Abstract
With the rising prevalence of antimicrobial resistance, the failure rate of the standard triple therapy for Helicobacter pylori infection is increasing. Sequential therapy and concomitant therapy have been recommended to replace standard triple therapy for H. pylori eradication in regions with high clarithromycin resistance. The aim of this prospective, randomized, and controlled study was to simultaneously assess the efficacies of 10-day sequential and 7-day concomitant therapies versus a 7-day standard triple therapy for treating H. pylori infection. Consecutive H. pylori-infected subjects were randomly assigned to a 7-day standard triple therapy (pantoprazole, clarithromycin, and amoxicillin for 7 days), a 10-day sequential therapy (pantoprazole and amoxicillin for 5 days, followed by pantoprazole, clarithromycin, and metronidazole for a further 5 days), or a 7-day quadruple therapy (pantoprazole, clarithromycin, amoxicillin, and metronidazole for 7 days). H. pylori status was confirmed 6 weeks after therapy. Three hundred seven H. pylori-infected participants were randomized to receive triple (n = 103), sequential (n = 102), or concomitant (n = 102) therapies. The eradication rates by an intention-to-treat analysis in the three treatment groups were 81.6% (95% confidence interval [CI], 74.1% to 89.0%), 89.2% (95% CI, 83.2% to 95.2%), and 94.1% (95% CI, 89.5% to 98.7%). The seven-day concomitant therapy had a higher eradication rate than did the 7-day triple therapy (difference, 12.5%; 95% CI, 3.7% to 21.3%). There were no significant differences in the eradication rates between the sequential and standard triple therapies. All three treatments exhibited similar frequencies of adverse events (8.7%, 8.8%, and 13.7%, respectively) and drug compliance (99.0%, 98.0%, and 100.0%, respectively). In conclusion, the seven-day concomitant therapy is superior to the 7-day standard triple therapy for H. pylori eradication. Additionally, it is less complex than the 10-day sequential therapy because the drugs are not changed halfway through the treatment course. (This study has been registered at ClinicalTrials.gov under registration no. NCT1769365.)
INTRODUCTION
Helicobacter pylori infects >50% of humans globally. It is the principal cause of chronic gastritis, gastric ulcer, duodenal ulcer, gastric adenocarcinoma, and gastric mucosa-associated lymphoid tissue lymphoma (MALToma) (1, 2). H. pylori eradication has become the standard and most widely adopted therapy for curing peptic ulcer disease (3, 4). This therapy is also strongly recommended for the treatment of H. pylori-related MALToma (5). In regions with a high incidence of gastric adenocarcinoma, the eradication of H. pylori is advocated as a preventative measure (6, 7).
In most international guidelines (6–9), standard triple therapy consisting of a proton pump inhibitor (PPI), clarithromycin, and amoxicillin (or metronidazole) for 7 to 14 days is recommended as the first-line therapy of H. pylori infection, especially in areas of low clarithromycin resistance (<10%). Recently, the eradication rates of standard triple therapy have declined to <80% in many countries, largely owing to emerging organism resistances (10–13). Some European studies even reported very poor treatment outcomes of the standard therapy, with failure rates of 25 to 60% (14–16). Several strategies, including bismuth-containing quadruple therapy and non-bismuth-containing quadruple therapy (either sequential or concomitant therapy), have therefore been proposed to increase the eradication rate (17–19).
The efficacy of sequential therapy consisting of a PPI and amoxicillin for the first 5 days, followed by a PPI plus clarithromycin and metronidazole for another 5 days, is less affected by clarithromycin resistance than standard triple therapy (17). It has been shown to be more effective than standard triple therapy for 7 or 10 days in clinical trials in Italy (18–20). However, two recent large-scale, multicenter, open-label, and randomized trials from Latin America and Taiwan revealed no significant differences in the treatment efficacies between a 10-day sequential therapy and a 14-day standard triple therapy (21, 22). Possible explanations for the discrepancies included the different antibiotic resistances of H. pylori strains and the different treatment durations of the standard triple therapy. Besides the regional variations in eradication efficacies, sequential therapy is much more complex in terms of medication requirements than standard triple therapy because of switching drugs halfway through the course. In clinical practice, changing drugs during a treatment course might reduce patient compliance and the inclination of a physician to prescribe and illustrate the regimen.
Concomitant therapy is another novel nonbismuth quadruple therapy proven to be successful in the presence of clarithromycin resistance (18). It is a 4-drug regimen containing a PPI, clarithromycin, amoxicillin, and metronidazole, which are all given for the entire duration of therapy. A head-to-head noninferiority trial of 10-day sequential and 10-day concomitant therapy by our group showed they were equivalent (23). It is less complex than sequential therapy and has excellent potential to replace standard triple therapy as the first-line treatment for H. pylori infection, especially in regions with high clarithromycin resistance. However, whether shortening the duration of concomitant therapy from 10 days to 7 days can still achieve a high success rate is unclear. To address this issue, we conducted a randomized controlled trial (registered at ClinicalTrials.gov under registration no. NCT1769365) to compare the efficacies of 7-day concomitant therapy and 10-day sequential therapy with that of 7-day standard triple therapy as a first-line anti-H. pylori therapy. Special attention was also paid to the impacts of H. pylori resistance to clarithromycin and metronidazole on the efficacies of the three therapies.
MATERIALS AND METHODS
Participants.
The open-labeled randomized trial was conducted at the Kaohsiung Veterans General Hospital in Taiwan in accordance with the principles of good clinical practice from the Declaration of Helsinki. Consecutive H. pylori-infected outpatients ≥20 years of age with endoscopically proven peptic ulcer diseases or gastritis were prospectively recruited for this study. The criteria for exclusion included (i) previous H. pylori eradication therapy, (ii) ingestion of antibiotics or bismuth within the prior 4 weeks, (iii) patients with allergic history to the medications used, (iv) patients with previous gastric surgery, (v) the coexistence of serious concomitant illness (for example, decompensated liver cirrhosis or uremia), and (vi) pregnant women.
All participants gave written informed consent before enrollment. The Medical Committee of the Kaohsiung Veterans General Hospital approved the trial. All authors had access to the study data and had reviewed and approved the final manuscript.
Randomization and treatment.
Using a computer-generated number sequence, the eligible H. pylori-infected patients were randomly assigned to a 7-day concomitant therapy (40 mg pantoprazole twice daily, 500 mg clarithromycin twice daily, 1 g amoxicillin twice daily, and 500 mg metronidazole twice daily for 7 days), a 10-day sequential therapy (40 mg pantoprazole twice daily and 1 g amoxicillin twice daily for 5 days, followed by 40 mg pantoprazole twice daily, 500 mg clarithromycin twice daily, and 500 mg metronidazole twice daily for a further 5 days), or a 7-day standard triple therapy (40 mg pantoprazole twice daily, 500 mg clarithromycin twice daily, and 1 g amoxicillin twice daily for 7 days). An independent research assistant generated the computerized random number sequence. The sequence was concealed in an opaque envelope until the intervention was assigned. After the written informed consents were obtained from the participants, an independent research assistant assigned the therapies according to the treatment allocations kept in the envelopes.
All drugs were taken 1 h before breakfast and dinner. Patients were asked to return at the 2nd week to assess drug compliance and adverse events. The patients with peptic ulcers found on the initial endoscopy received an additional 3-week monotherapy with 40 mg pantoprazole orally once daily, while patients with gastritis took only three weeks of antacid following eradication therapy.
Procedures.
Before enrollment, the status of H. pylori infection was determined by a rapid urease test (24), histology, and/or culture. Patients with positive results in at least two of these tests were eligible for enrollment. The patients were requested to complete a standard questionnaire for complete demographic data, including age, sex, medical history, history of smoking, and alcohol, coffee, and tea consumption.
The patients were informed of the common adverse events from the study drugs before treatment and were asked to record these symptoms during treatment in provided diaries. The adverse events were assessed according to a 4-point scale system: none, mild (discomfort annoying but not interfering with daily life), moderate (discomfort sufficient to interfere with daily life), and severe (discomfort resulting in discontinuation of eradication therapy) (25). Drug compliance was assessed via pill counts. Good compliance was defined as taking ≥80% of the total medication.
Because of the possibility that gastric cancer might be missed in the initial endoscopy as a benign gastric ulcer, a repeated endoscopy with a rapid urease test, histological examination, and culture at the 6th week after the end of anti-H. pylori therapy was performed in the gastric ulcer patients to assess the eradication efficacy and the healing status of the ulcer lesions. Since there was no concern about the malignant changes in duodenal ulcers or gastritis, a urea breath test was conducted to assess H. pylori status in participants with duodenal ulcer or gastritis (24). A staff member who was blind to the eradication arm of each patient performed the urea breath tests. The cutoff value was set at 4.8‰ of δ13 CO2 (25). Eradication was defined as (i) negative results of all rapid urease test, histology, and culture, or (ii) a negative result from the urea breath test.
Biopsy specimens were cultured according to previously described methods (26). Antibiotic susceptibility was determined by Etest (AB Biodisk, Solna, Sweden). H. pylori subculturing was done by rubbing the specimens on the surface of a Campy-BAP agar plate (Brucella agar; Difco, Sparks, MD) plus IsoVitaleX (Gibco, Grand Island, NY) plus 10% whole sheep blood, followed by incubation at 37°C under microaerobic conditions (5% O2, 10% CO2, and 85% N2) for 4 to 5 days. H. pylori strains were tested for clarithromycin, amoxicillin, and metronidazole susceptibilities using the Etest (AB Biodisk). H. pylori strains with MICs of >1 μg/ml, >0.5 μg/ml, and >8 μg/ml were considered to be resistant to clarithromycin, amoxicillin, and metronidazole, respectively (26).
Statistical analysis.
The primary endpoint of the study was the H. pylori eradication rate. The second endpoints were the frequency of adverse events and drug compliance. A chi-square test with or without the Yates correction for continuity and Fisher's exact test were used when appropriate to compare the major outcomes between the groups. A P value of <0.05 was considered statistically significant.
According to our previous study, the eradication rate of standard triple therapy is 82% (27). It is estimated that we required ≥102 patients in each treatment group to demonstrate a 10% absolute difference in the eradication rate with a type I error of 0.05 and a type II error and power of 80%, assuming a 5% loss to follow-up. The eradication rates were evaluated by intention-to-treat (ITT) and per-protocol (PP) analyses. The ITT analysis included all randomized patients who had taken at least one dose of the study medication. The patients whose infection statuses were unknown following treatment were considered treatment failures for the purposes of the ITT analysis. The PP analysis excluded the patients with unknown H. pylori status following therapy and those with major protocol violations.
RESULTS
Characteristics of the study groups.
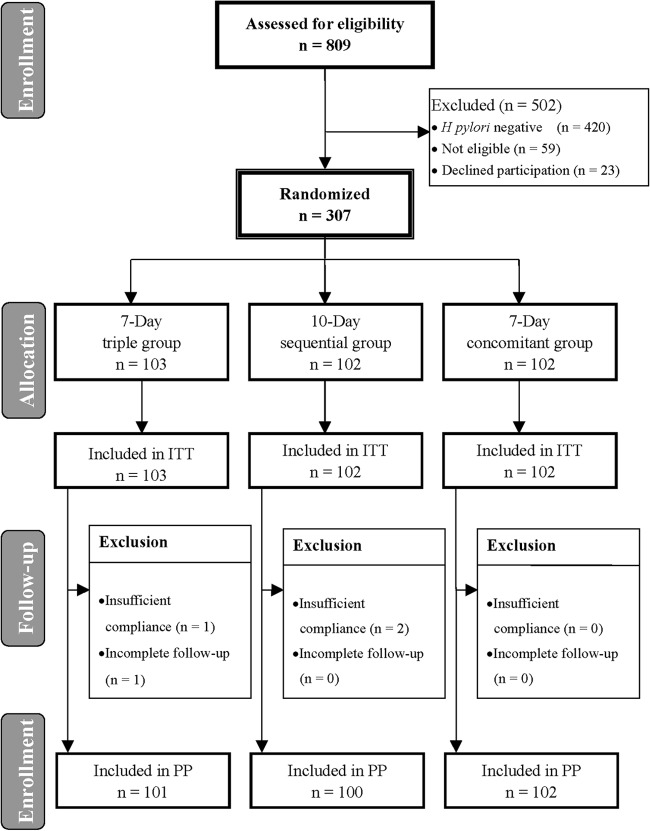
A total of 307 H. pylori-infected patients were randomly assigned to receive a 7-day concomitant (n = 102), 7-day standard triple (n = 103), or 10-day sequential therapy (n = 102). The subjects were all included in the ITT analysis for H. pylori eradication. The data regarding the clinical characteristics of the patients at entry are summarized in Table 1. The three groups had comparable ages, gender, history of smoking, alcohol, coffee, and tea consumption, and antibiotic resistances. Among the subjects, three with poor compliance and one with incomplete follow-up were excluded from the PP analysis for H. pylori eradication. Figure 1 summarizes the patient characteristics.
TABLE 1.
Baseline characteristics of the treated population
| Characteristica | Triple therapy (n = 103) | Sequential therapy (n = 102) | Concomitant therapy (n = 102) |
|---|---|---|---|
| Age (mean ± SD) (yr) | 56.08 ± 14.00 | 54.96 ± 12.00 | 53.85 ± 12.31 |
| Gender (no. of males/no. of females) | 62/41 | 52/50 | 61/41 |
| Smoking | 22 (21) | 22 (22) | 26 (26) |
| Alcohol consumption | 9 (9) | 8 (8) | 7 (7) |
| Ingestion of coffee | 23 (22) | 27 (27) | 28 (28) |
| Ingestion of tea | 35 (34) | 40 (39) | 43 (42) |
| NSAID user | 4 (4) | 2 (2) | 4 (4) |
| Underlying diseases | 27 (26) | 19 (19) | 21 (21) |
| Endoscopic findings | |||
| Gastritis | 25 (25) | 18 (18) | 23 (23) |
| Gastric ulcer | 34 (33) | 43 (42) | 29 (28) |
| Duodenal ulcer | 17 (17) | 18 (18) | 22 (22) |
| Gastric ulcer and duodenal ulcer | 27 (27) | 23 (23) | 28 (28) |
| Antibiotic sensitivity (no. of susceptible/no. of resistant)b | |||
| Clarithromycin (no. of susceptible/no. of resistant) | 32/7 | 39/5 | 41/5 |
| Amoxicillin (no. of susceptible/no. of resistant) | 39/0 | 44/0 | 46/0 |
| Metronidazole (no. of susceptible/no. of resistant) | 28/11 | 31/13 | 25/21 |
Values are shown as no. (%), unless otherwise indicated.
A total of 127 strains were isolated.
FIG 1.
Flowchart of the patients in the study.
Eradication of H. pylori.
Table 2 displays the major outcomes of the eradication therapies. The ITT analysis demonstrated that the standard triple, sequential, and concomitant therapies achieved eradication rates of 81.6% (95% confidence interval [CI], 74.1% to 89.0%), 89.2% (95% CI, 83.2% to 95.2%), and 94.1% (95% CI, 89.5% to 98.7%), respectively. The 7-day concomitant therapy had a higher eradication rate than did the 7-day standard triple therapy (difference, 12.5%; 95% CI, 3.7% to 21.3%; P = 0.006). However, the eradication rates did not differ significantly between the sequential and standard triple therapies or between the sequential and concomitant therapies. The PP analysis yielded similar results (eradication rates, 82.2%, 90.0%, and 94.1%, respectively). The eradication rates were greater for the 7-day concomitant therapy than that for 7-day standard triple therapy (95% CI, 3.2% to 20.6%; P = 0.003). There were no statistically significant differences in the treatment efficacies between the 10-day sequential and 7-day triple therapies.
TABLE 2.
Major outcomes of the three therapies
| Outcome characteristic | Rate (% [no./total no.]) (95% CI) |
||
|---|---|---|---|
| Standard triple therapy (n = 103) | Sequential therapy (n = 102) | Concomitant therapy (n = 102) | |
| Eradication rate | |||
| Intention-to-treat | 81.6 (84/103) (74.1–89.0) | 89.2 (91/102) (83.2–95.2) | 94.1 (96/102) (89.5–98.7)a |
| Per-protocol | 82.2 (83/101) (74.8–89.6) | 90.0 (90/100) (84.1–95.9) | 94.1 (96/102) (89.5–98.7)a |
| Adverse events | 8.7 (9/103) (3.3–14.2) | 8.8 (9/102) (3.3–14.3) | 13.7 (14/102) (7.0–20.4) |
| Compliance | 99.0 (102/103) (97.1–100.9) | 98.0 (100/102) (95.3–100.7) | 100.0 (102/102) |
Significant difference compared with standard triple therapy.
Univariate analysis revealed that clinical factors, including age, gender, smoking, alcohol, coffee, or tea consumption, nonsteroidal anti-inflammatory drug (NSAID) use, underlying disease, and initial endoscopic diagnosis did not affect the eradication efficacy. The eradication rates in the patients with gastritis, gastric ulcer, duodenal ulcer, and both gastric and duodenal ulcers were 86.4% (57/66), 89.3% (92/103), 89.5% (51/57), and 89.6% (69/77), respectively. There were also no statistically significant differences in the treatment efficacies between the assessed final H. pylori status of a patient by endoscopy and the urea breath test results (87.8% versus 89.4%, P = 0.657).
Impact of antibiotic resistance on eradication rates.
H. pylori strains were successfully isolated from 129 (80.6%) of the 160 patients receiving endoscopy and bacterial culture on enrollment. The rates of strains resistant to clarithromycin, amoxicillin, and metronidazole were 13.2% (16/129), 0.0% (0/129), and 34.9% (45/129), respectively.
In the 7-day standard therapy group, the eradication rates of the strains with nonresistance, clarithromycin resistance only, metronidazole resistance only, and dual resistances were 91.7%, 50%, 87.5%, and 66.7%, respectively (Table 3). The PP analysis found significantly lower eradication rates in the clarithromycin-resistant strains than those of the clarithromycin-susceptible strains (57.1% [4/7] versus 90.6% [29/32], respectively; P = 0.026) (Fig. 2). However, the eradication rates were comparable in the metronidazole-resistant and -sensitive strains (81.8% [9/11] versus 85.7% [24/28], respectively) (Fig. 3). In the 10-day sequential group, the eradication rates of the strains with nonresistance, clarithromycin resistance only, metronidazole resistance only, and dual resistances were 100.0%, 66.7%, 90.9%, and 50.0%, respectively (Table 3). The clarithromycin-resistant strains also exhibited lower eradication rates than did the clarithromycin-sensitive strains (60.0% [3/5] versus 97.4% [38/39], P = 0.030) (Fig. 2). The eradication rates did not differ between the metronidazole-resistant and -sensitive strains (84.6% [11/13] versus 96.8% [30/31], P = 0.204) (Fig. 3). In the 7-day concomitant therapy group, the eradication rates of the strains with nonresistance, clarithromycin resistance only, metronidazole resistance only, and dual resistances were 100.0%, 100.0%, 100.0%, and 66.7%, respectively (Table 3). No differences in the eradication rates existed between the clarithromycin-resistant and -sensitive strains (80.0% [4/5] versus 100.0% [41/41], P = 0.110) (Fig. 2) and between the metronidazole-resistant and -sensitive strains (95.2% [20/21] versus 100.0% [25/25], P = 0.478) (Fig. 3).
TABLE 3.
Eradication rates by PP analysis of the three therapies according to antibiotic susceptibility pattern of H. pylori
| Susceptibility pattern | No. eradicated/total no. (%) for therapy type: |
||
|---|---|---|---|
| Triple (n = 39) | Sequential (n = 44) | Concomitant (n = 46) | |
| Clas Mets | 22/24 (91.7) | 28/28 (100.0) | 23/23 (100.0) |
| Clas Metr | 7/8 (87.5) | 10/11 (90.9) | 18/18 (100.0) |
| Clar Mets | 2/4 (50.0) | 2/3 (66.7) | 2/2 (100.0) |
| Clar Metr | 2/3 (66.7) | 1/2 (50.0) | 2/3 (66.7) |
FIG 2.
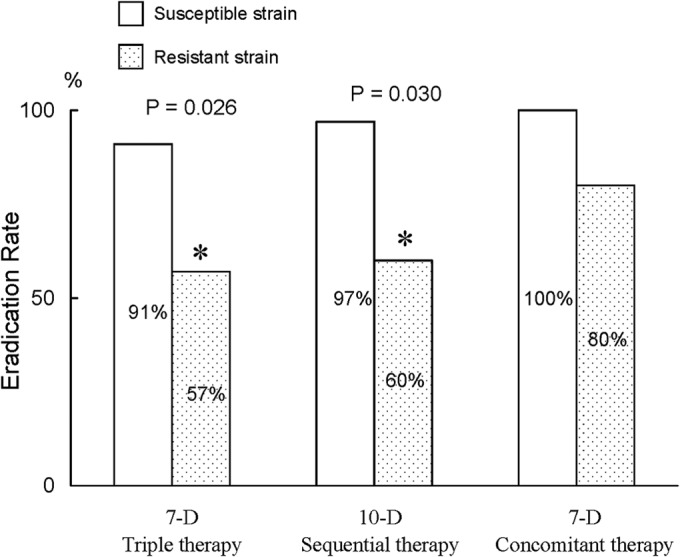
Impact of clarithromycin resistance on the eradication rates in the three treatment groups. The asterisks indicate a significant difference at a P value of <0.05. 10-D, 10-day; 7-D, 7-day.
FIG 3.
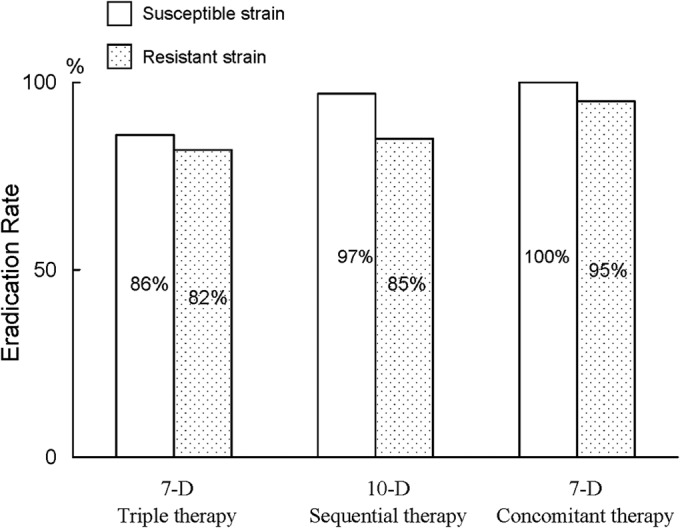
Impact of metronidazole resistance on the eradication rates in the three treatment groups. 10-D, 10-day; 7-D, 7-day.
Adverse events and compliance.
All of the patients received at least one dose of eradication medication and were included in the adverse event analysis. The incidences of adverse events in the participants receiving 7-day standard triple, 10-day sequential, and 7-day concomitant therapies were 8.7% (95% CI, 3.3% to 14.2%), 8.8% (95% CI, 3.3% to 14.3%), and 13.7% (95% CI, 7.0% to 20.4%), respectively. The three therapies exhibited similar frequencies of adverse events (Table 2, P = 0.182).
Table 4 lists the profiles of adverse events of the three eradication therapies. There were no significant differences in the frequencies of each adverse event between the three treatment groups. Vomiting was the most common adverse event in all three treatment groups (the incidences in standard triple, sequential, and concomitant, therapies were 3.9%, 4.9%, and 6.8%, respectively). In the standard triple group, one patient discontinued treatment due to palpitations and constipation. One patient in the concomitant group stopped the anti-H. pylori medication because of abdominal pain and headache. One of the patients in the sequential group stopped the medications due to constipation.
TABLE 4.
Adverse events of the treated population
| Adverse event | Total patients (no. of patients with mild/moderate/severe adverse events) for indicated therapy |
||
|---|---|---|---|
| Triple (n = 103) | Sequential (n = 102) | Concomitant (n = 102) | |
| Abdominal pain | 0 (0/0/0) | 0 (0/0/0) | 1 (0/0/1) |
| Constipation | 1 (0/0/1) | 1 (0/0/1) | 0 (0/0/0) |
| Diarrhea | 3 (3/0/0) | 0 (0/0/0) | 1 (1/0/0) |
| Dizziness | 0 (0/0/0) | 0 (0/0/0) | 0 (0/0/0) |
| Taste perversion | 1 (0/1/0) | 2 (0/2/0) | 4 (0/0/0) |
| Headache | 0 (0/0/0) | 1 (1/0/0) | 5 (3/1/1) |
| Anorexia | 0 (0/0/0) | 1 (1/0/0) | 1 (1/0/0) |
| Nausea | 3 (3/0/0) | 5 (0/5/0) | 7 (4/3/0) |
| Vomiting | 4 (4/0/0) | 5 (0/5/0) | 7 (4/3/0) |
| Skin rash | 1 (1/0/0) | 0 (0/0/0) | 0 (0/0/0) |
| Fatigue | 0 (0/0/0) | 1 (0/1/0) | 0 (0/0/0) |
| Other | 1 (0/0/1) | 1 (1/0/0) | 3 (3/0/0) |
All patients but three (one in the standard triple group and two in the sequential group) complied with the eradication therapies and took >80% of the assigned tablets. All the 7-day standard triple, 10-day sequential, and 7-day concomitant groups displayed similar compliance rates (99.0% [95% CI, 97.1% to 100.9%], 98.0% [95% CI, 95.3% to 100.7%], and 100.0%, respectively; Table 2).
DISCUSSION
The Maastricht IV/Florence Consensus Report recommended both sequential and concomitant therapies as a first-line empirical treatment for H. pylori infection in areas of high clarithromycin resistance (8). This study conducted the first, head-to-head, randomized, and controlled trial to simultaneously assess the efficacies of 7-day standard triple therapy, 10-day sequential therapy, and 7-day concomitant therapy for H. pylori eradication. The data clearly demonstrate that the 7-day concomitant therapy achieved a markedly higher eradication rate than did the 7-day triple therapy, whether using the ITT (94.1% versus 81.6%) or PP analyses (94.1% versus 82.2%). The eradication rate of the 7-day concomitant therapy was comparable with that of 10-day sequential therapy (94.1% versus 89.2% by ITT analysis and 94.1% versus 90.0% by PP analysis). The data presented here are consistent with those of our previous work showing similar eradication rates for the 10-day concomitant and 10-day sequential therapies (93.0% versus 92.3% by ITT analysis) (23). From the perspective of clinical practice, the concomitant regimen is much less complex than the sequential one, which is a two-step therapy with a switch in drugs halfway through the treatment course. Additionally, the treatment duration of the concomitant therapy in this study was shorter than that of the sequential therapy, and the treatments displayed similar frequencies of adverse events. Taken together, our findings lend support to the use of 7-day concomitant therapy as the standard first-line treatment for H. pylori infection in regions with moderate clarithromycin resistance rates (13.2%).
Our data are consistent with those of an independent study from Greece demonstrating that a 10-day concomitant regimen achieved a significantly higher eradication rate than did the 10-day standard triple therapy (28). However, another clinical trial by Greenberg et al. (21) showed that the eradication rate of a 14-day triple therapy was higher than that of a 5-day concomitant therapy (82% versus 74%, respectively) in Latin America. The reasons for the contradictory results were unclear, although different treatment durations are a possible explanation. A pilot study to identify the optimum duration of concomitant H. pylori eradication therapy from Thailand revealed that a 5-day concomitant therapy achieved an eradication rate of <90%, but a 10-day concomitant regimen provided excellent treatment success (eradication rate, >95%) (29). Two meta-analyses by Fuccio et al. and Ford and Moayyedi also revealed that the eradication rates of standard triple therapy were higher with a 14-day regimen than with a 7-day regimen (30, 31). Besides treatment duration, the difference in the prevalence of antibiotic resistances between the different geographic areas is probably another important explanation. In the current study, the frequencies of antibiotic resistance to clarithromycin and metronidazole were 13.2% and 34.9%, respectively. The reported prevalences of clarithromycin and metronidazole resistances in Latin America were 3.8% and 82%, respectively (32). The extremely high resistance to metronidazole in Latin America might decrease the benefits of adding metronidazole to the concomitant regimen.
Antibiotic resistance is a crucial determinant of treatment outcome in bacterial eradication. Clarithromycin resistance has been identified as the main reason for the failure of standard triple therapy (10–13). In the current study, patients with clarithromycin-resistant strains receiving standard triple therapy also exhibited a lower eradication rate than those with clarithromycin-susceptible strains (90.6% versus 57.1%, respectively). Although several randomized trials in Italy showed that clarithromycin resistance did not affect eradication with sequential treatment, our study demonstrated significant differences in the eradication rates of sequential therapy between clarithromycin-sensitive and -resistant strains (97.4% and 60.0%, respectively). Another independent randomized controlled trial from Taiwan supported this finding and showed that clarithromycin resistance decreased the efficacy of sequential therapy (22). Possible explanations for the discrepancies in the impact of clarithromycin resistance on the eradication rate included different metronidazole use in sequential therapy, different prevalences of metronidazole resistance, and different frequencies of CYP2C19 genotypes.
This study found poor efficacies of all the standard triple, sequential, and concomitant therapies for eradicating H. pylori strains with dual resistance to clarithromycin and metronidazole (eradication rates, 66.7%, 50.0%, and 66.7%, respectively). A randomized controlled trial by Malfertheiner et al. (32) showed that a 10-day quadruple therapy containing bismuth, metronidazole, tetracycline, and a PPI effectively eradicated H. pylori harboring dual resistance to clarithromycin and metronidazole (eradication rate, 92%). Recently, our study group reported a hybrid (dual-concomitant) therapy consisting of dual therapy with a PPI and amoxicillin for 7 days, followed by a concomitant quadruple therapy with a PPI, amoxicillin, clarithromycin, and metronidazole for 7 days (33). This novel therapy also achieved an excellent eradication rate (100%) for the treatment of H. pylori strains harboring dual resistance to clarithromycin and metronidazole. Sardarian et al. (34) recently demonstrated that hybrid therapy was superior to sequential therapy for first-line H. pylori eradication in Iran. The prolonged treatment duration of amoxicillin to 14 days in hybrid therapy might account for the high eradication rate for H. pylori strains with dual resistance.
The current study shows that all the three therapies were well tolerated and shared comparable adverse event profiles and frequencies. Drug compliance is an important determinant for the treatment outcome in bacterial eradication, particularly for the therapies with a short treatment duration. To improve patient compliance, all participants were given both verbal and written instructions regarding the importance of full compliance with taking medications and were recommended not to stop medication even in the event of mild to moderate side effects. Furthermore, the participants were given the telephone numbers of the study nurses so they could make inquiries during the daytime on weekdays if they had any questions concerning eradication therapy or wanted to stop their medication. This considerate service might contribute to the excellent compliance (all, ≥98%) of all the study regimens.
The strengths of this study include the comparison of three treatment groups and the use of a large sample size (>100 in each group). Additionally, this study provided the impacts of antibiotic resistances on the eradication results. Therefore, our findings are useful for assessing the PP eradication rates of 7-day standard triple, 10-day sequential, and 7-day concomitant therapies in different geographic areas by taking into consideration the prevalence of antibiotic resistance and the eradication outcomes of the different resistant strains (shown in Table 3). Our study has several limitations. First, antibiotic susceptibility data were available in only 42% of the patients. Second, the sample size was insufficient to detect small differences in the eradication rates between the treatment groups. Nonetheless, this study is the first randomized controlled trial simultaneously assessing the efficacies of 10-day sequential and 7-day concomitant therapies versus a 7-day standard triple therapy for treating H. pylori infection.
In conclusion, 7-day concomitant therapy is superior to 7-day standard triple therapy for H. pylori eradication. Additionally, it is simpler than sequential therapy because no change in drugs occurs halfway through the treatment course. The novel therapy can be recommended as the standard first-line treatment for H. pylori infection in areas with a high prevalence of clarithromycin resistance.
ACKNOWLEDGMENTS
We thank W. L. Tsai, H. H. Chan, K. H. Lin, and C. A. Shih for recruiting the patients, the study nurses at the Kaohsiung Veterans General Hospital, and L. P. Ger for statistical calculations.
This study was funded by research grant NSC 99-2314-B-075B-009-MY2 from the National Science Council.
P.-I. Hsu and D.-C. Wu recruited and followed up the patients, analyzed the data, and wrote the manuscript; F.-W. Tsay designed the study, recruited and followed up the patients, analyzed the data, and reviewed the manuscript; W.-C. Chen, H.-C. Yu, H.-M. Wang, S.-S. Kao, and K.-H. Lai performed endoscopies; H.-H. Tseng interpreted the pathology slides; and A. Chen performed the bacterial culture and urease test.
We have no conflicts of interest to disclose.
Footnotes
Published ahead of print 28 July 2014
REFERENCES
- 1.Suerbaum S, Michetti P. 2002. Helicobacter pylori infection. N. Engl. J. Med. 347:1175–1186. 10.1056/NEJMra020542 [DOI] [PubMed] [Google Scholar]
- 2.Malfertheiner P, Chan FK, McColl KE. 2009. Peptic ulcer disease. Lancet 374:1449–1461. 10.1016/S0140-6736(09)60938-7 [DOI] [PubMed] [Google Scholar]
- 3.Graham DY, Lew GM, Klein PD, Evans DG, Evans DJ, Jr, Saeed ZA, Malaty HM. 1992. Effect of treatment of Helicobacter pylori infection on the long-term recurrence of gastric or duodenal ulcer. A randomized controlled study. Ann. Intern. Med. 116:705–708 [DOI] [PubMed] [Google Scholar]
- 4.Sung JJ, Chung SC, Ling TK, Yung MY, Leung VK, Ng EK, Li MK, Cheng AF, Li AK. 1995. Antibacterial treatment of gastric ulcer associated with Helicobacter pylori. N. Engl. J. Med. 332:139–142. 10.1056/NEJM199501193320302 [DOI] [PubMed] [Google Scholar]
- 5.Zucca E, Dreyling M, ESMO Guidelines Working Group 2009. Gastric marginal zone lymphoma of MALT type: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann. Oncol. 20(Suppl 4):S113–S114. 10.1093/annonc/mdp146 [DOI] [PubMed] [Google Scholar]
- 6.Fock KM, Katelaris P, Sugano K, Ang TL, Hunt R, Talley NJ, Lam SK, Xiao SD, Tan HJ, Wu CY, Jung HC, Hoang BH, Kachintorn U, Goh KL, Chiba T, Rani AA, Second Asia-Pacific Conference 2009. Second Asia-Pacific Consensus Guidelines for Helicobacter pylori infection. J. Gastroenterol. Hepatol. 24:1587–1600. 10.1111/j.1440-1746.2009.05982.x [DOI] [PubMed] [Google Scholar]
- 7.Asaka M, Kato M, Takahashi S, Fukuda Y, Sugiyama T, Ota H, Uemura N, Murakami K, Satoh K, Sugano K, Japanese Society for Helicobacter Research 2010. Guidelines for the management of Helicobacter pylori infection in Japan: 2009 revised edition. Helicobacter 15:1–20. 10.1111/j.1523-5378.2009.00738.x [DOI] [PubMed] [Google Scholar]
- 8.Malfertheiner P, Megraud F, O'Morain CA, Atherton J, Axon AT, Bazzoli F, Gensini GF, Gisbert JP, Graham DY, Rokkas T, El-Omar EM, Kuipers EJ, The European Helicobacter Study Group (EHSG) 2012. Management of Helicobacter pylori infection—the Maastricht IV/Florence Consensus Report. Gut 61:646–664. 10.1136/gutjnl-2012-302084 [DOI] [PubMed] [Google Scholar]
- 9.Chey WD, Wong BC, Practice Parameters Committee of the American College of Gastroenterology 2007. American College of Gastroenterology guideline on the management of Helicobacter pylori infection. Am. J. Gastroenterol. 102:1808–1825. 10.1111/j.1572-0241.2007.01393.x [DOI] [PubMed] [Google Scholar]
- 10.Graham DY, Shiotani A. 2008. New concepts of resistance in the treatment of Helicobacter pylori infections. Nat. Clin. Pract Gastroenterol. Hepatol. 5:321–331. 10.1038/ncpgasthep1138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mégraud F. 2004. H. pylori antibiotic resistance: prevalence, importance, and advances in testing. Gut 53:1374–1384. 10.1136/gut.2003.022111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luther J, Higgins PD, Schoenfeld PS, Moayyedi P, Vakil N, Chey WD. 2010. Empiric quadruple vs triple therapy for primary treatment of Helicobacter pylori infection: systematic review and meta-analysis of efficacy and tolerability. Am. J. Gastroenterol. 105:65–73. 10.1038/ajg.2009.508 [DOI] [PubMed] [Google Scholar]
- 13.De Francesco V, Margiotta M, Zullo A, Hassan C, Troiani L, Burattini O, Stella F, Di Leo A, Russo F, Marangi S, Monno R, Stoppino V, Morini S, Panella C, Ierardi E. 2006. Clarithromycin-resistant genotypes and eradication of Helicobacter pylori. Ann. Intern. Med. 144:94–100. 10.7326/0003-4819-144-2-200601170-00006 [DOI] [PubMed] [Google Scholar]
- 14.Gumurdulu Y, Serin E, Ozer B, Kayaselcuk F, Ozsahin K, Cosar AM, Gursoy M, Gur G, Yilmaz U, Boyacioglu S. 2004. Low eradication rate of Helicobacter pylori with triple 7–14 days and quadruple therapy in Turkey. World J. Gastroenterol. 10:668–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bigard MA, Delchier JC, Riachi G, Thibault P, Barthelemy P. 1998. One-week triple therapy using omeprazole, amoxycillin and clarithromycin for the eradication of Helicobacter pylori in patients with non-ulcer dyspepsia: influence of dosage of omeprazole and clarithromycin. Aliment. Pharmacol. Ther. 12:383–388. 10.1046/j.1365-2036.1998.00315.x [DOI] [PubMed] [Google Scholar]
- 16.De Francesco V, Margiotta M, Zullo A, Hassan C, Giorgio F, Burattini O, Stoppino G, Cea U, Pace A, Zotti M, Morini S, Panella C, Ierardi E. 2007. Prevalence of primary clarithromycin resistance in Helicobacter pylori strains over a 15 year period in Italy. J. Antimicrob. Chemother. 59:783–785. 10.1093/jac/dkm005 [DOI] [PubMed] [Google Scholar]
- 17.Zullo A, De Francesco V, Hassan C, Morini S, Vaira D. 2007. The sequential therapy regimen for Helicobacter pylori eradication: a pooled-data analysis. Gut 56:1353–1357. 10.1136/gut.2007.125658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Essa AS, Kramer JR, Graham DY, Treiber G. 2009. Meta-analysis: four-drug, three-antibiotic, non-bismuth-containing “concomitant therapy” versus triple therapy for Helicobacter pylori eradication. Helicobacter 14:109–118. 10.1111/j.1523-5378.2009.00671.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jafri NS, Hornung CA, Howden CW. 2008. Meta-analysis: sequential therapy appears superior to standard therapy for Helicobacter pylori infection in patients naive to treatment. Ann. Intern. Med. 148:923–931. 10.7326/0003-4819-148-12-200806170-00226 [DOI] [PubMed] [Google Scholar]
- 20.Vaira D, Zullo A, Vakil N, Gatta L, Ricci C, Perna F, Hassan C, Bernabucci V, Tampieri A, Morini S. 2007. Sequential therapy versus standard triple-drug therapy for Helicobacter pylori eradication: a randomized trial. Ann. Intern. Med. 146:556–563. 10.7326/0003-4819-146-8-200704170-00006 [DOI] [PubMed] [Google Scholar]
- 21.Greenberg ER, Anderson GL, Morgan DR, Torres J, Chey WD, Bravo LE, Dominguez RL, Ferreccio C, Herrero R, Lazcano-Ponce EC, Meza-Montenegro MM, Peña R, Peña EM, Salazar-Martínez E, Correa P, Martínez ME, Valdivieso M, Goodman GE, Crowley JJ, Baker LH. 2011. 14-day triple, 5-day concomitant, and 10-day sequential therapies for Helicobacter pylori infection in seven Latin American sites: a randomised trial. Lancet 378:507–514. 10.1016/S0140-6736(11)60825-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liou JM, Chen CC, Chen MJ, Chen CC, Chang CY, Fang YJ, Lee JY, Hsu SJ, Luo JC, Chang WH, Hsu YC, Tseng CH, Tseng PH, Wang HP, Yang UC, Shun CT, Lin JT, Lee YC, Wu MS, Taiwan Helicobacter Consortium 2012. Sequential versus triple therapy for the first-line treatment of Helicobacter pylori: a multicentre, open-label, randomised trial. Lancet 381:205–213. 10.1016/S0140-6736(12)61579-7 [DOI] [PubMed] [Google Scholar]
- 23.Wu DC, Hsu PI, Wu JY, Opekun AR, Kuo CH, Wu IC, Wang SS, Chen A, Hung WC, Graham DY. 2010. Sequential and concomitant therapy with four drugs are equally effective for eradication of H. pylori infection. Clin. Gastroenterol. Hepatol. 8:36–41. 10.1016/j.cgh.2009.09.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hsu PI, Lai KH, Hsu PN, Lo GH, Yu HC, Chen WC, Tsay FW, Lin HC, Tseng HH, Ger LP, Chen HC. 2007. Helicobacter pylori infection and the risk of gastric malignancy. Am. J. Gastroenterol. 102:1–6. 10.1111/j.1572-0241.2007.01057.x [DOI] [PubMed] [Google Scholar]
- 25.Wu DC, Hsu PI, Tseng HH, Tsay FW, Lai KH, Kuo CH, Wang SW, Chen A. 2011. Helicobacter pylori infection: a randomized, controlled study comparing 2 rescue therapies after failure of standard triple therapies. Medicine (Baltimore) 90:180–185. 10.1097/MD.0b013e31821c9d1c [DOI] [PubMed] [Google Scholar]
- 26.Hsu PI, Wu DC, Wu JY, Graham DY. 2011. Is there a benefit to extending the duration of Helicobacter pylori sequential therapy to 14 days? Helicobacter 16:146–152. 10.1111/j.1523-5378.2011.00829.x [DOI] [PubMed] [Google Scholar]
- 27.Hsu PI, Lai KH, Lin CK, Chen WC, Yu HC, Cheng JS, Tsay FW, Wu CJ, Lo CC, Tseng HH, Yamaoka Y, Chen JL, Lo GH. 2005. A prospective randomized trial of esomeprazole- versus pantoprazole-based triple therapy for Helicobacter pylori eradication. Am. J. Gastroenterol. 100:2387–2392. 10.1111/j.1572-0241.2005.00264.x [DOI] [PubMed] [Google Scholar]
- 28.Georgopoulos S, Papastergiou V, Xirouchakis E, Laoudi F, Lisgos P, Spiliadi C, Papantoniou N, Karatapanis S. 2013. Nonbismuth quadruple “concomitant” therapy versus standard triple therapy, both of the duration of 10 days, for first-line H. pylori eradication: a randomized trial. J. Clin. Gastroenterol. 47:228–232. 10.1097/MCG.0b013e31826015b0 [DOI] [PubMed] [Google Scholar]
- 29.Kongchayanun C, Vilaichone RK, Pornthisarn B, Amornsawadwattana S, Mahachai V. 2012. Pilot studies to identify the optimum duration of concomitant Helicobacter pylori eradication therapy in Thailand. Helicobacter 17:282–285. 10.1111/j.1523-5378.2012.00953.x [DOI] [PubMed] [Google Scholar]
- 30.Fuccio L, Minardi ME, Zagari RM, Grilli D, Magrini N, Bazzoli F. 2007. Meta-analysis: duration of first-line proton pump inhibitor based triple therapy for Helicobacter pylori eradication. Ann. Intern. Med. 147:553–562. 10.7326/0003-4819-147-8-200710160-00008 [DOI] [PubMed] [Google Scholar]
- 31.Ford A, Moayyedi P. 2003. How can the current strategies for Helicobacter pylori eradication be improved? Can. J. Gastroenterol. 17(Suppl B):36–40 [DOI] [PubMed] [Google Scholar]
- 32.Malfertheiner P, Bazzoli F, Delchier JC, Celiñski K, Giguère M, Rivière M, Mégraud F, Pylera Study Group 2011. Helicobacter pylori eradication with a capsule containing bismuth subcitrate potassium, metronidazole, and tetracycline given with omeprazole versus clarithromycin-based triple therapy: a randomised, open-label, non-inferiority, phase 3 trial. Lancet 377:905–913. 10.1016/S0140-6736(11)60020-2 [DOI] [PubMed] [Google Scholar]
- 33.Hsu PI, Wu DC, Wu JY, Graham DY. 2011. Modified sequential Helicobacter pylori therapy: proton pump inhibitor and amoxicillin for 14 days with clarithromycin and metronidazole added as a quadruple (hybrid) therapy for the final 7 days. Helicobacter 16:139–145. 10.1111/j.1523-5378.2011.00828.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sardarian H, Fakheri H, Hosseini V, Taghvaei T, Maleki I, Mokhtare M. 2013. Comparison of hybrid and sequential therapies for Helicobacter pylori eradication in Iran: a prospective randomized trial. Helicobacter 18:129–134. 10.1111/hel.12017 [DOI] [PubMed] [Google Scholar]