Abstract
We evaluated the in vitro killing activity of voriconazole (VRC) and posaconazole (PSC) against two clinical isolates of Candida guilliermondii. The two drugs showed fungistatic activity against both isolates and were effective in reducing kidney fungal burden in a neutropenic murine model of disseminated candidiasis in infected mice. PSC was significantly more effective than VRC against one of the strains. The serum levels of PSC and VRC were above the corresponding MICs for these isolates.
TEXT
Candida guilliermondii, an emerging pathogen that causes invasive infections mainly in patients who have malignancies and/or intravascular devices, accounts for 1% to 3% of candidemia cases (1) and is the fourth most frequent cause of candidemia in Latin America (2). Candida guilliermondii has poor in vitro susceptibility to echinocandins, the recommended first-line therapy, with MICs from 2- to 100-fold higher than those for other Candida spp. (3, 4), so treatment regimens often fail. Consequently, we need to look for alternative antifungals in the treatment of these infections (5). Numerous studies have demonstrated that posaconazole (PSC) and voriconazole (VRC) are efficacious in experimental infections due to some non-albicans Candida species (6–8), but no in vivo data exist regarding their effectiveness against C. guilliermondii.
We have evaluated the in vitro killing activity of PSC and VRC against C. guilliermondii and their in vivo efficacies in a neutropenic murine model of disseminated candidiasis.
Two clinical strains of C. guilliermondii identified by ribosomal DNA (rDNA) sequencing were included in the study. The MICs of PSC and VRC were determined following CLSI guidelines (9) with some modifications (i.e., inocula were adjusted by hemocytometer counts, and viability was assessed by serial plating onto potato dextrose agar [PDA]). The MIC values of PSC and VRC were 0.12 μg/ml and 0.12 μg/ml for strain UTHSC (University of Texas Health Science Center) 11-142 and 0.03 μg/ml and 0.06 μg/ml for strain UTHSC 11-685, respectively.
Time-kill curves of PSC and VRC were generated in duplicate as previously reported (10), and the concentrations for each drug assayed were 0.03, 0.12, 0.5, 1, 2, 8, and 32 μg/ml.
For the in vivo studies, 4-week-old male OF1 mice were immunosuppressed by a single intraperitoneal injection of 200 mg/kg of body weight of cyclophosphamide plus a single intravenous injection of 150 mg/kg of 5-fluorouracil (11) 1 day prior to the infection. The mice were infected intravenously via the lateral tail vein with 1 × 108 CFU in 0.2 ml of sterile saline (12, 13). All animal care procedures were supervised and approved by the Universitat Rovira i Virgili Animal Welfare and Ethics Committee.
Six groups (three for each strain) of eight infected animals were established. They received no treatment or 12.5 mg/kg PSC twice daily (BID) or 25 mg/kg VRC once a day (QD), both administered orally by gavage beginning 24 hours after challenge and lasting for 10 days. The doses selected were based on pharmacokinetic studies (14, 15). All the animals received 5 mg/kg ceftazidime subcutaneously once daily until the end of the experiment, and from 3 days before infection, the mice treated with VRC were given diluted (50%) grapefruit juice instead of water (16). Efficacy was evaluated by a reduction of fungal burden in the kidneys on day 11 postinfection. Kidneys from euthanized mice were weighed, homogenized in 1 ml of sterile saline, diluted 10-fold, and placed on PDA plates for determining the number of CFU/g after incubation for 24 hours at 35°C.
Additionally, two groups of 5 mice infected with strain UTHSC 11-685 and treated as described above were anesthetized by inhalatory isoflurane 24 hours after the last dosing, and approximately 1 ml of blood from each mouse was extracted by cardiac puncture. Serum was obtained from centrifuged blood samples and used to determine the drug concentration of each sample by bioassay (17).
Colony counts from the tissue burden studies were analyzed using the Mann-Whitney U test, using GraphPad Prism 4.0 for Windows (GraphPad Software, San Diego, CA, USA). Differences were considered statistically significant at a P value of <0.05.
Regarding the starting inoculum, PSC and VRC showed fungistatic activities against the two strains, with 0.845- to 0.64-log10 and 0.795- to 1-log10 decreases in CFU/ml, respectively (Fig. 1 and 2).
FIG 1.
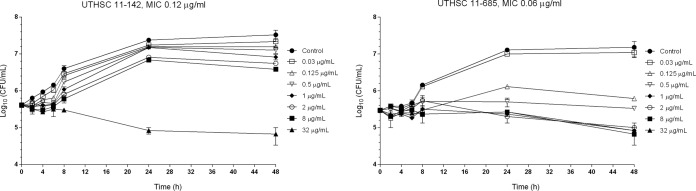
Time-kill kinetic assays of PSC at various concentrations against two strains of C. guilliermondii.
FIG 2.
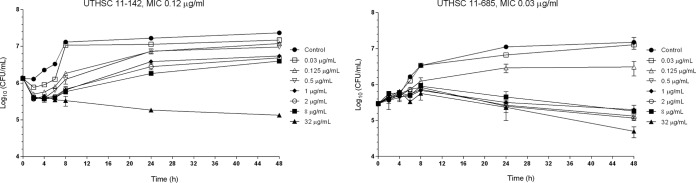
Time-kill kinetic assays of VRC at various concentrations against two strains of C. guilliermondii.
PSC and VRC significantly reduced the fungal load in the kidneys of mice infected with each of the two isolates in comparison to that of the control group (P = 0.0002) (Fig. 3). No statistically significant differences were found between the two drugs for strain UTHSC 11-142 (P = 0.0771); however, for the UTHSC 11-685 isolate, PSC worked better than did VRC (P = 0.0002). The mean (± the standard deviation) serum concentrations of PSC and VRC on day 11, 24 hours after the last dosing, were 5.4 ± 2.06 μg/ml and 5.65 ± 0.95 μg/ml, respectively (i.e., above the corresponding MIC values for the two strains tested).
FIG 3.
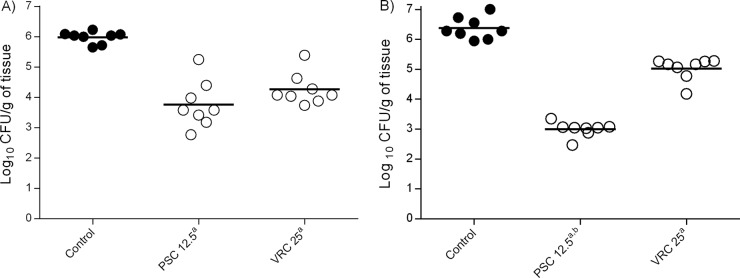
Effects of antifungal treatment on fungal loads in the kidneys of neutropenic mice infected with 1 × 108 CFU/animal of two clinical strains of C. guilliermondii, UTHSC 11-142 (A) and UTHSC 11-685 (B), 11 days postinfection. PSC and VRC were administered orally at 12.5 mg/kg BID and 25 mg/kg QD, respectively. a, P < 0.05 versus control; b, P < 0.05 versus VRC at 25 mg/kg QD.
In a previous experimental murine infection by C. guilliermondii, liposomal amphotericin B was the only effective treatment in reducing the fungal load in kidneys, in contrast to the low efficacies of fluconazole (FLC) and anidulafungin (AFG), although both of the tested strains were susceptible to these drugs (13). In this study, using the same fungal isolates, we have demonstrated the good in vivo efficacies of VRC and PSC. The MICs of PSC and VRC were within the ranges considered indicative of susceptibility and below the epidemiological cutoff values for C. guilliermondii (i.e., 0.5 and 0.12 μg/ml, respectively) (18). To our knowledge, there have been no reports of the efficacies of VRC and PSC in the treatment of invasive infections by C. guilliermondii in the clinical setting, but in the few clinical cases that reported infection by other non-albicans Candida species, both drugs demonstrated efficacy (19). In our study, the in vitro data were good predictors of the in vivo outcomes against strains, showing MIC values of PSC and VRC equal to those of the modal MICs reported in a recent multicenter study (18). To our knowledge, this is the first study to have established a relationship between the in vitro activities and in vivo efficacies of PSC and VRC against clinical isolates of C. guilliermondii.
In summary, our study demonstrated that PSC and VRC were experimentally effective in the treatment of an invasive infection by C. guilliermondii caused by isolates refractory to FLC and AFG in a neutropenic murine model in infected mice (13).
Footnotes
Published ahead of print 21 July 2014
REFERENCES
- 1.Savini V, Catavitello C, Onofrillo D, Masciarelli G, Astolfi D, Balbinot A, Febbo F, D'Amario C, D'Antonio D. 2011. What do we know about Candida guilliermondii? A voyage throughout past and current literature about this emerging yeast. Mycoses 54:434–441. 10.1111/j.1439-0507.2010.01960.x [DOI] [PubMed] [Google Scholar]
- 2.Santolaya ME, Alvarado Matute T, de Queiroz Telles F, Colombo AL, Zurita J, Tiraboschi IN, Cortes JA, Thompson-Moya L, Guzman-Blanco M, Sifuentes J, Echevarría J, Nucci M. 2013. Recommendations for the management of candidemia in neonates in Latin America. Rev. Iberoam. Micol. 30:158–170 (In Spanish.) 10.1016/j.riam.2013.06.002 [DOI] [PubMed] [Google Scholar]
- 3.Arendrup MC, Boekhout T, Akova M, Meis JF, Cornely OA, Lortholary O, ESCMID EFISG Study Group and ECMM 2014. ESCMID and ECMM joint clinical guidelines for the diagnosis and management of rare invasive yeast infections. Clin. Microbiol. Infect. 3:76–98. 10.1111/1469-0691.12360 [DOI] [PubMed] [Google Scholar]
- 4.Jensen RH, Arendrup MC. 2011. Candida palmioleophila: characterization of a previously overlooked pathogen and its unique susceptibility profile in comparison with five related species. J. Clin. Microbiol. 49:549–556. 10.1128/JCM.02071-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beyda ND, Lewis RE, Garey KW. 2012. Echinocandin resistance in Candida species: mechanisms of reduced susceptibility and therapeutic approaches. Ann. Pharmacother. 46:1086–1096. 10.1345/aph.1R020 [DOI] [PubMed] [Google Scholar]
- 6.Majithiya J, Sharp A, Parmar A, Denning DW, Warn PA. 2009. Efficacy of isavuconazole, voriconazole and fluconazole in temporarily neutropenic murine models of disseminated Candida tropicalis and Candida krusei. J. Antimicrob. Chemother. 63:161–166. 10.1093/jac/dkn431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mariné M, Pastor FJ, Serena C, Guarro J. 2009. Efficacy of triazoles in a murine disseminated infection by Candida krusei. Antimicrob. Agents Chemother. 53:3585–3588. 10.1128/AAC.00293-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mariné M, Pastor FJ, Guarro J. 2010. Efficacy of posaconazole in a murine disseminated infection by Candida tropicalis. Antimicrob. Agents Chemother. 54:530–532. 10.1128/AAC.01136-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clinical and Laboratory Standards Institute. 2008. Reference method for broth dilution antifungal susceptibility testing of yeasts; approved standard—3rd ed. CLSI M27-A3 Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 10.Cantón E, Pemán J, Valentín A, Bosch M, Espinel-Ingroff A, Gobernado M. 2008. Comparison of posaconazole and voriconazole in vitro killing against Candida krusei. Diagn. Microbiol. Infect. Dis. 62:177–181. 10.1016/j.diagmicrobio.2008.07.001 [DOI] [PubMed] [Google Scholar]
- 11.Graybill JR, Bocanegra R, Najvar LK, Loebenberg D, Luther MF. 1998. Granulocyte colony-stimulating factor and azole antifungal therapy in murine aspergillosis: role of immune suppression. Antimicrob. Agents Chemother. 42:2467–2473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arendrup M, Horn T, Frimodt-Møller N. 2002. In vivo pathogenicity of eight medically relevant Candida species in an animal model. Infection 30:286–291. 10.1007/s15010-002-2131-0 [DOI] [PubMed] [Google Scholar]
- 13.Paredes K, Pastor FJ, Capilla J, Sutton AD, Mayayo E, Fothergill WA, Guarro J. 2014. Therapies against murine Candida guilliermondii infection, relationship between in vitro antifungal pharmacodynamics and outcome. Rev. Iberoam. Micol. (In Spanish.) 10.1016/j.riam.2013.10.008 [DOI] [PubMed] [Google Scholar]
- 14.Oakley KL, Morrissey G, Denning DW. 1997. Efficacy of SCH-56592 in a temporarily neutropenic murine model of invasive aspergillosis with an itraconazole-susceptible and an itraconazole-resistant isolate of Aspergillus fumigatus. Antimicrob. Agents Chemother. 41:1504–1507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Warn PA, Sharp A, Mosquera J, Spickermann J, Schmitt-Hoffmann A, Heep M, Denning DW. 2006. Comparative in vivo activity of BAL4815, the active component of the prodrug BAL8557, in a neutropenic murine model of disseminated Aspergillus flavus. J. Antimicrob. Chemother. 58:1198–1207. 10.1093/jac/dkl396 [DOI] [PubMed] [Google Scholar]
- 16.Sugar AM, Liu XP. 2000. Effect of grapefruit juice on serum voriconazole concentration in the mouse. Med. Mycol. 38:209–212. 10.1080/mmy.38.3.209.212 [DOI] [PubMed] [Google Scholar]
- 17.Rodríguez MM, Calvo E, Serena C, Mariné M, Pastor FJ, Guarro J. 2009. Effects of double and triple combinations of antifungal drugs in a murine model of disseminated infection by Scedosporium prolificans. Antimicrob. Agents Chemother. 53:2153–2155. 10.1128/AAC.01477-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Espinel-Ingroff A, Pfaller MA, Bustamante B, Canton E, Fothergill A, Fuller J, Gonzalez GM, Lass-Flörl C, Lockhart SR, Martin-Mazuelos E, Meis JF, Melhem MS, Ostrosky-Zeichner L, Pelaez T, Szeszs MW, St-Germain G, Bonfietti LX, Guarro J, Turnidge J. 2014. Multilaboratory study of epidemiological cutoff values for detection of resistance in eight Candida species to fluconazole, posaconazole, and voriconazole. Antimicrob. Agents Chemother. 58:2006–2012. 10.1128/AAC.02615-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zaragoza R, Pemán J. 2012. Antifungal treatment options in the critically ill patient. Rev. Iberoam. Micol. 29:108–113 (In Spanish.) 10.1016/j.riam.2012.03.009 [DOI] [PubMed] [Google Scholar]


