Abstract
We conducted a retrospective cohort study of patients with MRC grade II/III tuberculous meningitis (TBM) who accepted a background antitubercular regimen (BR) with or without linezolid (LZD). At the 4th week, the LZD-BR group achieved a faster and higher percentage of Glasgow coma scale recovery and temperature recovery, a higher cerebrospinal fluid (CSF)/blood glucose ratio, and lower CSF white blood cell counts than did the BR group. Short-term linezolid supplementation may be a more effective treatment for life-threatening TBM.
TEXT
Tuberculous meningitis (TBM) is one of the most common forms of central nervous system (CNS) infections, especially in developing countries, where tuberculosis (TB) is highly epidemic (1). The incidence of TBM is directly related to the prevalence of TB infection and comprises approximately 10% of all TB cases (2). Despite the advent of newer antitubercular agents and imaging techniques, TBM still causes high mortality rates and severe neurologic deficiencies (3, 4).
New TB drugs are required to manage patients with TBM who face an increasing threat of drug resistance. A recent study from southwestern China found a rate of 32.14% of multidrug-resistant tuberculosis (MDR-TB) in TBM patients, which is higher than the reported resistances for pulmonary tuberculosis (5). First approved in 2000 for treating drug-resistant Gram-positive bacterial infections (6), linezolid (LZD) has shown antituberculosis potential in recent years. A number of case reports and retrospective studies suggest that linezolid may be effective in treating MDR and extensively drug-resistant tuberculosis (XDR-TB) (7–15). Furthermore, a phase 2a randomized two-group study showed that linezolid was effective at achieving culture conversion among patients with treatment-refractory pulmonary XDR-TB, but patients must be monitored carefully for adverse events (16).
Diagnosis and management differ significantly between TBM and pulmonary TB (1, 17). Acid-fast bacilli (AFB) have been found in fewer than 20% of patients with TBM (18), and the culture of cerebrospinal fluid (CSF), although considered the gold standard for diagnosis, is positive in only about 40% of cases (19). For most patients with TBM, pathogenic evidence and drug susceptibility testing (DST) results are not available in the initial phase of treatment when patients present with serious manifestations, such as conscious disturbance, headache, and fever. Thus, CSF parameters (Glasgow coma scale [GCS] scores, and temperature, which are closely related to TBM severity), other than culture, are routinely used to evaluate the therapeutic effect of antitubercular regimens in the initial phase of treatment. Although primarily bacteriostatic, linezolid has been employed successfully for treating CNS infections caused by multiresistant organisms. A case report showed good results with linezolid for the treatment of CNS infections in 10 patients, among whom three were caused by mycobacteria (20). However, the efficacy and adverse effects of linezolid in treating TBM have not been evaluated in sufficient detail. Here, we compared the treatment outcomes of linezolid use in the initiation phase of treatment and evaluated its adverse events in a cohort of patients with life-threatening TBM.
This retrospective cohort study was performed at Huashan Hospital, a tertiary hospital in eastern China. Ethics approval was obtained from the Institutional Review Board of Huashan Hospital, Fudan University. All patients who met the inclusion criteria from January 2010 to December 2012 were included (see Table S1 in the supplemental material). Cases of TBM were diagnosed as confirmed TBM, highly probable TBM, probable TBM, or possible TBM. Confirmed TBM was defined by results in the CSF culture that were positive for Mycobacterium tuberculosis. Highly probable TBM and probable TBM were diagnosed according to CSF criteria and supporting criteria. The CSF criteria included three parameters, (i) a CSF glucose level of <50%, (ii) >50% lymphocytes in the CSF, and (iii) a CSF protein level of >1.5 g/liter. Supporting criteria contained two items, (i) enhanced magnetic resonance imaging (MRI) brain scan features consistent with TBM and (ii) evidence of extra-CNS tuberculosis or positive T-SPOT.TB assay results. Highly probable TBM was diagnosed when at least 2 CSF criterion parameters and 2 CSF criterion items or 3 CSF criterion parameters and 1 CSF criterion item were fulfilled, and probable TBM was diagnosed when 2 CSF criterion items and 1 CSF criterion item were fulfilled. A diagnosis of possible TBM was made if patients did not fulfill the above criteria but a diagnosis of active TB could not be excluded. TBM severity was graded according to the modified MRC system: (i) grade I, a GCS score of 15, no focal neurology, (ii) grade II, a GCS score of 11 to 14 or a GCS score of 15 with focal neurology, and (iii) grade III, a GCS score of ≤10 (21).
The patients included in our study were divided into one of two groups based on whether their antitubercular regimen contained linezolid. We compared the following results associated with recovery in the first 4 weeks between these two groups, (i) CSF changes, including higher blood glucose ratio, lower white blood cell counts, and lower protein concentrations, (ii) consciousness recovery, indicated by a GCS score that increased to 15 and did not decrease later, and (iii) temperature recovery, indicated by a patient's oral temperature decreasing to no higher than 37.2°C and not increasing thereafter.
The regular protocol for the management of tuberculous meningitis and the data collection process are described in File S1 in the supplemental material. Statistical analysis was performed using GraphPad Prism version 5 and SPSS version 17.0. Categorical variables were compared using the Fisher exact test. Continuous variables were compared between the groups using the Wilcoxon rank sum test. Survival curves were compared between the groups using the log-rank test. Significance testing was done using 2-sided P values, with a P value of <0.05 considered statistically significant.
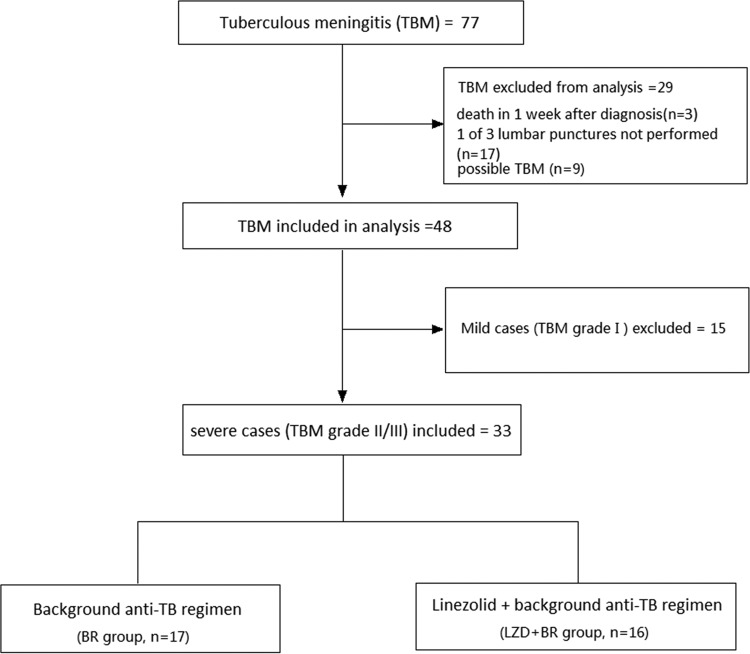
The final diagnoses and reasons for the inclusion and exclusion of the cases are shown in Fig. 1. Among the 33 grade II and III TBM patients, 16 were assigned to the background regimen with linezolid (LZD-BR) group and 17 to the BR group. Table 1 shows the demographic and baseline characteristics of patients in these two groups. The frequencies of baseline characteristics, such as clinical presentation, grade, and enhanced brain MRI abnormalities, were similar and did not show significant differences. Detailed information about the diagnosis and treatment regimens of all included cases is shown in Table S2 in the supplemental material.
FIG 1.
Patient enrollment and treatment group assignment. Among 77 patients with TBM, 29 were excluded, as they did not meet the criteria; they died, lacked one of three lumbar punctures, or had an uncertain diagnosis. Among the remaining 48 patients with TBM, 15 were excluded due to mild grade I disease. Thirty-three patients with grade II/III TBM were assigned to the background regimen group (17 patients) or the linezolid-plus-background-regimen group (16 patients). Three lumbar punctures were performed, one at tuberculous meningitis diagnosis, one 2 weeks after treatment, and one 4 weeks after treatment. TB, tuberculosis; TBM, tuberculous meningitis.
TABLE 1.
Demographic and baseline characteristics and background treatment of TBM patients from the LZD-BR and BR control groupsa
| Demographic or baseline characteristicb | LZD-BR group (n = 16) datac | BR group (n = 17) datac | P valued |
|---|---|---|---|
| Age (median [IQR]) (yr) | 41 (25–54) | 36 (26–49) | NS |
| Female | 10 (63) | 7 (41) | NS |
| Presentations | |||
| Temperature ≥ 37.8°C | 14 (88) | 13 (76) | NS |
| Headache | 11 (69) | 13 (76) | NS |
| Consciousness change | 16 (100) | 17 (100) | NS |
| Visual disturbances | 4 (25) | 4 (24) | NS |
| Neck pain/stiffness | 14 (88) | 14 (82) | NS |
| Focal neurological signs | 4 (25) | 5 (29) | NS |
| TBM diagnosis | |||
| Confirmed TBM | 5 (31) | 2 (12) | NS |
| Highly probable TBM | 9 (56) | 13 (76) | NS |
| Probable TBM | 2 (13) | 2 (12) | NS |
| TBM grade | |||
| II | 14 (88) | 15 (88) | NS |
| III | 2 (12) | 2 (12) | NS |
| Other investigations | |||
| Lung CT abnormalities | 2 (13) | 1 (6) | NS |
| Enhanced brain MRI abnormalities | 10 (63) | 13 (76) | NS |
| Features of extra-CNS tuberculosis | 3 (19) | 1 (6) | NS |
| T-SPOT.TB assay (n = 30)e | 10/13 | 15/17 | NS |
LZD, linezolid; BR, background regimen; TBM, tuberculous meningitis.
IQR, interquartile range; CT, computed tomography; CNS, central nervous system; TB, tuberculosis.
Data are shown as no. (%) unless otherwise indicated.
NS, not statistically significant.
Values indicate no. of positive results/total no. of patients with available assay results.
The baseline CSF findings were similar between the LZD-BR and the BR groups. Serial CSF changes are shown in Table 2. At the 2nd week, glucose ratios and white blood cell counts were not significantly different between these two groups. However, at the 4th week, the glucose ratios of the patients in the LZD-BR group were significantly higher than those of the patients in the BR group (median, 0.40 versus 0.34; P = 0.04). The white blood cell counts of the patients in the LZD-BR group were significantly lower than those of the patients in the BR group (median, 17 versus 42 cells × 106/liter; P = 0.02). The protein concentrations were not significantly different between these two groups. Kaplan-Meier curve analysis showed that the LZD-BR group achieved a faster and higher percentage of patients with GCS recovery (P = 0.032; Fig. 2) and temperature recovery (P = 0.032; Fig. 3) than did the BR group in the first 4 weeks of treatment. Of the 16 patients who received linezolid, only two (12.5%) episodes of linezolid-attributed adverse events (i.e., an episode of myelosuppression and an episode of optic neuropathy) occurred in the first 4 weeks of treatment. These two events resolved relatively quickly after linezolid was discontinued.
TABLE 2.
Serial CSF findings in TBM patients from the LZD-BR and BR groupsa
| Finding | LZD-BR group data (median [IQR]c) | BR group data (median [IQR]) | P valueb |
|---|---|---|---|
| CSF/blood glucose ratio | |||
| Baseline | 0.26 (0.17–0.36) | 0.28 (0.19–0.33) | 0.79 |
| After 2-wk treatment | 0.29 (0.26–0.33) | 0.33 (0.25–0.40) | 0.35 |
| After 4-wk treatment | 0.40 (0.35–0.47) | 0.34 (0.27–0.36) | 0.04 |
| CSF white blood cell count (×106/liter) | |||
| Baseline | 110 (50–255) | 130 (52–250) | 0.97 |
| After 2-wk treatment | 42 (9–80) | 86 (25–120) | 0.14 |
| After 4-wk treatment | 17 (8–40) | 42 (23–105) | 0.02 |
| CSF protein concentration (g/liter) | |||
| Baseline | 2.23 (1.56–3.87) | 1.55 (1.37–2.16) | 0.10 |
| After 2-wk treatment | 1.44 (1.21–2.13) | 1.33 (0.95–1.75) | 0.55 |
| After 4-wk treatment | 1.07 (0.64–1.81) | 1.41 (0.73–1.62) | 0.52 |
LZD, linezolid; BR, background regimen; TBM, tuberculous meningitis.
A P value of <0.05 was considered statistically significant.
IQR, interquartile range.
FIG 2.
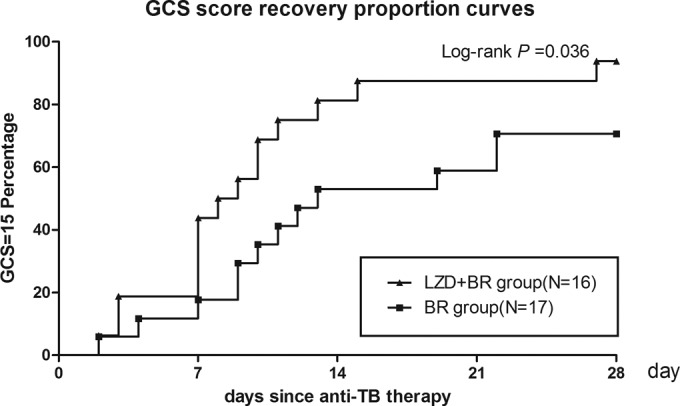
Kaplan-Meier curves for GCS score recovery according to time since anti-TB therapy. All patients included in the analysis had a decreased GCS score at diagnosis. By 4 weeks, 14 of 16 (88%) patients in the LZD-BR group and 12 of 17 (71%) patients in the BR group experienced consciousness recovery.
FIG 3.
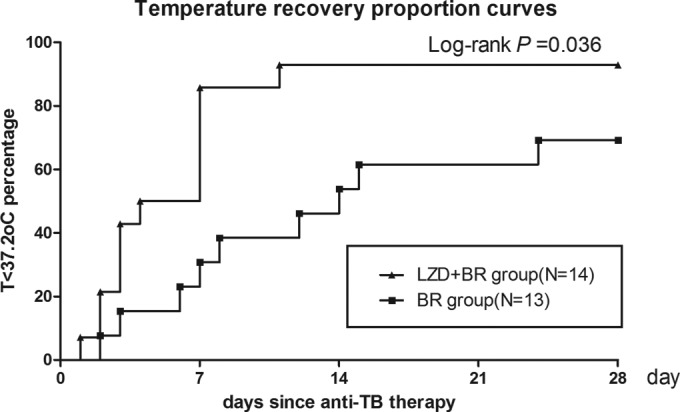
Kaplan-Meier curves for temperature recovery according to time since anti-TB therapy. At diagnosis, 14 of 16 patients in the LZD-BR group and 13 of 17 patients in the BR group had a fever (temperature [T] of >37.8°C). By 4 weeks, 13 of 14 (93%) patients in the LZD-BR group and 9 of 13 (71%) patients in the BR group experienced temperature recovery.
Previous studies suggested that linezolid might be useful in most complicated cases of pulmonary MDR/XDR-TB when other treatment alternatives are not available (7, 12, 13, 16). In our retrospective cohort study involving 33 patients with severe TBM, we found that the short-term addition of linezolid at a dosage of 1,200 mg per day to the background regimen showed significant beneficial effects on CSF improvement, consciousness recovery, and temperature recovery compared to the background regimen without linezolid. In particular, we noticed that the LZD-BR group showed a higher percentage of patients with consciousness recovery just after 1 or 2 weeks of treatment than did the BR group, which did not show significant improvement in CSF parameters. It seems that consciousness recovery occurred faster than did CSF parameter improvement. Considering that consciousness is a very important manifestation related to TBM severity, our finding that linezolid has a remarkable therapeutic effect on improving consciousness may prove to be critical for saving the lives of patients with life-threatening TBM. To our knowledge, this is the first report that demonstrates the dramatic therapeutic effects of linezolid on improving life-threatening TBM, which saves valuable time that is critical for the survival of such patients and should have implications for clinical care.
Several factors may explain this remarkable recovery from life-threatening TBM conferred by linezolid. First, a previous early bactericidal activity (EBA) study showed that linezolid has early bactericidal activity against rapidly dividing tubercle bacilli in patients during the first 2 days of administration (22). In addition, linezolid is active against drug-sensitive and drug-resistant TB strains (23, 24). Second, due to its amphiphilic properties, 80% to 100% of the linezolid administered penetrates the CSF (25), with an area under the concentration-time curve for CSF (AUCCSF)/AUC for serum (AUCS) ratio close to 1 (26). The linezolid dosage in our study, 600 mg twice a day, is the highest daily dosage at present, which ensures an effective therapeutic concentration in the CSF.
Most previous studies evaluated the efficacy of linezolid against pulmonary tuberculosis, with few reports on TBM. A meta-analysis showed that times for smear and culture conversions were 43.5 and 61 days, respectively, and that 81.8% of patients with MDR-TB were successfully treated with individualized regimens containing linezolid (15). A randomized controlled trial in patients with pulmonary XDR-TB also showed that linezolid was effective at achieving culture conversion, with a high conversion proportion of 87% (16). Although the treatment responses of pulmonary TB and TBM were evaluated differently, our results support the use of linezolid for treating severe cases of TBM due to its dramatic and rapid efficacy.
A previous meta-analysis showed a high proportion (50.5%) of major linezolid-attributed adverse events, which included anemia, peripheral neuropathy, gastrointestinal disorders, optic neuritis, and thrombocytopenia (15). However, we observed only two (12.5%) episodes that occurred in the first 4 weeks of treatment, indicating a much lower proportion of major linezolid-attributed adverse events. In our study, linezolid was administered at 1,200 mg per day with a median duration of 32 days, while a meta-analysis showed the median exposure time to linezolid (>600 mg per day) was 252 days for pulmonary TB (15). The duration was also much shorter than that of most previous studies on MDR/XDR pulmonary TB (8–10, 13, 27, 28). Our results suggest that high-dosage linezolid is relatively safe with a duration of <4 weeks, which was sufficient to show a significant beneficial effect when added to the background regimen for patients with life-threatening TBM. This is also in line with the label of the drug, which states that it can be used in a usual adult dose of 600 mg every 12 h for up to 28 days (29, 30).
This study had some limitations, such as the relatively small number of cases available and unclear long-term prognosis results. DST and pharmacokinetic evaluation may be helpful to objectivate the results in future studies. Thus, further prospective randomized blind controlled studies are needed to confirm the efficacy and determine the appropriate dosage and duration of linezolid for optimal TBM treatment.
In conclusion, our study demonstrated that a linezolid supplementation regimen has a remarkable therapeutic benefit for life-threatening TBM, as shown by rapid consciousness recovery, temperature recovery, and CSF improvement in the first 4 weeks of treatment. Although a small number of patients (12.5%) developed linezolid-attributed adverse events, these effects were generally mild and reversible upon drug discontinuation or dose reduction. We conclude that short-term linezolid supplementation is likely a more effective treatment for patients with life-threatening TBM than the current regimen without linezolid and warrants further clinical evaluation with more patients in future studies.
Supplementary Material
ACKNOWLEDGMENTS
We thank all the physicians from the Department of Infectious Diseases of Huashan Hospital for providing related data and information about cases.
We declare no conflicts of interest.
This work was supported by National Major Science and Technology Special Project 2013ZX10003008-003-001.
Footnotes
Published ahead of print 4 August 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.02784-14.
REFERENCES
- 1.Galimi R. 2011. Extrapulmonary tuberculosis: tuberculous meningitis new developments. Eur. Rev. Med. Pharmacol. Sci. 15:365–386 [PubMed] [Google Scholar]
- 2.Chatterjee S. 2011. Brain tuberculomas, tubercular meningitis, and post-tubercular hydrocephalus in children. J. Pediatr. Neurosci. 6:S96–S100. 10.4103/1817-1745.85725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lu CH, Chang WN, Chang HW. 2001. The prognostic factors of adult tuberculous meningitis. Infection 29:299–304. 10.1007/s15010-001-1100-3 [DOI] [PubMed] [Google Scholar]
- 4.Girgis NI, Sultan Y, Farid Z, Mansour MM, Erian MW, Hanna LS, Mateczun AJ. 1998. Tuberculosis meningitis, Abbassia Fever Hospital-Naval Medical Research Unit No. 3-Cairo, Egypt, from 1976 to 1996. Am. J. Trop. Med. Hyg. 58:28–34 [DOI] [PubMed] [Google Scholar]
- 5.Duo L, Ying B, Song X, Lu X, Ye Y, Fan H, Xin J, Wang L. 2011. Molecular profile of drug resistance in tuberculous meningitis from southwest China. Clin. Infect. Dis. 53:1067–1073. 10.1093/cid/cir663 [DOI] [PubMed] [Google Scholar]
- 6.Leach KL, Brickner SJ, Noe MC, Miller PF. 2011. Linezolid, the first oxazolidinone antibacterial agent. Ann. N. Y. Acad. Sci. 1222:49–54. 10.1111/j.1749-6632.2011.05962.x [DOI] [PubMed] [Google Scholar]
- 7.Anger HA, Dworkin F, Sharma S, Munsiff SS, Nilsen DM, Ahuja SD. 2010. Linezolid use for treatment of multidrug-resistant and extensively drug-resistant tuberculosis, New York City, 2000–06. J. Antimicrob. Chemother. 65:775–783. 10.1093/jac/dkq017 [DOI] [PubMed] [Google Scholar]
- 8.Condos R, Hadgiangelis N, Leibert E, Jacquette G, Harkin T, Rom WN. 2008. Case series report of a linezolid-containing regimen for extensively drug-resistant tuberculosis. Chest 134:187–192. 10.1378/chest.07-1988 [DOI] [PubMed] [Google Scholar]
- 9.Fortún J, Martin-Davila P, Navas E, Perez-Elias MJ, Cobo J, Tato M, De la Pedrosa EG, Gomez-Mampaso E, Moreno S. 2005. Linezolid for the treatment of multidrug-resistant tuberculosis. J. Antimicrob. Chemother. 56:180–185. 10.1093/jac/dki148 [DOI] [PubMed] [Google Scholar]
- 10.Park IN, Hong SB, Oh YM, Kim MN, Lim CM, Lee SD, Koh Y, Kim WS, Kim DS, Kim WD, Shim TS. 2006. Efficacy and tolerability of daily-half dose linezolid in patients with intractable multidrug-resistant tuberculosis. J. Antimicrob. Chemother. 58:701–704. 10.1093/jac/dkl298 [DOI] [PubMed] [Google Scholar]
- 11.Schecter GF, Scott C, True L, Raftery A, Flood J, Mase S. 2010. Linezolid in the treatment of multidrug-resistant tuberculosis. Clin. Infect. Dis. 50:49–55. 10.1086/648675 [DOI] [PubMed] [Google Scholar]
- 12.Singla R, Caminero JA, Jaiswal A, Singla N, Gupta S, Bali RK, Behera D. 2012. Linezolid: an effective, safe and cheap drug for patients failing multidrug-resistant tuberculosis treatment in India. Eur. Respir. J. 39:956–962. 10.1183/09031936.00076811 [DOI] [PubMed] [Google Scholar]
- 13.Migliori GB, Eker B, Richardson MD, Sotgiu G, Zellweger JP, Skrahina A, Ortmann J, Girardi E, Hoffmann H, Besozzi G, Bevilacqua N, Kirsten D, Centis R, Lange C. 2009. A retrospective TBNET assessment of linezolid safety, tolerability and efficacy in multidrug-resistant tuberculosis. Eur. Respir. J. 34:387–393. 10.1183/09031936.00009509 [DOI] [PubMed] [Google Scholar]
- 14.Cobelens FG, Heldal E, Kimerling ME, Mitnick CD, Podewils LJ, Ramachandran R, Rieder HL, Weyer K, Zignol M. 2008. Scaling up programmatic management of drug-resistant tuberculosis: a prioritized research agenda. PLoS Med. 5:e150. 10.1371/journal.pmed.0050150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sotgiu G, Centis R, D'Ambrosio L, Alffenaar JW, Anger HA, Caminero JA, Castiglia P, De Lorenzo S, Ferrara G, Koh WJ, Schecter GF, Shim TS, Singla R, Skrahina A, Spanevello A, Udwadia ZF, Villar M, Zampogna E, Zellweger JP, Zumla A, Migliori GB. 2012. Efficacy, safety and tolerability of linezolid containing regimens in treating MDR-TB and XDR-TB: systematic review and meta-analysis. Eur. Respir. J. 40:1430–1442. 10.1183/09031936.00022912 [DOI] [PubMed] [Google Scholar]
- 16.Lee M, Lee J, Carroll MW, Choi H, Min S, Song T, Via LE, Goldfeder LC, Kang E, Jin B, Park H, Kwak H, Kim H, Jeon HS, Jeong I, Joh JS, Chen RY, Olivier KN, Shaw PA, Follmann D, Song SD, Lee JK, Lee D, Kim CT, Dartois V, Park SK, Cho SN, Barry CE., III 2012. Linezolid for treatment of chronic extensively drug-resistant tuberculosis. N. Engl. J. Med. 367:1508–1518. 10.1056/NEJMoa1201964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brancusi F, Farrar J, Heemskerk D. 2012. Tuberculous meningitis in adults: a review of a decade of developments focusing on prognostic factors for outcome. Future Microbiol. 7:1101–1116. 10.2217/fmb.12.86 [DOI] [PubMed] [Google Scholar]
- 18.Garg RK. 1999. Tuberculosis of the central nervous system. Postgrad. Med. J. 75:133–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lachnik J, Ackermann B, Bohrssen A, Maass S, Diephaus C, Puncken A, Stermann M, Bange FC. 2002. Rapid-cycle PCR and fluorimetry for detection of mycobacteria. J. Clin. Microbiol. 40:3364–3373. 10.1128/JCM.40.9.3364-3373.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rupprecht TA, Pfister HW. 2005. Clinical experience with linezolid for the treatment of central nervous system infections. Eur. J. Neurol. 12:536–542. 10.1111/j.1468-1331.2005.01001.x [DOI] [PubMed] [Google Scholar]
- 21.Thwaites GE, Nguyen DB, Nguyen HD, Hoang TQ, Do TT, Nguyen TC, Nguyen QH, Nguyen TT, Nguyen NH, Nguyen TN, Nguyen NL, Vu NT, Cao HH, Tran TH, Pham PM, Nguyen TD, Stepniewska K, White NJ, Farrar JJ. 2004. Dexamethasone for the treatment of tuberculous meningitis in adolescents and adults. N. Engl. J. Med. 351:1741–1751. 10.1056/NEJMoa040573 [DOI] [PubMed] [Google Scholar]
- 22.Dietze R, Hadad DJ, McGee B, Molino LP, Maciel EL, Peloquin CA, Johnson DF, Debanne SM, Eisenach K, Boom WH, Palaci M, Johnson JL. 2008. Early and extended early bactericidal activity of linezolid in pulmonary tuberculosis. Am. J. Respir. Crit. Care Med. 178:1180–1185. 10.1164/rccm.200806-892OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bressler AM, Zimmer SM, Gilmore JL, Somani J. 2004. Peripheral neuropathy associated with prolonged use of linezolid. Lancet Infect. Dis. 4:528–531. 10.1016/S1473-3099(04)01109-0 [DOI] [PubMed] [Google Scholar]
- 24.Singh R, Manjunatha U, Boshoff HI, Ha YH, Niyomrattanakit P, Ledwidge R, Dowd CS, Lee IY, Kim P, Zhang L, Kang S, Keller TH, Jiricek J, Barry CE., III 2008. PA-824 kills nonreplicating Mycobacterium tuberculosis by intracellular NO release. Science 322:1392–1395. 10.1126/science.1164571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marx GE, Chan ED. 2011. Tuberculous meningitis: diagnosis and treatment overview. Tuberc. Res. Treat. 2011:798764. 10.1155/2011/798764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nau R, Sorgel F, Eiffert H. 2010. Penetration of drugs through the blood-cerebrospinal fluid/blood-brain barrier for treatment of central nervous system infections. Clin. Microbiol. Rev. 23:858–883. 10.1128/CMR.00007-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yew WW, Chau CH, Wen KH. 2008. Linezolid in the treatment of “difficult” multidrug-resistant tuberculosis. Int. J. Tuberc. Lung Dis. 12:345–346 [PubMed] [Google Scholar]
- 28.von der Lippe B, Sandven P, Brubakk O. 2006. Efficacy and safety of linezolid in multidrug resistant tuberculosis (MDR-TB): a report of ten cases. J. Infect. 52:92–96. 10.1016/j.jinf.2005.04.007 [DOI] [PubMed] [Google Scholar]
- 29.Di Paolo A, Malacarne P, Guidotti E, Danesi R, Del Tacca M. 2010. Pharmacological issues of linezolid: an updated critical review. Clin. Pharmacokinet. 49:439–447. 10.2165/11319960-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 30.Bishop E, Melvani S, Howden BP, Charles PG, Grayson ML. 2006. Good clinical outcomes but high rates of adverse reactions during linezolid therapy for serious infections: a proposed protocol for monitoring therapy in complex patients. Antimicrob. Agents Chemother. 50:1599–1602. 10.1128/AAC.50.4.1599-1602.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.