Abstract
Doxycycline is widely used for malaria prophylaxis by international travelers. However, there is limited information on doxycycline efficacy in Kenya, and genetic polymorphisms associated with reduced efficacy are not well defined. In vitro doxycycline susceptibility profiles for 96 Plasmodium falciparum field isolates from Kenya were determined. Genetic polymorphisms were assessed in P. falciparum metabolite drug transporter (Pfmdt) and P. falciparum GTPase tetQ (PftetQ) genes. Copy number variation of the gene and the number of KYNNNN amino acid motif repeats within the protein encoded by PftetQ were determined. Reduced in vitro susceptibility to doxycycline was defined by 50% inhibitory concentrations (IC50s) of ≥35,000 nM. The odds ratio (OR) of having 2 PfTetQ KYNNNN amino acid repeats in isolates with IC50s of >35,000 nM relative to those with IC50s of <35,000 nM is 15 (95% confidence interval [CI], 3.0 to 74.3; P value of <0.0002). Isolates with 1 copy of the Pfmdt gene had a median IC50 of 6,971 nM, whereas those with a Pfmdt copy number of >1 had a median IC50 of 9,912 nM (P = 0.0245). Isolates with 1 copy of PftetQ had a median IC50 of 6,370 nM, whereas isolates with a PftetQ copy number of >1 had a median IC50 of 3,422 nM (P < 0.0007). Isolates with 2 PfTetQ KYNNNN motif repeats had a median IC50 of 26,165 nM, whereas isolates with 3 PfTetQ KYNNNN repeats had a median IC50 of 3,352 nM (P = 0.0023). PfTetQ sequence polymorphism is associated with a reduced doxycycline susceptibility phenotype in Kenyan isolates and is a potential marker for susceptibility testing.
INTRODUCTION
Plasmodium resistance to antimalarials is one of the major obstacles in global malaria control efforts (1). The emergence and spread of resistance to antimalarials such as chloroquine and sulfadoxine-pyrimethamine, which were once first-line drugs, have rendered these drugs ineffective in most areas of malaria endemicity. This has compromised control programs and malaria elimination efforts (2). The emergence of resistant Plasmodium falciparum encouraged efforts to find an alternative class of drugs, including antibiotics such as tetracycline which was shown to have antimalarial activity. Treatment and prophylactic efficacy of tetracyclines such as doxycycline, tetracycline, and minocycline against chloroquine-resistant P. falciparum have been investigated (3, 4). Doxycycline has been shown to be a slower-acting blood schizonticide during the course of an acute Plasmodium infection than other antimalarials. However, it achieves good treatment efficacy when given with rapidly acting schizontocidal drugs such as quinine or quinidine (5). Doxycycline has also been shown to be an effective prophylactic drug when administered as a monotherapy given at 100 mg once a day starting 1 to 2 days before travel to an area of malaria endemicity, daily during travel, and for 4 weeks after return from a malarious region (5). As a prophylaxis, it is recommended for travelers visiting countries where malaria is endemic, especially in regions with chloroquine- and multidrug-resistant Plasmodium parasites (6). Chemoprophylaxis is useful in limiting the spread of drug-resistant parasite strains within and between different localities. The emergence of resistant parasites involves evolutionary recombination events within the parasite genome which give rise to de novo polymorphisms and a subsequent selection process, in which survival advantage in the presence of the drug leads to preferential transmission, propagation, and the spread of resistance-conferring gene alleles within parasite populations (7, 8).
The Centers for Disease Control and Prevention (CDC) estimates that 1,500 malaria cases are diagnosed in the United States annually among tourists and deployed personnel such as the military returning from countries of malaria endemicity. Malaria is also imported to Europe, with more than 6,000 such cases reported in 2010 according to the World Health Organization (WHO). Therefore, it is important that parasite drug resistance profiles be monitored in areas of malaria endemicity that are of interest to international travelers and nations. Chemoprophylaxis and drug regimens to which there is a considerable level of resistance within the indigenous population should not be recommended for international travelers and deployed personnel.
Clinical failure of doxycycline therapy and prophylaxis has been attributed to administration of a suboptimal dosage (9) and poor compliance (10). This was evident in 2009 when a Navy Seabee deployed to Liberia died of malaria; his death was attributed to poor compliance to mandated doxycycline prophylaxis of all U.S. personnel deployed in regions of malaria endemicity (11). Clinical failure could also be attributed to cycline resistance in Plasmodium. This has been examined in a murine malaria model where Plasmodium berghei-infected mice were exposed to increasing concentrations of minocycline, resulting in a resistant parasite strain with a 50% inhibitory concentration of (IC50) of 600 mg/kg of body weight/day (12).
Studies have shown a link between specific gene polymorphisms and malaria drug treatment outcomes. Allele variations in codons 86, 184, and 1246 in P. falciparum multidrug resistance gene 1 (Pfmdr1; PFE1150w) is an example of such a genetic link which renders parasites resistant to several drugs, including mefloquine (13) and lumefantrine (14). An increase in Pfmdr1 gene copy number also renders parasites resistant to mefloquine (15). However, there is limited information on doxycycline cellular targets within P. falciparum. Using 90 isolates from 14 African countries (central and west Africa), Briolant et al. demonstrated that copy number increases of P. falciparum metabolite drug transporter (Pfmdt; PFE0825w) and P. falciparum GTPase TetQ (PftetQ; PFL1710c) genes as well as sequence polymorphism within the latter are associated with reduced susceptibility to doxycycline (16). The correlation of increased copy numbers of the Pfmdt and PftetQ genes with reduced doxycycline susceptibility was later confirmed (17). However, a study by Gaillard et al. did not show a significant association between doxycycline in vitro susceptibility and increased copy numbers of Pfmdt and PftetQ (18). To further investigate the role of the Pfmdt and PftetQ genes in susceptibility of P. falciparum to doxycycline, the correlation between doxycycline in vitro susceptibility to Pfmdt and PftetQ gene polymorphisms in P. falciparum field isolates from western Kenya was assessed.
MATERIALS AND METHODS
Study sites and subjects.
Samples used in this study were collected from Kisumu District Hospital (KDH) located in Kisumu town, in Kisumu county, western Kenya. Patients who were 6 months of age and older attending the outpatient clinic with uncomplicated malaria at KDH were enrolled into the study under an approved protocol by the Kenya Medical Research Institute (KEMRI) and Walter Reed Army Institute of Research (WRAIR) Ethical Review boards. Samples analyzed in this study were collected between 2010 and 2013. Written informed consent was obtained from adult subjects (≥18 years old), and consent from legal guardians was obtained for subjects of <18 years old. The study excluded patients who had been treated for malaria in the 2 weeks preceding the visit to the clinic or who had severe P. falciparum malaria.
Sample collection and preparation.
Microscopy and a rapid diagnostic test (RDT) (Parascreen Pan/Pf; Zephyr Biomedicals, Verna Goa, India) were used to screen and confirm that the patient was infected with P. falciparum malaria. Upon patient consent, 2 to 3 ml of whole blood from eligible participants was collected for in vitro culture, and 100 μl was spotted onto FTA filter paper (Whatman Inc., Bound Brook, NJ, USA). Some of the whole blood was also aliquoted and cryopreserved. DNA was extracted from either whole blood or FTA filter paper using a QIAamp DNA minikit (Qiagen, Valencia, CA), as recommended by the manufacturer.
Doxycycline in vitro drug susceptibility testing.
A previously described SYBR green I-based assay (19, 20) was used to test P. falciparum field isolates for in vitro susceptibility to doxycycline hyclate. Doxycycline was obtained from a commercial supplier (Sigma-Aldrich, Co., St. Louis, MO). Reference P. falciparum clone 3D7 obtained from the Malaria Research and Reference Reagent Resource Center (Manassas, VA, USA) was assayed together with the study isolates for internal control against the test drug. To prepare test drugs, 19.53 M doxycycline stock solution was prepared in 5 ml of 100% dimethyl sulfoxide. Prior to initiation of the drug-testing assay, the stock solution was lowered to a concentration of 48,828 nM and transferred to the first well of a 96-well microculture plate. Using a Biomek 2000 robotic work station (Beckman Coulter, CA, USA), the drug was further serially diluted in complete RPMI 1640 medium to 10 different concentrations ranging between 48,828 and 95.4 nM. The drug plates were prepared and used immediately or stored at −80°C for not longer than 1 month before use. Basic medium and complete RPMI 1640 culture medium enriched with glucose (0.2%), human serum (10%), and hypoxanthine (0.004%) were prepared as necessary for in vitro culture. Briefly, in vitro culturing was carried out at 5% hematocrit for 7 to 30 days to establish parasite growth of between 3 and 8% parasitemia. Before drug susceptibility testing was carried out, culture hematocrit was adjusted to 2%, and parasitemia was adjusted to 1%. To carry out susceptibility testing, 12.5 μl of doxycycline at concentrations ranging from 48,828 to 95.4 nM were transferred onto 96-well microculture plates. On the same plates, culture-adapted sample at 2% hematocrit and 1% parasitemia was added, and the plates were incubated for 72 h as previously described (19). After this duration, lysis buffer was added, and plates were incubated for a further 24 h in the dark. Parasite growth inhibition was quantified by measuring the per well relative fluorescence units (RFU) of SYBR green I dye using a Tecan Genios Plus plate reader with excitation and emission wavelengths of 485 nm and 535 nm, respectively, and with the gain set at 60. Data in the form of RFU counts was outputted directly to MS Excel. For analysis, the readouts were aligned with corresponding drug doses, and analysis was performed by GraphPad Prism software. The drug concentrations (x values) were transformed using the equation x = log(x) and plotted against the counts (y values), and the data were analyzed by nonlinear regression (sigmoid dose-response/variable slope equation) to yield the IC50.
Pfmdt and PftetQ copy number determination.
Pfmdt and PftetQ copy numbers were estimated using a 7500 Fast real-time PCR system (Applied Biosystems Inc., Foster City, CA) relative to the single-copy P. falciparum tubulin gene (PF10_0084). Oligonucleotide primer and probe sequences used for the assay were as previously described (21). The PCR efficiencies of all the primer pairs were evaluated on a dilution series of P. falciparum 3D7 genomic DNA and were found to be sufficiently close to obviate the need for any correction factor. Individual PCRs were performed using 1× Quantifast probe (Qiagen, Inc., Valencia, CA) and a final concentration of 0.5 μM (each) forward primer, reverse primer, and probes and 1 μl of template DNA in a final volume of 20 μl. The reaction mixtures were prepared at 4°C in a 96-well optical reaction plate (Applied Biosystems Inc., Foster City, CA) covered with optical adhesive covers (Applied Biosystems Inc., Foster City, CA). The thermal cycling conditions were 95°C for 10 min and 40 cycles of 95°C for 15 s and 60°C for 1 min. Each sample was assayed in triplicate and analyzed with the SDS software, version 2.2.1 (Applied Biosystems Inc., Foster City, CA). The 2−ΔΔCT (where CT is threshold cycle) method of relative quantification was used to estimate copy number variation in the PftetQ and Pfmdt genes according to the following formula: ΔΔCT = (CT(PftetQ or Pfmdt) − CT(tubulin))sample − (CT(PftetQ or Pfmdt) − CT(tubulin))calibrator, where the same genes are used for both the sample and calibrator (clone 3D7) and where tubulin represents the P. falciparum tubulin gene.
Genomic DNA extracted from P. falciparum 3D7, which has a single copy of each gene, was used for calibration, whereas the P. falciparum tubulin gene served as the control housekeeping gene.
Genotyping for PftetQ sequence polymorphisms.
Extracted DNA from P. falciparum-positive isolates was used as templates for determining the variation in the number of PfTetQ KYNNNN motif repeats. This was done by conventional PCR using the primers PftetQ forward (5′-TCACGACAAATGTGCTAGATAC-3′) and PftetQ reverse (5′-ATCATCATTTGTGGTGGATAT-3′), which were designed using primer 3 software, version 4.0.0, as previously described (21). The final concentrations of PCR reactants were as follows: a 0.5 μM concentration of each primer, 1× PCR buffer, 4 mM magnesium chloride (MgCl2), 0.02 units of Taq polymerase, 100 μM (25 μM each) deoxyribonucleotide mix. The final reaction volume was 20 μl including 1 μl of extracted P. falciparum DNA. PCR products were purified by use of a QIAquick PCR purification kit (Qiagen, Inc., Valencia, CA), as per the manufacturer's instructions. The resultant purified PCR products were then sequenced using an ABI 3500xL genetic analyzer (Applied Biosystems Inc., Foster City, CA).
Statistical analysis.
The raw DNA PftetQ sequences generated after sequencing were assembled into contigs using DNA Baser, version 3X (Heracle BioSoft S.R.L., Pitesti, Romania). BioEdit software, version 7.1.3 (Ibis Biosciences, Carlsbad, CA), was used to perform sequence alignment for the generated PftetQ contigs with the published 3D7 reference strain for the PftetQ gene. A Kruskal-Wallis test was used to test for differences in doxycycline in vitro susceptibility between isolates with and without the specified Pfmdt and PftetQ polymorphisms. Chi-square and Fisher's exact tests were used as appropriate to determine an association between Pfmdt and PftetQ genotypes and doxycycline susceptibility profiles for all the study isolates. Significance was defined as a P value of <0.05.
RESULTS
Determination of Pfmdt and PftetQ copy numbers and PfTetQ KYNNNN motif repeat variations.
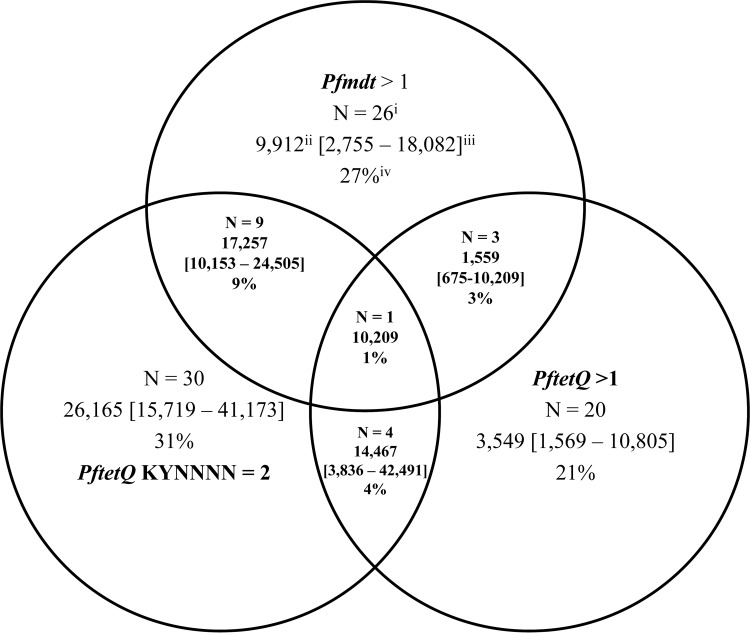
A previous study observed a correlation between reduced doxycycline in vitro susceptibility and increased copy numbers of both the Pfmdt and PftetQ genes as well as a reduction in PfTetQ KYNNNN amino acid motif repeats from 3 to 2 copies or 1 copy (16). The PfTetQ KYNNNN motif repeats are situated between amino acid positions 816 and 833 (nucleotide positions 2448 to 2499). Figure 1 shows the prevalence of polymorphism in Pfmdt and PftetQ genes in all 96 analyzed sample isolates. The frequencies of Pfmdt and PftetQ copy numbers of >1 were 27% (26/96) and 21% (20/96), respectively. Sixty-nine percent (66/96) of the sample isolates had 3 copies of the KYNNNN motif repeat (wild type), whereas 31% (30/96) had 2 copies (mutant). None of the isolates had 1 copy of the KYNNNN repeat. A haplotype combination of Pfmdt and/or PftetQ with a gene copy number of >1 and 2 copies of the PfTetQ KYNNNN motif repeats was observed in a subset of the isolates. As shown in Fig. 1, nine isolates had a Pfmdt gene copy number of >1 and 2 copies of PfTetQ KYNNNN repeats, four isolates had >1 copy of the PftetQ gene and 2 copies of PfTetQ KYNNNN repeats, and one isolate contained both Pfmdt and PftetQ with copy numbers of >1 and 2 copies of the PfTetQ KYNNNN motif repeats.
FIG 1.
Description of Plasmodium falciparum isolates with Pfmdt and PftetQ polymorphisms and their doxycycline susceptibilities showing (i) the number of isolates, (ii) the median 50% inhibitory concentration (IC50), (iii) the median interquartile range (IQR), and (iv) frequencies according to copy numbers of Pfmdt and PftetQ and occurrence of PfTetQ KYNNNN repeats.
Doxycycline susceptibility testing.
Doxycycline susceptibility testing was performed on 96 clinical isolates. The median IC50 was 7,341 nM (interquartile range [IQR], 2,032 to 20,719) for the field isolates and 749.4 nM (IQR, 607.4 to 763.8 nM) for the 3D7 clone. Reduced in vitro susceptibility to doxycycline has been defined by an IC50 of ≥35,000 nM (22). In our study, 85% (82/96) of sample isolates had an IC50 of <35,000 nM, with a median of 4,739 nM (IQR, 1,744 to 12,775 nM), whereas 15% (14/96) of the sample isolates had reduced in vitro susceptibility, with a median IC50 of 49,952 nM (IQR, 40,212 to 70,580 nM). The highest observed doxycycline IC50 was 119,805 nM. There was a reduction in the doxycycline median IC50 over time in sample isolates collected during the study period of 2010 to 2013 (Fig. 2).
FIG 2.
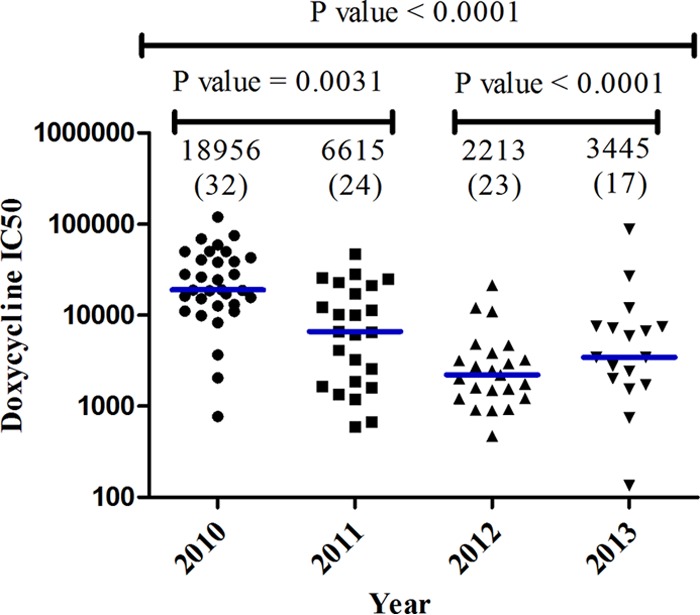
Doxycycline median 50% inhibitory concentrations (IC50; values in nM), indicated above each data plot, during the 2010 to 2013 study period. The number of isolates analyzed in each year is shown in parentheses. There was a significant decline in median IC50 from 18,956 nM in 2010 to 3,445 nM in 2013.
Association between PftetQ and Pfmdt gene polymorphisms and doxycycline in vitro susceptibility.
We further sought to correlate the obtained parasite IC50s (phenotypic characteristics) to the parasite genotype as described previously. Kruskal-Wallis analysis revealed significant differences in chemosusceptibility between wild-type isolates having 1 copy each of Pfmdt and PftetQ and 3 PfTetQ KYNNNN motif repeats relative to those with 2 or 3 copies of both genes and 2 PfTetQ KYNNNN motif repeats. Isolates with 1 copy of Pfmdt had a median IC50 of 6,972 nM (IQR, 1,723 to 25,028 nM), whereas isolates with >1 copy of Pfmdt had a median IC50 of 9,912 nM (IQR, 2,755 to 18,082 nM) (P = 0.0245). Unexpectedly, isolates with 1 copy of PftetQ had a median IC50 of 6,370 nM (IQR, 2,005 to 20,695 nM), whereas isolates with >1 copy of PftetQ had a median IC50 of 3,422 nM (IQR, 1,240 to 10,606 nM) (P < 0.0007). Isolates with 2 PfTetQ KYNNNN motif repeats had a median IC50 of 26,165 nM (IQR, 15,719 to 41,173 nM), whereas isolates with 3 PfTetQKYNNNN repeats had a median IC50 of 3,352 nM (IQR, 1,554 to 10,987 nM) (P = 0.0023). Chi-square analysis revealed a significant correlation between isolates with an IC50 of >35,000 nM and occurrence of 2 PfTetQ KYNNNN motif repeats relative to those with IC50s of <35,000 nM (OR, 15;95% CI, 3.0 to 74.3; P < 0.0002) (Table 1). The odds ratios of having >1 copy number of Pfmdt and PftetQ within isolates with IC50s of >35,000 nM relative to those with IC50s of <35,000 nM were 0.41 (95% CI, 0.04–3.7; P value < 0.423) and 0.205 (95% CI, 0.02–1.6; P value = 0.140), respectively.
TABLE 1.
Pfmdt and PftetQ gene polymorphism odds ratios of isolates with doxycycline IC50s of >35,000 nM relative to those with IC50s of <35,000 nM
| Gene copy number and repeat frequency | No. (%) of isolates with the indicated doxycycline IC50 |
OR (95% CI)a | P value | |
|---|---|---|---|---|
| <35,000 nM (n = 82) | >35,000 nM (n = 14) | |||
| No. of Pfmdt copies | ||||
| 1 | 57 (71) | 12 (92) | 1* | |
| >1 | 23 (29) | 1 (8) | 0.205 (0.02–1.6) | 0.172 |
| No. of PftetQ copies | ||||
| 1 | 41 (67) | 5 (83) | 1* | |
| >1 | 20 (33) | 1 (17) | 0.4100 (0.04–3.7) | 0.65 |
| No. of PfTetQ KYNNNN repeats | ||||
| 3 | 60 (77) | 2 (17) | 1* | |
| 2 | 20 (25) | 10 (83) | 15 (3.0–74.3) | <0.0002b |
Odds ratio (OR) with 95% confidence interval (CI). *, reference group.
The probability of having two PfTetQ KYNNNN motif repeats within isolates with IC50s of >35,000 nM relative to those with IC50s of <35,000 nM was significant at a P value of <0.0002.
DISCUSSION
In this study, we have shown that sample isolates with 2 PfTetQKYNNNN motif repeats are associated with reduced doxycycline susceptibility and with a significantly higher probability of having an IC50 above the doxycycline resistance threshold of 35,000 nM. This is in line with previous studies in which Briolant et al. showed correlation of <3 PfTetQKYNNNN motif repeat(s) with reduced doxycycline susceptibility (16). Interestingly, the PftetQ gene copy number was inversely associated with the isolate IC50. The median IC50 of isolates with 1 copy of the PftetQ gene was significantly higher than that of isolates with >1 copy of PftetQ. However, the median IC50 for isolates with 1 or 2 copies of the Pfmdt and PftetQ genes was >35,000 nM. These data suggest that although copy numbers of the Pfmdt and PftetQ genes might influence the parasite IC50, this genotype does not significantly influence the probability of a parasite having an IC50 above the doxycycline resistance threshold.
Briolant et al. found sample isolates with more than 1 copy of the Pfmdt or PftetQ gene to be independently associated with decreased susceptibility to doxycycline (16), which was later confirmed (17). However, Gaillard et al. found that there was no association between copy numbers of the Pfmdt or PftetQ gene with susceptibility to doxycycline (18). In our study, in line with the study of Briolant et al. (16), isolates with more than 1 copy of the Pfmdt gene had a significantly higher median IC50. However, the reverse was observed for the PftetQ gene. The discrepancy observed might be explained by the location where the samples were obtained and the period during which they were collected. Briolant et al. (16) analyzed 90 sample isolates from 14 different African countries, mostly in west and central Africa collected between 1997 and 2006. Gaillard et al. (18) analyzed 113 sample isolates collected from Senegal between 2009 and 2010, whereas in our study, sample isolates were obtained in Kenya and collected between 2010 and 2013. Further, the difference in data obtained between the studies might also be explained by the difference in methods used for parasite IC50 analysis ([3H]hypoxanthine based-assay versus SYBR green I-based assay), data interpretation and analysis (geometric mean versus mean versus median), the threshold which data were based on, sample size, parasite genetic background, population structure, and much more. Additional studies with more consistent, representative analytical methods using larger sample sizes and covering more representative regions of sub-Saharan Africa would eliminate regional bias and give an in-depth perspective that is more conclusive of the role of Pfmdt and PftetQ copy numbers in conferring doxycycline resistance.
Tetracyclines such as doxycycline inhibit protein synthesis of bacteria and eukaryotes such as Plasmodium spp. Studies have shown that they attach at the proteins S4, S7, and S9 of the small ribosomal subunit 30S and with various ribonucleic acids of the rRNA 16S subunit (23), preventing the binding of aminoacyl tRNA to site A of the ribosome and thus blocking elongation of protein translation. However, these mechanisms of action for Plasmodium are not clearly identified. There are three recognized mechanisms of bacterial cycline resistance: tetracycline modification, efflux, and ribosomal protection. Cycline efflux is mediated by tet efflux membrane-associated proteins, which export cyclines from the cytoplasm, thereby reducing drug intracellular concentrations. Analogues of tet efflux proteins have been found in P. falciparum (24). Ribosomal protection proteins are cytoplasmic proteins that protect ribosomes from the action of tetracycline in a GTP-dependent way (25) and display sequence similarity to translation elongation factors EF-G/EF-2 and EF-Tu/EF-1 (26). The P. falciparum tetQ GTPase protein, the PftetQ gene product analogue, was discovered in Bacteroides by Nikolich et al. (27). A previous study observed homology between amino acid sequences of five bacterial ribosomal protection proteins with the PftetQ gene product (24). It is not well understood how reduction in the number of PfTetQ KYNNNN amino acid motif repeats enhances its protective effect on the ribosome; however, similar sequence polymorphisms such as increase in number of DNNND and DDNHNDNHNNDD microsatellite repeats within the Plasmodium falciparum sodium hydrogen exchanger (Pfnhe) have been linked to a reduction in the in vitro activity of quinine (28).
The decline in doxycycline IC50s during the study period of 2010 to 2013 is indicative of increased susceptibility to the antibiotic. The use of doxycycline for malaria chemoprophylaxis by the local community is limited. However, it is used for treatment of Neisseria gonorrhea and Chlamydia trachomatis infections (29). A reduction in the doxycycline IC50 could also be explained by declining usage of the drug due to reduced prevalence of gonorrhea, which decreased from 3.2% to 2.7% between 2002 and 2010 (30).
To further analyze the prevalence of P. falciparum reduced susceptibility to doxycycline in Kenya, it would be ideal to conduct both in vivo and in vitro surveillance of doxycycline protective efficacy against malaria among the international travelers who are on doxycycline prophylaxis while visiting Kenya and/or among expatriates who are temporarily residents in regions of malaria endemicity, such as western Kenya. It is important to continue monitoring the P. falciparum drug resistance profiles to doxycycline because this drug is still widely used as the drug of choice for chemoprophylaxis, especially for U.S. international travels and military personnel.
We have confirmed the association of a reduction in PfTetQ KYNNNN amino acid repeats with the doxycycline resistance phenotype (IC50 of >35,000 nM) in Kenyan isolates. Pfmdt and PftetQ gene amplification was observed; however, this polymorphism was not strongly associated with the doxycycline resistance phenotype. This finding warrants further confirmatory studies to determine the role of this polymorphism in conferring resistance to doxycycline before it can be used as a reference molecular marker for reduced doxycycline susceptibility.
ACKNOWLEDGMENTS
We thank Duke Omariba, our study coordinator, and Eunice Awiti, the study site clinician, for their contribution in recruitment of participants and sample collection. We also thank the Director of the Kenya Medical Research Institute for permission to publish this work.
This work was supported by the U.S. Department of Defense, Global Emerging Infections System, Silver Spring, MD.
The opinions and assertions contained herein are the private opinions of the authors and are not to be construed as reflecting the views of the U.S. Army Medical Research Unit-Kenya or the U.S. Department of Defense.
Footnotes
Published ahead of print 28 July 2014
REFERENCES
- 1.Roll Back Malaria Partnership. 2010. Roll back malaria, key malaria facts. World Health Organization, Geneva, Switzerland [Google Scholar]
- 2.Picot S, Olliaro P, de Monbrison F, Bienvenu AL, Price RN, Ringwald P. 2009. A systematic review and meta-analysis of evidence for correlation between molecular markers of parasite resistance and treatment outcome in falciparum malaria. Malar. J. 8:89. 10.1186/1475-2875-8-89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Willerson D, Jr, Rieckmann KH, Carson PE, Frischer H. 1972. Effects of minocycline against chloroquine-resistant falciparum malaria. Am. J. Trop. Med. Hyg. 21:857–862 [DOI] [PubMed] [Google Scholar]
- 4.Colwell EJ, Hickman RL, Kosakal S. 1972. Tetracycline treatment of chloroquine-resistant falciparum malaria in Thailand. JAMA 220:684–686. 10.1001/jama.1972.03200050026007 [DOI] [PubMed] [Google Scholar]
- 5.Tan KR, Magill AJ, Parise ME, Arguin PM. 2011. Doxycycline for malaria chemoprophylaxis and treatment: report from the CDC expert meeting on malaria chemoprophylaxis. Am. J. Trop. Med. Hyg. 84:517–531. 10.4269/ajtmh.2011.10-0285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.WHO. 2006. Guidelines for the treatment of malaria. Document WHO/HTM/MAL/2006.1108 World Health Organization, Geneva, Switzerland [Google Scholar]
- 7.Vinayak S, Alam MT, Mixson-Hayden T, McCollum AM, Sem R, Shah NK, Lim P, Muth S, Rogers WO, Fandeur T, Barnwell JW, Escalante AA, Wongsrichanalai C, Ariey F, Meshnick SR, Udhayakumar V. 2010. Origin and evolution of sulfadoxine resistant Plasmodium falciparum. PLoS Pathog. 6:e1000830. 10.1371/journal.ppat.1000830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alam MT, Vinayak S, Congpuong K, Wongsrichanalai C, Satimai W, Slutsker L, Escalante AA, Barnwell JW, Udhayakumar V. 2011. Tracking origins and spread of sulfadoxine-resistant Plasmodium falciparum dhps alleles in Thailand. Antimicrob. Agents Chemother. 55:155–164. 10.1128/AAC.00691-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pang L, Limsomwong N, Singharaj P. 1988. Prophylactic treatment of vivax and falciparum malaria with low-dose doxycycline. J. Infect. Dis. 158:1124–1127. 10.1093/infdis/158.5.1124 [DOI] [PubMed] [Google Scholar]
- 10.Wallace MR, Sharp TW, Smoak B, Iriye C, Rozmajzl P, Thornton SA, Batchelor R, Magill AJ, Lobel HO, Longer CF, Burans JP. 1996. Malaria among United States troops in Somalia. Am. J. Med. 100:49–55. 10.1016/S0002-9343(96)90011-X [DOI] [PubMed] [Google Scholar]
- 11.Ollikainen R. 29 December 2009. Malaria claims Navy Seabee from Port Angeles. In Peninsula Daily News. Black Press, Ltd., Port Angeles, WA: http://www.peninsuladailynews.com/article/20091230/news/312309994/0/SEARCH [Google Scholar]
- 12.Jacobs RL, Koontz LC. 1976. Plasmodium berghei: development of resistance to clindamycin and minocycline in mice. Exp. Parasitol. 40:116–123. 10.1016/0014-4894(76)90073-4 [DOI] [PubMed] [Google Scholar]
- 13.Pickard AL, Wongsrichanalai C, Purfield A, Kamwendo D, Emery K, Zalewski C, Kawamoto F, Miller RS, Meshnick SR. 2003. Resistance to antimalarials in Southeast Asia and genetic polymorphisms in pfmdr1. Antimicrob. Agents Chemother. 47:2418–2423. 10.1128/AAC.47.8.2418-2423.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dokomajilar C, Nsobya SL, Greenhouse B, Rosenthal PJ, Dorsey G. 2006. Selection of Plasmodium falciparum pfmdr1 alleles following therapy with artemether-lumefantrine in an area of Uganda where malaria is highly endemic. Antimicrob. Agents Chemother. 50:1893–1895. 10.1128/AAC.50.5.1893-1895.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Price RN, Uhlemann AC, Brockman A, McGready R, Ashley E, Phaipun L, Patel R, Laing K, Looareesuwan S, White NJ, Nosten F, Krishna S. 2004. Mefloquine resistance in Plasmodium falciparum and increased pfmdr1 gene copy number. Lancet 364:438–447. 10.1016/S0140-6736(04)16767-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Briolant S, Wurtz N, Zettor A, Rogier C, Pradines B. 2010. Susceptibility of Plasmodium falciparum isolates to doxycycline is associated with pftetQ sequence polymorphisms and pftetQ and pfmdt copy numbers. J. Infect. Dis. 201:153–159. 10.1086/648594 [DOI] [PubMed] [Google Scholar]
- 17.Gaillard T, Briolant S, Houze S, Baragatti M, Wurtz N, Hubert V, Lavina M, Pascual A, Travaille C, Le Bras J, Pradines B. 2013. pftetQ and pfmdt copy numbers as predictive molecular markers of decreased ex vivo doxycycline susceptibility in imported Plasmodium falciparum malaria. Malar. J. 12:414. 10.1186/1475-2875-12-414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gaillard T, Fall B, Tall A, Wurtz N, Diatta B, Lavina M, Fall KB, Sarr FD, Baret E, Dieme Y, Wade B, Bercion R, Briolant S, Pradines B. 2012. Absence of association between ex vivo susceptibility to doxycycline and pftetQ and pfmdt copy numbers in Plasmodium falciparum isolates from Dakar, Senegal. Clin. Microbiol. Infect. 18:E238–E240. 10.1111/j.1469-0691.2012.03889.x [DOI] [PubMed] [Google Scholar]
- 19.Johnson JD, Dennull RA, Gerena L, Lopez-Sanchez M, Roncal NE, Waters NC. 2007. Assessment and continued validation of the malaria SYBR green I-based fluorescence assay for use in malaria drug screening. Antimicrob. Agents Chemother. 51:1926–1933. 10.1128/AAC.01607-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Akala HM, Eyase FL, Cheruiyot AC, Omondi AA, Ogutu BR, Waters NC, Johnson JD, Polhemus ME, Schnabel DC, Walsh DS. 2011. Antimalarial drug sensitivity profile of western Kenya Plasmodium falciparum field isolates determined by a SYBR Green I in vitro assay and molecular analysis. Am. J. Trop. Med. Hyg. 85:34–41. 10.4269/ajtmh.2011.10-0674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koressaar T, Remm M. 2007. Enhancements and modifications of primer design program Primer3. Bioinformatics 23:1289–1291. 10.1093/bioinformatics/btm091 [DOI] [PubMed] [Google Scholar]
- 22.Briolant S, Baragatti M, Parola P, Simon F, Tall A, Sokhna C, Hovette P, Mamfoumbi MM, Koeck JL, Delmont J, Spiegel A, Castello J, Gardair JP, Trape JF, Kombila M, Minodier P, Fusai T, Rogier C, Pradines B. 2009. Multinormal in vitro distribution model suitable for the distribution of Plasmodium falciparum chemosusceptibility to doxycycline. Antimicrob. Agents Chemother. 53:688–695. 10.1128/AAC.00546-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pioletti M, Schlunzen F, Harms J, Zarivach R, Gluhmann M, Avila H, Bashan A, Bartels H, Auerbach T, Jacobi C, Hartsch T, Yonath A, Franceschi F. 2001. Crystal structures of complexes of the small ribosomal subunit with tetracycline, edeine and IF3. EMBO J. 20:1829–1839. 10.1093/emboj/20.8.1829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Briolant S, Fusai T, Rogier C, Pradines B. 2008. Tetracycline antibiotics in malaria. Open Trop. Med. J. 1:31–46. 10.2174/1874315300801010031 [DOI] [Google Scholar]
- 25.Dantley KA, Dannelly HK, Burdett V. 1998. Binding interaction between Tet(M) and the ribosome: requirements for binding. J. Bacteriol. 180:4089–4092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kobayashi TNL, Maruyama F, Suzuki S. 2007. Molecular evidence for the ancient origin of the ribosomal protection protein that mediates tetracycline resistance in bacteria. J. Mol. Evol. 65:228–235. 10.1007/s00239-007-9006-z [DOI] [PubMed] [Google Scholar]
- 27.Nikolich MP, Shoemaker NB, Salyers AA. 1992. A Bacteroides tetracycline resistance gene represents a new class of ribosome protection tetracycline resistance. Antimicrob. Agents Chemother. 36:1005–1012. 10.1128/AAC.36.5.1005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Okombo J, Kiara SM, Rono J, Mwai L, Pole L, Ohuma E, Borrmann S, Ochola LI, Nzila A. 2010. In vitro activities of quinine and other antimalarials and pfnhe polymorphisms in Plasmodium isolates from Kenya. Antimicrob. Agents Chemother. 54:3302–3307. 10.1128/AAC.00325-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Workowski KA, Berman S, CDC 2010. Sexually transmitted diseases treatment guidelines, 2010. MMWR Recomm. Rep. 59(RR-12):1–110 [PubMed] [Google Scholar]
- 30.Otieno-Nyunya B, Bennett E, Bunnell R, Dadabhai S, Gichangi AA, Mugo N, Wanyungu J, Baya I, Kaiser R, Kenys AIDS Indicator Survey Study Team 2011. Epidemiology of syphilis in Kenya: results from a nationally representative serological survey. Sex. Transm. Infect. 87:521–525. 10.1136/sextrans-2011-050026 [DOI] [PubMed] [Google Scholar]