The thiosulfate dehydrogenase TsdA from A. vinosum was recombinantly expressed, purified and crystallized. The crystals belonged to space group C2, with unit-cell parameters a = 79.2, b = 69.9, c = 57.9 Å, β = 129.3°, and diffracted to a resolution of 1.98 Å.
Keywords: thiosulfate dehydrogenase, Allochromatium vinosum, c-type cytochrome, sulfur metabolism
Abstract
The ability to perform the very simple oxidation of two molecules of thiosulfate to tetrathionate is widespread among prokaryotes. Despite the prevalent occurrence of tetrathionate formation and its well documented significance within the sulfur cycle, little is known about the enzymes that catalyze the oxidative condensation of two thiosulfate anions. To fill this gap, the thiosulfate dehydrogenase (TsdA) enzyme from the purple sulfur bacterium Allochromatium vinosum was recombinantly expressed in Escherichia coli, purified and crystallized, and a crystallographic data set was collected. The crystals belonged to the monoclinic space group C2, with unit-cell parameters a = 79.2, b = 69.9, c = 57.9 Å, β = 129.3°, contained one monomer per asymmetric unit and diffracted to a resolution of 1.98 Å.
1. Introduction
Thiosulfate dehydrogenases (TsdA) catalyze the oxidation of two thiosulfate molecules to tetrathionate, thereby releasing two electrons (2S2O3 2−→S4O6 2− + 2e−). Formation of tetrathionate occurs not only in specialized sulfur oxidizers but also in many chemoorganoheterotrophic bacteria, suggesting a widespread occurrence of TsdA homologues among bacteria (Denkmann et al., 2012 ▶). In the purple sulfur bacterium Allochromatium vinosum, thiosulfate dehydrogenase is a periplasmic, monomeric 27.2 kDa dihaem c-type cytochrome with an activity optimum at pH 4.0. TsdA comprises two typical haem c binding sites with the characteristic CXXCH sequence motif, indicating two covalently bound haems. Electron paramagnetic resonance (EPR) suggested structural flexibility in TsdA affecting both haems and revealed cysteine and methionine to be possible distal axial ligands (Denkmann et al., 2012 ▶). Axial His/Cys ligation is rare among prokaryotic c-type cytochromes and evidence is accumulating that it is of special importance in sulfur-based energy metabolism (Bamford et al., 2002 ▶; Grein et al., 2010 ▶).
In A. vinosum, TsdA operates in the direction of tetrathionate formation. Interestingly, the closely related protein from the human gut pathogen Campylobacter jejuni has been identified as a bifunctional tetrathionate reductase/thiosulfate dehydrogenase appearing to be especially adapted to catalyze tetrathionate reduction (Liu et al., 2013 ▶). Thus, TsdA enzymes may not have the same role in different bacteria, but rather are optimized for thiosulfate oxidation or tetrathionate reduction according to the physiological requirements and lifestyle of the particular bacteria concerned.
Here, we report the production, crystallization and preliminary crystallographic analysis of A. vinosum TsdA. The protein crystallized in space group C2 with one molecule in the asymmetric unit. Initial crystallization trials rendered multiple, urchin-like crystals with no diffraction ability. Using iodide as an additive significantly improved the X-ray diffraction quality of the crystals. A crystallographic data set was collected to 1.98 Å resolution at a synchrotron source and further analysis is under way to determine the phases.
2. Materials and methods
2.1. Cloning, overexpression and purification
The construction of expression plasmids, overproduction and purification of recombinant TsdA protein was performed as described in Denkmann et al. (2012 ▶). Escherichia coli BL21(DE3) cells containing the pPR-IBAAvtsdAs expression plasmid (Denkmann et al., 2012 ▶) and pEC86 (Arslan et al., 1998 ▶) (Table 1 ▶) were cultured in 700 ml NZCYM medium containing 100 mg ml−1 ampicillin and 25 mg ml−1 chloramphenicol in 1 l Erlenmeyer flasks at 37°C and 180 rev min−1. When the OD600 reached 0.6, the temperature was lowered to 25°C and the cells were harvested after further incubation for 16–20 h. It is important to note that protein production was not induced by the addition of isopropyl β-d-1-thiogalactopyranoside (IPTG). The absence of IPTG and the low growth temperature guaranteed slow production of TsdA, which is essential for efficient and complete loading of the enzyme with haem by the Ccm (cytochrome c maturation) proteins encoded on the pEC86 plasmid. Cells were resuspended in 50 mM Tris–HCl pH 8.0 and lysed by sonication. After removal of the insoluble cell material by centrifugation (10 000g for 30 min at 4°C), TsdA protein was purified by affinity chromatography on Strep-Tactin Superflow (300 nmol ml−1 resin, IBA GmbH) according to the manufacturer’s instructions. The washing buffer consisted of 100 mM Tris–HCl, 150 mM NaCl pH 8.0; the elution and regeneration buffers were similar to the washing buffer but were supplemented with 2.5 mM desthiobiotin and 1 mM 2-(4-hydroxyphenylazo)-benzoic acid, respectively. Gel filtration was performed on a HiLoad Superdex 75 column (GE Healthcare) with 50 mM Tris–HCl pH 8.0, 150 mM NaCl as buffer using an ÄKTApurifier system (GE Healthcare) according to the manufacturer’s instructions. After gel filtration the buffer was exchanged to 20 mM Tris–HCl pH 8.0 using a 5 ml HiTrap desalting column (GE Healthcare). The protein was then concentrated to 8 mg ml−1 with Vivaspin 500 columns (Sartorius) and used at this concentration for crystallization trials.
Table 1. Macromolecule-production information.
| Source organism | A. vinosum DSM 180T |
| DNA source | Genomic |
| Forward primer | CCACAAAGAAACATATGCGCGGTG |
| Reverse primer | CCGACGGGTGATATCGCGTCG |
| Cloning vector | pPR-IBA 1 |
| Expression vector | pPR-IBA 1 |
| Expression host | E. coli BL21 (DE3) |
| Complete amino-acid sequence of construct produced | MEPPTVALTVPAAALLPDGALGESIVRGRRYLSDTPAQLPDFVGNGLACRHCHPGRDGEVGTEANAAPFVGVVGRFPQYSARHGRLITLEQRIGDCFERSLNGRALALDHPALIDMLAYMSWLSQGVPVGAVVAGHGIPTLTLEREPDGVHGEALYQARCLACHGADGSGTLDADGRYLFPPLWGPRSFNTGAGMNRQATAAGFIKHKMPLGADDSLSDEEAWDVAGFVLTHPRPLFQEPTGDAWSHPQFEK |
SDS–PAGE was performed as described in Dahl et al. (2005 ▶) and haem staining in acrylamide gels was performed as described in Thomas et al. (1976 ▶). Protein concentration was determined with the BCA Kit from Pierce. UV–Vis absorbance spectra were recorded with a Specord 210 spectrophotometer (Analytik Jena). Macromolecule-production information is summarized in Table 1 ▶.
2.2. Crystallization
Initial TsdA crystallization trials were carried out at 20°C using the sitting-drop vapour-diffusion method and a Cartesian robot dispensing system (Mini-Bee, Genomic Solutions). Two commercially available crystallization screens (Structure Screen 1 and 2 from Molecular Dimensions and Index HT from Hampton Research) were used; 200 nl drops of a 1:1 ratio of protein and precipitant solutions were set up in 96-well flat-bottom plates (Greiner Bio-One) and equilibrated against a reservoir consisting of 100 µl precipitant solution. Crystalline material appeared in several conditions, most of them containing PEG 3350 as precipitant, in a pH range from 5.5 to 7.5. The buffer of the protein sample was further exchanged to 20 mM Bis-Tris–HCl pH 6.5 using a HiTrap desalting column (GE Healthcare) and protein concentrated to 8 mg ml−1. A grid screen was performed around 25%(w/v) PEG 3350, 0.2 M ammonium sulfate, 0.1 M Bis-Tris pH 6.5 using home-made solutions and the sitting-drop vapour-diffusion technique. Drops consisted of 1.5 µl protein solution and 1.5 µl precipitant solution and were equilibrated against 500 µl reservoir solution in 24-well EasyXtal Tool plates (Qiagen) at room temperature (20°C). Additionally, the Additive Screen (Hampton Research) was tested. Final optimization conditions comprised 23.5%(w/v) PEG 3350, 0.2 M ammonium sulfate, 0.1 M Bis-Tris pH 6.28, 0.1 M NaI (Table 2 ▶), and drops were set up using an Oryx 6 dispensing robot (Douglas Instruments).
Table 2. Crystallization conditions and experimental setup.
| Method | Vapour diffusion (sitting-drop) |
| Plate type | MRC Maxi 48-well (Swissci) |
| Temperature (C) | 20 |
| Protein concentration (mgml1) | 8 |
| Buffer composition of protein solution | 20mM Bis-TrisHCl pH 6.5 |
| Composition of reservoir solution | 23.5%(w/v) PEG 3350, 0.2M (NH4)2SO4, 0.1M Bis-Tris pH 6.28, 0.1M NaI |
| Volume and ratio of drop | 3l (1.2l protein + 1.5l precipitant + 0.3l additive) |
| Volume of reservoir (l) | 120 |
Cryoprotection conditions for diffraction experiments were achieved by transferring crystals to a new drop with a higher PEG 3350 concentration [25.4%(w/v)] supplemented with 5%(v/v) PEG 400. Crystallization information is summarized in Table 2 ▶.
2.3. Data collection, processing and phasing
A single crystal of approximate dimensions 0.2 × 0.1 × 0.02 mm was used for data collection at cryogenic temperatures by flash-cooling at −173°C in a nitrogen-gas stream (Oxford Cryosystems 700). X-ray diffraction images were collected at a wavelength of 1.722 Å (Fe edge) using a PILATUS 6M detector (Dectris) on the XALOC beamline at the ALBA synchrotron (Barcelona, Spain). The data were indexed, integrated and scaled using XDS and converted to MTZ format with XDSCONV (Kabsch, 2010 ▶). The crystals belonged to the monoclinic space group C2, with unit-cell parameters a = 79.2, b = 69.9, c = 57.9 Å, β = 129.3°, which yields a Matthews coefficient of 2.31 Å3 Da−1, corresponding to a solvent content of ∼46.8% and assuming one monomer per asymmetric unit (Matthews, 1968 ▶). A data set was collected to 1.98 Å resolution with an overall R merge of 9.6% and 92.7% completeness (Table 3 ▶).
Table 3. Data-collection and processing statistics.
Values in parentheses are for the highest resolution shell.
| Diffraction source | XALOC, ALBA |
| Wavelength () | 1.722 |
| Space group | C2 |
| a, b, c () | 79.2, 69.9, 57.9 |
| , , () | 90.0, 129.3, 90.0 |
| Resolution range () | 46.11.98 (2.061.98) |
| Total reflections | 52517 (4007) |
| Unique reflections | 15751 (1266) |
| Completeness (%) | 92.70 (75.40) |
| Multiplicity | 3.3 (3.2) |
| I/(I) | 13.1 (5.3) |
| R merge † (%) | 9.6 (30.3) |
| CC1/2 ‡ (%) | 99.1 (91.9) |
| CC*§ (%) | 99.8 (97.9) |
| Matthews coefficient (3Da1) | 2.31 |
| V solv (%) | 46.8 |
R
merge = 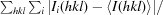
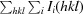 , where Ii(hkl) is the ith intensity measurement of reflection hkl, including symmetry-related reflections, and I(hkl) is its average.
, where Ii(hkl) is the ith intensity measurement of reflection hkl, including symmetry-related reflections, and I(hkl) is its average.
CC1/2 is the half data set correlation coefficient (Diederichs Karplus, 2013 ▶).
CC* is the correlation of an observed data set with the underlying (not measurable) true signal (Karplus Diederichs, 2012 ▶).
3. Results and discussion
The recombinant TsdA protein was produced in and purified from E. coli. Although Coomassie staining showed only one band after affinity chromatography, the more sensitive staining for covalently bound haem revealed the presence of additional contaminating proteins. These were finally removed by gel-permeation chromatography (Fig. 1 ▶). The pure Strep-tag-fused TsdA protein migrated as a single band in Coomassie-stained as well as in haem-stained gels. Its apparent molecular mass coincided with the calculated mass of 27.2 kDa (Fig. 1 ▶). For the reduced protein, E 419.8 nm/E 280 nm increased significantly from 2.89 to 3.32 after the final gel-filtration step. A molar extinction coefficient of ∊407 nm = 124 400 M −1 cm−1 was calculated for the pure, fully haem-loaded, oxidized protein.
Figure 1.
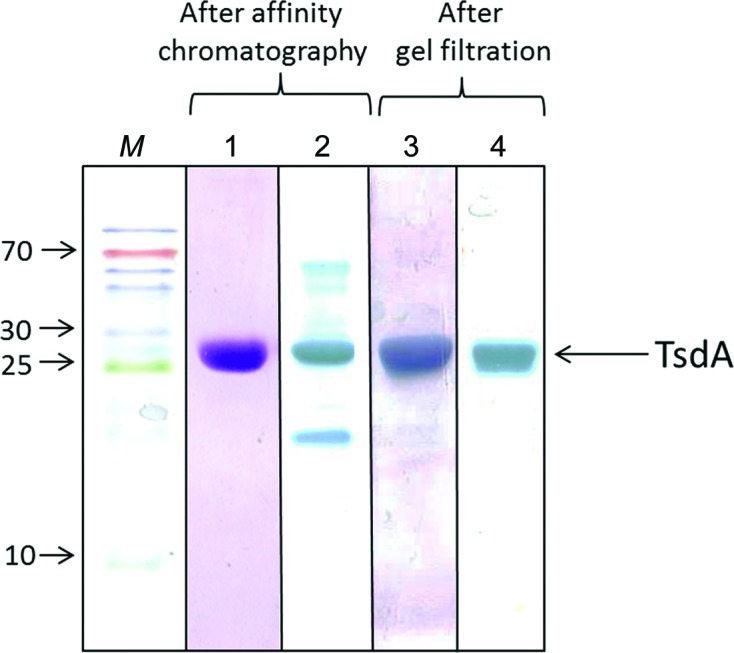
Coomassie (lanes 1 and 3) and haem (lanes 2 and 4) staining of SDS–PAGE gels. TsdA preparations obtained after affinity chromatography (lanes 1 and 2) and after gel filtration (lanes 3 and 4) were analyzed. Protein loaded per lane: 3.5 µg in lanes 1 and 2, and 5 µg in lanes 3 and 4. Lane M contains molecular-mass markers (labelled in kDa).
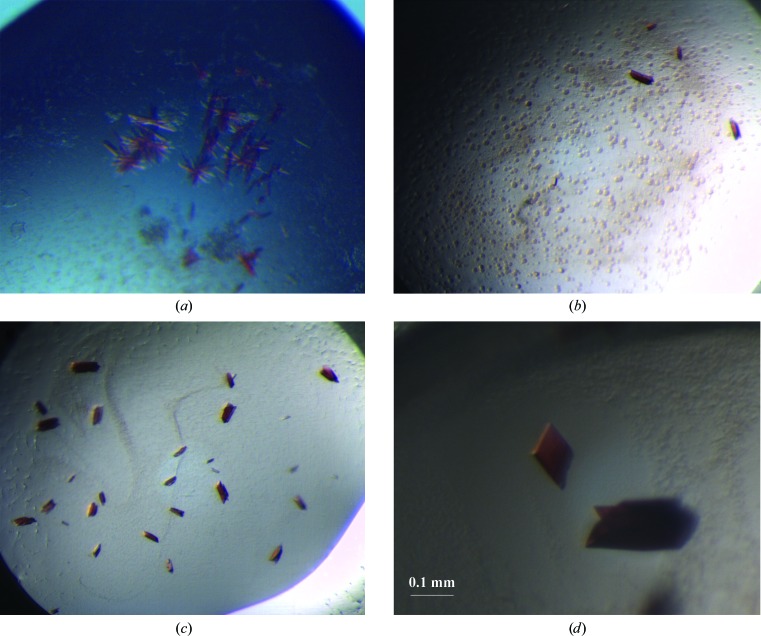
Crystals appeared over the course of 10 d in the initial crystallization trials using a Cartesian robot dispensing system with the protein at 8 mg ml−1 in 20 mM Tris–HCl pH 8.0. However, most crystals appeared as sea-urchin agglomerates (Fig. 2 ▶ a) and spherulites (Fig. 2 ▶ b). Since most crystallization conditions occurred at a pH lower than 7.0, a buffer-exchange protocol was carried out to lower the pH. Thus, the new batch of protein was concentrated to 8 mg ml−1 in 20 mM Bis-Tris–HCl buffer pH 6.5 and the crystallization conditions were further optimized. No urchin-like agglomerates and spherulites were observed, but the crystals still looked like toothpicks tied together (Fig. 2 ▶ c). Notably, the addition of sodium iodide (NaI) from Additive Screen (Hampton Research) acted as a ‘silver bullet’ yielding single, large crystals with good X-ray diffraction quality (Fig. 2 ▶ d).
Figure 2.
TsdA crystals obtained (a), (b) during initial crystallization trials using the Mini-Bee Cartesian robot, (c) on scaling up after buffer exchange and (d) on scaling up after buffer exchange and using NaI as crystallization additive.
A crystallographic data set was collected at a wavelength of 1.722 Å (Fe K edge) from a single crystal on the XALOC beamline at the ALBA synchrotron, Barcelona, Spain. The crystals diffracted to 1.98 Å resolution and further analysis is under way to determine the phases. Simultaneously, we are also attempting to co-crystallize TsdA with substrate and product, as well as obtaining the structure in different redox states. This analysis will provide valuable insights into the reaction mechanism of the TsdA enzyme.
Acknowledgments
The authors acknowledge the Deutsche Forschungsgemeinschaft (grant DA 351/7-1, Germany), Fundação para a Ciência e a Tecnologia (FCT, grants PTDC/QUI-BIQ/100591/2008, PTDC/BIA-PRO/118535/2010 and PEst-OE/EQB/LA0004/2011, Portugal), the German Academic Exchange Service (DAAD, Germany), Conselho de Reitores das Universidades Portuguesas (CRUP, Portugal) and BioStruct-X (proposal 1493) for funding. JAB is the recipient of FCT fellowship SFRH/BPD/79224/2011. The authors thank Pedro Matias and Célia Romão for collecting the Fe-SAD diffraction data and Jordi Juanhuix from the XALOC beamline at the ALBA synchrotron (Barcelona, Spain) for technical support during data collection.
References
- Arslan, E., Schulz, H., Zufferey, R., Künzler, P. & Thöny-Meyer, L. (1998). Biochem. Biophys. Res. Commun. 251, 744–747. [DOI] [PubMed]
- Bamford, V. A., Bruno, S., Rasmussen, T., Appia-Ayme, C., Cheesman, M. R., Berks, B. C. & Hemmings, A. M. (2002). EMBO J. 21, 5599–5610. [DOI] [PMC free article] [PubMed]
- Dahl, C., Engels, S., Pott-Sperling, A. S., Schulte, A., Sander, J., Lübbe, Y., Deuster, O. & Brune, D. C. (2005). J. Bacteriol. 187, 1392–1404. [DOI] [PMC free article] [PubMed]
- Denkmann, K., Grein, F., Zigann, R., Siemen, A., Bergmann, J., van Helmont, S., Nicolai, A., Pereira, I. A. & Dahl, C. (2012). Environ. Microbiol. 14, 2673–2688. [DOI] [PubMed]
- Diederichs, K. & Karplus, P. A. (2013). Acta Cryst. D69, 1215–1222. [DOI] [PMC free article] [PubMed]
- Grein, F., Venceslau, S. S., Schneider, L., Hildebrandt, P., Todorovic, S., Pereira, I. A. & Dahl, C. (2010). Biochemistry, 49, 8290–8299. [DOI] [PubMed]
- Kabsch, W. (2010). Acta Cryst. D66, 125–132. [DOI] [PMC free article] [PubMed]
- Karplus, P. A. & Diederichs, K. (2012). Science, 336, 1030–1033. [DOI] [PMC free article] [PubMed]
- Liu, Y. W., Denkmann, K., Kosciow, K., Dahl, C. & Kelly, D. J. (2013). Mol. Microbiol. 88, 173–188. [DOI] [PubMed]
- Matthews, B. W. (1968). J. Mol. Biol. 33, 491–497. [DOI] [PubMed]
- Thomas, P. E., Ryan, D. & Levin, W. (1976). Anal. Biochem. 75, 168–176. [DOI] [PubMed]