Abstract
Mycobacterium leprae and Mycobacterium tuberculosis antimicrobial resistance has been followed with great concern during the last years, while the need for new drugs able to control leprosy and tuberculosis, mainly due to extensively drug-resistant tuberculosis (XDR-TB), is pressing. Our group recently showed that M. leprae is able to induce lipid body biogenesis and cholesterol accumulation in macrophages and Schwann cells, facilitating its viability and replication. Considering these previous results, we investigated the efficacies of two statins on the intracellular viability of mycobacteria within the macrophage, as well as the effect of atorvastatin on M. leprae infections in BALB/c mice. We observed that intracellular mycobacteria viability decreased markedly after incubation with both statins, but atorvastatin showed the best inhibitory effect when combined with rifampin. Using Shepard's model, we observed with atorvastatin an efficacy in controlling M. leprae and inflammatory infiltrate in the BALB/c footpad, in a serum cholesterol level-dependent way. We conclude that statins contribute to macrophage-bactericidal activity against Mycobacterium bovis, M. leprae, and M. tuberculosis. It is likely that the association of statins with the actual multidrug therapy effectively reduces mycobacterial viability and tissue lesion in leprosy and tuberculosis patients, although epidemiological studies are still needed for confirmation.
INTRODUCTION
Tuberculosis (TB) and leprosy are chronic infections caused by Mycobacterium tuberculosis and Mycobacterium leprae, the most important facultative pathogen and an obligate intracellular pathogen, respectively; they target macrophages for replication and persistence, while M. leprae also infects Schwann cells in nerves. Tuberculosis is the most frequent bacterial infection in humans, resulting in 1.4 million deaths around the world in 2011 (1). Leprosy may evolve to cause permanent nerve damage and incapacities, and in 2011, it was diagnosed in 219,075 patients worldwide, but it is usually not fatal. On the other hand, M. leprae infection was responsible for 12,225 cases of motor disability and deformity, which are the hallmarks of the infection (2).
Leprosy and TB are bacterial infections treatable with effective combined multidrug therapy, but very long treatment is necessary: 12 or 6 months for leprosy (depending on the clinical form) and 6 months for TB. In fact, the introduction of multidrug therapy (MDT) represented a great achievement in the control of the disease, which has been declining in incidence in the past 20 years around the world (2). Nevertheless, adherence to treatment is an important issue where the emergence of multidrug resistance (MDR) cases is increasing quickly, especially with TB. However, M. leprae resistance has been followed with great concern during the last few years (3). Over the last decades, the WHO has identified clinical isolates of M. leprae resistant to rifampin, dapsone, or ofloxacin and has reported that the number of these isolates is growing (4).
It is well known that after M. leprae or M. tuberculosis phagocytosis by host cells, an alteration in cellular lipid metabolism takes place, resulting in an increase in cholesterol and aliphatic lipid uptake and de novo synthesis (5–7). Parihar and colleagues (8) recently demonstrated that cholesterol reduction by statins reduces M. tuberculosis viability in vitro and in vivo, a strategy already proposed in the literature (9). This metabolic remodeling by the mycobacterial infection is responsible for an increase in different classes of lipids in a systemic way, as demonstrated in leprosy patient serum metabolomics studies, in which a correlation was established between the bacilloscopy index (BI) (the bacillary load in skin biopsy samples expressed in a logarithmic scale from 0 to 6) and the abundance of some polyunsaturated fatty acids and phospholipids, such as arachidonic acid, eicosapentaenoic acid, and docosahexaenoic acid (10). Our group also demonstrated an increase in cholesterol ester in multibacillary skin biopsy samples compared to paucibacillary ones (11). Additionally, it has been shown that M. leprae suppresses lipid degradation through the inhibition of hormone-sensitive lipase expression, contributing to lipid accumulation in infected cells (12), and this process plays a central role in bacterial survival (11). Although only two lipases and one phospholipase were encoded by its genome, recent proteomic analyses indicate an active glyoxylate cycle in M. leprae, in which fatty acid beta oxidation generates succinate for the synthesis of carbohydrates (13, 14). For that reason, the metabolism of host lipids represents an important mechanism for this bacterium to cause and sustain infection. This is best illustrated by M. tuberculosis, which presents a large number of coding genes involved with lipogenesis and lipolysis (15). Controversially, many of these genes are pseudogenes in the M. leprae genome (14). Most likely, enzymes from the host cells complement the bacterial genes, as suggested by a study describing induction by the infection of cellular lipases and phospholipases in the tissues of lepromatous patients (16). Although cholesterol metabolism is still not completely understood in M. leprae, accumulation in the M. tuberculosis cell wall was previously shown to be responsible for decreasing tritiated rifampin permeability in vitro (17), as well as being involved in mycobacterial avoidance of macrophage vacuolar fusion and, consequently, the immune response (18); this represents the most important carbon source inside gamma interferon (IFN-γ)-activated macrophages in vitro (19).
Based on an accumulation of data involving the essential role of cholesterol and lipids in the intracellular survival of mycobacteria, in this study, we investigated the use of statins to control M. leprae infection both in vitro and in vivo. Statins are a class of drugs largely used in the treatment of cholesterol-induced atherosclerotic cardiovascular disease. They are structural analogs of mevalonate that are able to inhibit the rate-limiting enzyme 3-hydroxy-3-methyl-glutaryl–coenzyme A (HMG-CoA) reductase, which is responsible for cholesterol synthesis in mammals. Mainly used in the treatment of hypercholesterolemia, statins are now recognized as immunomodulatory drugs, presenting satisfactory pleiotropic effects in immune disorders (20, 21). By reducing cholesterol availability in the intracellular environment, we anticipate a reduction in mycobacterial persistence and multiplication in host cells.
In our present work, we tested the activity and additive effect of rifampin treatment in association with two statins, atorvastatin and simvastatin, in the control of M. leprae and M. tuberculosis in a macrophage in vitro infection model and in vivo in Shepard's mouse footpad M. leprae infection model. We demonstrated that both statins induce a bactericidal effect in M. tuberculosis and M. leprae infections. The bactericidal effect observed in cells infected by M. leprae is related to phagosomal arrest, and its combination with rifampin drastically reduces cellular infiltration in the leprosy mouse footpad model.
MATERIALS AND METHODS
Cell culture.
THP-1 cells were obtained from the American Type Culture Collection (ATCC) and maintained in RPMI medium 1640 (LCG Bioscience, São Paulo, Brazil), supplemented with 10% fetal bovine serum (Cultilab, Campinas, São Paulo, Brazil), without antibiotics. The cultures were kept at 37°C or 33°C within a humidified 5% CO2 atmosphere. The differentiation of monocytes to macrophages was achieved by exposing the cells to 200 ng/ml phorbol 12-myristate 13-acetate (PMA) (Sigma, St. Louis, MO) for 24 h.
Mycobacterial strains and staining.
Live M. leprae prepared from the footpads of athymic nude (nu/nu) mice was produced at the Lauro de Sousa Lima Institute, Bauru, São Paulo, Brazil. M. leprae was purified from iodine-disinfected hind footpads. Briefly, the skin and bones were removed, and the tissue was minced into small pieces with scissors and digested with a solution of 170 units of collagenase type I, 2 units of dispase (Life Technologies, NY), 5 mg/ml ampicillin (Sigma, St. Louis, MO), and 150 units of DNase (Life Technologies) for 2 h at 33°C. The digested tissue was homogenized by vortex and washed three times in water, one time in 0.1 N NaOH, and one time in RPMI medium, centrifuged at 10,000 × g for 5 min, and counted by acid-fast staining (Ziehl-Neelsen kit; Becton Dickinson). Part of the M. leprae suspension was sterilized by gamma irradiation at the Acelétron Facility (Rio de Janeiro, Brazil). M. bovis strain BCG Pasteur (ATCC 35734) and M. tuberculosis strain H37Rv were grown at 37°C in Middlebrook 7H9 base ADC enrichment medium (Becton Dickinson, Franklin Lakes, NJ), supplemented with 0.2% glycerol and 0.05% Tween 80 (Sigma).
M. tuberculosis and M. bovis BCG Pasteur viability determination.
M. tuberculosis and M. bovis BCG viability was measured after 2 × 105 THP-1 cells were infected and differentiated into macrophages, and after 24 h, these cells were infected with M. tuberculosis or M. bovis BCG at a multiplicity of infection (MOI) of 10:1 or 50:1, respectively, for 72 h at 37°C. The cultures were washed and lysed by 0.1% Triton X-100 in phosphate-buffered saline (PBS) for 10 min. The number of intracellular live bacteria was assessed by serial dilution of the lysate and seeding on Middlebrook 7H10 medium with 10% oleic acid-albumin-dextrose-catalase (OADC), with a determination of the CFU after 1 month of incubation at 37°C.
M. leprae viability determination.
We used two methods to determine M. leprae viability. The first one is a fluorimetric LIVE/DEAD staining protocol described elsewhere (22) using the LIVE/DEAD BacLight bacterial viability kit (Life Technologies, CA), performed according to the manufacturer's instructions, to be sure that the viability of bacilli from the nude mouse preparation was always >85%; otherwise, it was discarded. The second method is a molecular approach described elsewhere (23) to determine M. leprae viability in cellular cultures, in which the levels of labile M. leprae mRNA were normalized by its highly stable DNA. Briefly, 2 × 105 THP-1 cells were differentiated into macrophages within 24 h of incubation with 200 nM PMA. After this time, the cells were washed and allowed to rest. After 24 h of rest, the cells were infected with M. leprae at an MOI of 10:1. After testing at different times of infection, we observed that 7 days of infection generated the most consistent results on viability analysis. After 1 week of infection, M. leprae RNA and DNA were extracted using the TRIzol reagent (Invitrogen, CA) in FastPrep-24 tubes (MP Biomedicals, CA), as described previously (23). In RNA preparation, DNA was removed using the Turbo DNA-free kit (Ambion, CA). M. leprae RNA was reverse transcribed using random primer and SuperScript III according to the manufacturer's instructions (Invitrogen). The levels of M. leprae 16S rRNA, mRNA, and DNA were determined from cultured macrophages by real-time reverse transcription-PCR (RT-PCR), using the same primer pair: sense, 5′-GCA TGTCTTGTGGTGGAAAGC-3′, and antisense, 5′-CACCCCACCAACAAGCTGAT-3′. All samples presented cycle threshold (CT) values between 20 and 28. One hundred percent viability was arbitrarily assumed as 2−ΔCT of the infected control samples, and all other values were normalized as a percentage of this. The reactions were performed in an ABI StepOnePlus sequence detection system (Applied Biosystems, CA).
Lipid extraction and analysis.
A total of 8 × 105 THP-1 cells were differentiated into macrophages as described above, infected with M. leprae at an MOI of 10:1, and exposed to 2 μM simvastatin for 1 week. The cultures were washed twice with PBS, detached, and homogenized by three freeze-thaw cycles. The total cholesterol content was determined using the Amplex red cholesterol assay kit (Invitrogen, CA), according to the manufacturer's instructions. The total cholesterol levels were represented as μg of total cholesterol per mg of protein.
Fluorescence microscopy.
THP-1 cells were plated in 24-well plates containing coverslips at a density of 2 × 105 cell/well and treated with statins for 24 h. To measure the infection rate in the statin-treated cells, the cultures were exposed to irradiated M. leprae stained with PKH26 red fluorescence, as described elsewhere (24), at an MOI of 10:1 for 5 h, a time known to be sufficient to infect one-third of the THP-1 cells with M. leprae at this MOI. After that, the medium was removed by washing in PBS, and the cells were fixed in 4% (wt/vol) paraformaldehyde at 4°C for 20 min. Images were taken in a Zeiss Axio Observer fluorescence microscope, where 10 fields from three biological replicates were analyzed.
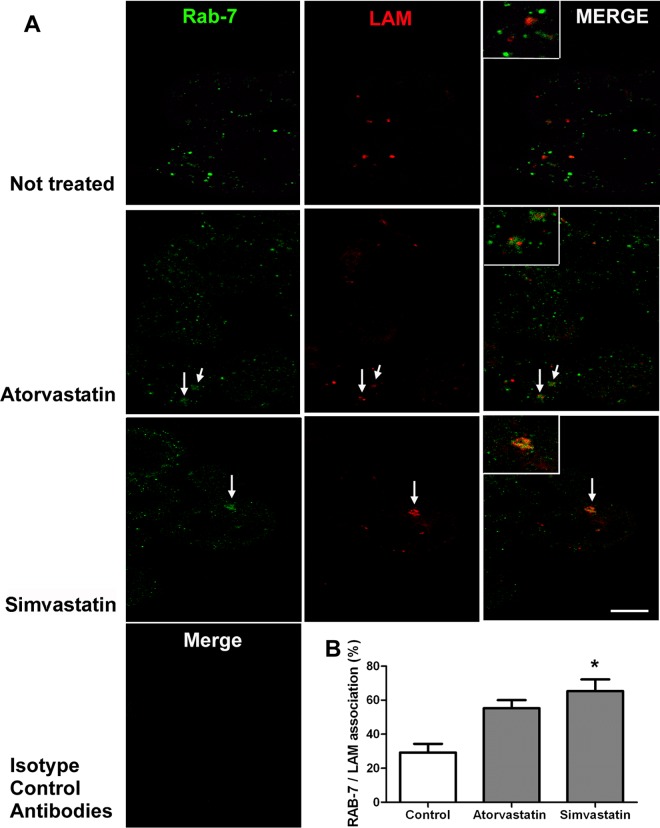
For immunocytochemistry by confocal microscopy, the cultures were infected with live M. leprae at an MOI of 1:10 for 24 h, a time known to be sufficient to observe M. tuberculosis escape from the phagosome (25). The slides were permeabilized and blocked by 30 min incubation with 0.01% Triton X-100 and 10% fetal bovine serum in PBS (pH 7.2). The cells were incubated for 2 h with rabbit IgG antilipoarabinomannan (anti-LAM) antibodies (1:50 [vol/vol]), which were kindly donated by John S. Spencer (BEI Resources Repository, NIAID, NIH), to identify M. leprae, and mouse IgG anti-human Rab7 (1:500 [vol/vol]; Abcam, MA) to identify late endosomes. Secondary antibodies conjugated with Alexa Fluor 488 (IgG anti-mouse) and Alexa Fluor 555 (IgG anti rabbit) (Invitrogen, CA) were incubated with the samples for 2 h. The slides were observed in an LSM 710 confocal laser scanning microscope (Carl Zeiss, Heidenheim, Germany). We analyzed 100 cells from three biological replicates, plotting the percentage of late phagosomes (Rab7+ vesicles) containing M. leprae.
In vivo atorvastatin efficacy test against M. leprae in Shepard's BALB/c mouse model.
A suspension containing 1 × 104 live M. leprae cells in 10 μl was inoculated in each hind footpad of the BALB/c mice, as described in Shepard's model. One month after inoculation, the mice were divided into 6 groups. The control group was inoculated and not treated; two other groups were treated with atorvastatin added to the daily feed at doses of 40 and 80 mg/kg of body weight/day. The other three groups received rifampin at 10 mg/kg, rifampin at 1 mg/kg by gavage weekly, or rifampin at 1 mg/kg of body weight and atorvastatin at 80 mg/kg of body weight/day in the feed. The mice were treated for 5 months. Six months after inoculation, each mouse was sacrificed and both footpads were excised one was macerated for bacillary counting, and the contralateral was fixed in 10% buffered formalin, paraffinized, and sectioned for histopathological examination with hematoxylin and eosin and Fite-Faraco staining for acid-fast bacilli (AFB).
Determination of cholesterol and transaminase activity in mouse plasma.
Each BALB/c mouse was sacrificed under anesthesia, and plasma was collected in heparin directly from the heart. Cholesterol, aspartate aminotransferase (AS), and alanine aminotransferase (ALT) were measured using the assay kit Bioclin/Quibasa (Belo Horizonte, Minas Gerais, Brazil), according to the manufacturer's instructions.
Statistical analysis.
All numerical data were statistically analyzed using nonparametric Kruskal-Wallis test and Dunn's posttest to compare relevant groups, with the GraphPad Prism software.
Ethics statement.
The animal protocols were in agreement with the animal care guidelines of the National Institutes of Health and were approved by the Animal Welfare Committee of Sagrado Coração University (São Paulo, Brazil).
RESULTS
Intracellular mycobacterial viability is reduced by statins.
Since host cholesterol accumulation in the M. tuberculosis cell wall is involved in the decrease in rifampin permeability in vitro (17), we hypothesize that cholesterol de novo synthesis inhibition might not only kill mycobacteria by carbon deprivation but also make them more vulnerable to rifampin.
We first observed the potential of atorvastatin and simvastatin in the control of in vitro THP-1 mycobacterial infection. After 72 h of infection, both atorvastatin and simvastatin were able to inactivate intracellular BCG in a dose-dependent fashion (Fig. 1A). With the higher dose (2 μM), both drugs were able to reduce the number of viable intracellular bacilli by about 75%, and atorvastatin showed an additive effect in combination with 1 μg/ml rifampin. M. tuberculosis displayed a very similar response to statins as that of BCG, presenting a reduction in viability in a dose-dependent manner (Fig. 1B). One distinction between BCG and M. tuberculosis was that in the pathogenic strain, the additive effect of statins with rifampin was seen in both combinations (1 μg/ml rifampin plus 0.2 μM atorvastatin or simvastatin), with a P value of <0.05.
FIG 1.
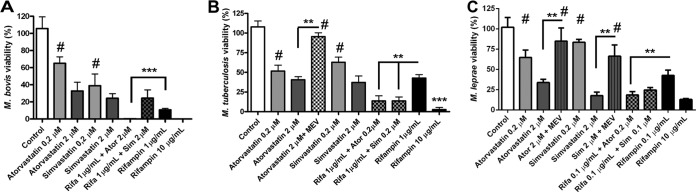
Statins increase rifampin mycobactericidal effect. THP-1 cells were differentiated to macrophages with PMA, and after 24 h of rest, the cultures were treated with different regimes and were concomitantly infected with M. bovis (BCG Pasteur) (A), M. tuberculosis H37Rv (B), and M. leprae strain Thai-53 (C). The viability in relation to the controls was measured after 72 h from the infection by CFU determination (A and B) or after 1 week by real-time PCR (C). It was observed that despite simvastatin displaying a stronger mycobactericidal effect, only atorvastatin showed an additive effect when associated with rifampin against all three mycobacteria tested. The effects were reverted by mevalonate at 150 μM (MEV). Means and standard errors of the mean (SEM) were generated from four normalized independent biological replicates, where two and three asterisks mean P < 0.01 and P < 0.001, respectively. #, treatment was not statistically different from control. Rifa, rifampin; Ator, atorvastatin; Sim, simvastatin.
We determined M. leprae viability by RT-PCR, the most sensitive method available, being chosen due to its capacity to determine the viability of a small amount of bacilli (106) (23). After 1 week of infection at 33°C, the M. leprae RNA copy number, which is rapidly degraded after bacillus inactivation, was quantified after normalization. As shown in Fig. 1C, both atorvastatin and simvastatin caused a bactericidal effect in a dose-dependent fashion. Here again, only atorvastatin showed an additive effect when combined with rifampin (0.1 μg/ml rifampin plus 0.2 μM atorvastatin). None of these effects are related to the cytotoxicity of statins (see Fig. S1 in the supplemental material).
Statin treatment does not alter mycobacterial invasion.
To observe if statins were able to reduce cholesterol levels in our model and through that mechanism prevent mycobacterial infectivity instead of reducing mycobacterial viability, we treated infected THP-1 cells for 1 week with 2 μM simvastatin. Our group has already shown that M. leprae-infected macrophages accumulate cholesterol as lipid bodies through Toll-like receptor 2/6 (TLR2/6) signaling (7). In this study, we observed that simvastatin was able to prevent M. leprae-infected cells from accumulating cholesterol, restoring normal cholesterol levels (Fig. 2A). Since the importance of 9-O-acetyl GD3, a cholesterol-anchored ganglioside located in cellular lipid rafting, to M. leprae invasion in Schwann cells was already demonstrated by our group (26), we investigated if the changes in cholesterol levels observed in the treated cells could interfere in the ability of M. leprae to infect cells. We observed the rates of infection between the untreated and statin-treated cells using irradiated PKH26-stained M. leprae and determined that there was no difference (Fig. 2B to F). Thus, we conclude that the lower number of viable mycobacteria after treatment with statins is not due to either THP-1 cell mortality or differences in the infection rate.
FIG 2.
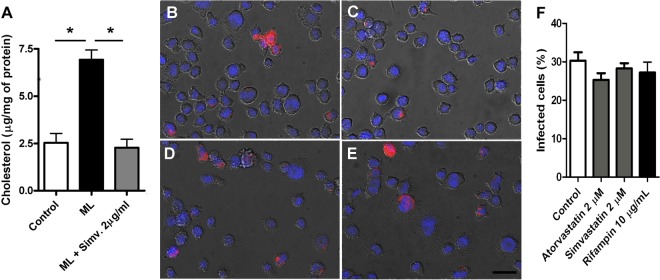
THP-1 mycobacterial infection and cholesterol levels after treatment with statins. (A) Total cellular free cholesterol and cholesteryl ester levels were measured by the fluorometric method after 1 week of infection with concomitant treatment. Atorvastatin treatment decreased cholesterol levels even in the presence of M. leprae (ML). Means and SEM are representative of the results from three independent biological replicates (* indicates P < 0.01). PKH-stained M. leprae was used to infect THP-1 cells during 5 h without treatment (B) or after 24 h pretreatment with 2 μM atorvastatin (C) or simvastatin (D) or 10 μg/ml rifampin (E). The nuclei were stained in blue by 4′,6-diamidino-2-phenylindole (DAPI). (F) Quantification of the percentage of infected cells, where it can be observed that the treatments did not interfere with infection rate. Forty representative images from three biological replicates were used to perform this analysis. The scale bar corresponds to 20 μm.
Statins increase association between M. leprae and late endosomes.
Cholesterol also plays a role in the ability of mycobacteria to escape the phagosome, as it serves as an anchor for early secretory antigenic target 6 (ESAT-6), a secreted mycobacterial protein able to disrupt cellular membranes and to consequently release engulfed mycobacteria to the cytosol (A. B. Robottom-Ferreira et al., unpublished data) (27). Other authors showed that only in cholesterol-depleted macrophages did Mycobacterium avium-containing phagosomes fuse with lysosomes, generating phagolysosomes (18). In addition, it was recently demonstrated that macrophages isolated from simvastatin-treated mice more efficiently kill M. tuberculosis through phagosomal maturation and autophagy (8). It has been shown that the dissociation of Rab7 from M. tuberculosis phagosomes, which occurs around 6 h, is important for the maintenance of the phagosomal environment, which matures to a phagolysosome after fusion with lysosomes (25). Corroborating the literature, we show a close association of the transient phagosomal membrane protein Rab7 with M. leprae lipoarabinomannan (LAM) within the phagosomal space 24 h after statin treatment of the infected cells. We observed that Rab7 and LAM were colocalized within the phagosome 24 h after M. leprae infection only in THP-1 cells treated with statins (Fig. 3A, arrows and insets). Simvastatin induced higher mycobacterial LAM/Rab7 colocalization than did atorvastatin, in agreement with its higher bactericidal activity against M. leprae, as shown in Fig. 1C. However, since atorvastatin had a similar activity when used at a higher dose (2 μM) and this drug was the only one to present an additive effect in association with rifampin, we chose it for the in vivo tests.
FIG 3.
Confocal analysis of M. leprae phagosomal arrest in THP-1 statin-treated cells. (A) Immunolocalization of Rab7, a late-endosome marker (Alexa Fluor 488) and M. leprae lipoarabinomannan (LAM) (Alexa Fluor 633), in THP-1 cells after 24 h of M. leprae infection with 2 μM atorvastatin or simvastatin treatment, demonstrating antigen association by arrows and in higher magnification by insets. Merge images of both channels' signals produced by isotype control antibodies are also presented. (B) The percentage of intracellular LAM signal associated with Rab7 is shown as mean and SEM, demonstrating a higher mycobacterial arrest in mature phagosomes due to exposure to simvastatin. Images are representative from 360 randomly chosen cells of four experiments from two independent biological replicates. The scale bar corresponds to 10 μm in panels and 5 μm in insets. Asterisk indicates a difference with a P value of <0.05.
Atorvastatin synergizes with rifampin in its antibacterial effect in vivo.
Due to the previous studies showing that host cholesterol deposition in the mycobacterial cell wall inhibits rifampin permeability (17), our hypothesis is that the application of statins in tuberculosis and leprosy treatment involves its association with the actual multidrug therapy. For this reason, we evaluated whether atorvastatin was able to potentiate the effect of rifampin in vivo. To test this, we infected BALB/c mouse footpads and, after 1 month of infection, the mice were subjected to five different treatments for 5 months (Fig. 4). After 6 months, atorvastatin alone at the higher dose (80 mg/kg/day) effectively reduced M. leprae replication (Fig. 4A, triangles). In addition, only groups treated with the higher dose of atorvastatin showed a significant reduction in plasma cholesterol levels (Fig. 4B). More interestingly, atorvastatin combined with rifampin (1 mg/kg/week) induced a larger decrease than rifampin alone (P < 0.01). We also demonstrate that none of the treatment schemes increased muscle tissue damage or led to detectable hepatotoxic effects (Fig. 4C and D). We examined if atorvastatin at the higher dose could not only prevent infection but also eliminate an established 4-month Foxn1nu (nude) mouse infection. After 1 month of treatment, we observed that 2 of 3 treated nude mice had a higher number of dead bacilli recovered from their footpads (data not shown).
FIG 4.
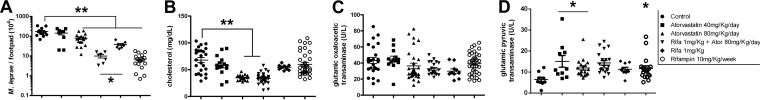
Atorvastatin activity in BALB/c mouse M. leprae infection. BALB/c mouse footpads were infected with 104 M. leprae bacilli. After 1 month, different groups of animals were untreated (control) or treated with 40 mg/kg/day or 80 mg/kg/day of atorvastatin, the combination of 80 mg/kg/day of atorvastatin (Ator) with 1 mg/kg/week of rifampin (Rifa), 1 mg/kg/week of rifampin alone, or 10 mg/kg/week of rifampin. (A) Number of bacilli in footpad suspensions after 6 months of infection, followed by 5 months of treatment. Plasma cholesterol (B), glutamic oxaloacetic (C), and pyruvic (D) transaminase activities were measured in the same groups of mice. Means and SEM were generated from two independent biological replicates, where one and two asterisks mean P < 0.05 and P < 0.0001, respectively.
The representative histological characteristics from contralateral footpads from the same groups of mice analyzed in Fig. 4 are shown in Fig. 5. As seen at a higher magnification, the untreated group of animals (Fig. 5A and B, asterisks) presents a predominantly macrophage inflammatory infiltrate in the dermis, around blood vessels, and involving skeletal muscle fibers. The macrophages showed features of activated cells, with eosinophilic cytoplasm and round or oval nuclei containing one or more nucleoli. There were a small number of lymphocytes, rare neutrophils, and eosinophils mixed with the macrophages. The bacilloscopic index (determined by Fite-Faraco stain) was high, at 5/6+, with the fragmented bacilli inside irregularly distributed macrophages in the entire extension of the inflammatory infiltrate. In some areas of Fig. 5F (1 mg/kg/week rifampin), it can be seen that the infiltrate was composed of granulomas in regression, characterized by vacuolated macrophages and nuclei without nucleoli, as well as lymphocytes. Figure 5G and H (1 mg/kg/week rifampin plus 80 mg/kg/day atorvastatin) and I and J (10 mg/kg/week rifampin) show a small and discrete inflammatory infiltrate, consisting predominantly of loosely arranged macrophages located in the dermis and perivascular area, and involving the skeletal muscle fibers. The granulomas are more regressive, and some muscle fibers show recovered areas. The bacilloscopic index (Fite-Faraco stain) was lower, at only 2+, with multifragmented weakly stained bacilli inside the macrophages. This supports an important and previously described beneficial pleiotropic effect of statins and their possible immunomodulatory role, which might be involved in the reduction in tissue damage promoted by statin treatment in tuberculosis animal infections (8, 21), a desired effect in the control of immune-related tissue inflammation and damage in leprosy.
FIG 5.
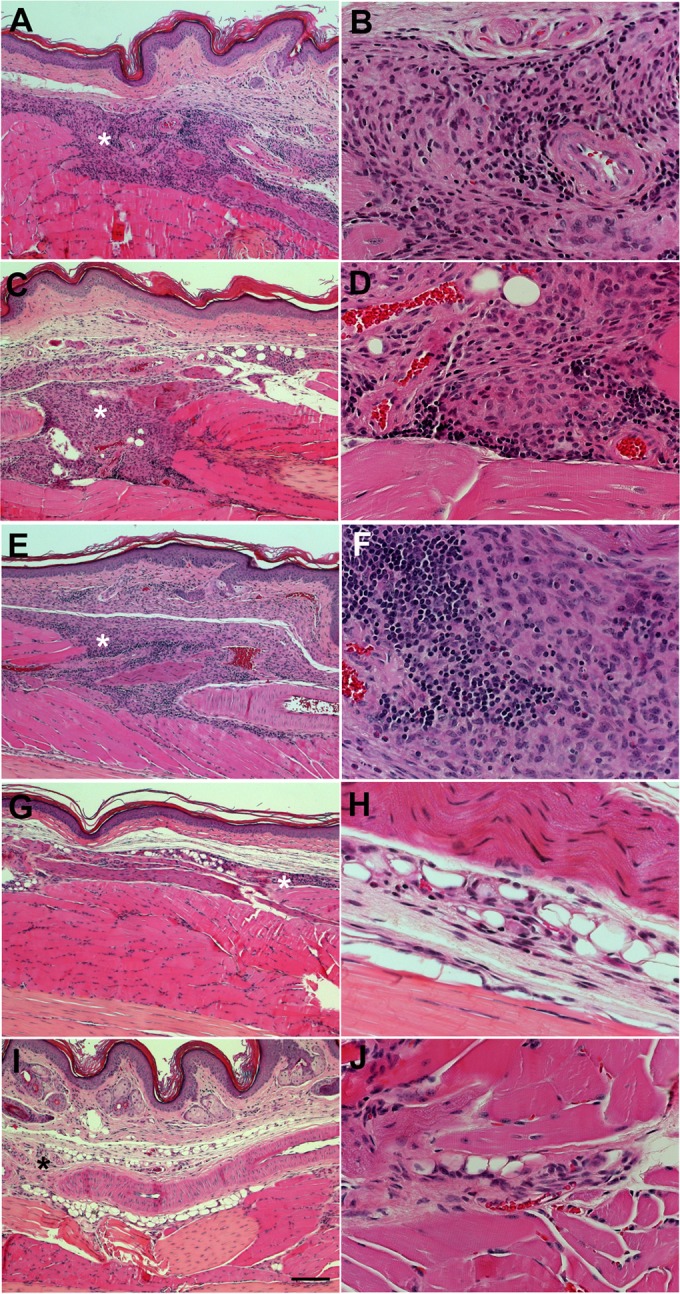
Histopathological analysis of footpads from M. leprae-infected mice after different treatment regimes. Contralateral footpads from the same groups of mice analyzed in Fig. 4 were fixed, paraffinized, sectioned, and stained with hematoxylin and eosin after 6 months of infection and 5 months of treatment. (A and B) Untreated controls. (C and D) Representative images of a lesion area from an animal treated with 80 mg/kg/day of atorvastatin. (E and F) Treatment with 1 mg/kg/week rifampin (subtherapeutic dose). (G and H) Combination of 80 mg/kg/day atorvastatin and 1 mg/kg/week rifampin. (I and J) Rifampin at 10 mg/kg/week. The asterisks show inflammatory cellular infiltrates, which were virtually absent in panels G and I. The scale bar represents 100 μm in panels A, C, E, G, and I and 25 μm in panels B, D, F, H, and J.
DISCUSSION
Our group recently described the mechanism by which M. leprae induces lipid droplet formation in macrophages and Schwann cells (5, 7, 28), showing that host-derived cholesterol represents the major lipid component inside these organelles. The importance of lipids in M. leprae energetic metabolism is further supported by the high prevalence of genes involved in lipid anabolism and catabolism, despite the huge reductive evolution that occurred in its genome, producing essentially a minimal gene set for survival (14). It has already been shown in in vitro studies that host cholesterol accumulation in the M. tuberculosis cell wall is related to the decrease in rifampin permeability (17); this is a possible explanation for the additive effect observed between statins and rifampin. The hypothesis that mycobacterial HMG-CoA reductase would be inhibited by statins is not valid, since the similarity of M. tuberculosis to the human enzyme is <40%. Moreover, this enzyme is not expressed in M. leprae, which displayed a similar sensitivity to statins as those of M. tuberculosis and BCG. More importantly, the higher concentrations of atorvastatin and simvastatin used in this work (2 μM) failed to kill M. tuberculosis H37Rv cultivated in 7H9 medium. On the other hand, the results observed in the infected THP-1 cells could not be ascribed to the cytotoxicity of statins, since cellular viability was not altered under all conditions employed in the study (see Fig. S1 in the supplemental material). Concerning the isolated effect of statins against mycobacteria inside macrophages, other events must be taken into consideration. For example, the metabolism of M. tuberculosis isolated from infected mouse lungs is stimulated by fatty acids and is unresponsive to carbohydrates (29), and the generation of M. tuberculosis mutants with knockouts of some enzymes involved in cholesterol metabolism, such as KshA, KshB, FadA5, ChoD, and KstD, leads to inefficient mouse and macrophage infection, demonstrating the pivotal relevance of this pathway to tuberculosis infection and persistence inside the host (30–33). Since statins were already described as generators of oxidative stress in hepatocytes (34), we measured nitrite production and observed no significant difference between all conditions used in our study (see Fig. S2 in the supplemental material). Therefore, we also discarded the possibility that statins would kill mycobacteria by increasing the oxidative stress in our model. Another hypothesis, different from carbon restriction, might explain the mycobactericidal effect of statins, such as the capacity of a statin to disrupt signaling cascades originated at lipid rafts, a phenomenon already described in T lymphocytes (35). Recently, Parihar and collaborators (8) showed that statins can control M. tuberculosis infection, and they suggested that this effect is due to phagolysosomal arrest of M. tuberculosis. We conclude that atorvastatin and simvastatin display similar effects in our study, achieving effective mycobactericidal activities against BCG, M. tuberculosis, and M. leprae reverted by mevalonate, the product of HMG-CoA reductase, as shown in Fig. 1. We successfully observed M. leprae and mature endosome association increasing after statin treatment (Fig. 3). It has been clearly shown that the mechanism used by M. tuberculosis to escape from the endosome compartment involves the expression of a complex of proteins, including ESX-1, which is involved in the transport of proteins able to bind cholesterol-rich membranes, such as ESAT-6, which is involved in membrane destabilization and rupture (27). Statins efficiently prevent M. tuberculosis-induced inhibition of macrophage phagosomal maturation (8). ESAT-6 is also expressed by M. leprae during THP-1 infection (Robottom-Ferreira et al., unpublished data). Although simvastatin was not able to reduce the cholesterol levels of the infected cell below that of the control, we hypothesize that the avoidance of cholesterol accumulation induced by M. leprae in treated THP-1 cells is sufficient to inhibit ESAT-6 disruption of the phagosome, which had matured in the late endosome with the mycobacteria arrested inside (Fig. 3, insets).
Our in vivo study corroborates the in vitro findings, with significant differences from the control groups observed only in mice receiving the highest dose of statin (80 mg/kg) in their daily diet. An equivalent dose in humans is 390 mg/day for a 60-kg adult (36). This very high dose of atorvastatin could be avoided if we were to use daily gavage or intraperitoneal injections as the administration route, but this would be impractical for a 5-month period of treatment. This subject will be addressed in a future work.
Taken together, our results show that statins are able to inactivate M. leprae and M. tuberculosis in vitro, as well as potentiate the antimicrobial effect of rifampin against both pathogens. We can see that the atorvastatin-rifampin combination presents very different results than those with each drug used separately, increasing rifampin mycobactericidal activity (Fig. 1 and 4A) while decreasing the inflammatory response and tissue damage (Fig. 5G and H). We believe that this decrease in M. leprae viability contributed to the reduction in the inflammatory infiltrate observed in Fig. 5, but this decrease alone does not explain everything, since the viability observed under both conditions, rifampin alone and the rifampin-atorvastatin combination, shows only a small difference, while the inflammatory infiltrate reduction is much more evident.
The inflammatory infiltrate reduction observed in Fig. 5 can be explained by the well-known pleiotropic effect of the statins already described in other models (20, 21, 37–39). Recent studies have demonstrated that statins may be beneficial in the treatment of T-cell–mediated autoimmune diseases (40), due to the involvement of cholesterol in the maintenance of lipid raft structures and its importance in T-cell activation (41). By reducing isoprenoids, statins also inhibit protein prenylation, blocking small GTPase Ras superfamily tethering and activity (42), leading to the inhibition of T-cell activation (43), antigen uptake, processing, and presentation by antigen-presenting cells, as well as their maturation (44).
The immunomodulatory pleiotropic effect of the combination of statin and rifampin might be particularly beneficial to the inhibition of leprosy reactional episodes during treatment. The combination of statins with MDT might reduce the occurrence of reactional episodes, which are characterized by an intense and sudden activation of the host immune response with high levels of tumor necrosis factor alpha (TNF-α), affecting about half of the patients under treatment (45). The use of statins to control mycobacteriosis might be a low-cost, efficient, and fast way to provide an entirely new class of drugs to aid tuberculosis and leprosy treatment efforts, being of extreme importance if used in association with the MDT regime against MDR or extensively drug-resistant (XDR) mycobacterial strains.
Supplementary Material
ACKNOWLEDGMENTS
We thank BEI Resources Repository, NIAID, NIH for providing the M. leprae anti-LAM antibody. We also thank Karina Garcia and Evandro Gomes de Souza from Acéletron (Acelétrica Comércio e Representações Ltda., Rio de Janeiro, Rio de Janeiro, Brazil) for technical assistance with M. leprae irradiation. We also thank Andre Pedrosa, Yraima Cordeiro, and John S. Spencer for critical reading of the manuscript.
We certify that we have no affiliations with or involvement in any organization or entity with any financial or nonfinancial interest in the subject matter or materials discussed in this paper.
This work was supported by the Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ) (APQ-1 E-26/111.718/2012) and the Programa de Inovação Tecnológica INOVATEC (from Fiocruz Foundation). The sources of funds had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Published ahead of print 21 July 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.01826-13.
REFERENCES
- 1.WHO. 2012. Global tuberculosis report 2012. World Health Organization, Geneva, Switzerland: http://apps.who.int/iris/bitstream/10665/75938/1/9789241564502_eng.pdf [Google Scholar]
- 2.WHO. 2012. Global leprosy situation, 2012. Wkly. Epidemiol. Rec. 87:317–328 [PubMed] [Google Scholar]
- 3.Williams DL, Gillis TP. 2012. Drug-resistant leprosy: monitoring and current status. Lepr. Rev. 83:269–281 [PubMed] [Google Scholar]
- 4.WHO. 2011. Surveillance of drug resistance in leprosy: 2010. Wkly. Epidemiol. Rec. 86:237–240 [PubMed] [Google Scholar]
- 5.Mattos KA, Lara FA, Oliveira VG, Rodrigues LS, D'Avila H, Melo RC, Manso PP, Sarno EN, Bozza PT, Pessolani MC. 2011. Modulation of lipid droplets by Mycobacterium leprae in Schwann cells: a putative mechanism for host lipid acquisition and bacterial survival in phagosomes. Cell. Microbiol. 13:259–273. 10.1111/j.1462-5822.2010.01533.x [DOI] [PubMed] [Google Scholar]
- 6.Kim MJ, Wainwright HC, Locketz M, Bekker LG, Walther GB, Dittrich C, Visser A, Wang W, Hsu FF, Wiehart U, Tsenova L, Kaplan G, Russell DG. 2010. Caseation of human tuberculosis granulomas correlates with elevated host lipid metabolism. EMBO Mol. Med. 2:258–274. 10.1002/emmm.201000079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mattos KA, D'Avila H, Rodrigues LS, Oliveira VG, Sarno EN, Atella GC, Pereira GM, Bozza PT, Pessolani MC. 2010. Lipid droplet formation in leprosy: Toll-like receptor-regulated organelles involved in eicosanoid formation and Mycobacterium leprae pathogenesis. J. Leukoc. Biol. 87:371–384. 10.1189/jlb.0609433 [DOI] [PubMed] [Google Scholar]
- 8.Parihar SP, Guler R, Khutlang R, Lang DM, Hurdayal R, Mhlanga MM, Suzuki H, Marais AD, Brombacher F. 2013. Statin therapy reduces Mycobacterium tuberculosis infection in human macrophages and in mice by enhancing autophagy and phagosome maturation. J. Infect. Dis. 209:754–763. 10.1093/infdis/jit550 [DOI] [PubMed] [Google Scholar]
- 9.Pohl PC, Klafke GM, Junior JR, Martins JR, da Silva Vaz I, Jr, Masuda A. 2012. ABC transporters as a multidrug detoxification mechanism in Rhipicephalus (Boophilus) microplus. Parasitol. Res. 111:2345–2351. 10.1007/s00436-012-3089-1 [DOI] [PubMed] [Google Scholar]
- 10.Al-Mubarak R, Vander Heiden J, Broeckling CD, Balagon M, Brennan PJ, Vissa VD. 2011. Serum metabolomics reveals higher levels of polyunsaturated fatty acids in lepromatous leprosy: potential markers for susceptibility and pathogenesis. PLoS Negl. Trop. Dis. 5:e1303. 10.1371/journal.pntd.0001303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mattos KA, Oliveira VC, Berrêdo-Pinho M, Amaral JJ, Antunes LC, Melo RC, Acosta CC, Moura DF, Olmo R, Han J, Rosa PS, Almeida PE, Finlay BB, Borchers CH, Sarno EN, Bozza PT, Atella GC, Pessolani MC. 2014. Mycobacterium leprae intracellular survival relies on cholesterol accumulation in infected macrophages: a potential target for new drugs for leprosy treatment. Cell. Microbiol. 16:797–815. 10.1111/cmi.12279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tanigawa K, Degang Y, Kawashima A, Akama T, Yoshihara A, Ishido Y, Makino M, Ishii N, Suzuki K. 2012. Essential role of hormone-sensitive lipase (HSL) in the maintenance of lipid storage in Mycobacterium leprae-infected macrophages. Microb. Pathog. 52:285–291. 10.1016/j.micpath.2012.02.003 [DOI] [PubMed] [Google Scholar]
- 13.Marques MA, Neves-Ferreira AG, da Silveira EK, Valente RH, Chapeaurouge A, Perales J, da Silva Bernardes R, Dobos KM, Spencer JS, Brennan PJ, Pessolani MC. 2008. Deciphering the proteomic profile of Mycobacterium leprae cell envelope. Proteomics 8:2477–2491. 10.1002/pmic.200700971 [DOI] [PubMed] [Google Scholar]
- 14.Cole ST, Eiglmeier K, Parkhill J, James KD, Thomson NR, Wheeler PR, Honoré N, Garnier T, Churcher C, Harris D, Mungall K, Basham D, Brown D, Chillingworth T, Connor R, Davies RM, Devlin K, Duthoy S, Feltwell T, Fraser A, Hamlin N, Holroyd S, Hornsby T, Jagels K, Lacroix C, Maclean J, Moule S, Murphy L, Oliver K, Quail MA, Rajandream MA, Rutherford KM, Rutter S, Seeger K, Simon S, Simmonds M, Skelton J, Squares R, Squares S, Stevens K, Taylor K, Whitehead S, Woodward JR, Barrell BG. 2001. Massive gene decay in the leprosy bacillus. Nature 409:1007–1011. 10.1038/35059006 [DOI] [PubMed] [Google Scholar]
- 15.Cole ST, Brosch R, Parkhill J, Garnier T, Churcher C, Harris D, Gordon SV, Eiglmeier K, Gas S, Barry CE, III, Tekaia F, Badcock K, Basham D, Brown D, Chillingworth T, Connor R, Davies R, Devlin K, Feltwell T, Gentles S, Hamlin N, Holroyd S, Hornsby T, Jagels K, Krogh A, McLean J, Moule S, Murphy L, Oliver K, Osborne J, Quail MA, Rajandream MA, Rogers J, Rutter S, Seeger K, Skelton J, Squares R, Squares S, Sulston JE, Taylor K, Whitehead S, Barrell BG. 1998. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 393:537–544. 10.1038/31159 [DOI] [PubMed] [Google Scholar]
- 16.Cruz D, Watson AD, Miller CS, Montoya D, Ochoa MT, Sieling PA, Gutierrez MA, Navab M, Reddy ST, Witztum JL, Fogelman AM, Rea TH, Eisenberg D, Berliner J, Modlin RL. 2008. Host-derived oxidized phospholipids and HDL regulate innate immunity in human leprosy. J. Clin. Invest. 118:2917–2928. 10.1172/JCI34189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brzostek A, Pawelczyk J, Rumijowska-Galewicz A, Dziadek B, Dziadek J. 2009. Mycobacterium tuberculosis is able to accumulate and utilize cholesterol. J. Bacteriol. 191:6584–6591. 10.1128/JB.00488-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Chastellier C, Thilo L. 2006. Cholesterol depletion in Mycobacterium avium-infected macrophages overcomes the block in phagosome maturation and leads to the reversible sequestration of viable mycobacteria in phagolysosome-derived autophagic vacuoles. Cell. Microbiol. 8:242–256. 10.1111/j.1462-5822.2005.00617.x [DOI] [PubMed] [Google Scholar]
- 19.Pandey AK, Sassetti CM. 2008. Mycobacterial persistence requires the utilization of host cholesterol. Proc. Natl. Acad. Sci. U. S. A. 105:4376–4380. 10.1073/pnas.0711159105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamashita M, Otsuka F, Mukai T, Otani H, Inagaki K, Miyoshi T, Goto J, Yamamura M, Makino H. 2008. Simvastatin antagonizes tumor necrosis factor-alpha inhibition of bone morphogenetic proteins-2-induced osteoblast differentiation by regulating Smad signaling and Ras/Rho-mitogen-activated protein kinase pathway. J. Endocrinol. 196:601–613. 10.1677/JOE-07-0532 [DOI] [PubMed] [Google Scholar]
- 21.Pedersen TR. 2010. Pleiotropic effects of statins: evidence against benefits beyond LDL-cholesterol lowering. Am. J. Cardiovasc. Drugs 10(Suppl 1):S10–S17. 10.2165/1158822-S0-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 22.Pharmazie in unserer Zeit. 2003. High therapeutic effect and safety of fluvastatin. Pharm. Unserer Zeit 32:516–517 (In German.) 10.1002/pauz.200390134 [DOI] [PubMed] [Google Scholar]
- 23.Martinez AN, Lahiri R, Pittman TL, Scollard D, Truman R, Moraes MO, Williams DL. 2009. Molecular determination of Mycobacterium leprae viability by use of real-time PCR. J. Clin. Microbiol. 47:2124–2130. 10.1128/JCM.00512-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lahiri R, Randhawa B, Krahenbuhl JL. 2005. Effects of purification and fluorescent staining on viability of Mycobacterium leprae. Int. J. Lepr. Other Mycobact. Dis. 73:194–202 [PubMed] [Google Scholar]
- 25.Seto S, Matsumoto S, Ohta I, Tsujimura K, Koide Y. 2009. Dissection of Rab7 localization on Mycobacterium tuberculosis phagosome. Biochem. Biophys. Res. Commun. 387:272–277. 10.1016/j.bbrc.2009.06.152 [DOI] [PubMed] [Google Scholar]
- 26.Ribeiro-Resende VT, Ribeiro-Guimarães ML, Lemes RM, Nascimento IC, Alves L, Mendez-Otero R, Pessolani MC, Lara FA. 2010. Involvement of 9-O-acetyl GD3 ganglioside in Mycobacterium leprae infection of Schwann cells. J. Biol. Chem. 285:34086–34096. 10.1074/jbc.M110.147272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Jonge MI, Pehau-Arnaudet G, Fretz MM, Romain F, Bottai D, Brodin P, Honoré N, Marchal G, Jiskoot W, England P, Cole ST, Brosch R. 2007. ESAT-6 from Mycobacterium tuberculosis dissociates from its putative chaperone CFP-10 under acidic conditions and exhibits membrane-lysing activity. J. Bacteriol. 189:6028–6034. 10.1128/JB.00469-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mattos KA, Oliveira VG, D'Avila H, Rodrigues LS, Pinheiro RO, Sarno EN, Pessolani MC, Bozza PT. 2011. TLR6-driven lipid droplets in Mycobacterium leprae-infected Schwann cells: immunoinflammatory platforms associated with bacterial persistence. J. Immunol. 187:2548–2558. 10.4049/jimmunol.1101344 [DOI] [PubMed] [Google Scholar]
- 29.Ahmad Z, Sharma S, Khuller GK. 2005. Inhalable alginate nanoparticles as antitubercular drug carriers against experimental tuberculosis. Int. J. Antimicrob. Agents 26:298–303. 10.1016/j.ijantimicag.2005.07.012 [DOI] [PubMed] [Google Scholar]
- 30.Hu Y, Van Der Geize R, Besra GS, Gurcha SS, Liu A, Rohde M, Singh M, Coates A. 2010. 3-Ketosteroid 9α-hydroxylase is an essential factor in the pathogenesis of Mycobacterium tuberculosis. Mol. Microbiol. 75:107–121. 10.1111/j.1365-2958.2009.06957.x [DOI] [PubMed] [Google Scholar]
- 31.Nesbitt NM, Yang X, Fontán P, Kolesnikova I, Smith I, Sampson NS, Dubnau E. 2010. A thiolase of Mycobacterium tuberculosis is required for virulence and production of androstenedione and androstadienedione from cholesterol. Infect. Immun. 78:275–282. 10.1128/IAI.00893-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Klink M, Brzezinska M, Szulc I, Brzostek A, Kielbik M, Sulowska Z, Dziadek J. 2013. Cholesterol oxidase is indispensable in the pathogenesis of Mycobacterium tuberculosis. PLoS One 8:e73333. 10.1371/journal.pone.0073333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brzezinska M, Szulc I, Brzostek A, Klink M, Kielbik M, Sulowska Z, Pawelczyk J, Dziadek J. 2013. The role of 3-ketosteroid 1(2)-dehydrogenase in the pathogenicity of Mycobacterium tuberculosis. BMC Microbiol. 13:43. 10.1186/1471-2180-13-43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abdoli N, Heidari R, Azarmi Y, Eghbal MA. 2013. Mechanisms of the statins cytotoxicity in freshly isolated rat hepatocytes. J. Biochem. Mol. Toxicol. 27:287–294. 10.1002/jbt.21485 [DOI] [PubMed] [Google Scholar]
- 35.Lu HZ, Li BQ. 2009. Effect of HMG-CoA reductase inhibitors on activation of human gammadeltaT cells induced by Mycobacterium tuberculosis antigens. Immunopharmacol. Immunotoxicol. 31:485–491. 10.1080/08923970902806505 [DOI] [PubMed] [Google Scholar]
- 36.Reagan-Shaw S, Nihal M, Ahmad N. 2008. Dose translation from animal to human studies revisited. FASEB J. 22:659–661. 10.1096/fj.07-9574LSF [DOI] [PubMed] [Google Scholar]
- 37.Benati D, Ferro M, Savino MT, Ulivieri C, Schiavo E, Nuccitelli A, Pasini FL, Baldari CT. 2010. Opposite effects of simvastatin on the bactericidal and inflammatory response of macrophages to opsonized S. aureus. J. Leukoc. Biol. 87:433–442. 10.1189/jlb.0409273 [DOI] [PubMed] [Google Scholar]
- 38.Wu BQ, Luo JM, Wang YH, Shi YF, Liu H, Ba JH, Zhang TT. 2013. Inhibitory effects of simvastatin on Staphylococcus aureus lipoteichoic acid-induced inflammation in human alveolar macrophages. Clin. Exp. Med. 14:151–160 [DOI] [PubMed] [Google Scholar]
- 39.Zhang S, Rahman M, Zhang S, Qi Z, Thorlacius H. 2011. Simvastatin antagonizes CD40L secretion, CXC chemokine formation, and pulmonary infiltration of neutrophils in abdominal sepsis. J. Leukoc. Biol. 89:735–742. 10.1189/jlb.0510279 [DOI] [PubMed] [Google Scholar]
- 40.Vollmer T, Key L, Durkalski V, Tyor W, Corboy J, Markovic-Plese S, Preiningerova J, Rizzo M, Singh I. 2004. Oral simvastatin treatment in relapsing-remitting multiple sclerosis. Lancet 363:1607–1608. 10.1016/S0140-6736(04)16205-3 [DOI] [PubMed] [Google Scholar]
- 41.Triantafilou M, Miyake K, Golenbock DT, Triantafilou K. 2002. Mediators of innate immune recognition of bacteria concentrate in lipid rafts and facilitate lipopolysaccharide-induced cell activation. J. Cell Sci. 115:2603–2611 [DOI] [PubMed] [Google Scholar]
- 42.Greenwood J, Steinman L, Zamvil SS. 2006. Statin therapy and autoimmune disease: from protein prenylation to immunomodulation. Nat. Rev. Immunol. 6:358–370. 10.1038/nri1839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ghittoni R, Patrussi L, Pirozzi K, Pellegrini M, Lazzerini PE, Capecchi PL, Pasini FL, Baldari CT. 2005. Simvastatin inhibits T-cell activation by selectively impairing the function of Ras superfamily GTPases. FASEB J. 19:605–607. 10.1096/fj.04-2702fje [DOI] [PubMed] [Google Scholar]
- 44.Ghittoni R, Napolitani G, Benati D, Ulivieri C, Patrussi L, Laghi Pasini F, Lanzavecchia A, Baldari CT. 2006. Simvastatin inhibits the MHC class II pathway of antigen presentation by impairing Ras superfamily GTPases. Eur. J. Immunol. 36:2885–2893. 10.1002/eji.200636567 [DOI] [PubMed] [Google Scholar]
- 45.Sarno EN, Grau GE, Vieira LM, Nery JA. 1991. Serum levels of tumour necrosis factor-alpha and interleukin-1 beta during leprosy reactional states. Clin. Exp. Immunol. 84:103–108 [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.