Dipeptidyl peptidase‐4 (DPP‐4) inhibitors are widely used in the management of type 2 diabetes worldwide. Despite amelioration of insulin secretion from pancreatic β‐cells, DPP‐4 inhibitors have very little hypoglycemia risk and do not promote bodyweight gain when compared with other insulin secretagogues, such as sulfonylureas (SU) and glinides1. Furthermore, accumulating data from clinical trials shows that DPP‐4 inhibitors exert greater glycated hemoglobin (HbA1c)‐lowering effects in non‐obese Asian type 2 diabetes when compared with diabetes in other ethnicities1. This could be explained by the fact that Asian type 2 diabetes is characterized by impaired insulin secretion, particularly in the early phase after ingestion of glucose or mixed meals. Enhancement of the incretin system by DPP‐4 inhibitors was found to ameliorate impaired early phase insulin secretion in clinical studies using DPP‐4 inhibitors in patients with type 2 diabetes1.
Although DPP‐4 inhibitors alone are considered to have very little hypoglycemia risk based on the results of clinical trials, cases of severe hypoglycemia with DPP‐4 inhibitor and SU combinations were reported when the first DPP‐4 inhibitor sitagliptin emerged in Japanese clinical practice in November 2009 (Figure 1). The estimated incidence of hypoglycemic coma with sitagliptin was 16.3 per million patients who received sitagliptin during the first 6 months after its launch in Japan (Sitagliptin Japanese early post‐marketing vigilance data, Pharmaceuticals and Medical Devices Agency and National Prescription Audit™; IMS Japan K.K., Tokyo, Japan), and was approximately 6.4‐fold higher than that of the USA in the corresponding period (Food and Drug Administration Adverse Event Reporting System, Food and Drug Administration and National Prescription Audit™; IMS Health Inc., Danbury, CT, USA). The cases in Japan were mostly elderly, and were found to have renal insufficiency and high HbA1c, even with use of high‐dose SU. Because of their impaired insulin secretion, SU such as glibenclamide, glimepiride and gliclazide have been widely used for glycemic control of Japanese type 2 diabetes patients rather than metformin, which is frequently used as the first‐line drug in the USA and European countries. Furthermore, the SU dose tends to be increased to the maximal levels in patients with high HbA1c, and even then does not fully improve glycemic control, a state called ‘SU secondary failure’. Based on the characteristics of the cases with severe hypoglycemia by DPP‐4 inhibitor treatment, a committee of experts in the field (Chair, Y Seino of Kansai Electric Power Hospital; T Kadowaki of University of Tokyo; N Inagaki of Kyoto University; T Iwakura of Kobe City Hospital; Y Iwamoto of Tokyo Women's Medical University; S Seino of Kobe University) urged physicians to reduce the doses of preprescribed SU drugs, especially in the elderly and/or patients with renal insufficiency, before co‐administration of DPP‐4 inhibitors as follows: glibenclamide ≤1.25 mg, glimepiride ≤2.0 mg and gliclazide ≤40 mg. Incidences of severe hypoglycemia have been drastically reduced by this recommendation (Figure 1). However, the mechanism of the phenomenon was unclear until recently.
Figure 1.
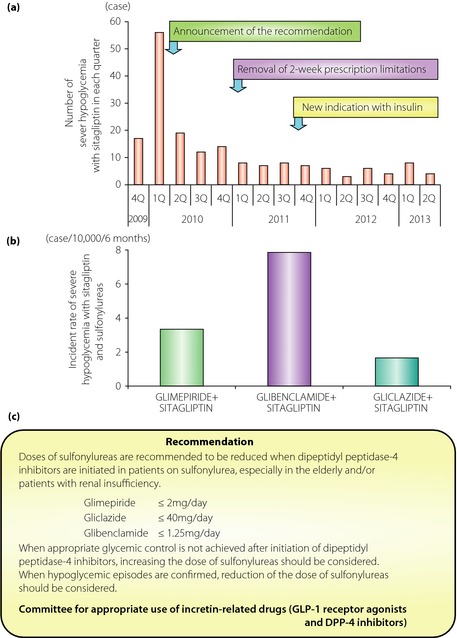
(a) The number of cases of severe hypoglycemia in patients treated with dipeptidyl peptidase‐4 (DPP‐4) inhibitor sitagliptin in each quarter (Sitagliptin Japanese early post‐marketing vigilance data, Pharmaceuticals and Medical Devices Agency). The number was drastically reduced on announcement of the recommendation from the committee for appropriate use of incretin‐related drugs (glucagon‐like peptide‐1 [GLP‐1] receptor agonists and DPP‐4 inhibitors). (b) Estimated incidence of severe hypoglycemia with sitagliptin and indicated sulfonylureas. The number of cases of severe hypoglycemia caused by sitagliptin and sulfonylurea combinations during the 6 months after the launch of sitagliptin was divided by the number of patients who received indicated sulfonylureas and sitagliptin simultaneously in the same period (Sitagliptin Japanese early post‐marketing vigilance data between November 2009 and May 2010; National Prescription Audit™, IMS Japan K.K.). (c) An extract of the recommendation from the committee. 1Q, first quarter; 2Q, second quarter; 3Q, third quarter; 4Q, fourth quarter.
Clues to understand the mechanism of severe hypoglycemia on initiation of DPP‐4 inhibitors in SU‐treated patients came from two key investigations on incretin signaling in pancreatic β‐cells. A study showed that activation of incretin signaling by the glucagon‐like peptide‐1 (GLP‐1) receptor agonist, exendin‐4, ameliorates glucose metabolism in pancreatic β‐cells of non‐obese diabetic model Goto‐Kakizaki rats, thereby improving glucose‐induced insulin secretion2. Chronic hyperglycemia is known to enhance the production of reactive oxygen species, which then impairs glucose metabolism and reduces adenosine triphosphate (ATP) production in pancreatic β‐cells. It was previously shown that SU‐induced closure of KATP channels is affected significantly by intracellular ATP levels3. Reduced ATP production as a result of chronic hyperglycemia could thus make pancreatic β‐cells less sensitive to SU, partly explaining ‘SU secondary failure’. The Goto‐Kakizaki rat study clearly showed that activation of incretin signaling reduces production of reactive oxygen species in islets and increases ATP, an exchange protein directly activated by cyclic adenosine monophosphate (cAMP) 2A (EPAC2A) dependently2. Thus, initiation of DPP‐4 inhibitors in patients with ‘SU secondary failure’ could result in hypoglycemia as a result of improved sensitivity of pancreatic β‐cells to SU.
Another clue came from a study showing novel cross‐talk between SU and incretin signaling through EPAC2A4. Activation of incretin signaling in pancreatic β‐cells increases intracellular levels of cAMP, which binds to EPAC2A and triggers its conformational change, thereby activating its downstream target Ras‐related protein 1 (RAP1) to enhance insulin secretion. They had previously shown that SU also bind to and activate EPAC2A, thereby promoting insulin secretion through activation of RAP1. Recently, they extended their study and showed through molecular docking simulation and fluorescence resonance energy transfer experiments using mutagenized EPAC2 that binding of SU to EPAC2A depends on both the concentration of cAMP and the structure of SU. Importantly, glibenclamide and glimepiride, but not gliclazide, binds to EPAC2A and induces a conformational change that activates RAP15. These results are suggestive of the cases in which SU is responsible for severe hypoglycemia. Among the 62 cases of severe hypoglycemia after initiation of sitagliptin (Sitagliptin Japanese early post‐marketing vigilance data between November 2009 and May 2010), most of the patients were taking glimepiride (67.7%) and glibenclamide (30.6%), whereas very few were taking gliclazide (3.2%). The estimated incident rates of severe hypoglycemia in patients who received sitagliptin with glimepiride (3.35 per 10,000) and glibenclamide (7.86 per 10,000) were more than twofold higher than in those who received sitagliptin with gliclazide (1.66 per 10,000) between November 2009 and May 2010 (Sitagliptin Japanese early post‐marketing vigilance data between November 2009 and May 2010; National Prescription Audit™, IMS Japan K.K.). Although numerous factors including reduced glucose counter‐regulation might affect the incident rates of severe hypoglycemia by the combinations of sitagliptin and each SU, these data complement the original observations in clinical settings and provide insight on the suitability of the various SU to be used in combination with DPP‐4 inhibitors. These observations also have important implications for SU and GLP‐1 receptor agonist use in combination. As was shown, a high level of cAMP inhibits binding of SU to EPAC2A, so cross‐talk of SU and incretin signaling through EPAC2A might not be expected with GLP‐1 receptor agonists, which appear to increase levels of cAMP in pancreatic β‐cells more strongly than DPP‐4 inhibitors. Indeed, no severe hypoglycemia cases were reported for combinations of SU and the GLP‐1 receptor agonist liraglutide during the first 6 months after its launch in Japan (Liraglutide Japanese early post‐marketing vigilance data from Pharmaceuticals and Medical Devices Agency). Although greater awareness of the recommendations among Japanese physicians at the time of launching the first GLP‐1 receptor agonist, liraglutide, in Japan and the 21.6‐fold higher number of prescriptions for sitagliptin as a combination drug with SU during 6 months after their launch (National Prescription Audit™, IMS Japan K.K.) must be taken into account, the very rare incident of severe hypoglycemia in combinations of SU and GLP‐1 receptor agonists is in good accordance with these observations5.
Taken together, these critical findings explain why activation of incretin signaling by DPP‐4 inhibitors enhances SU‐induced insulin secretion from pancreatic β‐cells, even in patients with ‘SU secondary failure’. With careful titration of SU doses and appropriate patient education on hypoglycemia, a combination of DPP‐4 inhibitors and SU drugs can be effective therapy for type 2 diabetes. However, careful consideration is required when such combination therapy is initiated in the elderly and/or patients with renal insufficiency.
References
- 1.Seino Y, Yabe D. GIP and GLP‐1: incretin actions beyond pancreas. J Diabetes Invest 2013; 4: 108–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mukai E, Fujimoto S, Sato H, et al Exendin‐4 suppresses SRC activation and reactive oxygen species production in diabetic Goto‐Kakizaki rat islets in an Epac‐dependent manner. Diabetes 2010; 60: 218–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mukai E, Ishida H, Kato S, et al Metabolic inhibition impairs ATP‐sensitive K+ channel block by sulfonylurea in pancreatic beta‐cells. Am J Physiol 1998; 274: E38–E44 [DOI] [PubMed] [Google Scholar]
- 4.Zhang CL, Katoh M, Shibasaki T, et al The cAMP sensor Epac2 is a direct target of antidiabetic sulfonylurea drugs. Science 2009; 325: 607–610 [DOI] [PubMed] [Google Scholar]
- 5.Takahashi T, Shibasaki T, Takahashi H, et al Antidiabetic Sulfonylureas and cAMP Cooperatively Activate Epac2A. Sci Signal 2013; 6: ra94. [DOI] [PubMed] [Google Scholar]


