reveals pathovar-specific conservation. Infect. Immun. 79:950–960. 10.1128/IAI.00932-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Angiuoli SV, Salzberg SL. 2011. Mugsy: fast multiple alignment of closely related whole genomes. Bioinformatics 27:334–342. 10.1093/bioinformatics/btq665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stamatakis A. 2006. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22:2688–2690. 10.1093/bioinformatics/btl446 [DOI] [PubMed] [Google Scholar]
- 25.Lin AC, Liao TL, Lin YC, Lai YC, Lu MC, Chen YT. 2012. Complete genome sequence of Klebsiella pneumoniae 1084, a hypermucoviscosity-negative K1 clinical strain. J. Bacteriol. 194:6316. 10.1128/JB.01548-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu P, Li P, Jiang X, Bi D, Xie Y, Tai C, Deng Z, Rajakumar K, Ou HY. 2012. Complete genome sequence of Klebsiella pneumoniae subsp. pneumoniae HS11286, a multidrug-resistant strain isolated from human sputum. J. Bacteriol. 194:1841–1842. 10.1128/JB.00043-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dolejska M, Villa L, Poirel L, Nordmann P, Carattoli A. 2013. Complete sequencing of an IncHI1 plasmid encoding the carbapenemase NDM-1, the ArmA 16S RNA methylase and a resistance-nodulation-cell division/multidrug efflux pump. J. Antimicrob. Chemother. 68:34–39. 10.1093/jac/dks357 [DOI] [PubMed] [Google Scholar]
- 28.Villa L, Poirel L, Nordmann P, Carta C, Carattoli A. 2012. Complete sequencing of an IncH plasmid carrying the blaNDM-1, blaCTX-M-15 and qnrB1 genes. J. Antimicrob. Chemother. 67:1645–1650. 10.1093/jac/dks114 [DOI] [PubMed] [Google Scholar]
- 29.Hu H, Hu Y, Pan Y, Liang H, Wang H, Wang X, Hao Q, Yang X, Yang X, Xiao X, Luan C, Yang Y, Cui Y, Yang R, Gao GF, Song Y, Zhu B. 2012. Novel plasmid and its variant harboring both a blaNDM-1 gene and type IV secretion system in clinical isolates of Acinetobacter lwoffii. Antimicrob. Agents Chemother. 56:1698–1702. 10.1128/AAC.06199-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.García-Fernández A, Villa L, Carta C, Venditti C, Giordano A, Venditti M, Mancini C, Carattoli A. 2012. Klebsiella pneumoniae ST258 producing KPC-3 identified in Italy carries novel plasmids and OmpK36/OmpK35 porin variants. Antimicrob. Agents Chemother. 56:2143–2145. 10.1128/AAC.05308-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Woodford N, Carattoli A, Karisik E, Underwood A, Ellington MJ, Livermore DM. 2009. Complete nucleotide sequences of plasmids pEK204, pEK499, and pEK516, encoding CTX-M enzymes in three major Escherichia coli lineages from the United Kingdom, all belonging to the international O25:H4-ST131 clone. Antimicrob. Agents Chemother. 53:4472–4482. 10.1128/AAC.00688-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Frasson I, Lavezzo E, Franchin E, Toppo S, Barzon L, Cavallaro A, Richter SN, Palù G. 2012. Antimicrobial treatment and containment measures for an extremely drug-resistant Klebsiella pneumoniae ST101 isolate carrying pKPN101-IT, a novel fully sequenced blaKPC-2 plasmid. J. Clin. Microbiol. 50:3768–3772. 10.1128/JCM.01892-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clark TA, Lu X, Luong K, Dai Q, Boitano M, Turner SW, He C, Korlach J. 2013. Enhanced 5-methylcytosine detection in single-molecule, real-time sequencing via Tet1 oxidation. BMC Biol. 11:4. 10.1186/1741-7007-11-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Onmus-Leone F, Hang J, Clifford RJ, Yang Y, Riley MC, Kuschner RA, Waterman PE, Lesho EP. 2013. Enhanced de novo assembly of high throughput pyrosequencing data using whole genome mapping. PLoS One 8:e61762. 10.1371/journal.pone.0061762 [DOI] [PMC free article] [PubMed] [Google Scholar]