Abstract
Accelerating rates of health care-associated infections caused by Clostridium difficile, with increasing recurrence and rising antibiotic resistance rates, have become a serious problem in recent years. This study was conducted to explore whether a combination of antibiotics with human antimicrobial peptides may lead to an increase in antibacterial activity. The in vitro activities of the antimicrobial peptides HBD1 to HBD3, HNP1, HD5, and LL-37 and the antibiotics tigecycline, moxifloxacin, piperacillin-tazobactam, and meropenem alone or in combination against 10 toxinogenic and 10 nontoxinogenic C. difficile strains were investigated. Bacterial viability was determined by flow cytometry and toxin production by enzyme-linked immunosorbent assay (ELISA). When combined at subinhibitory concentrations, antimicrobial peptides and antibiotics generally led to an additive killing effect against toxinogenic and nontoxinogenic C. difficile strains. However, LL-37 and HBD3 acted in synergism with all the antibiotics that were tested. Electron microscopy revealed membrane perturbation in bacterial cell walls by HBD3. In 3 out of 10 toxinogenic strains, HBD3, LL-37, piperacillin-tazobactam, and meropenem administration led to an increased toxin release which was not neutralized by the addition of HNP1. Antimicrobial peptides increase the bacterial killing of antibiotics against C. difficile regardless of the antibiotics' mode of action. Membrane perturbation in or pore formation on the bacterial cell wall may enhance the uptake of antibiotics and increase their antibacterial effect. Therefore, a combination of antibiotics with antimicrobial peptides may represent a promising novel approach to the treatment of C. difficile infections.
INTRODUCTION
Clostridium difficile infections (CDIs) have become an increasing health care problem, occurring often during or after antibiotic therapy. The transmission of C. difficile in the nosocomial environment is primarily caused by the ingestion of spores, which may be acquired directly from other patients, via the hands of health care workers, or indirectly from inanimate objects. The severity of the infections, increasing recurrence rates, and the development of resistance against antibiotics have limited the effectiveness of antibiotic therapy for C. difficile infections in recent years (1). While most antibiotics act on the vegetative bacterial cell of C. difficile, only a few antibiotics, such as vancomycin or fidaxomicin, may have an inhibitory effect on sporulation. However, there are still recurrence rates of 15% with fidaxomicin and 25% with vancomycin (2).
C. difficile infections can lead to symptoms from diarrhea to severe pseudomembranous colitis and toxic megacolon. In these gastrointestinal infections, the proper balance between the gut flora and host defense is disturbed. Although the colonic mucosa is constantly exposed to a high number of bacteria, bacterial infections are rare. One important mechanism for the prevention of microbial invasion of the epithelium is the production and secretion of antimicrobial peptides such as defensins or the cathelicidin LL-37. The family of defensins comprises small cationic peptides with low molecular masses of 3 to 6 kDa. Due to the different positions of three intramolecular disulfide bonds, human defensins are categorized as alpha- or beta-defensins (3). The alpha-defensins derive mainly from intestinal Paneth cells (HD5 and -6) or neutrophils (HNP1 to -4), whereas the beta-defensins (HBD1 to -4) are predominantly of epithelial origin (4–7).
The only human cathelicidin, LL-37, is released by proteolytic cleavage from the precursor hCAP-18, which is synthesized from epithelial cells, and also from a variety of immune cells (8).
The main mode of action of antimicrobial peptides is the formation of pores in the bacterial cell membrane, but they can also act on intracellular targets, such as nucleic acid and protein biosynthesis (9). It is conceivable that antimicrobial peptides augment the activity of antibiotics which share some of their targets with them.
Despite numerous studies investigating the effects of antimicrobial peptides on a broad range of bacterial species, information on their impact on the viability of C. difficile is limited. McQuade et al. recently examined the effect of the cathelicidin LL-37 on C. difficile strains and found strain-specific susceptibility (10). In addition, it was shown by Giesemann et al. (11) that human alpha-defensins inhibit toxin B production in C. difficile. In this study, synergism between selected antibiotics and the human beta-defensins HBD1, -2, and -3, the alpha-defensin HD5, and the cathelicidin LL-37 against 10 toxin-positive and 10 toxin-negative clinical strains of C. difficile was assessed employing flow cytometry. This assay was developed for viability assessment of other anaerobic bacteria based on membrane polarization (12). Bacterial strains were first preincubated with the various antimicrobial peptides and then exposed to subinhibitory concentrations of the antibiotics tigecycline, moxifloxacin, piperacillin-tazobactam, or meropenem. Subsequently, bacterial membrane depolarization was measured by flow cytometry, and toxin release was controlled by an enzyme-linked immunosorbent assay (ELISA).
MATERIALS AND METHODS
Bacterial strains.
We obtained 10 nontoxinogenic and 9 toxinogenic clinical isolates from material submitted for routine laboratory testing at the Institute of Laboratory Medicine, Hospital Alb Fils Kliniken. C. difficile DSM 1296 was included as the 10th toxinogenic strain. All strains were recovered on Columbia agar with 5% defibrinated sheep blood (BD, Sparks, MD, USA). Toxin production of the toxinogenic strains was verified by toxin A/B ELISA (Ridascreen Clostridium difficile; R-Biopharm, Darmstadt, Germany). The strains were characterized molecular genetically with GenoType CDiff (Hain Lifescience, GmbH, Nehren, Germany).
MIC determination.
The MICs of the C. difficile isolates against tigecycline, moxifloxacin, piperacillin-tazobactam, and imipenem were determined by Etest (bioMérieux, Nürtingen, Germany) according to the manufacturer's instructions. The control strains were Staphylococcus aureus ATCC 25923, Bacteroides fragilis ATCC 25285, and Bacteroides thetaiotaomicron ATCC 29741. Briefly, an inoculum of about 1.5 × 107 CFU was plated on Mueller-Hinton agar supplemented with 5% sheep blood (BD, Sparks, MD, USA). The Etest strips were placed on the agar, and the plates were incubated for 48 h under anaerobic conditions (AnaeroGen; Oxoid Limited, Hampshire, United Kingdom). The MIC was recorded as the concentration at which the elliptical inhibition zone met the Etest strip. To determine subinhibitory concentrations with the flow cytometric test, 1.5 × 106 CFU/ml of mid-logarithmic-phase C. difficile was incubated with moxifloxacin at 64, 32, 16, 8, and 4 μg/ml, tigecycline at 2, 1, 0.512, 0.256, 0.128, and 0.0064 μg/ml, piperacillin (combined with 4 μg tazobactam) at 6, 3, 1.5, 0.75, and 0.375 μg/ml, and meropenem at 512, 256, 128, 64, 16, and 4 μg/ml for 8 h at 37°C under anaerobic conditions (AnaeroGen). Subsequently, the suspensions were incubated for 10 min with 1 μg/ml of the membrane potential-sensitive dye bis-(1,3-dibutylbarbituric acid) trimethine oxonol [DiBAC4(3)] (Invitrogen, Carlsbad, CA). Cell damage leads to the breakdown of the membrane potential, followed by the uptake of the dye in the bacterial cells and to increasing green fluorescence (13). The suspensions were centrifuged for 10 min at 4,500 × g, and the bacterial pellets were resuspended in 300 μl phosphate-buffered saline (PBS) and analyzed by flow cytometry as described below. For several strains, the flow cytometric results were confirmed by plating.
Effect of defensins and LL-37 on C. difficile.
For a total of 10 toxinogenic or nontoxinogenic C. difficile strains, the subinhibitory concentrations of the antimicrobial peptides were determined. C. difficile suspensions with a concentration of 1.5 × 106 CFU/ml were incubated with the antimicrobial peptides HBD1, HBD2, HBD3, HD5 (all peptides from Peptide Institute, Osaka, Japan), and LL-37 (Innovagen, Lund, Sweden) at various concentrations ranging from 0.5 to 15 μg/ml for 90 min at 37°C under anaerobic conditions (AnaeroGen). Then, 1 μg/ml DiBAC4(3) (Invitrogen, Carlsbad, CA) was added, and after 10 min, the suspensions were centrifuged for 10 min at 4,500 × g, and the pellets were resuspended in 300 μl PBS and analyzed by flow cytometry.
Activities of reduced antimicrobial peptides.
To reduce the antimicrobial peptides, they were preincubated with 10 mM dithiothreitol (DTT) at 37°C for 2 h (13). The peptides were incubated in the native/oxidized or reduced form with 5 C. difficile strains at a concentration of 15 μg/ml as described above and were analyzed by flow cytometry.
Combined effect of antibiotics and antimicrobial peptides.
Synergism studies were performed using a concentration of 1 μg/100 μl of the various antimicrobial peptides, since at this peptide concentration alone, no significant depolarization of bacteria was observed. Based on pilot experiments, we used tigecycline at 0.512 μg/ml, moxifloxacin at 32 μg/ml, piperacillin-tazobactam at 6 μg/ml and 4 μg/ml, and meropenem at 64 μg/ml because these concentrations do not lead to significant depolarization.
Suspensions with 1.5 × 106 CFU/ml were incubated with the antimicrobial peptides for 30 min at 37°C. Subsequently, the antimicrobials were added. After another 8 h of incubation at 37°C under anaerobic conditions (AnaeroGen; Oxoid Limited, Hampshire, United Kingdom), the suspensions were incubated for 10 min with 1 μg/ml DiBAC4(3). The suspensions were centrifuged for 10 min at 4,500 × g, and the bacterial pellets were each resuspended in 300 μl PBS. The percentage of depolarized fluorescent bacteria in the suspension was determined by flow cytometry as described below.
All the experiments were performed at least twice.
Flow cytometry.
In each sample, 10,000 events were analyzed on a FACSCalibur flow cytometer (BD, Sparks, MD) using Cell Quest software (BD). With the parameters forward scatter and side scatter referring to relative cell size and granularity, respectively, the bacterial population was gated for the evaluation of fluorescence 1 (DiBAC4[3]) in a corresponding histogram. Antibacterial activity was determined as the percentage of fluorescent bacteria with respect to that of the untreated bacterial control.
Electron microscopy.
To visualize the effect of antimicrobial peptides on C. difficile cells, 1.5 × 107 bacteria/ml were exposed to HBD3 at a concentration of 200 μg/ml and incubated for 4 h at 37°C. (Due to the 10-fold bacterial concentration used for microscopy, the concentration of antimicrobial peptides was also increased 10-fold.) Untreated bacteria served as controls. After centrifugation, the bacterial pellets were fixed with Karnovsky's fixative. The pellets were embedded in 3.5% agarose at 37°C, coagulated at room temperature, and fixed again in Karnovsky's fixative. Postfixation was carried out with 1% osmium tetroxide containing 1.5% K-ferrocyanide in 0.1 M cacodylate buffer for 2 h. Subsequently, the specimens were embedded in glycid ether. Ultrathin sections, 20 to 30 nm thick, were mounted on uncoated copper grids and imaged by electron microscopy with a Zeiss LIBRA 120 microscope (Carl Zeiss, Oberkochen, Germany).
Influence of antimicrobial peptides and antibiotics on toxin release.
For the antimicrobial peptides that exhibited synergism with the antibiotics, HBD3 and LL-37, and for HNP1, we investigated the influence on toxin release by C. difficile.
The 10 toxinogenic strains were grown overnight in Schaedler broth and adjusted to 4.5 × 106 cells/ml in Schaedler broth (BD, Sparks, MD, USA), which was diluted 1:3 in distilled water (dH2O). The dilution was performed because the salt content in undiluted Schaedler broth has an inhibitory effect on the activity of some antimicrobial peptides. The suspensions were incubated with the respective antimicrobial peptide at a concentration of 10 μg/ml for 30 min at 37°C. Tigecycline at 0.512 μg/ml, moxifloxacin at 32 μg/ml, piperacillin-tazobactam at 4 and 6 μg/ml, and meropenem at 64 μg/ml were added. The bacterial suspensions were incubated for 8 h under anaerobic conditions (AnaeroGen). One hundred microliters of the suspension was used in a toxin A and B ELISA, which was performed according to the manufacturer's instructions (R-Biopharm, Darmstadt, Germany).
Statistics.
Graphing and statistical analyses were carried out using Prism 5.0 software. The data are presented as means plus the standard deviation (SD). For the comparison of bacterial killing with or without preincubation with antimicrobial peptides, the Mann-Whitney test was used. P values of <0.05 were considered statistically significant.
A depolarization rate of the combination of antimicrobial peptide and an antibiotic equivalent to the sum of their individual killing rates was defined as additive, whereas a higher percentage than the sum of their individual rates was considered synergistic.
RESULTS
MIC determination.
For the control strains S. aureus ATCC 25923, B. fragilis ATCC 25285, and B. thetaiotaomicron ATCC 29741, the MICs determined by the Etest were, for all antibiotics tested (tigecycline, piperacillin-tazobactam, moxifloxacin, and imipenem), within the ranges provided by the manufacturer. For the C. difficile strains, the MICs for tigecycline were ≤0.016 mg/liter. The MICs for piperacillin-tazobactam ranged from 0.75 mg/liter to >32 mg/liter, with the MICs of most strains (n = 13) between 3 and 6 mg/liter. For 14 strains, the MICs for moxifloxacin were >32 mg/liter, and only 6 strains were sensitive (MIC, 0.125 or 0.75 mg/liter). For imipenem, 17 strains had a MIC of >32 mg/liter, and only three strains were sensitive (MICs, 2, 6, and 12 mg/liter) (see Table S1 in the supplemental material).
Effect of defensins and LL-37 on C. difficile.
With a concentration of 0.5 or 1 μg/ml, no antibacterial effects were seen with any of the peptides tested. Using higher concentrations of 2.5 to 15 μg/ml, no effects on C. difficile integrity were observed when incubating the strains with the constitutive defensin HBD1. Some strains were marginally affected by the inducible HBD2 or by HD5. In contrast, with concentrations of >5 μg/ml, LL-37 and HBD3 effectively depolarized vegetative cells (Fig. 1A) and reduced significantly the number of CFU in cultures of all C. difficile strains examined (data not shown).
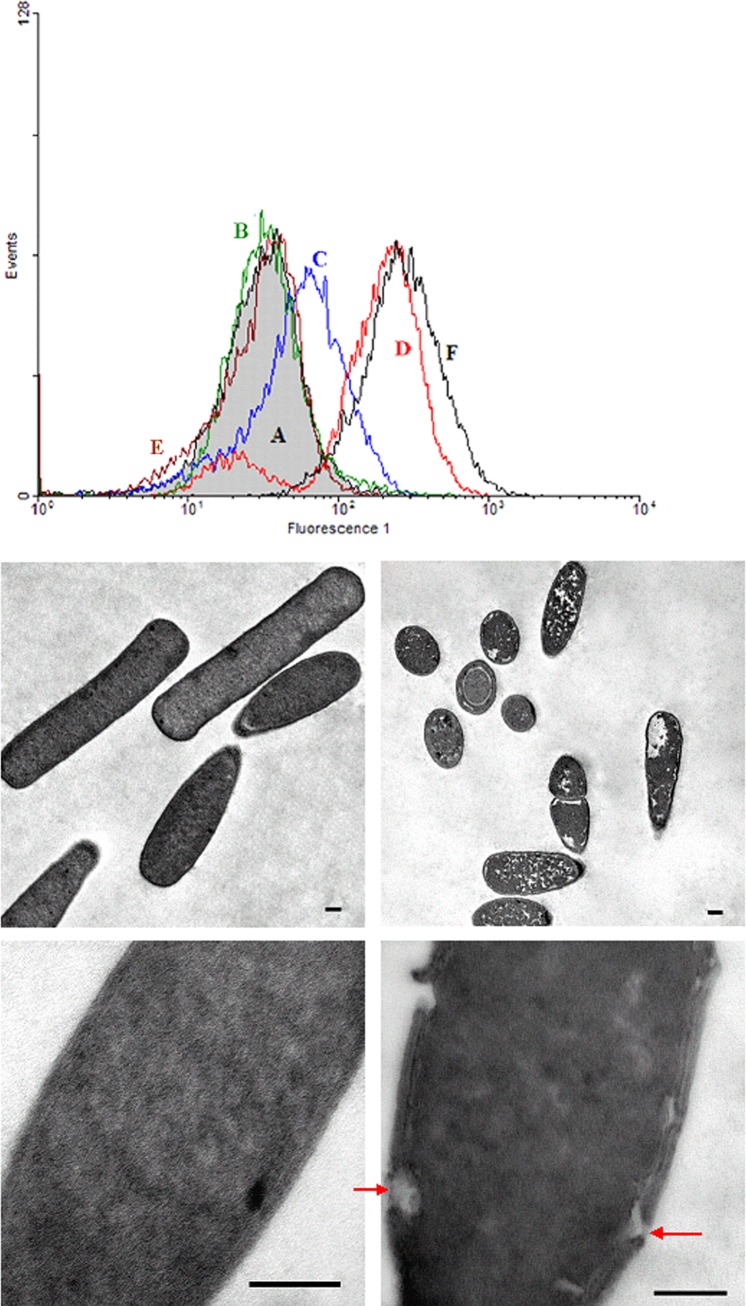
FIG 1.
(A) Flow cytometric analysis of the antimicrobial activities of the defensins HBD2 and HBD3 and the cathelicidin LL-37 against vegetative cells of C. difficile strain 109. The gray-filled histogram indicates the green fluorescence of untreated bacteria, and the lines indicate the fluorescence of bacteria incubated with antimicrobial peptides at a concentration of 15 μg/ml (green line, HBD1; blue line, HBD2; red line, HBD3; brown line, HD5; black line, LL-37). Cell damage leads to an uptake of the membrane potential-sensitive dye DiBAC4(3) in the bacteria and therefore to an increasing fluorescence 1 compared to that of the untreated control. (B) Electron microscopy of C. difficile cells incubated with 200 μg HBD3/ml. Shown are the untreated control (left panels) and pore formation after treatment with HBD3 (right panels) (scale bars, 0.2 μm). HBD3 led to disruptions in the cell membrane (arrows) and a decrease in the density of the cytoplasm.
Electron microscopy performed on C. difficile with the defensin HBD3 showed disruptions of the cell wall and less dense cytoplasmic regions or even dissolution of the cytoplasm (Fig. 1B). After reduction of the antimicrobial peptides, the activity of HBD3 was lower than the activity of the native (oxidized) form. This effect was not observed after reducing LL-37. No significant differences occurred in the activities of reduced HBD1, HBD2, or HD5 against C. difficile compared to those of the native form of these peptides (Fig. 2).
FIG 2.
Activities of antimicrobial peptides in their native (oxidized) or reduced forms at a concentration of 15 μg/ml against 5 C. difficile strains (the values are means + SD). The reduction of HBD3 led to diminished antimicrobial activity (P < 0.05, indicated by an asterisk), whereas the antibacterial activity of LL-37 was not significantly different with either of the two forms.
Synergism between antibiotics and antimicrobial peptides.
With an average of 3.83% (range, 0.89 to 9.73%) no significant depolarization of bacterial cells was found in the untreated controls. Incubated with subinhibitory concentrations of the various antibiotics, the average percentage of depolarized cells was 9.02% (ranges, 3.69 to 29.6% [tigecycline], 1.03 to 12.69% [moxifloxacin], 1.07 to 12.69% [piperacillin-tazobactam], and 0.29 to 26.5% [meropenem]), and there were no statistically significant differences among the different antibiotics. The subinhibitory concentrations of HBD1 to -3, HD5, and LL-37 revealed average depolarization rates of 11.16% (range, 0.02 to 39.12%).
When combined, the antibiotics exerted an additive effect on nearly all of the antimicrobial peptides. In nontoxinogenic strains, HD5 had an additive effect only in combination with tigecycline but not with moxifloxacin, piperacillin-tazobactam, or meropenem. Furthermore, in combination with HBD3 or LL-37, all the antibiotics exhibited significant synergistic effects (Table 1; see also Fig. S1 in the supplemental material). In 15 out of the 20 strains tested, a synergistic effect was observed after adding HBD3, with an average increase in depolarized cells from 9.02% with the various antibiotics alone to 41.42% in combination with HBD3. With LL-37, this effect was seen in 16 strains, but the average increase in depolarized cells was generally lower, with 29.12% depolarized cells after the addition of LL-37. Therefore, the degree of additive or synergistic effects of the antibiotics combined with antimicrobial peptides was mainly strain specific.
TABLE 1.
Bacterial killing of toxinogenic (n = 10) and nontoxinogenic (n = 10) C. difficile strains after incubation with antimicrobial peptides, antibiotics, or a combination of both
| Peptide | Strain type | Bacterial killinga with: |
||||
|---|---|---|---|---|---|---|
| No antibiotic | Tigecycline | Moxifloxacin | Piperacillin-tazobactam | Meropenem | ||
| None (control) | Toxinogenic | 3.8 (2.67) | 10.8 (7.90) | 5.5 (3.70) | 14.3 (11.16) | 9.6 (8.41) |
| Nontoxinogenic | 6.9 (1.93) | 13.8 (13.76) | 8.2 (11.77) | 13.2 (16.37) | 14.4 (12.38) | |
| LL-37 | Toxinogenic | 11.7 (8.60) | 27.2 (20.21)b*; 6 | 31.9 (17.62)****; 7 | 37.7 (21.61)**; 7 | 31.6 (19.93)**; 7 |
| Nontoxinogenic | 13.6 (19.91) | 39.3 (22.25)**; 9 | 33.9 (24.31)**; 7 | 37.9 (23.52)**; 9 | 34.6 (23.62)*; 8 | |
| HBD1 | Toxinogenic | 12.7 (6.99) | 21.8 (19.32); 2 | 17.3 (20.21); 2 | 22.5 (17.14); 4 | 19.5 (19.37); 4 |
| Nontoxinogenic | 10.1 (11.78) | 19.2 (19.45); 2 | 12.0 (18.69); 0 | 22.3 (18.44)*; 5 | 19.5 (19.26); 2 | |
| HBD2 | Toxinogenic | 8.1 (5.63) | 29.2 (23.44); 6 | 13.3 (10.16); 2 | 29.2 (29.49); 5 | 27.0 (19.58)*; 7 |
| Nontoxinogenic | 12.7 (10.87) | 21.9 (22.79); 4 | 17.7 (19.65)*; 3 | 21.6 (19.55)*; 2 | 19.5 (18.24); 1 | |
| HBD3 | Toxinogenic | 16.9 (16.94) | 49.9 (30.49)**; 7 | 41.8 (34.07)**; 8 | 56.4 (32.22)**; 8 | 49.4 (30.49)**; 8 |
| Nontoxinogenic | 15.7 (22.83) | 52.8 (26.41)***; 9 | 38.0 (28.74)***; 8 | 49.3 (25.71)**; 7 | 44.0 (27.69)**; 7 | |
| HD5 | Toxinogenic | 13.6 (9.39) | 23.1 (21.68); 4 | 17.9 (20.00)*; 3 | 20.0 (19.71); 3 | 38.9 (24.26)**; 8 |
| Nontoxinogenic | 13.7 (16.45) | 21.4 (23.65); 2 | 13.8 (21.71); 1 | 17.4 (19.30); 1 | 17.4 (20.29); 2 | |
Values are mean (+SD) percentage of depolarized bacteria; number of strains out of 10 with synergistic antimicrobial killing.
Statistically significant differences: *, P < 0.05; **, P < 0.005; ***, P < 0.0005; ****, P < 0.0001.
There were no significant differences in the averages of depolarized bacteria among the various antibiotic-LL-37 and antibiotic-HBD3 combinations.
In contrast to LL-37 and HBD3, the peptides HBD1 and HBD2 showed no synergistic effects in the toxigenic or nontoxigenic strains. HD5 demonstrated statistically significant synergism in toxin-positive strains with meropenem only (P < 0.01).
Influence of antibiotics and antimicrobial peptides on toxin production.
When tested at subinhibitory concentrations, none of the antibiotics inhibited toxin production of the toxinogenic strains, and no conversions were seen in the toxin-negative strains. An increase in detectable toxin was seen in three toxinogenic strains in response to subinhibitory concentrations of piperacillin-tazobactam and meropenem. The same strains reacted similarly when HBD3 or LL-37 alone was added. The addition of HBD3 or LL-37 to piperacillin-tazobactam or meropenem did not further increase the toxin release. In other strains, no significant increased toxin amounts were measured after the addition of antibiotics or antimicrobial peptides. C. difficile toxins were not detectable by toxin ELISA in any strains when incubated with the alpha-defensin HNP1 alone (Table 2). This was also observed for all strains tested with moxifloxacin or tigecycline in combination with HNP1. However, when treated with piperacillin-tazobactam or meropenem, the toxin release of those strains which showed an induction were not neutralized by the applied HNP1 concentration.
TABLE 2.
Levels of toxin A and B of 10 toxinogenic C. difficile strains after incubation with antimicrobial peptides, antibiotics, or a combination of both
| Antimicrobial and/or antibiotic | Total (n = 10) | Mean (SD) level for strains (OD450)a: |
|
|---|---|---|---|
| Without increased toxin levels (n = 7) | With increased toxin levels (n = 3) | ||
| None (control) | 0.425 | 0.282 (0.23) | 0.761 (0.63) |
| LL-37 | 1.264 | 0.390 (0.25) | 3.302 (0.21) |
| HBD3 | 1.113 | 0.300 (0.22) | 3.011 (0.39) |
| HNP1 | 0.180 | 0.005 (0.02) | 0.587 (0.49) |
| Moxifloxacin | 0.365 | 0.251 (0.22) | 0.628 (0.46) |
| Tigecycline | 0.413 | 0.236 (0.20) | 0.824 (0.57) |
| Piperacillin-tazobactam | 0.954 | 0.293 (0.24) | 2.495 (0.54) |
| Meropenem | 0.892 | 0.261 (0.20) | 2.365 (0.28) |
| LL-37 plus moxifloxacin | 1.158 | 0.342 (0.21) | 3.061 (0.54) |
| LL-37 plus tigecycline | 1.177 | 0.338 (0.22) | 3.138 (0.32) |
| LL-37 plus piperacillin | 1.190 | 0.349 (0.21) | 3.152 (0.34) |
| LL-37 plus meropenem | 1.212 | 0.354 (0.23) | 3.215 (0.36) |
| HBD3 plus moxifloxacin | 1.178 | 0.347 (0.21) | 3.117 (0.33) |
| HBD3 plus tigecycline | 1.135 | 0.327 (0.21) | 3.021 (0.38) |
| HBD3 plus piperacillin | 1.178 | 0.337 (0.25) | 3.141 (0.40) |
| HBD3 plus meropenem | 1.077 | 0.285 (0.17) | 2.295 (0.56) |
| HNP1 plus moxifloxacin | 0.042 | 0.000 (0.00) | 0.140 (0.13) |
| HNP1 plus tigecycline | 0.713 | 0.005 (0.03) | 0.566 (0.29) |
| HNP1 plus piperacillin | 0.471 | 0.008 (0.01) | 1.553 (0.23) |
| HNP1 plus meropenem | 0.564 | 0.004 (0.01) | 1.869 (0.49) |
OD450, optical density at 450 nm.
DISCUSSION
In this investigation of the bacterial killing effect of antimicrobial peptides against C. difficile, HBD3 had the strongest activity, followed by LL-37 and HBD2, whereas HBD1 had little or no antimicrobial effect. To our knowledge, this is the first report which describes the antimicrobial activities of defensins on vital C. difficile cells, whereas the activity of LL-37 against C. difficile was recently reported (10).
In a preceding study, the reduced form of HBD1 showed higher antimicrobial activity against Bifidobacterium spp. and Candida albicans than the native form (13). In this study, a reduction of the disulfide bonds led not to a pronounced effect of HBD1 but to decreased activity of the defensin HBD3 against C. difficile strains. The activity of LL-37 was not influenced by pretreatment with DTT, because in contrast to the defensins, the cathelicidin LL-37 is a linear helical peptide without disulfide bonds in its secondary structure.
We found that the flow cytometric test often required higher concentrations of the different antibiotics for depolarization of the bacteria than the MICs causing growth inhibition found using the Etest. This is in concordance with prior findings (14) with other bacterial species, because the Etest measures growth inhibition, but the flow cytometric test measures killing reflected by depolarization of the bacterial membrane. Nevertheless, subinhibitory antibiotic concentrations were adapted to the concentrations showing little or no antibacterial effect in the flow cytometric assay. Defensins or the cathelicidin LL-37 led to additive or synergistic effects with meropenem and moxifloxacin, even against the C. difficile strains, which were mostly resistant to these two antibiotics. The increased bacterial killing effect was most evident for the combination of antibiotics with the defensin HBD3 or the cathelicidin LL-37. As the electron microscopic investigations with HBD3 showed (Fig. 1B), it may be assumed that access of antibiotics into the bacterial cell may be supported by membrane perturbation by antimicrobial peptides. The observed effects were independent from the mode of action of the different antibiotics and independent of whether the antibiotic was bactericidal or bacteriostatic, such as tigecycline.
Concerning the toxin release of C. difficile strains incubated with antibiotics or antimicrobial peptides, none of the antibiotics tested at subinhibitory concentrations had an inhibiting effect on toxin production. Tigecycline and moxifloxacin provided antimicrobial efficacy without the induction of toxin release. This is in concordance with the findings of Baines et al. (15), who showed that tigecycline does not lead to increased cytotoxin production in a human gut model. Therefore, the combination of tigecycline and antimicrobial peptides is assumed to be a therapy option for C. difficile colitis, whereas the synergistic effect of defensins with piperacillin-tazobactam and meropenem may contribute to an adverse outcome due to an increased release of toxin from C. difficile cells in some strains.
Onderdonk et al. demonstrated that toxin production was affected by external stress factors (16). They measured increased amounts of toxin in response to alterations of the oxidation-reduction potential and subinhibitory concentrations of vancomycin and penicillin in a C. difficile strain, whereas clindamycin had no increasing effect. Comparative analysis from the supernatant and sonicated cells led to the hypothesis that C. difficile released more rather than produced more toxin in the medium. Inhibition of cell wall synthesis with subsequent membrane perturbation or the formation of pores on the cell wall may have contributed to an increased release of C. difficile toxin, which then was not neutralized by the added HNP1. The C. difficile toxin release from the bacterial cell is also supported by the fact that a similar amount of increased toxin was detected using the antimicrobial peptides HBD3 and LL-37 alone, which pass through pore formation or through the inhibition of protein biosynthesis for cell wall synthesis to kill the bacterial cell. Many studies have shown the effects of antibiotics on toxin production in C. difficile, but these were partly strain specific and therefore partially contradictory.
Using ciprofloxacin at subinhibitory concentrations, Aldape et al. demonstrated a significant and dose-dependent increase of toxin A gene expression and a shift of its expression to the earlier growth cycle in a highly ciprofloxacin-resistant isolate (17). TcdB gene expression was also increased but was less sensitive to low-dose ciprofloxacin. Nevertheless, moxifloxacin at subinhibitory concentrations did not lead to any alterations in toxin release in our strains.
Prophage carriage seems to be common in clinically relevant strains of C. difficile (18). Goh et al. suggested that lysogens carrying temperate phages can modify toxin production in C. difficile; furthermore, Sekulovic et al. were able to detect 1.6- to 2.1-fold more TcdA and TcdB in a NAP1/O27 strain infected with ΦCD38-2 than in the wild-type strain (19, 20). Therefore, prophage carriage may contribute to the strain-specific behavior when exposed to various antimicrobials and defensins, but in particular cases, this will have to be further verified.
It is expected that few new antimicrobials are in developmental stages to feasibly come onto the market in the coming years. Cationic peptides, like defensins or the cathelicidin LL-37, are substances that have found little application in practice. However, as combination partners that show synergistic effects at subinhibitory concentrations, they provide the opportunity to increase the bacterial killing effects of antibiotics even at low dosages.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by a grant from Pfizer Pharma GmbH.
We thank Birgit Fehrenbacher (Department of Dermatology, University of Tübingen, Germany) for excellent technical assistance and Edith Porter (Department of Biological Sciences, California State University, Los Angeles, CA) for critical revision of the manuscript.
We have no conflicts of interest to declare.
Footnotes
Published ahead of print 14 July 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.02542-14.
REFERENCES
- 1.Venugopal AA, Johnson S. 2012. Current state of Clostridium difficile treatment options. Clin. Infect. Dis. 55(Suppl 2):S71–S76. 10.1093/cid/cis355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Louie TJ, Miller MA, Mullane KM, Weiss K, Lentnek A, Golan Y, Gorbach S, Sears P, Shue YK, OPT-80-003 Clinical Study Group 2011. Fidaxomicin versus vancomycin for Clostridium difficile infection. N. Engl. J. Med. 64:422–431. 10.1056/NEJMoa0910812 [DOI] [PubMed] [Google Scholar]
- 3.Ganz T. 2003. Defensins: antimicrobial peptides of innate immunity. Nat. Rev. Immunol. 3:710–720. 10.1038/nri1180 [DOI] [PubMed] [Google Scholar]
- 4.Klotman ME, Chang TL. 2006. Defensins in innate antiviral immunity. Nat. Rev. Immunol. 6:447–456. 10.1038/nri1860 [DOI] [PubMed] [Google Scholar]
- 5.Ouellette AJ. 2011. Paneth cell a-defensins in enteric innate immunity. Cell. Mol. Life Sci. 68:2215–2229. 10.1007/s00018-011-0714-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wehkamp J, Harder J, Weichenthal M, Mueller O, Herrlinger KR, Fellermann K, Schroeder JM, Stange EF. 2003. Inducible and constitutive beta-defensins are differentially expressed in Crohn's disease and ulcerative colitis. Inflamm. Bowel Dis. 9:215–223. 10.1097/00054725-200307000-00001 [DOI] [PubMed] [Google Scholar]
- 7.Fahlgren A, Hammarström S, Danielsson A, Hammarstrom ML. 2004. Beta-defensin-3 and -4 in intestinal epithelial cells display increased mRNA expression in ulcerative colitis. Clin. Exp. Immunol. 137:379–385. 10.1111/j.1365-2249.2004.02543.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kai-Larsen Y, Agerberth B. 2008. The role of the multifunctional peptide LL-37 in host defense. Front. Biosci. 13:3760–3767 [DOI] [PubMed] [Google Scholar]
- 9.Brogden KA. 2005. Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nat. Rev. Microbiol. 3:238–250. 10.1038/nrmicro1098 [DOI] [PubMed] [Google Scholar]
- 10.McQuade R, Roxas B, Viswanathan VK, Vedantam G. 2012. Clostridium difficile clinical isolates exhibit variable susceptibility and proteome alterations upon exposure to mammalian cationic antimicrobial peptides. Anaerobe 18:614–620. 10.1016/j.anaerobe.2012.09.004 [DOI] [PubMed] [Google Scholar]
- 11.Giesemann T, Guttenberg G, Aktories K. 2008. Human alpha-defensins inhibit Clostridium difficile toxin B. Gastroenterology 134:2049–2058. 10.1053/j.gastro.2008.03.008 [DOI] [PubMed] [Google Scholar]
- 12.Nuding S, Zabel LT, Enders C, Porter E, Fellermann K, Wehkamp J, Mueller HA, Stange EF. 2009. Antibacterial activity of human defensins on anaerobic intestinal bacterial species: a major role of HBD-3. Microbes Infect. 11:384–393. 10.1016/j.micinf.2009.01.001 [DOI] [PubMed] [Google Scholar]
- 13.Schroeder BO, Wu Z, Nuding S, Groscurth S, Marcinowski M, Beisner J, Buchner J, Schaller M, Stange EF, Wehkamp J. 2011. Reduction of disulfide bonds unmasks potent antimicrobial activity of human β-defensin 1. Nature 469:419–423. 10.1038/nature09674 [DOI] [PubMed] [Google Scholar]
- 14.Nuding S, Zabel LT. 2013. Detection, identification, and susceptibility testing of bacteria by flow cytometry. J. Bacteriol. Parasitol. S5:e00l. 10.4172/2155-9597 [DOI] [Google Scholar]
- 15.Baines SD, Saxton K, Freeman J, Wilcox MH. 2006. Tigecycline does not induce proliferation or cytotoxin production by epidemic Clostridium difficile strains in a human gut model. J. Antimicrob. Chemother. 58:1062–1065. 10.1093/jac/dkl364 [DOI] [PubMed] [Google Scholar]
- 16.Onderdonk AB, Lowe BR, Bartlett JG. 1979. Effect of environmental stress on Clostridium difficile toxin levels during continuous cultivation. Appl. Environ. Microbiol. 38:637–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aldape MJ, Packham AE, Nute DW, Bryant AE, Stevens DL. 2013. Effects of ciprofloxacin on the expression and production of exotoxins by Clostridium difficile. J. Med. Microbiol. 62:741–747. 10.1099/jmm.0.056218-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shan J, Patel KV, Hickenbotham PT, Nale JY, Hargreaves KR, Clokie MR. 2012. Prophage carriage and diversity within clinically relevant strains of Clostridium difficile. Appl. Environ. Microbiol. 78:6027–6034. 10.1128/AEM.01311-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goh S, Chang BJ, Riley TV. 2005. Effect of phage infection on toxin production by Clostridium difficile. J. Med. Microbiol. 54:129–135. 10.1099/jmm.0.45821-0 [DOI] [PubMed] [Google Scholar]
- 20.Sekulovic O, Meessen-Pinard M, Fortier L-C. 2011. Prophage-stimulated toxin production in Clostridium difficile NAP1/O27 lysogens. J. Bacteriol. 193:2726–2734. 10.1128/JB.00787-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.