Abstract
Renal ischaemia reperfusion injury (IRI) is a common cause of acute kidney injury (AKI) in patients and occlusion of renal blood flow is unavoidable during renal transplantation. Experimental models that accurately and reproducibly recapitulate renal IRI are crucial in dissecting the pathophysiology of AKI and the development of novel therapeutic agents. Presented here is a mouse model of renal IRI that results in reproducible AKI. This is achieved by a midline laparotomy approach for the surgery with one incision allowing both a right nephrectomy that provides control tissue and clamping of the left renal pedicle to induce ischaemia of the left kidney. By careful monitoring of the clamp position and body temperature during the period of ischaemia this model achieves reproducible functional and structural injury. Mice sacrificed 24 hr following surgery demonstrate loss of renal function with elevation of the serum or plasma creatinine level as well as structural kidney damage with acute tubular necrosis evident. Renal function improves and the acute tissue injury resolves during the course of 7 days following renal IRI such that this model may be used to study renal regeneration. This model of renal IRI has been utilized to study the molecular and cellular pathophysiology of AKI as well as analysis of the subsequent renal regeneration.
Keywords: Medicine, Issue 88, Murine, Acute Kidney Injury, Ischaemia, Reperfusion, Nephrectomy, Regeneration, Laparotomy
Introduction
Ischaemia reperfusion injury (IRI) is a common mode of injury for multiple organs including the kidney, heart and brain. Renal IRI may lead to acute kidney injury (AKI) in patients and no specific treatment is available. AKI as a result of IRI has a complicated pathogenesis involving both the innate and adaptive immune response1. An experimental model of renal IRI offers the potential to dissect the key cells and mediators involved in the pathogenesis of AKI as well as the subsequent renal regeneration that ensues over subsequent days. Furthermore the effects of novel therapeutic agents upon disease processes can be assessed.
The overall goal of the experimental model of renal IRI described here is to induce both acute functional and structural kidney injury. Some investigators have utilized a model that involves the induction of bilateral IRI2. Although the bilateral renal IRI model is of use, the unilateral renal IRI model has the advantage of a right nephrectomy being undertaken at the time of surgery. The right nephrectomy tissue serves as valuable control tissue in studies involving a pretreatment step that either induces or suppresses the expression of a gene or protein. For example, we have used this model to assess the preconditioning effects of heme arginate (HA) injection 24 hours before renal IRI3. The successful induction of the cytoprotective enzyme heme-oxygenase-1 (HO-1) by HA before IRI was confirmed in the right nephrectomy control tissue4. HA reduced renal IRI in aged mice in part via an HO-1 dependent mechanism. Similarly, we have used the model in macrophage depletion studies to examine the role of macrophages in renal IRI5. Immunohistochemical analysis of the right nephrectomy tissue can be used to confirm the efficacy of the ablation methodology. The right nephrectomy tissue can therefore be used to both confirm and quantify the level of induction or inhibition of the molecule of interest in each individual experimental animal. This model will be of interest to researchers who are using drugs or other compounds to modulate the expression of genes or proteins etc. prior to the induction of renal IRI.
Other studies have used flank incisions to access the kidneys. The model described here uses a single midline abdominal surgery to perform both the right nephrectomy and induce ischaemia reperfusion of the left kidney. This surgical approach provides excellent visualization of the surgical field including the renal pedicles and color changes that follow renal pedicle clamping. Our published experience with the model4-6 indicates that mice quickly recover from the surgery with a near 100% survival rate.
Lastly, kinetic analysis of the model over a period of 7 days indicates that this model exhibits restoration of both renal function and tubular integrity, with significant tubular cell proliferation.
Protocol
NOTE: Animal experiments were performed in accordance with the guidelines and regulations imposed by the Animals (Scientific Procedures) Act 1986. Procedures were conducted using sterile (autoclaved) surgical instruments and consumables. Whilst the murine model of renal IRI presented here was performed on an 8-week-old male Balb/c mouse it can be reproducibly performed on a variety of murine strains of either gender typically aged between 7 – 15 weeks, with the optimum age being 8 weeks. The data presented in the representative results section was obtained from both Balb/c and FVB mice. The application of warmed saline is used to keep the intestines and surgical area moist but it should be carefully monitored and kept to a minimum as the excessive application of fluids can lead to an artifactual lowering of the serum or plasma creatinine levels, which is an important experimental readout.
1. Animal Preparation and Laparotomy
Anesthetize the mouse with ketamine hydrochloride (70 mg/kg) and medetomidine hydrochloride (1 mg/kg) intraperitoneally and confirm the depth of anesthesia by loss of reflexes e.g. toe pinch. The duration of the resulting anesthetic plane is 4 hours. This is sufficient to perform the entire surgical procedure and no supplemental anesthesia is required.
Remove the hair surrounding the incision area, ensure that the area is free of loose hair, and sanitize the abdominal skin using a dilute chlorhexidine solution.
Place the mouse on a heated surgical pad in a supine position and fix the upper and lower limbs to the pad using low-tack adhesive tape. Ensure that the upper extremities are maintained in a normal position to prevent lung compression. Throughout the procedure monitor the mouse for thermal burns. If possible it is recommended that a non-electric heat source be used.
Administer the analgesic buprenorphine hydrochloride (0.06 mg/kg) subcutaneously and apply eye lubricant to the eyes to prevent corneal drying. Analgesics are administered pre-operatively in order to aid the post-surgical recovery.
Using tissue separating scissors or a scalpel blade make a midline laparotomy incision and bluntly separate the skin from the peritoneum. This allows the skin and peritoneum to be sutured separately during wound closure. An incision of the avascular linea alba is made giving access to the peritoneal cavity.
To create a clear view of the surgical area insert a retractor and drape the mouse.
2. Ureter Division and Right Nephrectomy
Gently push the intestines towards the left side of the abdominal cavity using autoclaved sterilized cotton swabs moistened with saline to expose the right kidney and ureter. Cover the intestines with moistened drapes to prevent drying.
Lift the right ureter with angled forceps. Ligate the right ureter twice using 6/0 silk-braided suture. For long term experiments, use absorbable sutures for all abdominal surgeries.
Divide the ureter between the sutures. Whilst the vesico-ureteric junction should prevent urine from leaking into the peritoneum from the bladder the ureter is ligated as a safeguard against any leakage post-operatively during long-term experiments.
To allow easier access when performing the right nephrectomy, gently push the liver up and to the right using a moistened cotton swab and hold in place using moistened gauze to expose the right renal artery and vein.
Bluntly dissect the connective tissue and fat along the medial aspect of the right kidney as far as the right renal artery and vein.
Create a channel underneath the renal artery and vein by carefully sliding the angled forceps underneath the blood vessels. Guide the forceps underneath with the tips closed and gently remove with the tips open in order to facilitate the formation of the channel.
Repeat step 2.6 until the tips of the forceps are visible through the connective tissues just above the renal artery and vein. Once visible gently rub the tips of the forceps against the tips of another set of angled forceps to gently break the connective tissue.
With the renal artery and vein clearly accessible they can now be occluded. Carefully slide angled forceps underneath the renal artery and vein with the tips closed. Once in place open the tips of the forceps and guide the haemostatic clip applicator between the tips and firmly apply a haemostatic clip onto the renal artery and vein close to the kidney. Alternatively the renal artery and vein can be ligated using 6/0 silk braided suture.
Divide the occluded renal artery and vein close to the kidney, which can now be removed with any remaining adherent connective tissue.
Remove any gauze used to hold the liver and replace the intestines with cotton swabs.
3. Left Kidney Ischaemia and Reperfusion
Gently push the intestines towards the right side of the abdominal cavity with cotton swabs to expose the left kidney and ureter and cover with moistened drapes. If necessary the pancreas can be deflected with moistened gauze to allow easier access.
Use blunt dissection to gently break the connective tissues anterior and posterior to the left renal artery and vein, and then create a channel underneath the vessels in a similar manner to that described in Ureter Division and Right Nephrectomy step 2.4.
Induce ischaemia of the left kidney by applying a micro serrafine clip onto the renal artery and vein. Successful ischaemia can be visually confirmed by a gradual uniform darkening of the kidney. Blood vessels in addition to the left renal artery and vein may occasionally supply the kidney. If present, these additional blood vessels will also need to be occluded using micro serrafine clips in order to successfully induce ischaemia of the entire kidney.
Replace the intestines and carefully ensure that there are no abrupt twists that may lead to intestinal ischaemia compromising results. Temporarily close the peritoneum with a single suture.
Following ischaemia induction immediately place the mouse on a homeothermic blanket with a rectal thermistor attached to a control unit that will maintain the body temperature at 37 °C for the required duration of ischaemia. To define the appropriate length of ischaemia necessary to induce the desired degree of renal injury and kidney failure it is recommended that a titration be performed for each strain and surgical operator.
Shortly before the end of the ischaemic period re-open the peritoneum and carefully position the intestines to allow access to the clamp and view the kidney. Insertion of the retractor, as shown in the video, is not necessary and was solely performed for presentational purposes.
Remove the clamp after the period of ischaemia has concluded. Immediately after removal the kidney will rapidly change color from a dark maroon to a healthy dark pink indicating successful reperfusion.
Again ensure that the intestines are not twisted prior to closing the peritoneum with a blanket stitch using 6/0 braided silk suture. Then close the skin using metallic skin clips.
To minimize the risk of post-operative infection, apply an antiseptic such as iodine/alcohol solution to the surgical area.
4. Post-operative Recovery and Care
Partially reverse anesthesia with atipamezole hydrochloride (2 mg/kg) subcutaneously and administer fluids with a subcutaneous injection of 1 ml warmed saline to prevent dehydration following surgery.
Carefully monitor mice until they have recovered consciousness, appear alert and are able to right themselves.
Allow mice to recover in a heated box kept at 29 °C, located in a quiet environment, for 24 hr. Mice will have an impaired ability to thermoregulate due to the anesthesia and it is therefore crucial that they are housed at an elevated temperature to enable effective recovery. Moistened food can also be provided to encourage fluid and nutrition intake.
For long-term recovery experiments provide ongoing analgesics and remove skin clips 7 days following surgery.
5. Assessing Functional and Structural Kidney Injury and Regeneration
Blood may be collected from the tail vein during experiments or by cardiac puncture at the time of euthanasia at the end of the experiment. Measure renal function by serum creatinine, using a creatinase based method, and blood urea nitrogen (BUN) on a biochemical centrifugal analyzer as described previously7. The renal function of normal healthy mice needs to be determined before establishing a model of renal failure as this can vary markedly depending on the analysis method used e.g. the Jaffe reaction and creatinase based methods for measuring creatinine give different results.
Examine renal structure by histopathology on paraffin embedded kidney tissue sections stained with Haematoxylin and Eosin (H&E) or Periodic Acid Schiff (PAS). Capture between 5 – 10 images at 200x magnification within the outer stripe of the outer medulla (OSOM) for each mouse. Assess the level of injury in the OSOM as this region is injured in this model because it is highly vulnerable to hypoxia.
Use either of the two scoring systems detailed below to measure acute tubular necrosis (ATN) or regeneration. Representative morphology of the classifications used in these systems is illustrated in Figure 1.
- Use a simple binary system to score ATN. On the basis of cellular integrity and morphology mark and count tubules as either viable (intact cell morphology) or necrotic (compromised cell integrity, abnormal cell morphology or loss of cells).
- Express the number of necrotic tubules as a percentage of the total number of tubules (necrotic tubules %).
- Classify regeneration using another system based on cell integrity, morphology and nuclei number. Mark and count tubules as healthy, necrotic, injured or recovering according to the criteria listed in the following steps.
- As with the ATN scoring system (detailed in step 5.4) mark tubules with intact normal cells as healthy whilst tubules showing compromised cell integrity, abnormal cell morphology or overt cell loss should be scored as necrotic.
- Classify tubules as injured if they have a thinned cytoplasm containing few nuclei. In contrast, designate tubules containing more nuclei with more normal cell morphology as recovering.
- Express each tubule classification as a percentage of the total tubule number.
Representative Results
Tubular injury and recovery may be assessed by H&E or PAS staining of tissue sections following renal IRI. Tubules located within the OSOM are classified as healthy, injured, necrotic or recovering according to cell morphology, integrity and nuclei number (Figure 1). The functional and structural injury in this model is dependent upon the duration of ischaemia. A progressive increase in the severity of renal dysfunction, assessed by plasma creatinine and BUN, is evident as the duration of ischaemia is increased by 2min increments (Figure 2). The extent of structural renal injury, inferred from the ATN score, follows a similar trend with more severe ATN accompanying more prolonged ischaemia (Figure 3). Following an initial titration of varying durations of ischaemia, this model of renal IRI should achieve a near 100% success rate, with all mice developing both functional and structural injury following surgery. This model of renal IRI also enables a fine level of control over the degree of injury with limited variation observed between animals (Figures 2 and 3). The use of different durations of ischaemia enables therapeutic interventions to be examined for their ability to modulate different levels of injury severity. For example on the basis of the length of ischaemia the resulting injury can be classed as mild (20 min), moderate (22 min) or high (24 min). These values are for male Balb/c mice aged 8 weeks and are included as guidance only. Investigators should establish their own experimental conditions as these will differ according to mouse strain, age, sex and the biochemical assay used to assess renal function.
This model has been successfully used to study regeneration following renal IRI with both renal function and structure recovering over the ensuing days. A gradual decline in plasma creatinine and BUN levels are observed, with plasma creatinine returning to basal levels by day 7 (Figure 4). An assessment of H&E stained tissue sections indicates that an increased number of tubules are classified as healthy or recovering at day 7 compared to day 4 indicating that tissue regeneration is taking place (Figure 5). The administration of 5-bromo-2'-deoxyuridine (BrdU) prior to sacrifice facilitates the immunostaining of kidney tissue for BrdU and subsequent quantification of tubular cell proliferation. Dramatic nuclear BrdU expression is observed 4 days following renal IRI indicating that the tubules are undergoing marked cell proliferation in order to restore tubule integrity and function (Figure 6). The combination of renal function assessment, scoring for structural improvement and BrdU immunostaining enables this model to be used to study regeneration following renal IRI. This allows the long-term effects of therapeutic interventions to be investigated.
Lastly, the utility of this model is dependent upon the induction of global ischaemia to the entire kidney and this may be jeopardized by the presence of blood vessels supplying the kidney in addition to the main left renal artery. If these additional vessels are not clamped then part of the kidney will not be subjected to ischaemic injury (Figure 7).
 Figure 1. Scoring of structural tubular injury and regeneration – Representative morphology of healthy, recovering, injured and necrotic tubules. Tissue sections from a kidney removed 4 days following renal IRI are stained with PAS for assessment. Tubules located within the OSOM are classified as healthy, injured, necrotic or recovering according to cell morphology, integrity and nuclei number with representative examples highlighted. Healthy tubules are intact with a normal cellular morphology. Necrotic tubules exhibit a compromised monolayer with evident cell loss and loss of cell morphology. Injured tubules exhibit a thinned cellular monolayer and contain fewer nuclei. In contrast, recovering tubules exhibit a more normal cellular morphology and a similar number of nuclei to healthy tubules. Magnification: 400x. Please click here to view a larger version of this figure.
Figure 1. Scoring of structural tubular injury and regeneration – Representative morphology of healthy, recovering, injured and necrotic tubules. Tissue sections from a kidney removed 4 days following renal IRI are stained with PAS for assessment. Tubules located within the OSOM are classified as healthy, injured, necrotic or recovering according to cell morphology, integrity and nuclei number with representative examples highlighted. Healthy tubules are intact with a normal cellular morphology. Necrotic tubules exhibit a compromised monolayer with evident cell loss and loss of cell morphology. Injured tubules exhibit a thinned cellular monolayer and contain fewer nuclei. In contrast, recovering tubules exhibit a more normal cellular morphology and a similar number of nuclei to healthy tubules. Magnification: 400x. Please click here to view a larger version of this figure.
 Figure 2. Injury – Renal ischaemia impairs renal function. Male Balb/c mice aged 8 weeks underwent a right nephrectomy and the left renal pedicle was clamped for 20, 22 or 24 min (n = 4 per group). Mice were sacrificed at 24 hr following renal IRI. Plasma creatinine and blood urea nitrogen show an increasing trend of severity as the length of ischaemia increased. The dashed line represents the levels of plasma creatinine and blood urea nitrogen found in normal control mice. Data presented as mean ± SEM and analyzed by one-way ANOVA. Please click here to view a larger version of this figure.
Figure 2. Injury – Renal ischaemia impairs renal function. Male Balb/c mice aged 8 weeks underwent a right nephrectomy and the left renal pedicle was clamped for 20, 22 or 24 min (n = 4 per group). Mice were sacrificed at 24 hr following renal IRI. Plasma creatinine and blood urea nitrogen show an increasing trend of severity as the length of ischaemia increased. The dashed line represents the levels of plasma creatinine and blood urea nitrogen found in normal control mice. Data presented as mean ± SEM and analyzed by one-way ANOVA. Please click here to view a larger version of this figure.
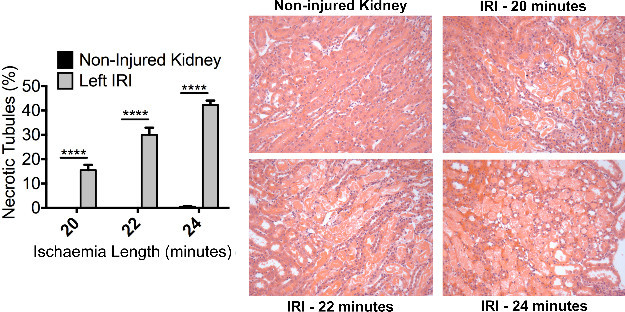 Figure 3. Injury – Renal ischaemia induces significant acute tubular necrosis. Male Balb/c mice aged 8 weeks underwent a right nephrectomy and the left renal pedicle was clamped for 20, 22 or 24 min (n = 4 per group). Mice were sacrificed at 24 hr following renal IRI. Representative photomicrographs (Magnification: 200x) of the OSOM from H&E stained kidney sections of control and injured kidneys illustrate ATN. Formal scoring of ATN (expressed as the proportion of necrotic tubules) confirms the increased level of injury with increasing duration of ischaemia. Data presented as mean ± SEM and analyzed by one-way ANOVA (**** = P ≤ 0.0001). Please click here to view a larger version of this figure.
Figure 3. Injury – Renal ischaemia induces significant acute tubular necrosis. Male Balb/c mice aged 8 weeks underwent a right nephrectomy and the left renal pedicle was clamped for 20, 22 or 24 min (n = 4 per group). Mice were sacrificed at 24 hr following renal IRI. Representative photomicrographs (Magnification: 200x) of the OSOM from H&E stained kidney sections of control and injured kidneys illustrate ATN. Formal scoring of ATN (expressed as the proportion of necrotic tubules) confirms the increased level of injury with increasing duration of ischaemia. Data presented as mean ± SEM and analyzed by one-way ANOVA (**** = P ≤ 0.0001). Please click here to view a larger version of this figure.
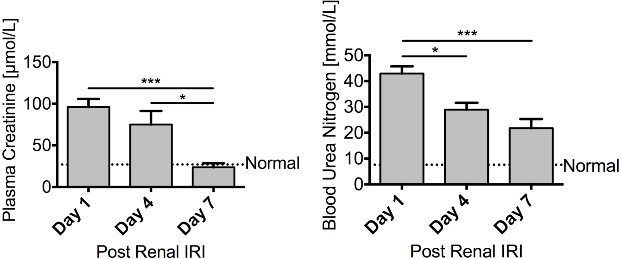 Figure 4. Regeneration – Renal function recovers following renal ischaemia. Male FVB mice aged 8 – 10 weeks underwent a right nephrectomy prior to 25 min of ischaemia to the left kidney. Mice were sacrificed 1 day (n = 10), 4 days (n = 10) or 7 days (n = 6) following renal IRI. Both plasma creatinine and blood urea nitrogen steadily decline over the course of 7 days, with plasma creatinine returning to basal levels, illustrating a recovery in renal function. Data presented as mean ± SEM and analyzed by one-way ANOVA (*** = P ≤ 0.001, * = P ≤ 0.05). Please click here to view a larger version of this figure.
Figure 4. Regeneration – Renal function recovers following renal ischaemia. Male FVB mice aged 8 – 10 weeks underwent a right nephrectomy prior to 25 min of ischaemia to the left kidney. Mice were sacrificed 1 day (n = 10), 4 days (n = 10) or 7 days (n = 6) following renal IRI. Both plasma creatinine and blood urea nitrogen steadily decline over the course of 7 days, with plasma creatinine returning to basal levels, illustrating a recovery in renal function. Data presented as mean ± SEM and analyzed by one-way ANOVA (*** = P ≤ 0.001, * = P ≤ 0.05). Please click here to view a larger version of this figure.
 Figure 5. Regeneration – Renal tissue shows signs of recovery following renal ischaemia. Male FVB mice aged 8 – 10 weeks underwent a right nephrectomy prior to 25 min of ischaemia to the left kidney. Mice were sacrificed 4 days (n = 10) or 7 days (n = 6) following renal IRI. Representative photomicrographs (Magnification: 200x) of the OSOM following the induction of ischaemia are shown. Formal scoring of the number of healthy, recovering, injured or necrotic tubules within the OSOM was assessed in PAS stained paraffin sections. At day 4 following renal IRI a large proportion of tubules within the OSOM still appear injured. However by 7 days there is a considerable increase in the proportion of tubules that are classified as healthy or recovering indicative of renal regeneration. Data presented as mean ± SEM. Please click here to view a larger version of this figure.
Figure 5. Regeneration – Renal tissue shows signs of recovery following renal ischaemia. Male FVB mice aged 8 – 10 weeks underwent a right nephrectomy prior to 25 min of ischaemia to the left kidney. Mice were sacrificed 4 days (n = 10) or 7 days (n = 6) following renal IRI. Representative photomicrographs (Magnification: 200x) of the OSOM following the induction of ischaemia are shown. Formal scoring of the number of healthy, recovering, injured or necrotic tubules within the OSOM was assessed in PAS stained paraffin sections. At day 4 following renal IRI a large proportion of tubules within the OSOM still appear injured. However by 7 days there is a considerable increase in the proportion of tubules that are classified as healthy or recovering indicative of renal regeneration. Data presented as mean ± SEM. Please click here to view a larger version of this figure.
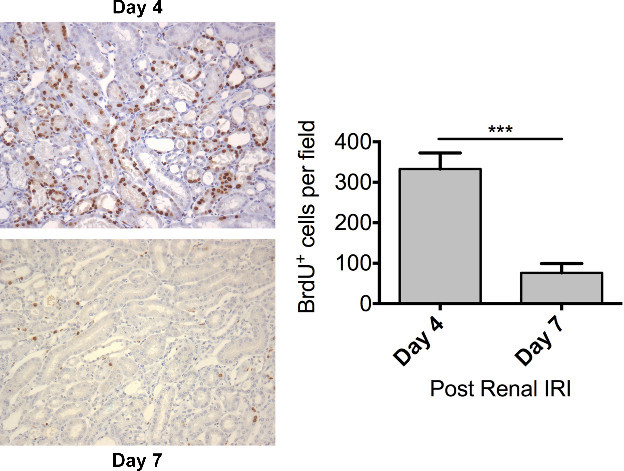 Figure 6. Regeneration – Dramatic tubular proliferation is evident 4 days following renal ischaemia. Male FVB mice aged 8 – 10 weeks underwent a right nephrectomy prior to 25 min of ischaemia to the left kidney. BrdU (50 mg/kg) was administered by intraperitoneal injection 24 hours prior to sacrifice. Mice were sacrificed 4 days (n = 10) or 7 days (n = 6) following renal IRI. Representative photomicrographs (Magnification: 200x) of the OSOM from mice sacrificed at day 4 and day 7 following the induction of ischaemia are shown. Tubular cell proliferation was assessed by immunohistochemical staining for BrdU on paraffin embedded kidney sections. Tubular cell proliferation was quantified by counting the number of BrdU positive nuclei in the OSOM with increased cell proliferation evident at day 4. Data presented as mean ± SEM and analyzed by students t-test (*** = P ≤ 0.001). Please click here to view a larger version of this figure.
Figure 6. Regeneration – Dramatic tubular proliferation is evident 4 days following renal ischaemia. Male FVB mice aged 8 – 10 weeks underwent a right nephrectomy prior to 25 min of ischaemia to the left kidney. BrdU (50 mg/kg) was administered by intraperitoneal injection 24 hours prior to sacrifice. Mice were sacrificed 4 days (n = 10) or 7 days (n = 6) following renal IRI. Representative photomicrographs (Magnification: 200x) of the OSOM from mice sacrificed at day 4 and day 7 following the induction of ischaemia are shown. Tubular cell proliferation was assessed by immunohistochemical staining for BrdU on paraffin embedded kidney sections. Tubular cell proliferation was quantified by counting the number of BrdU positive nuclei in the OSOM with increased cell proliferation evident at day 4. Data presented as mean ± SEM and analyzed by students t-test (*** = P ≤ 0.001). Please click here to view a larger version of this figure.
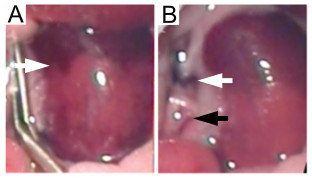 Figure 7. Left ischaemic kidney with non-occluded additional renal blood vessels. Failure to occlude additional blood vessels supplying the kidney leads to inconsistent ischaemia. (A) The absence of global kidney ischaemia is indicated by an uneven color change (white arrow) when the micro serrafine clip is in position. (B) Following removal of the micro serrafine clip, the main renal artery and vein are visible (white arrow) together with the additional blood vessel supplying the middle and lower pole of the kidney (black arrow). Please click here to view a larger version of this figure.
Figure 7. Left ischaemic kidney with non-occluded additional renal blood vessels. Failure to occlude additional blood vessels supplying the kidney leads to inconsistent ischaemia. (A) The absence of global kidney ischaemia is indicated by an uneven color change (white arrow) when the micro serrafine clip is in position. (B) Following removal of the micro serrafine clip, the main renal artery and vein are visible (white arrow) together with the additional blood vessel supplying the middle and lower pole of the kidney (black arrow). Please click here to view a larger version of this figure.
Discussion
Renal IRI is an important cause of AKI with no specific treatment available. The experimental study of renal IRI has been highly informative with previous work demonstrating the role of macrophages, dendritic cells, lymphocytes, regulatory T-cells as well as other cells and mediators in the induction of both the acute injury and healing phase5,8-16. In addition, experimental renal IRI has been used to assess the effect of various therapeutic agents4,17-19.
The model of renal IRI detailed here uses a midline laparotomy approach to perform a right nephrectomy and induce ischaemia in the left kidney using clamps. As illustrated by the representative results, modifying the duration of ischaemia can control the severity of injury. Therefore this model can be adjusted to induce mild, moderate or a high level of kidney injury as required by the experimental question posed. However, a limitation of this model is that it is not suitable for the induction of very severe kidney injury by prolonged renal ischaemia. The resultant severe acute kidney dysfunction may lead to significant mortality.
There are several aspects of this model that are crucial in order to achieve predictable and reproducible renal injury. One major source of variability with this model can originate from the body temperature during the ischaemic period20. It is therefore essential that body temperature is maintained at a constant level and monitored throughout the ischaemic period. In this protocol a control unit with temperature probes and homoeothermic blanket was used to auto-regulate body temperature at 37 °C. The influence of body temperature on the susceptibility of the kidney to ischaemic injury is well described. Previous work has shown that body temperature affects the severity of renal IRI20 and variation in the body temperature of the mice is a potential confounding factor that may affect the interpretation of the results. Another important consideration is that mice may exhibit anatomical variation with additional blood vessels that supply the left kidney. It is critical that such additional blood vessels are identified and occluded when performing the right nephrectomy and inducing global ischaemia in the left kidney. Failure to do so will result in inconsistent ischaemia throughout the kidney. Similarly, the color change of the left kidney following clamping of the left renal pedicle should be carefully scrutinized as the absence of a global color changes suggests the presence of an additional renal artery that had not initially been identified.
In contrast to models that utilize bilateral kidney ischaemia, this model will be of interest to researchers who are using the prophylactic administration of pharmaceutical drugs or other agents to modulate the expression of genes, proteins etc. prior to renal injury. The right nephrectomy tissue can be used to both confirm and quantify the level of induction or inhibition of the molecule of interest in each individual experimental animal. This model will also be of interest to researchers studying renal regeneration as the acute injury phase is followed by prominent tubular proliferation.
Some experimental models of renal inflammation are limited as significant disease can only be induced in certain strains of mice. In contrast, the model of renal IRI described here is versatile and can be applied to both male5 and female4 mice, different strains of mice as well as aged mice4.
Disclosures
The authors have no competing or conflicting interests to disclose.
Acknowledgments
The present study was supported by grants from Kidney Research UK (ST4/2011), the Cunningham Trust (CT11/14) and the Mrs EA Hogg's Charitable Trust.
References
- Kinsey GR, Li L, Okusa MD. Inflammation in acute kidney injury. Nephron Exp Nephrol. 2008;109 doi: 10.1159/000142934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Q, Dong Z. Mouse model of ischemic acute kidney injury: technical notes and tricks. Am J Physiol Renal Physiol. 2012;303 doi: 10.1152/ajprenal.00352.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferenbach DA, Kluth DC, Hughes J. Hemeoxygenase-1 and renal ischaemia-reperfusion injury. Nephron Exp Nephrol. 2010;115 doi: 10.1159/000313828. [DOI] [PubMed] [Google Scholar]
- Ferenbach DA, et al. The induction of macrophage hemeoxygenase-1 is protective during acute kidney injury in aging mice. Kidney Int. 2011;79:966–976. doi: 10.1038/ki.2010.535. [DOI] [PubMed] [Google Scholar]
- Ferenbach DA, et al. Macrophage/monocyte depletion by clodronate, but not diphtheria toxin, improves renal ischemia/reperfusion injury in mice. Kidney Int. 2012;82:928–933. doi: 10.1038/ki.2012.207. [DOI] [PubMed] [Google Scholar]
- Ferenbach DA, et al. Macrophages expressing heme oxygenase-1 improve renal function in ischemia/reperfusion injury. Mol Ther. 2010;18:1706–1713. doi: 10.1038/mt.2010.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keppler A, et al. Plasma creatinine determination in mice and rats: an enzymatic method compares favorably with a high-performance liquid chromatography assay. Kidney Int. 2007;71:74–78. doi: 10.1038/sj.ki.5001988. [DOI] [PubMed] [Google Scholar]
- Vinuesa E, et al. Macrophage involvement in the kidney repair phase after ischaemia/reperfusion injury. J Pathol. 2008;214:104–113. doi: 10.1002/path.2259. [DOI] [PubMed] [Google Scholar]
- Friedewald JJ, Rabb H. Inflammatory cells in ischemic acute renal failure. Kidney Int. 2004;66:486–491. doi: 10.1111/j.1523-1755.2004.761_3.x. [DOI] [PubMed] [Google Scholar]
- Gandolfo MT, et al. Foxp3+ regulatory T cells participate in repair of ischemic acute kidney injury. Kidney Int. 2009;76:717–729. doi: 10.1038/ki.2009.259. [DOI] [PubMed] [Google Scholar]
- Burne MJ, et al. Identification of the CD4+ T cell as a major pathogenic factor in ischemic acute renal failure. J Clin Invest. 2001. pp. 1283–1290. [DOI] [PMC free article] [PubMed]
- Burne-Taney MJ, et al. B cell deficiency confers protection from renal ischemia reperfusion injury. J Immunol. 2003;171:3210–3215. doi: 10.4049/jimmunol.171.6.3210. [DOI] [PubMed] [Google Scholar]
- Kinsey GR, et al. Regulatory T cells suppress innate immunity in kidney ischemia-reperfusion injury. J Am Soc Nephrol. 2009;20:1744–1753. doi: 10.1681/ASN.2008111160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Okusa MD. Macrophages, dendritic cells, and kidney ischemia-reperfusion injury. Semin Nephrol. 2010;30:268–277. doi: 10.1016/j.semnephrol.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SL, et al. Macrophage Wnt7b is critical for kidney repair and regeneration. Proc Natl Acad Sci U S A. 2010;107:4194–4199. doi: 10.1073/pnas.0912228107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, et al. Distinct macrophage phenotypes contribute to kidney injury and repair. J Am Soc Nephrol. 2011;22:317–326. doi: 10.1681/ASN.2009060615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, et al. Dexamethasone ameliorates renal ischemia-reperfusion injury. J Am Soc Nephrol. 2009;20:2412–2425. doi: 10.1681/ASN.2008080868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharples EJ, et al. Erythropoietin protects the kidney against the injury and dysfunction caused by ischemia-reperfusion. J Am Soc Nephrol. 2004;15:2115–2124. doi: 10.1097/01.ASN.0000135059.67385.5D. [DOI] [PubMed] [Google Scholar]
- Gueler F, et al. Statins attenuate ischemia-reperfusion injury by inducing heme oxygenase-1 in infiltrating macrophages. Am J Pathol. 2007;170:1192–1199. doi: 10.2353/ajpath.2007.060782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delbridge MS, Shrestha BM, Raftery AT, ElNahas AM, Haylor JL. The effect of body temperature in a rat model of renal ischemia-reperfusion injury. Transplant Proc. 2007;39:2983–2985. doi: 10.1016/j.transproceed.2007.04.028. [DOI] [PubMed] [Google Scholar]


