Abstract
Toxoplasmosis and amebiasis are important public health concerns worldwide. The drugs currently available to control these diseases have proven limitations. Therefore, innovative approaches should be adopted to identify and develop new leads from novel scaffolds exhibiting novel modes of action. In this paper, we describe results from the screening of compounds in the Medicines for Malaria Venture (MMV) open access Malaria Box in a search for new anti-Toxoplasma and anti-Entamoeba agents. Standard in vitro phenotypic screening procedures were adopted to assess their biological activities. Seven anti-Toxoplasma compounds with a 50% inhibitory concentration (IC50) of <5 μM and selectivity indexes (SI) of >6 were identified. The most interesting compound was MMV007791, a piperazine acetamide, which has an IC50 of 0.19 μM and a selectivity index of >157. Also, we identified two compounds, MMV666600 and MMV006861, with modest activities against Entamoeba histolytica, with IC50s of 10.66 μM and 15.58 μM, respectively. The anti-Toxoplasma compounds identified in this study belong to scaffold types different from those of currently used drugs, underscoring their novelty and potential as starting points for the development of new antitoxoplasmosis drugs with novel modes of action.
INTRODUCTION
Infectious diseases exert a very heavy toll on populations at risk worldwide. Of particular note is that infections caused by pathogenic protozoa affect the most vulnerable people, leading to tremendous economic and social burdens.
The diseases caused by protozoan parasites are responsible for considerable mortality and morbidity, affecting >500 million people worldwide (1). Currently, chemotherapy remains an essential component of both clinical management and disease control programs in areas where these diseases are endemic. A number of factors, such as high cost, poor compliance, drug resistance, low efficacy, and poor safety, limit the utility of antiprotozoan drugs.
Globally, toxoplasmosis is an important zoonosis with potentially devastating health impacts for humans and for a range of domestic and wild species (2). In humans, the causative agent (Toxoplasma gondii) has a simple life cycle consisting of two asexual parasite forms, tachyzoites and bradyzoites, that affect more than one-third of the world's population (3).Tachyzoites are rapidly growing obligate intracellular T. gondii forms that are commonly found in acutely infected individuals. Following a successful lytic phase in immunocompetent individuals, T. gondii tachyzoites differentiate into slowly growing bradyzoites that then establish latent infection as tissue cysts in vital organs, such as the brain, heart, and kidneys. When a cyst ruptures, a stage transition from latent bradyzoites back to rapidly growing tachyzoites occurs, causing destruction of the surrounding tissue. The reasons for recrudescence of eye toxoplasmosis have not been completely defined, but it is a lifelong problem, particularly in congenitally infected patients and in patients who acquired the infection shortly after birth (4, 5). Toxoplasmosis is a widely distributed parasitic disease, with seroprevalence rates as high as 98% in some regions of the world (6, 7). In the United States, for instance, about 1 million new infections occur each year, resulting in approximately 20,000 cases of retinal infection (8) and 750 deaths, making it the second most common cause of death related to food-borne diseases (9). Although infections in most animals and humans are asymptomatic, toxoplasmosis can cause severe illness in congenitally infected children, leading to severe sequelae that include complete blindness and various neurological impairments in developing fetuses and newborns, and in most immunologically depressed subjects, particularly organ transplant and AIDS patients (10).
Overall, studies on Toxoplasma should be spurred on for two important reasons. First, Toxoplasma can cause severe and life-threatening disease in developing fetuses and in immunocompromised patients. Second, although available drugs can treat Toxoplasma infections, they are poorly tolerated and are ineffective against chronic Toxoplasma infections. Clearly, all existing antitoxoplasmosis therapies, including the antimalarial compound pyrimethamine and the antibiotics clindamycin and sulfadiazine, are ineffective against bradyzoites. In addition, resistance to some of these drugs was recently reported (10–12), highlighting the urgent need for new and improved antitoxoplasmosis agents. An ideal anti-Toxoplasma drug would be potent and nontoxic and would eliminate latent infection (bradyzoites).
Amebiasis caused by Entamoeba histolytica is often associated with high morbidity and mortality. It continues to be a major public health problem throughout the world, especially in sub-Saharan Africa, where sanitation infrastructures and health facilities are usually inadequate (13, 14). The clinical features of amebiasis range from asymptomatic colonization to amebic colitis (dysentery or diarrhea) and invasive extraintestinal amebiasis, which commonly manifests as liver abscesses (15). With 40 to 50 million cases of amebic colitis and amebic liver abscess and up to 100,000 deaths annually, WHO estimates place amebiasis as a major public health threat throughout the world, second only to malaria in terms of mortality (16–18). Recent data showed an increase in the occurrence of E. histolytica among HIV patients (19–22). Although many drugs destroy E. histolytica within the colonic lumen, the number of tissue amebicides used to treat invasive amebiasis is still relatively limited. Metronidazole (MTZ), which is the drug of choice for invasive amebiasis, and other nitroimidazoles have greatly simplified chemotherapy for this disease. However, decades after the introduction of these drugs for the therapy of amebiasis, there have been few innovations in treating this infection. The toxic effects of MTZ and recent failures in the treatment of several intestinal protozoan parasites have led to a search for alternative amebicidal drugs (23).
Globally, the burden of infectious diseases is such that innovative drugs are greatly needed, especially for the control of protozoan infections. The aim of this work was to repurpose the 400 blood-stage-active anti-Plasmodium hits from the open access Malaria Box (24) to discover active chemical entities against Toxoplasma gondii and Entamoeba histolytica using standard in vitro drug susceptibility assays. This strategy is similar to the one used for extending the indications of existing treatments for other human and animal ailments to tropical diseases (25–27). Importantly, this fast-track approach has been successful, resulting in some of the most important currently used antiparasitic drugs, such as ivermectin for filariasis/onchocerciasis and praziquantel for schistosomiasis, and it continues to have a major role in the global drug discovery and development strategy for tropical diseases (28–30).
MATERIALS AND METHODS
The compounds were obtained from the Medicines for Malaria Venture (MMV) (Geneva, Switzerland) and used for the experiments. The Malaria Box was supplied in V-shaped 96-well plates in 20 μl of a 10 mM dimethyl sulfoxide (DMSO) solution and shipped frozen. The chemical purity based on liquid chromatography-mass spectrometry (LC-MS) was >90%. Plate mapping and full data on the Malaria Box with the original GSK/St Jude/Novartis compound numbers, structures, canonical simplified molecular-input line-entry system (SMILES), biological data, and selected in silico physicochemical parameters are available (see Table S1 in reference 24 and http://www.mmv.org/research-development/malaria-box-supporting-information). A list of vendors used to supply compounds for the Malaria Box, including their website addresses and the number of compounds from each vendor in the Malaria Box as of December 2011, is also available (see Table S2 in reference 24). For compounds to be of most interest, a fresh solid sample was repurchased from the vendors. The compounds were used at a 30 μM top concentration and diluted as needed in respective culture media for individual experiments.
Human foreskin fibroblasts (HFFs) were purchased from the ATCC (HS68). Standard strains T. gondii TS-4 and E. histolytica Rahman were obtained from BEI Resources (Manassas, VA). According to the product sheets, T. gondii TS-4 (ATCC 40050) is a mutant of the RH strain, which was isolated in 1939 from a 6-year-old boy in Cincinnati, Ohio, with a lethal case of encephalitis (31), and E. histolytica Rahman (ATCC 30886) was isolated in 1972 from the feces of an adult human male asymptomatic cyst passer in England (32).
T. gondii cultures.
T. gondii TS-4 parasites optimally grow in human foreskin fibroblasts (HFFs). Following the product recommendations, HS68 HFFs (ATCC) were cultured in 75-cm2 cell culture flasks (Corning Incorporated, USA) using Dulbecco's modified Eagle medium (DMEM) supplemented with 10% heat-inactivated fetal bovine serum (HIFBS) and 1% penicillin-streptomycin (10,000 units and 10 mg/ml, respectively). At approximately 90% confluence, the cells were trypsinized using a 1:3 dilution of 1× trypsin-EDTA plus magnesium in calcium-free phosphate-buffered saline (PBS) (Sigma, Germany) and passaged into a new culture flask or used in the drug susceptibility studies as described below.
To propagate the T. gondii parasites, the thawed content of a parasite's vial was aseptically transferred to a tissue culture flask containing a fresh monolayer of HFFs and 10 ml of growth medium containing 3% (vol/vol) HIFBS and 1% penicillin-streptomycin (Sigma, Germany) and incubated at 37°C in a humidified chamber containing 5% CO2. T. gondii parasites were maintained in tissue culture by thrice-weekly passages in 75-cm2 cell culture flasks (Corning Incorporated, USA) in DMEM (ATCC, USA) supplemented with 1% penicillin-streptomycin and 3% FBS (Sigma).
In vitro cytotoxicity studies on human foreskin fibroblasts.
HFFs were used for cytotoxicity profiling of the Malaria Box compounds. Cytotoxicity tests were performed in 96-well microtiter culture plates (Costar, USA) using serial dilutions of the compounds and incubation at 37°C for 24 h in a humidified chamber containing 5% CO2. Thereafter, 20 μl of 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium/phenazinemethosulfate (MTS/PMS) (Promega) was introduced into each well and further incubated for 1.5 h at 37°C. Negative control wells consisted of cultures with equivalent concentrations of DMSO. The total absorbance in each well was recorded at 490 nm using a 96-well plate reader (EL800; BioTek, USA). The percent growth inhibition was calculated from the optical densities relative to the negative control, and 50% cytotoxic concentration (CC50) values were determined using GraphPad Prism 5.0. Selectivity indices (SIs) of the drugs were subsequently calculated on the basis of their antiparasitic activities (IC50) and host cell cytotoxicity (CC50) profiles, and the results were used to aid in compound selection for progression into the hit optimization phase.
Assessment of anti-Toxoplasma activity in vitro.
Drugs were tested for anti-Toxoplasma activity in vitro by targeting the parasite tachyzoite form as previously described (33), with some modifications. HFFs were harvested during exponential growth (day 2) and cultured in 96-well plates (100-μl suspension consisting of 6 × 104 cells/ml; Costar, USA). A total of 100 μl of inoculum (6 × 105 parasites/ml adjusted using a hemocytometer and trypan blue exclusion dye) was added to each well (parasite/cell ratio, ∼10:1; final volume, 200 μl). Six hours after inoculation, nonadherent parasites were removed, and 100 μl of complete DMEM (1% penicillin-streptomycin, 3% FBS) supplemented with inhibitors at different concentrations (2-fold serial dilutions starting from 30 μM) was added to all except the negative-control wells. Positive controls, consisting of pyrimethamine (PYR) and sulfadiazine (SDZ) (20-mg/ml stocks in DMSO; Sigma-Aldrich), were tested at a 2-mg/ml final concentration. Each test was performed in triplicate. Culture plates were incubated at 37°C in a humidified 5% CO2 atmosphere for 24 h. A total of 20 μl of MTS/PMS (Promega) was then introduced into each well of the 96-well assay plate and incubated at 37°C for 1.5 h in a humidified 5% CO2 atmosphere. Thereafter, the absorbances were immediately recorded at 490 nm using a 96-well plate reader (EL800; BioTek, USA). These data were normalized to percent control activity, and 50% inhibitory concentrations (IC50s) were calculated using Prism 5.0 software (GraphPad) per the following variable slope sigmoidal dose-response formula with data fitted by nonlinear regression and the bottom and top values constrained to 0 and 100, respectively: y = 100/1 + 10(logIC50 − x)H (where y = percent inhibition; 100 = bottom + [top − bottom]; x = drug concentration; and H = hill slope [largest absolute value of the slope of the curve]). For sigmoidal dose-response analysis data, see Data S3 in the supplemental material.
Entamoeba histolytica cultures.
The E. histolytica Rahman strain (ATCC 30886) was primarily cultured in Eagle's minimum essential medium (EMEM) (Sigma, Germany) supplemented with various concentrations of heat-inactivated fetal bovine serum (HIFBS) and 1% (vol/vol) of the commercial penicillin-streptomycin solution (Sigma, Germany) in sterile 15-ml screw-cap tubes but showed poor growth and no significant difference. In contrast, the replacement of FBS with adult bovine serum (ABS) resulted in optimal trophozoite growth. EMEM supplemented with ABS and 1% (vol/vol) of the commercial penicillin-streptomycin solution was then used successfully to maintain E. histolytica Rahman in culture. The parasites were subcultured twice weekly. Parasites at the log phase of growth were used for drug susceptibility assays and were grown for 1 day following regular subculturing procedures.
In vitro anti-Entamoeba assay.
Anti-E. histolytica drug activities were determined as previously described (34) with the following modifications. Primary screening was performed with each compound at a 30 μM final concentration and in triplicate in sealed 96-well polystyrene microtiter plates (Costar, USA). Parasite inocula (100 μl) comprising 2 × 104 parasites/ml (adjusted using trypan blue exclusion dye [Sigma] and hemocytometer-based counts) were added to each well and grown at 37°C in a humidified atmosphere of 5% CO2 for 72 h. Positive controls consisted of metronidazole at 1 mg/ml. The growth of E. histolytica trophozoites in each well was determined microscopically by measuring the diameters of the confluent cells in drug-containing wells relative to those in the negative-control wells.
Based on the confluence and proportion of motile parasites, the growth inhibition in each triplicate well was scored as follows: 4+, <20% confluent cells and 100% of parasites dead; 3+, <50% confluent cells and <50% parasite motility; 2+, >50% confluent cells and >50% parasite motility; 1+, 100% confluent cells and 100% parasite motility (0 growth inhibition). Only the compounds with scores of 4+, 3+, or 2+ were considered for IC50 determination to confirm the compound activities.
For the dose-response experiments, 100 μl of a 2-fold serial dilution of each selected compound was added to 100 μl of E. histolytica trophozoite inocula (2 × 104 cells/ml) in complete EMEM supplemented with 10% ABS in a 96-well microtiter plate. Plates were sealed with expanded polystyrene and incubated as described above at 37°C for 72 h. Positive-control wells contained metronidazole at the highest concentration (0.5 mg/ml), whereas the negative-control wells contained culture medium with equivalent numbers of trophozoites. The number of viable parasites in each well was determined using trypan blue exclusion dye, and the percent inhibition was calculated with respect to the negative control. IC50s were determined as described above using GraphPad Prism 5.0. For sigmoidal dose-response analysis data, see Data S3 in the supplemental material.
RESULTS
In the screening of the Malaria Box content, 7 compounds showed potent anti-Toxoplasma gondii activity (IC50, <5 μM) and 2 compounds moderately inhibited Entamoeba histolytica (IC50s, ∼10 to 15 μM). The results are summarized below and discussed in later sections. Additional information on the moderately active compounds that represent alternative options for anti-Toxoplasma structure-activity relationship (SAR)-based optimization studies are presented in the supplemental material.
Cellular cytotoxicity of drugs.
From the cytotoxicity assays against HFFs, none of the compounds showed significant toxic effects on cells after 72 h of incubation. Few compounds showed limited cell growth inhibition (overall <40% inhibition) at a 30 μM final concentration, which was several orders of magnitude above the IC50s of the promising compounds. Therefore, the CC50 values were >30 μM against HFFs (Table 1).
TABLE 1.
Hits with potential for further extensive medicinal chemistry/biological screenings against T. gondii and E. histolytica
| Compounda | Mol wtb | AlogPb | IC50 (μM)c | CC50 against HFFs (μM)d | SIe |
|---|---|---|---|---|---|
| Anti-Toxoplasma gondii TS-4 | |||||
| MMV007791 | 357.40362 | 1.565 | 0.19 | >30 | >157 |
| MMV007881 | 378.48574 | 3.784 | 1.07 | >30 | >28.04 |
| MMV007363 | 232.70872 | 3.303 | 1.49 | >30 | >20.13 |
| MMV006704 | 291.39014 | 3.91 | 1.95 | >30 | >15.38 |
| MMV666095 | 280.16035 | 4.203 | 3.05 | >30 | >9.83 |
| MMV020548 | 413.51478 | 3.765 | 3.85 | >30 | >7.79 |
| MMV085203 | 362.42167 | 3.719 | 4.54 | >30 | >6.60 |
| PYR | 3.62 | ||||
| SDZ | 26.05 | ||||
| Anti-Entamoeba histolytica Rahman | |||||
| MMV666600 | 459.92087 | 6.819 | 10.66 | >30 | >2.81 |
| MMV006861 | 355.43233 | 4.324 | 15.58 | >30 | >1.92 |
| MTZ | 5.83 |
Compounds are designated by their MMV identifier codes. PYR, pyrimethamine; SDZ, sulfadiazine; MTZ, metronidazole.
Molecular weight and AlogP values were obtained from Malaria Box supporting information.
Compounds were serially diluted and tested in culture. Results are means from triplicate experiments.
Cytotoxicity against HFFs was evaluated in culture. Results are means from triplicate experiments.
Selectivity indices were calculated based on the ratio CC50 (HFF)/IC50 test drugs.
Anti-Toxoplasma activity of drugs.
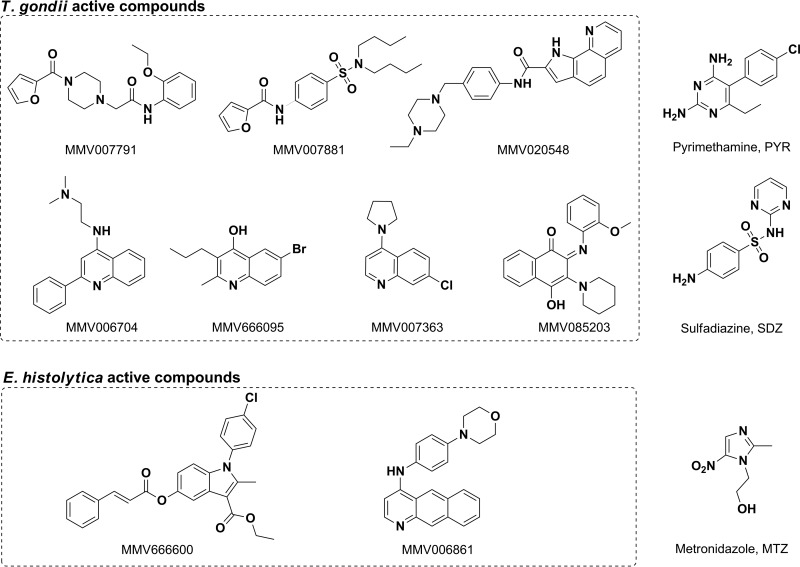
Serially diluted compounds were screened for in vitro activity against T. gondii. Of the 200 drug-like and 200 probe-like compounds tested, 45 (27 drug-like and 18 probe-like) inhibited the growth of T. gondii as determined by the MTS/PMS-based cell viability assay. Based on our activity cutoff criteria (IC50, <5 μM; SI, >6), 7 compounds showed potent activities (Table 1 and Fig. 1), whereas 38 others moderately inhibited the parasites, with IC50s ranging from 5.85 to 28.99 μM (see Table S1 and Fig. S2 in the supplemental material). These results indicate that among the most potent inhibitors (IC50, <5 μM), the drug-like compound MMV007791 inhibited the parasites with an IC50 of 190 nM and an SI of >157. The 6 others exhibited anti-T. gondii activities with an IC50 of <5 μM and an SI of >6.
FIG 1.
Scaffolds and compounds with high potential for anti-Toxoplasma drug discovery and anti-Entamoeba SAR-based studies. MMV identifiers and structures were provided by the MMV as part of the supporting information for the open access Malaria Box. Triplicate concentrations of serially diluted compounds were tested in vitro against Toxoplasma gondii TS-4 strain tachyzoites and Entamoeba histolytica Rahman strain trophozoites. IC50 (50% inhibitory concentration) values were calculated from sigmoidal dose-response curves. Structure and MMV identifiers are shown for each listed compound. Scaffolds were obtained from SAR studies. Anti-Toxoplasma compounds: MMV007791, N-(2-ethoxy-phenyl)-2-[4-(furan-2-carbonyl)-piperazin-1-yl]-acetamide; MMV085203, 4-hydroxy-2-(2-methoxy-phenyl imino)-3-piperidin-1-yl-2H-naphthalen-1-one; MMV007881, furan-2-carboxylic acid (4-dibutylsulfamoyl-phenyl)-amide; MMV020548, 1H-pyrrolo[3,2-h]quinoline-2-carboxylic acid [4-(4-ethyl-piperazin-1-ylmethyl)-phenyl]-amide; MMV007363, 7-chloro-4-pyrrolidin-1-yl-quinoline; MMV006704, N,N-dimethyl-N′-(2-phenyl-quinolin-4-yl)-ethane-1,2-diamine; MMV666095, 6-bromo-2-methyl-3-propyl-quinolin-4-ol. Anti-Entamoeba compounds: MMV666600, 1-(4-chloro-phenyl)-2-methyl-5-(3-phenyl-acryloyloxy)-1H-indole-3-carboxylic acid ethyl ester; MMV006861, benzo[g]quinolin-4-yl-(4-morpholin-4-yl-phenyl)-amine.
Anti-Entamoeba activity.
The 400 Malaria Box compounds were primarily screened at a single concentration of 30 μM to select promising candidates for IC50 determination. E. histolytica trophozoite growth and viability were evaluated by microscopic estimation of the diameters of confluent cells and by standard trypan blue exclusion assays, respectively. Following our hit selection criteria, 5 compounds, MMV006861 and MMV666600 (scored 4+), MMV006303 (scored 3+), and MMV006172 and MMV006087 (scored 2+), were selected for IC50 determination. By dose-response analyses, only two (MMV006861 and MMV666600) of these five compounds displayed promising potency, with IC50s of 15.58 μM and 10.66 μM, respectively. These results are summarized in Table 1, and their structures are presented in Fig. 1.
DISCUSSION
According to our hit criteria (IC50, <5 μM and SI, >6), 7 anti-Toxoplasma compounds were identified in this study (Fig. 1). This represents a 1.75% hit rate, a value that seems to be above the standard 0.1% observed in other screens. A putative reason could be that these compounds already have properties favorable for them to reach their biological target in a whole-cell assay, as all compounds are known to be active against the blood-stage form of Plasmodium falciparum in vitro. The potential commonality of a target shared between the two protozoan parasites may be another explanation for the improved hit rate.
First, 6 of the compounds showed an activity range similar to that of pyrimethamine, with IC50s going from 1.07 to 4.54 μM with moderate to good selectivities.
However, the structures seemed novel, with the possible exception of MMV007881, which has a 4-aminobenzenesulfonamide group embedded in the structure, similar to that in sulfadiazine. MMV020548 is composed of piperazine and a pyrroloquinoline carboxamide with an IC50 of 3.85 μM and an SI of >7. Three compounds, MMV007363, MMV006704, and MMV666095, contain a quinolone fragment with an IC50 of 1.49 μM, 1.95 μM, or 3.05 μM and an SI of >20, 15, or 9, respectively. Of interest is the close structural proximity of MMV007363 and MMV006704 to the marketed antimalarial chloroquine. Also, quinoline and derivatives tested with diverse pharmacological-activity functional groups constitute an important class of compounds for new drug development. For example, a recent study described highly efficacious endochin-like quinolones against acute and latent experimental toxoplasmosis (35). The study showed that this scaffold contains highly potent derivatives against acute and latent toxoplasmosis, with IC50s of <1 nM in vitro, that are also effective in mouse models. Overall, the quinolone scaffold represents a target group for detailed medicinal chemistry for anti-Toxoplasma drug discovery. MMV085203 is a 2,3-diaminonaphthoquinone with an IC50 of 4.54 μM and can be compared to the P. falciparum mitochondrial cytochrome bc1 complex (1) inhibitor atovaquone, which has known anti-Toxoplasma activity (36).
More interestingly, MMV007791 showed a significantly higher potency (IC50, 190 nM) and an excellent selectivity index (SI, >157) against HFFs. This chemotype is novel for its anti-Toxoplasma activity, and the structure is composed of a piperazine acetamide moiety. Additional data on this compound are reported in the open access database powered by the European Bioinformatics Institute (ChEMBL website [see https://www.ebi.ac.uk/chembl/malaria]), where the compound is reported to have good selectivity over those of other pathogens, as it has no activity against kinetoplastids (IC50, >32 μM), no effect on the nonreplicating form of Mycobacterium tuberculosis at 25 μM, no activity against newly transformed schistosomula at 2.5 μM, and good overall SI profiles, as no overt toxicities were seen against MRC-5 (CC50, >32 μM) and Huh7 (CC50, >100 μM) cells. Hence, compounds with the closely related 2-phenoxy-N-phenylacetamide core structure have attracted considerable research interest, as these entities have demonstrated a variety of biological activities, e.g., antiparasitic (37), anticancer (38), and antiviral (39) effects. Of particular interest is their activity against Toxoplasma gondii enoyl reductase (37). These derivatives have also shown P-glycoprotein and Mycobacterium tuberculosis H37Rv inhibition in vitro (40, 41). Also, in vitro drug metabolism and pharmacokinetic (DMPK) data suggest that these scaffolds may have some liability, as they have high intrinsic clearance rates (46 and 906 μl/min/mg in humans and mice, respectively) in liver microsomes. Efforts in SAR-based studies to address the metabolism and the potential genotoxicity risk represented by the anilines are considered.
In a very recent study, a strategy similar to ours was adopted to repurpose the open access Malaria Box to identify chemical series active against Cryptosporidium parvum (42). The authors showed in a subsidiary objective that the 2,4-diamino-quinazoline-based compounds were also potent growth inhibitors of the related apicomplexan parasite T. gondii of the RH strain from which the TS-4 strain used in our study was derived. Compounds of this series from commercial origin exerted anti-Toxoplasma activity, with IC50s ranging from 0.55 to 7.3 μM. From our findings, two derivatives (MMV019199 and MMV080034) of this series showed moderate activity (28.12 μM and 28.99 μM, respectively) compared to the other study's results. Given that the T. gondii TS-4 strain used in our study is a mutant of the RH strain used by these authors, it is likely that significant differences in the susceptibilities of different T. gondii strains exist and might justify the observed differences in activity levels.
Currently recommended drugs for the treatment of toxoplasmosis act primarily against the tachyzoite form of T gondii; thus, they do not eradicate the encysted form (bradyzoites) that maintains the parasite in a latent state in tissues. Pyrimethamine is the most effective agent and is included in most drug regimens. The most effective available therapeutic combination is pyrimethamine plus sulfadiazine or the trisulfapyrimidines (e.g., a combination of sulfamerazine, sulfamethazine, and sulfapyrazine). These agents are active against tachyzoites and are synergistic when used in combination (43–45). These compounds belong to the pyrimidine scaffold. In contrast, all the compounds identified in our study belong to other scaffold types, underscoring their novelty and potential as starting points for the development of new antitoxoplasmosis drugs with novel modes of action.
In a recent work, the potent Malaria Box compound MMV008138 was tested against the T. gondii RH-HX-KO-YFP2-DHFR(m2m3) strain. The authors observed no effect of MMV008138 on T. gondii growth (46), similar to what we found.
Other similar studies attempting to identify novel hit compounds active against T. gondii were conducted recently. Among their findings, compounds of (benzaldehyde)-4-phenyl-3-thiosemicarbazone and (benzaldehyde)-(4 or 1)-phenylsemicarbazone scaffolds showed limited efficacies against intracellular tachyzoites (47). Also, a label extension strategy was adopted to assess the anti-Toxoplasma activities of nine antiretroviral drugs in vitro. Nucleoside analogs showed no effects on parasite growth, whereas ritonavir and nelfinavir were inhibitory for Toxoplasma, with IC50 values of 7.50 and 7.05 μM, respectively (48). Fusidic acid also showed efficacy against T. gondii in vitro but not in mice (49). In another study, 1-hydroxy-2-dodecyl-4(1H)quinolone exhibited growth inhibition of T. gondii at a nanomolar concentration, with the alternative (type II) NADH dehydrogenases as the specific target (50). Recently, two novel 1-hydroxyquinolones were shown to display low nanomolar anti-Toxoplasma activities (51).
From our study, two compounds were identified, but they have very modest activities against E. histolytica. They are MMV666600 (IC50, 10.66 μM), which is a 2-methyl-1H-indole derivative, and MMV006861 (IC50, 15.58 μM), which is a benzo[g]quinolin-4-ylamine derivative. Metronidazole, which is the mainstay therapy for invasive amebiasis (52, 53), belongs to the imidazole class.
From a similar approach, some investigators recently described the in vitro anti-Entamoeba activity of an FDA-approved drug for use in rheumatoid arthritis (auranofin). The IC50 of auranofin against E. histolytica trophozoites was <2 μM. It is notable that auranofin had much higher cysticidal activity on Entamoeba invadens cysts than the standard amebicide metronidazole, providing a potential therapeutic advantage (54, 55).
Our study identified compounds with low micromolar anti-Toxoplasma activities (IC50s, <5 μM) and acceptable physicochemical drug profiles. Ongoing studies based on the extensive medicinal chemistry and metabolism of these compounds will identify related scaffolds with improved biological properties. Compounds that showed moderate activity might be potential options for SAR-based optimization studies. In addition, detailed studies on the MMV Malaria Box novel scaffolds that were identified as anti-Entamoeba in this work might be fruitful in drug development against amebiasis.
Plasmodium falciparum, Toxoplasma gondii, and Entamoeba histolytica are parasitic protozoa. Within this family, apicomplexans are a large group of parasitic protists, most of which possess a unique organelle, a type of plastid called an apicoplast, an apical complex structure most likely involved in penetrating a host's cell that is currently among the prioritized targets for drug discovery. P. falciparum and T. gondii share this feature, as do many other metabolic drug targets. The Malaria Box compounds that inhibited T. gondii might therefore likely target the parasite apicoplast, in contrast to E. histolytica, which is not an apicomplexan. Further studies on the rates and mechanisms of drug action will elucidate these considerations.
We report here the results from repurposing the Malaria Box to identify hit compounds against T. gondii and E. histolytica. The findings are encouraging and support further investigations to find drug candidates with innovative and acceptable profiles.
Supplementary Material
ACKNOWLEDGMENTS
We are very grateful to the MMV, which supported this work through a challenge grant (MMV-12/0087) and provided the open access Malaria Box.
Toxoplasma gondii strain TS-4 and the Entamoeba histolytica Rahman strain were obtained from BEI Resources, NIAID, NIH.
We thank Lawrence Ayong for his critical review of the manuscript.
Footnotes
Published ahead of print 21 July 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.02541-14.
REFERENCES
- 1.Monzote L, Siddiq A. 2011. Drug development to protozoan diseases. Open Med. Chem. J. 5:1–3. 10.2174/1874104501105010001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chadwick EA, Cable J, Chinchen A, Francis J, Guy E, Kean EF, Paul SC, Perkins SE, Sherrard-Smith E, Wilkinson C, Forman DW. 2013. Seroprevalence of Toxoplasma gondii in the Eurasian otter (Lutra lutra) in England and Wales. Parasit. Vectors 6:75. 10.1186/1756-3305-6-75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klaren VN, Kijlstra A. 2002. Toxoplasmosis, an overview with emphasis on ocular involvement. Ocul. Immunol. Inflamm. 10:1–26. 10.1076/ocii.10.1.1.10330 [DOI] [PubMed] [Google Scholar]
- 4.Glasner PD, Silveira C, Kruszon-Moran D, Martins MC, Burnier M, Jr, Silveira S, Camargo ME, Nussenblatt RB, Kaslow RA, Belfort R., Jr 1992. An unusually high prevalence of ocular toxoplasmosis in southern Brazil. Am. J. Ophthalmol. 114:136–144 [DOI] [PubMed] [Google Scholar]
- 5.Roberts F, Mets MB, Ferguson DJ, O'Grady R, O'Grady C, Thulliez P, Brezin AP, McLeod R. 2001. Histopathological features of ocular toxoplasmosis in the fetus and infant. Arch. Ophthalmol. 119:1–58 [PubMed] [Google Scholar]
- 6.Shin DW, Cha DY, Hua QJ, Cha GH, Lee YH. 2009. Seroprevalence of Toxoplasma gondii infection and characteristics of seropositive patients in general hospitals in Daejeon, Korea. Korean J. Parasitol. 47:125–130. 10.3347/kjp.2009.47.2.125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Silveira C, Belfort R, Jr, Burnier M, Jr, Nussenblatt R. 1988. Acquired toxoplasmic infection as the cause of toxoplasmic retinochoroiditis in families. Am. J. Ophthalmol. 106:362–364. 10.1016/0002-9394(88)90382-0 [DOI] [PubMed] [Google Scholar]
- 8.Jones JL, Holland GN. 2010. Annual burden of ocular toxoplasmosis in the United States. Am. J. Trop. Med. Hyg. 82:464–465. 10.4269/ajtmh.2010.09-0664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scallan E, Hoekstra RM, Angulo FJ, Tauxe RV, Widdowson MA, Roy SL, Jones JL, Griffin PM. 2011. Foodborne illness acquired in the United States—major pathogens. Emerg. Infect. Dis. 17:7–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dannemann B, McCutchan JA, Israelski D, Antoniskis D, Leport C, Luft B, Nussbaum J, Clumeck N, Morlat P, Chiu J, Vilde JL, Orellana M, Feigal D, Bartok A, Heseltine P, Leedom J, Remington J. 1992. Treatment of toxoplasmic encephalitis in patients with AIDS: a randomized trial comparing pyrimethamine plus clindamycin to pyrimethamine plus sulfadiazine. Ann. Intern. Med. 116:33–43. 10.7326/0003-4819-116-1-33 [DOI] [PubMed] [Google Scholar]
- 11.Aspinall TV, Joynson DH, Guy E, Hyde JE, Sims PF. 2002. The molecular basis of sulfonamide resistance in Toxoplasma gondii and implications for the clinical management of toxoplasmosis. J. Infect. Dis. 185:1637–1643. 10.1086/340577 [DOI] [PubMed] [Google Scholar]
- 12.Baatz H, Mirshahi A, Puchta J, Gumbel H, Hattenbach LO. 2006. Reactivation of Toxoplasma retinochoroiditis under atovaquone therapy in an immunocompetent patient. Ocul. Immunol. Inflamm. 14:185–187. 10.1080/09273940600659740 [DOI] [PubMed] [Google Scholar]
- 13.Ximénez C, Morán P, Rojas L, Valadez A, Gómez A. 2009. Reassessment of the epidemiology of amebiasis: state of the art. Infect. Genet. Evol. 9:1023–1032. 10.1016/j.meegid.2009.06.008 [DOI] [PubMed] [Google Scholar]
- 14.Bansal D, Malla N, MahajanIndian RC. 2006. Drug resistance in amoebiasis. Indian J. Med. Res. 123:115–118 [PubMed] [Google Scholar]
- 15.Fotedar R, Stark D, Beebe N, Marriott D, Ellis J, Harkness J. 2007. Laboratory diagnostic techniques for Entamoeba species. Clin. Microbiol. Rev. 20:511–532. 10.1128/CMR.00004-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stanley SL., Jr 2003. Amoebiasis. Lancet 361:1025–1034. 10.1016/S0140-6736(03)12830-9 [DOI] [PubMed] [Google Scholar]
- 17.Ravdin JI, Stauffer WM. 2005. Entamoeba histolytica (amoebiasis), p 3097–3111 In Mandell GL, Bennett JE, Dolin R. (ed), Principles and practice of infectious diseases, vol 2 Churchill Livingstone, Philadelphia, PA [Google Scholar]
- 18.WHO. 1997. WHO/PAHO/UNESCO report: a consultation with experts on amoebiasis. Mexico City, Mexico 28-29 January 1997. Epidemiol. Bull. 18:13–14 [PubMed] [Google Scholar]
- 19.Moran P, Ramos F, Ramiro M, Curiel O, González E, Valadez A, Gómez A, García G, Melendro EI, Ximénez C. 2005. Entamoeba histolytica and/or Entamoeba dispar: infection frequency in HIV+/AIDS patients in Mexico City. Exp. Parasitol. 110:331–334. 10.1016/j.exppara.2005.03.023 [DOI] [PubMed] [Google Scholar]
- 20.Hung CC, Ji DD, Sun HY, Lee YT, Hsu SY, Ya TL, Shui YH, Sui YC, Cheng HW, Yun HC, Chin FH, Wen CL, Robert C. 2008. Increased risk for Entamoeba histolytica infection and invasive amoebiasis in HIV seropositive men who have sex with men in Taiwan. PLoS Negl. Trop. Dis. 2:e175. 10.1371/journal.pntd.0000175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Samie A, Barrett LJ, Bessong PO, Ramalivhana JN, Mavhandu LG, Njayou M, Gurreant RL. 2010. Seroprevalence of Entamoeba histolytica in the context of HIV and AIDS: the case of the Vhembe district, in South Africa's Limpopo province. Ann. Trop. Med. Parasitol. 104:55–63. 10.1179/136485910X12607012373911 [DOI] [PubMed] [Google Scholar]
- 22.Watanabe K, Gatanaga H, Escueta-de Cadiz A, Tanuma J, Nozaki T, Oka S. 2011. Amebiasis in HIV-1-infected Japanese men: clinical features and response to therapy. PLoS Negl. Trop. Dis. 5:e1318. 10.1371/journal.pntd.0001318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chacín-Bonilla L. 2012. Current pharmacotherapy of amebiasis, advances in new drugs, and design of a vaccine. Invest. Clin. 53:301–314 (In Spanish.) [PubMed] [Google Scholar]
- 24.Spangenberg T, Burrows JN, Kowalczyk P, McDonald S, Wells TNC, Willis P. 2013. The open access Malaria Box: a drug discovery catalyst for neglected diseases. PLoS One 8:e62906. 10.1371/journal.pone.0062906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pink R, Hudson A, Mouriès MA, Bendig M. 2005. Opportunities and challenges in antiparasitic drug discovery. Nat. Rev. Drug Discov. 4:727–740. 10.1038/nrd1824 [DOI] [PubMed] [Google Scholar]
- 26.Rosenthal PJ. 2003. Antimalarial drug discovery: old and new approaches. J. Exp. Biol. 206:3735–3744. 10.1242/jeb.00589 [DOI] [PubMed] [Google Scholar]
- 27.Witty M. 1999. Current strategies in the search for novel antiparasitic agents. Int. J. Parasitol. 29:95–103. 10.1016/S0020-7519(98)00193-3 [DOI] [PubMed] [Google Scholar]
- 28.Omura S, Crump A. 2004. The life and times of ivermectin—a success story. Nat. Rev. Microbiol. 2:984–989. 10.1038/nrmicro1048 [DOI] [PubMed] [Google Scholar]
- 29.Ridley RG. 2004. Research on infectious diseases requires better coordination. Nat. Med. 10:S137–S140. 10.1038/nm1153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Horton J. 2003. Drug development for tropical diseases—present situation, future perspectives. Trends Parasitol. 19:P06 [Google Scholar]
- 31.Sabin AB. 1941. Toxoplasmic encephalitis in children. JAMA 116:801–807 [Google Scholar]
- 32.Mattern CF, Keister DB. 1977. Experimental amebiasis. II. Hepatic amebiasis in the newborn hamster. Am. J. Trop. Med. Hyg. 26:402–411 [PubMed] [Google Scholar]
- 33.Jin C, Kaewintajuk K, Jiang J, Jeong W, Kamata M, Kim HS, Wataya Y, Park H. 2009. Toxoplasma gondii: a simple high-throughput assay for drug screening in vitro. Exp. Parasitol. 121:132–136. 10.1016/j.exppara.2008.10.006 [DOI] [PubMed] [Google Scholar]
- 34.Upcroft JA, Upcroft P. 2001. Drug susceptibility testing of anaerobic protozoa. Antimicrob. Agents Chemother. 45:1810–1814. 10.1128/AAC.45.6.1810-1814.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Doggett JS, Nilsen A, Forquer I, Wegmann KW, Jones-Brando L, Yolken RH, Bordón C, Charman SA, Katneni K, Schultz T, Burrows JN, Hinrichs DJ, Meunier B, Carruthers VB, Riscoe MK. 2012. Endochin-like quinolones are highly efficacious against acute and latent experimental toxoplasmosis. Proc. Natl. Acad. Sci. U. S. A. 109:15936–15941. 10.1073/pnas.1208069109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Djurković-Djaković O, Milenković V, Nikolić A, Bobić B, Grujić J. 2002. Efficacy of atovaquone combined with clindamycin against murine infection with a cystogenic (Me49) strain of Toxoplasma gondii. J. Antimicrob. Chemother. 50:981–987. 10.1093/jac/dkf251 [DOI] [PubMed] [Google Scholar]
- 37.Tipparaju SK, Muench SP, Mui EJ, Ruzheinikov SN, Lu JZ, Hutson SL, Kirisits MJ, Prigge ST, Roberts CW, Henriquez FL, Kozikowski AP, Rice DW, McLeod RL. 2010. Identification and development of novel inhibitors of Toxoplasma gondii enoyl reductase. J. Med. Chem. 53:6287–6300. 10.1021/jm9017724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sun A, Prussia A, Zhan W, Murray EE, Doyle J, Cheng LT, Yoon JJ, Radchenko EV, Palyulin VA, Compans RW, Liotta DC, Plemper RK, Snyder JP. 2006. Nonpeptide inhibitors of measles virus entry. J. Med. Chem. 49:5080–5092. 10.1021/jm0602559 [DOI] [PubMed] [Google Scholar]
- 39.Hedstrom LK, Striepen B. June 2007. IMP dehydrogenase inhibitors for treating mammalian gastrointestinal parasitic infections. Patent WO2007143557
- 40.Ang W, Lin YN, Tao Y, Yang JZ, Pi WY, Yang YH, Luo YF, Deng Y, Wei YQ. 2012. Synthesis and biological evaluation of 2-(3-fluoro-4-nitro phenoxy)-N-phenylacetamide derivatives as novel potential affordable antitubercular agents. Molecules 17:2248–2258. 10.3390/molecules17022248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee K, Roh SH, Xia Y, Kang KW. 2011. Synthesis and biological evaluation of phenoxy-N-phenylacetamide derivatives as novel P-glycoprotein inhibitors. Bull. Korean Chem. Soc. 32:3666–3674. 10.5012/bkcs.2011.32.10.3666 [DOI] [Google Scholar]
- 42.Bessoff K, Spangenberg T, Foderaro JE, Jumani RS, Ward GE, Huston CD. 2014. Identification of Cryptosporidium parvum active chemical series by repurposing the open access Malaria Box. Antimicrob. Agents Chemother. 58:2731–2739. 10.1128/AAC.02641-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sobrin L, Kump LI, Foster CS. 2007. Intravitreal clindamycin for toxoplasmic retinochoroiditis. Retina 27:952–957. 10.1097/IAE.0b013e31804b3f0d [DOI] [PubMed] [Google Scholar]
- 44.Soheilian M, Ramezani A, Azimzadeh A, Sadoughi MM, Dehghan MH, Shahghadami R, Yaseri M, Peyman GA. 2011. Randomized trial of intravitreal clindamycin and dexamethasone versus pyrimethamine, sulfadiazine, and prednisolone in treatment of ocular toxoplasmosis. Ophthalmology 118:134–141. 10.1016/j.ophtha.2010.04.020 [DOI] [PubMed] [Google Scholar]
- 45.Soheilian M, Sadoughi MM, Ghajarnia M, Dehghan MH, Yazdani S, Behboudi H, Anisian A, Peyman GA. 2005. Prospective randomized trial of trimethoprim/sulfamethoxazole versus pyrimethamine and sulfadiazine in the treatment of ocular toxoplasmosis. Ophthalmology 112:1876–1882. 10.1016/j.ophtha.2005.05.025 [DOI] [PubMed] [Google Scholar]
- 46.Bowman JD, Merino EF, Brooks CF, Striepen B, Carlier PR, Cassera MB. 2014. Anti-apicoplast and gametocytocidal screening to identify the mechanisms of action of compounds within the Malaria Box. Antimicrob. Agents Chemother. 58:811–819. 10.1128/AAC.01500-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gomes MAGB, Carvalho LP, Rocha BS, Oliveira RR, de Melo EJT, Maria EJ. 2013. Evaluating anti-Toxoplasma gondii activity of new series of phenylsemicarbazone and phenylthiosemicarbazones in vitro. Med. Chem. Res. 22:3574–3580. 10.1007/s00044-012-0347-9 [DOI] [Google Scholar]
- 48.Derouin F, Santillana-Hayat M. 2000. Anti-Toxoplasma activities of antiretroviral drugs and interactions with pyrimethamine and sulfadiazine in vitro. Antimicrob. Agents Chemother. 44:2575–2577. 10.1128/AAC.44.9.2575-2577.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Payne AJ, Neal LM, Knoll LJ. 2013. Fusidic acid is an effective treatment against Toxoplasma gondii and Listeria monocytogenes in vitro, but not in mice. Parasitol. Res. 112:3859–3863. 10.1007/s00436-013-3574-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Saleh A, Friesen J, Baumeister S, Gross U, Bohne W. 2007. Growth inhibition of Toxoplasma gondii and Plasmodium falciparum by nanomolar concentrations of 1-hydroxy-2-dodecyl-4(1H)quinolone, a high-affinity inhibitor of alternative (type II) NADH dehydrogenases. Antimicrob. Agents Chemother. 51:1217–1222. 10.1128/AAC.00895-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bajohr LL, Ma L, Platte C, Liesenfeld O, Tietze LF, Groß U, Bohne W. 2010. In vitro and in vivo activities of 1-hydroxy-2-alkyl-4(1H)quinolone derivatives against Toxoplasma gondii. Antimicrob. Agents Chemother. 54:517–521. 10.1128/AAC.01001-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kimura M, Nakamura T, Nawa Y. 2007. Experience with intravenous metronidazole to treat moderate-to-severe amebiasis in Japan. Am. J. Trop. Med. Hyg. 77:381–385 [PubMed] [Google Scholar]
- 53.Moon TD, Oberhelman RA. 2005. Antiparasitic therapy in children. Pediatr. Clin. North Am. 52:917–948. 10.1016/j.pcl.2005.02.012 [DOI] [PubMed] [Google Scholar]
- 54.Buckner FS, Waters NC, Avery VM. 2012. Recent highlights in anti-protozoan drug development and resistance research. Int. J. Parasitol. Drugs Drug Resist. 2:230–270. 10.1016/j.ijpddr.2012.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Debnath A, Parsonage D, Andrade RM, He C, Cobo ER, Hirata K, Chen S, García-Rivera G, Orozco E, Martínez MB, Gunatilleke SS, Barrios AM, Arkin MR, Poole LB, McKerrow JH, Reed SL. 2012. A high throughput drug screen for Entamoeba histolytica identifies a new lead and target. Nat. Med. 18:956–960. 10.1038/nm.2758 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.