Abstract
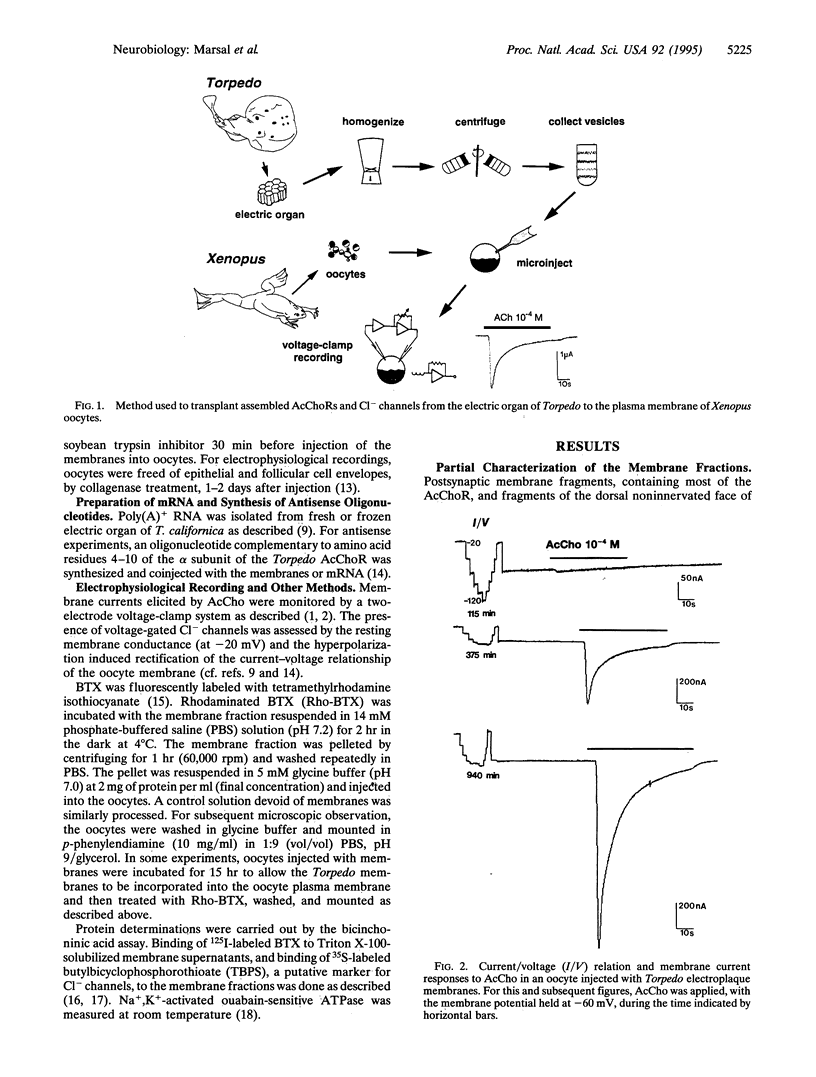
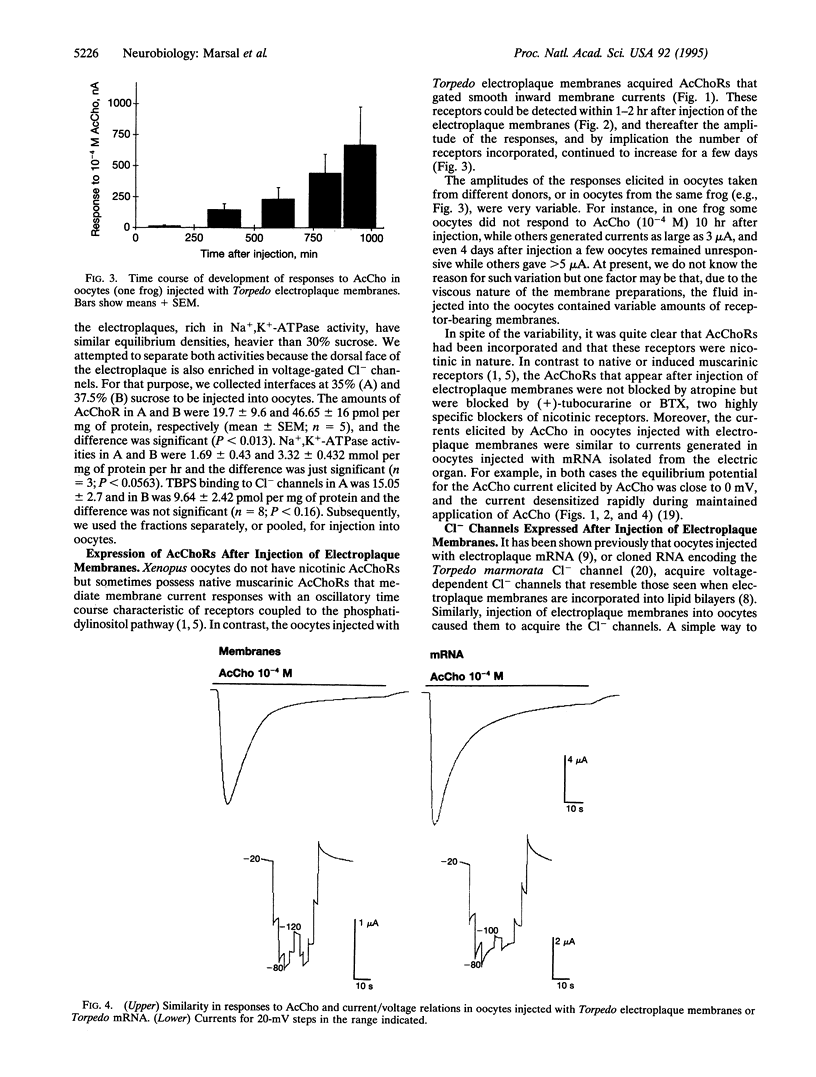
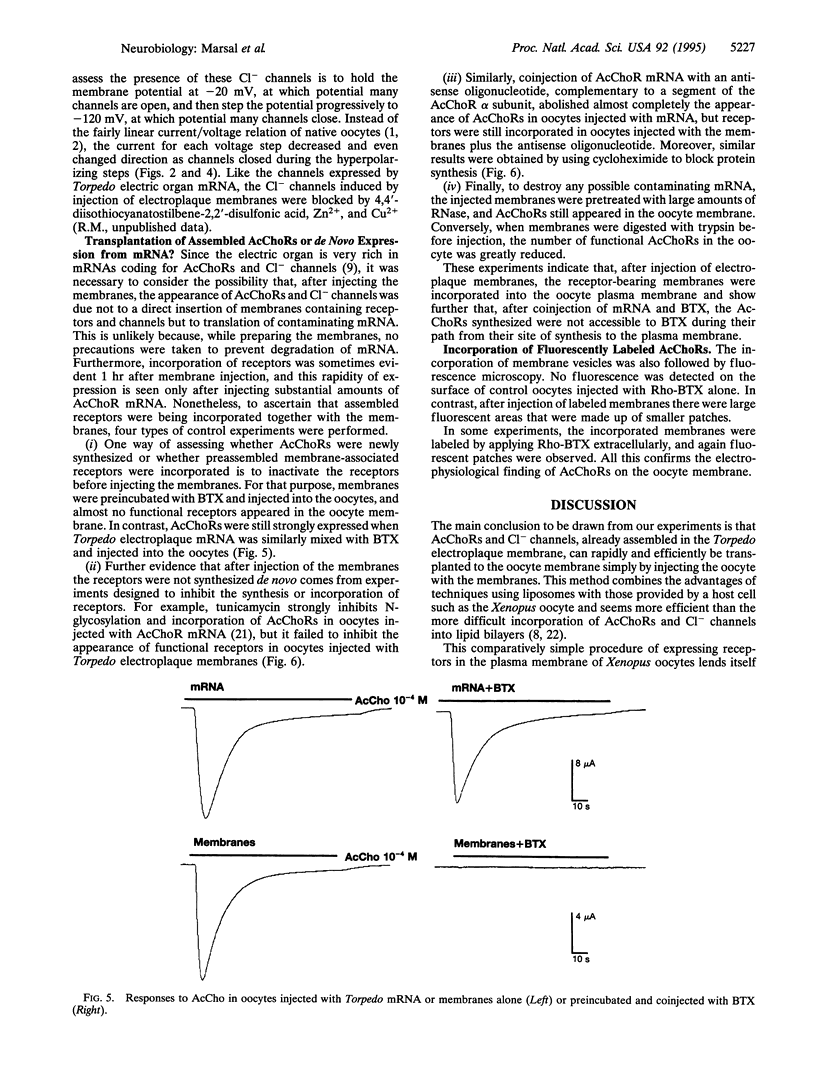
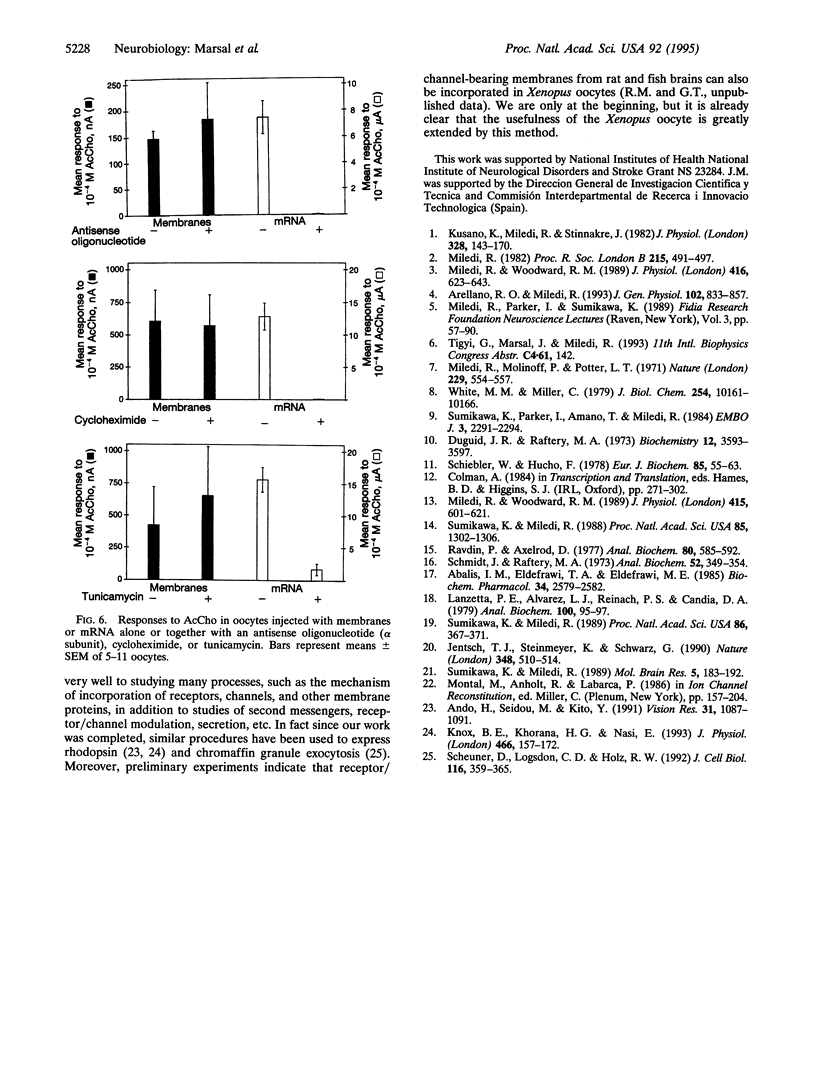
A method was developed to transplant assembled nicotinic acetylcholine receptors (AcChoRs) and Cl- channels from the electric organ of Torpedo to the membrane of Xenopus oocytes. Membrane vesicles from Torpedo electroplaques were injected into the oocytes and, within a few hours, the oocyte membrane acquired AcChoRs and Cl- channels. The mechanism of expression of these receptors and channels is very different from that which follows the injection of mRNA, since the appearance of receptors after membrane injection does not require de novo protein synthesis or N-glycosylation. This, and other controls, indicate that the foreign receptor-bearing membranes fuse with the oocyte membrane and cause the appearance of functional receptors and channels. All this makes the Xenopus oocyte an even more powerful tool for studies of the structure and function of membrane proteins.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abalis I. M., Eldefrawi M. E., Eldefrawi A. T. Binding of GABA receptor channel drugs to a putative voltage-dependent chloride channel in Torpedo electric organ. Biochem Pharmacol. 1985 Jul 15;34(14):2579–2582. doi: 10.1016/0006-2952(85)90550-7. [DOI] [PubMed] [Google Scholar]
- Ando H., Seidou M., Kito Y. Light-induced, GTP-binding protein mediated membrane currents of Xenopus oocytes injected with rhodopsin of cephalopods. Vision Res. 1991;31(7-8):1087–1091. doi: 10.1016/0042-6989(91)90034-3. [DOI] [PubMed] [Google Scholar]
- Arellano R. O., Miledi R. Novel Cl- currents elicited by follicle stimulating hormone and acetylcholine in follicle-enclosed Xenopus oocytes. J Gen Physiol. 1993 Nov;102(5):833–857. doi: 10.1085/jgp.102.5.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duguid J. R., Raftery M. A. Fractionation and partial characterization of membrane particles from Torpedo californica electroplax. Biochemistry. 1973 Sep 11;12(19):3593–3597. doi: 10.1021/bi00743a003. [DOI] [PubMed] [Google Scholar]
- Jentsch T. J., Steinmeyer K., Schwarz G. Primary structure of Torpedo marmorata chloride channel isolated by expression cloning in Xenopus oocytes. Nature. 1990 Dec 6;348(6301):510–514. doi: 10.1038/348510a0. [DOI] [PubMed] [Google Scholar]
- Knox B. E., Khorana H. G., Nasi E. Light-induced currents in Xenopus oocytes expressing bovine rhodopsin. J Physiol. 1993 Jul;466:157–172. [PMC free article] [PubMed] [Google Scholar]
- Kusano K., Miledi R., Stinnakre J. Cholinergic and catecholaminergic receptors in the Xenopus oocyte membrane. J Physiol. 1982 Jul;328:143–170. doi: 10.1113/jphysiol.1982.sp014257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanzetta P. A., Alvarez L. J., Reinach P. S., Candia O. A. An improved assay for nanomole amounts of inorganic phosphate. Anal Biochem. 1979 Nov 15;100(1):95–97. doi: 10.1016/0003-2697(79)90115-5. [DOI] [PubMed] [Google Scholar]
- Miledi R. A calcium-dependent transient outward current in Xenopus laevis oocytes. Proc R Soc Lond B Biol Sci. 1982 Jul 22;215(1201):491–497. doi: 10.1098/rspb.1982.0056. [DOI] [PubMed] [Google Scholar]
- Miledi R., Molinoff P., Potter L. T. Isolation of the cholinergic receptor protein of Torpedo electric tissue. Nature. 1971 Feb 19;229(5286):554–557. doi: 10.1038/229554a0. [DOI] [PubMed] [Google Scholar]
- Miledi R., Woodward R. M. Effects of defolliculation on membrane current responses of Xenopus oocytes. J Physiol. 1989 Sep;416:601–621. doi: 10.1113/jphysiol.1989.sp017780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R., Woodward R. M. Membrane currents elicited by prostaglandins, atrial natriuretic factor and oxytocin in follicle-enclosed Xenopus oocytes. J Physiol. 1989 Sep;416:623–643. doi: 10.1113/jphysiol.1989.sp017781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravdin P., Axelrod D. Fluorescent tetramethyl rhodamine derivatives of alpha-bungarotoxin: preparation, separation, and characterization. Anal Biochem. 1977 Jun;80(2):585–592. doi: 10.1016/0003-2697(77)90682-0. [DOI] [PubMed] [Google Scholar]
- Scheuner D., Logsdon C. D., Holz R. W. Bovine chromaffin granule membranes undergo Ca(2+)-regulated exocytosis in frog oocytes. J Cell Biol. 1992 Jan;116(2):359–365. doi: 10.1083/jcb.116.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiebler W., Hucho F. Membranes rich in acetylcholine receptor: characterization and reconstitution to excitable membranes from exogenous lipids. Eur J Biochem. 1978 Apr;85(1):55–63. doi: 10.1111/j.1432-1033.1978.tb12211.x. [DOI] [PubMed] [Google Scholar]
- Schmidt J., Raftery M. A. A simple assay for the study of solubilized acetylcholine receptors. Anal Biochem. 1973 Apr;52(2):349–354. doi: 10.1016/0003-2697(73)90036-5. [DOI] [PubMed] [Google Scholar]
- Sumikawa K., Miledi R. Assembly and N-glycosylation of all ACh receptor subunits are required for their efficient insertion into plasma membranes. Brain Res Mol Brain Res. 1989 May;5(3):183–192. doi: 10.1016/0169-328x(89)90034-x. [DOI] [PubMed] [Google Scholar]
- Sumikawa K., Miledi R. Change in desensitization of cat muscle acetylcholine receptor caused by coexpression of Torpedo acetylcholine receptor subunits in Xenopus oocytes. Proc Natl Acad Sci U S A. 1989 Jan;86(1):367–371. doi: 10.1073/pnas.86.1.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumikawa K., Miledi R. Repression of nicotinic acetylcholine receptor expression by antisense RNAs and an oligonucleotide. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1302–1306. doi: 10.1073/pnas.85.4.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumikawa K., Parker I., Amano T., Miledi R. Separate fractions of mRNA from Torpedo electric organ induce chloride channels and acetylcholine receptors in Xenopus oocytes. EMBO J. 1984 Oct;3(10):2291–2294. doi: 10.1002/j.1460-2075.1984.tb02128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White M. M., Miller C. A voltage-gated anion channel from the electric organ of Torpedo californica. J Biol Chem. 1979 Oct 25;254(20):10161–10166. [PubMed] [Google Scholar]



