Abstract
Aims/Introduction
The present study was designed to investigate from which tissues the decrease in retinol‐binding protein 4 (RBP4) expression could contribute to the improvement of serum RBP4 and insulin resistance (IR) after endurance training.
Materials and Methods
Male 7‐week‐old Wistar rats were randomly assigned into four groups including control (C), trained (T), diabetic control (DC) and trained diabetic (TD). At 8 weeks‐of‐age, diabetes was induced by a high‐fat diet and intraperitoneal injection of low‐dose streptozotocin (STZ; 35 mg/kg). Rats in the T and TD groups carried out a 7‐week exercise program on a motorized treadmill (15–20 m/min for 20 min/day for 5 weeks), whereas the C and DC remained sedentary in their cages. Tissues gene expression and protein levels of RBP4 were assessed by using real‐time polymerase chain reaction and western blot, respectively, while serum RBP4 was measured using an enzyme‐linked immunosorbent assay kit.
Results
Exercise significantly improved IR and reduced serum concentration of RBP4 in the TD group. This reduction of serum RBP4 was accompanied by decreased RBP4 protein expression in visceral fat tissue. In contrast, exercise had no significant effect on RBP4 expression in liver and subcutaneous fat tissue in the TD group. Exercise also significantly decreased RBP4 gene expression in visceral fat tissue and muscle, whereas the effect of exercise on liver RBP4 messenger ribonucleic acid expression was not significant.
Conclusions
The present study showed that the mechanism for RBP4 reducing the effect of endurance training could involve decreased RBP4 messenger ribonucleic acid expression and its protein level in adipose tissue in STZ‐induced diabetic rats.
Keywords: Endurance training, Insulin resistance, Retinol‐binding protein 4 gene and protein expression
Introduction
The beneficial effect of exercise training on reduced insulin resistance in streptozotocin (STZ)‐induced diabetes1 could be partly mediated by changing the secretion of adipokines. One of these adipokines is retinol‐binding protein 4 (RBP4), which is suggested to be involved in the development of insulin resistance2. Based on the evidence, increased serum RBP4 concentration reduces insulin‐dependent glucose uptake by muscle tissue through reducing phosphoinositide‐3‐kinase (PI‐3‐kinase) activity and subsequent phosphorylation of the insulin receptor substrate‐1 (IRS‐1), which are necessary components of the insulin signaling pathway2. In contrast, in the liver, RBP4 increases the expression of the enzyme, phosphoenolpyruvate carboxylase (PEPCK), which eventually leads to increased hepatic glucose output that serves to raise blood glucose3. In addition, a negative effect of RBP4 on the secreting function of β‐cells has been suggested6. Some studies consider the impact of exercise on insulin resistance and serum RBP4. The results of these studies showed that the insulin sensitizing effect of exercise was accompanied with reduced RBP4 concentration. A significant decrease of serum RBP4 and an improvement of insulin sensitivity were observed in a wide range of subjects with insulin resistance3. Similar findings have also been observed in obese children after exercise9. However some studies reported that normalization of insulin sensitivity was not mediated by changes in serum RBP4 in an exercised animal model10. Although serum RBP4 seems to be decreased by exercise in most cases, some studies could not find any association between RBP4 and insulin resistance10. Therefore, further investigation of the effect of exercise on insulin resistance and serum RBP4 is still necessary. In addition, the effects of exercise on RBP4 expression in organs, such as the liver and adipose tissue, have not been reported and it is not clear from which tissue decrease in RBP4 expression could contribute to the improvement of serum RBP4 and insulin resistance after endurance training. Thus, in the present study, we investigated whether endurance exercise could change circulating RBP4 in STZ‐induced diabetic rats, and whether these changes would be accompanied by changes in RBP4 messenger gene expression and its protein level in the liver, adipose tissue and muscles. Finally, we investigated in which tissue the degree of changes in RBP4 expression was higher.
Materials and Methods
Animals
Male 5‐week‐old Wistar rats were purchased from the Pasteur Institute (Tehran, Iran), and animals were maintained in an air‐conditioned room (temperature 22 ± 3°C) with a 12‐h light–dark cycle. All rats were fed chow diet and water ad libitum during the 2‐week acclimation period while their bodyweights were monitored daily. At the age of 7 weeks, the rats were randomly assigned to four groups including controls (C), trained (T), diabetic (DC) and trained diabetic (TD). The protocol of this experimental study was approved by the Ethical Committees on Animal Care at the Endocrinology and Metabolism Research Center of Tehran University of Medical Sciences.
Diabetes Induction Method
At the beginning of the rats' eighth week of age, all diabetic rats were fed a high‐fat diet (Table 1) for 2 weeks, and then diabetes was induced by intraperitoneal injection of low‐dose STZ (35 mg/kg) dissolved in citrate buffer (0.01 mol/L, pH 4.5). Blood samples were obtained from the orbital sinus and analyzed for non‐fasting glucose. Blood was collected through a heparinized tube and plasma separation was carried out by centrifuge at 3,000 g for 10 min at 4°C. Plasma glucose concentration was determined by the glucose oxidase method using an autoanalyzer12 (Hitachi, Hitachi, Japan). Animals with non‐fasting plasma glucose level of ≥300 mg/dL were considered diabetic, then housed in a separate cage and fed a high‐fat diet (HFD) for 7 weeks. The rats from the C and T groups were fed a conventional pellet diet.
Table 1. Composition of high fat diet.
| Ingredients | Diet (g/kg) |
|---|---|
| Powdered NPD | 365 |
| Fat | 310 |
| Casein | 250 |
| Cholesterol | 10 |
| Vitamin and mineral mix | 60 |
| DL‐methionine | 03 |
| Yeast powder | 01 |
| Sodium chloride | 01 |
NPD, normal pellet diet.
Training Intervention
Exercise training was started at 10 weeks‐of‐age. Endurance training was carried out every day for 7 weeks. Initially, the T and TD groups were familiarized with a motor‐driven treadmill running at low speeds (15–20 m/min) for 20 min/day for the first 5 days. Thereafter, the duration was increased gradually over the 7‐week period, until the animals were running for 35 min/day at 30 m/min for the last 2 weeks. Electrical shock was not used to force the rats to run. The C and D rats remained sedentary in their cages for the duration of the 7‐week training program.
Collection of Blood and Tissue Samples
At the end of the experimental period, the animals were fasted overnight, but they were allowed to take water. The next morning, animals were anesthetized (ketamin [90 mg/kg] and xylazin [10 mg/kg]), and the soleus and extensor digitorum longus (EDL) muscles, visceral and subcutaneous fat, and liver were excised rapidly, frozen in liquid nitrogen and stored at −80°C for further analysis. Blood samples were withdrawn by heart puncture, plasma or serum separated as aforementioned and stored at −80°C. These samples were used for measurement of fasting blood glucose, fasting plasma insulin, fasting serum RBP4 concentration and plasma lipid profile.
Insulin Resistance Confirmation
Type 2 diabetic rats were selected according to the following insulin resistance criteria: (i) fasting plasma insulin level above 60 pmol/L; and (ii) homeostasis model assessment of insulin resistance >2.513.
Biochemical Measurement
Plasma glucose was determined by the glucose oxidase method12. Serum RBP4 was quantitatively measured by enzyme‐linked immunosorbent assay (cat: RB0642EK; AdipoGen Inc., Seoul, Korea). The sensitivity of the assay was 60 pg/mL, and the intra‐assay and interassay coefficients were lower than 10%. Plasma insulin was measured by mouse/rat insulin enzyme‐linked immunosorbent assay kit (cat: EZRMI‐13K; Millipore, Billerica, MA, USA). The sensitivity of the assay was 0.1 ng/mL, and the intra‐assay and interassay coefficients were lower than 10%. Total cholesterol and triglycerides were determined by standard enzymatic procedures using an autoanalyzer (Hitachi).
Western Blotting
Approximately 150–200 mg of each tissue was powdered with a cold mortar and pestle in liquid nitrogen. Muscle and liver were homogenized in 1 mL of phosphate‐buffered saline (PBS) and centrifuged at 12,000 g for 15 min at 4°C, and supernatant was removed. Fat samples were lysed in Tris buffer (20 mmol/L Tris, 1% NP‐40; 137 mmol/L NaCl; 1 mmol/L CaCl2; 1 mmol/L MgCl2; 10% (v/v) glycerol; 1 mmol/L dithiothretol; 1 mmol/L phenylmethanesulphonyl fluoride; 2 mmol/L Na3VO4). Total protein was determined by the Bradford method using bovine serum albumin (BSA) as a standard. A total of 20 μg total protein of each sample was loaded and separated on 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred by electroblotting onto polyvinylidenedifluoride (PVDF) membranes (Amersham, Pharmacia Biotech, Upsala, Sweden). Membranes were incubated for 1 h at room temperature on an orbital shaker in blocking solution (150 mmol/L NaCl, 0.1% Tween 20 and 50 mmol/L Tris, pH 7.5 [TTBS], 5% skimmed milk) and incubated in primary antibody overnight at 4°C in Tris‐buffered saline. Membranes were washed once for 15 min and then twice for 5 min in TTBS, and then incubated for 90 min at room temperature with secondary antibody in Tris‐buffered saline. Membranes were washed as aforementioned, and protein expression was then detected by enhanced chemiluminescence according to the manufacturer's instructions. Autoradiographic film was exposed to membranes and developed. Molecular weight standards were used to identify appropriate antibody binding. Band densities were determined with image densitometer software. Rat RBP4 protein was used as a positive control and to fix an arbitrary unit to allow comparison between experiments (1 equals the RBP4 signal generated by 5 ng of RBP4 protein).
Real Time Polymerase Chain Reaction
Tissue was powdered with a cold mortar and pestle, and total ribonucleic acid (RNA) was isolated using Isol RNA‐Lysis reagent. Approximately 50 mg of powdered tissue was added to 1 mL ice‐cold Isol and homogenized. Homogenates were centrifuged at 12,000 g for 10 min at 4°C to remove the pellet. Chloroform (200 μL) was added to the supernatant fraction and shaken vigorously for 15 s. The organic and aqueous phases were separated by centrifugation at 12,000 g for 15 min. The aqueous phase was removed and 600 μL isopropanol was added, and RNA was isolated according to the manufacturer's instructions. RNA concentration and purity was estimated by OD 260/280.
Complementary deoxyribonucleic acid (cDNA) synthesis was carried out with 1 μg RNA in a total reaction volume of 20 μL using random hexamer oligonucleotides. Reverse transcriptase reactions were carried out according to the manufacturer's instructions. Quantitative real‐time polymerase chain reaction (PCR) was carried out using a 7300 Real Time PCR System (Applied Biosystems, Step One, Weiterstadt, Germany). The PCR reaction was carried out using Syber Green II, and ROX was used as a reference dye. The concentration of each primer and cDNA were 100 pm and 100 ng, respectively. The thermocycling conditions were: 10 min at 95°C, followed by 40 cycles at 95°C for 15 s and 60°C for 60 s. Table 2 shows the primer sequences used for real‐time PCR.
Table 2. Primer sequences used for real‐time polymerase chain reaction analysis.
| Gene | Forward primer (5′ to 3′) | Reverse primer (5′ to 3′) | Size (bp) |
|---|---|---|---|
| RBP4 | GGTGAGCAGCTTCAGAGTC | ACCATGTCTGCACACACTTC | 204 |
| 18S | GTTGGTTTTCGGAACTGAGGC | GTCGGCATCGTTTATGGTCG | 206 |
Gene expressions were expressed relative to the expression of the 18S housekeeping gene. To avoid detection of non‐specific PCR products, the purity of each amplified product was confirmed using a melting curve analysis. Data quantification was carried out using the 2−∆∆CT method14. Primer amplification efficiencies were determined using serial cDNA dilutions, and were determined to be approximately equal.
Statistical Analysis
All data are shown as means ± standard deviation. The differences were considered to be significant if P ≤ 0.05. Comparisons of variables between study groups were carried out by using one‐way analysis of variance (anova) test. When a significant effect was found, post‐hoc Scheffé analysis was carried out to determine which mean differences were significant. All analysis was carried out using SPSS version 16 (SPSS, Chicago, IL, USA).
Results
Induction of diabetes caused a significant increase in bodyweight, fasting glucose, insulin and homeostasis model assessment index. Diabetes also resulted in increased levels of triglyceride and cholesterol (Figure 1, Table 3). Exercise caused a significant decrease in bodyweight in the TD group. Fasting insulin and glucose were significantly lower in the TD group compared with the D group; however, they were still significantly higher compared with the C group. Lipid profile also significantly decreased in the TD group. The values of lipid profile in the TD group were still much higher than those in the C and T groups (Table 3).
Figure 1.
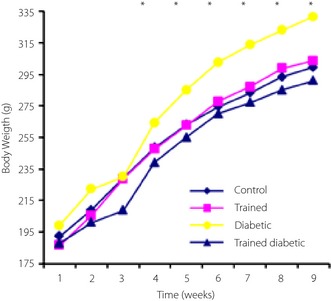
Weekly bodyweight changes during the course of the study among four experimental groups (n = 6 rats in each group)
Table 3. Effect of 7‐week endurance training on bodyweight and biochemical parameter.
| Variable | Control (n = 6) | Trained (n = 6) | Diabetic control (n = 6) | Trained diabetic (n = 6) |
|---|---|---|---|---|
| Weight (g) | 299.79 ± 30.81* | 303.82 ± 26.06* | 331.7 ± 18.14 | 291.17 ± 25.21* |
| Amount of food intake (g/kg weight) | 16.8 ± 2.5 | 14.2 ± 3.1 | 13.7 ± 2.7 | 13.8 ± 3.1 |
| Fasting glucose (mg/dL) | 106.4 ± 9.26 | 101.62 ± 9.02* | 394.44 ± 48.74 | 301.7 ± 47.95*,† |
| Fasting insulin (nmol/L) | 6.25 ± 1.15 | 5.73 ± 0.68 | 10.55 ± 1.12 | 8.95 ± 0.78*,† |
| HOMA‐IR index | 1.62 ± 0.24* | 1.45 ± 0.19* | 10.48 ± 1.44 | 6.68 ± 1.31*,† |
| Triglyceride (mg/dL) | 33.8 ± 4.4* | 35.7 ± 7.1* | 114.6 ± 12.4 | 46.14 ± 5.1* |
| Cholesterol (mg/dL) | 48.4 ± 5.3* | 43.5 ± 6.07* | 108.3 ± 10.1 | 47.6 ± 9.1* |
Values presented as mean ± standard deviation. *Significant differences with diabetic control. †Significant difference with control. HOMA‐IR, homeostasis model assessment of insulin resistance.
Change of Serum Concentration of RBP4 and RBP4 Protein Expression
Diabetes induced a significant increase in serum level of RBP4 (almost a threefold increase in the DC group compared with the C group). RBP4 expression in the liver, adipose tissues and muscle were significantly higher in the DC group than the other groups. Among them, the liver showed the highest increase in RBP4 expression (almost a 3.5‐fold increase in DC compared with the C group; Table 4). Exercise led to a reduction in serum concentration of RBP4 in the T group. However, a significantly higher reduction occurred in the TD group (Figure 2).
Table 4. Effect of 7‐week endurance training on retinol‐binding protein 4 protein and gene expression in different tissues.
| Control (n = 6) | Trained (n = 6) | Diabetic control (n = 6) | Trained diabetic (n = 6) | DC vs C (P) | DC vs TD (P) | |
|---|---|---|---|---|---|---|
| Serum RBP4 (ng/mL) | 3.52 ± 0.34 | 3.23 ± 0.27 | 10.22 ± 1.02 | 6.49 ± 0.69 | 0.000 | 0.000 |
| Liver RBP4 | 2.43 ± 0.37 | 2.47 ± 0.23 | 8.38 ± 0.83 | 7.91 ± 0.67 | 0.000 | 0.59 |
| Visceral fat RBP4 | 1.53 ± 0.32 | 1.44 ± 0.19 | 3.10 ± 0.34 | 2.50 ± 0.27 | 0.000 | 0.015 |
| SC fat RBP4 | 1.35 ± 0.18 | 1.24 ± 0.15 | 2.47 ± 0.21 | 2.35 ± 0.34 | 0.000 | 0.85 |
| EDL RBP4 | 0.41 ± 0.09 | 0.36 ± 0.06 | 0.93 ± 0.15 | 0.88 ± 0.21 | 0.000 | 0.9 |
| Liver RBP4 mRNA | 1.00 | 0.86 ± 0.30 | 2.33 ± 0.56 | 2.27 ± 0.41 | 0.000 | 0.10 |
| Visceral fat RBP4 mRNA | 1.00 | 0.69 ± 0.19 | 2.37 ± 0.53 | 1.31 ± 0.25 | 0.000 | 0.000 |
| SC fat RBP4 mRNA | 1.00 | 0.92 ± 0.41 | 1.83 ± 0.45 | 1.14 ± 0.31 | 0.005 | 0.02 |
| EDL RBP4 mRNA | 1.00 | 0.64 ± 0.13 | 2.04 ± 0.53 | 1.34 ± 0.45 | 0.000 | 0.000 |
Values presented in mean ± standard deviation. P‐value represents significant level between groups differences based on anova with post‐hoc Scheffé's test. C, control; DC, diabetic control; EDL, extensor digitorum longus; mRNA, messenger ribonucleic acid; RBP4, retinol‐binding protein 4; SC fat, subcutaneous fat; TD, trained diabetic.
Figure 2.
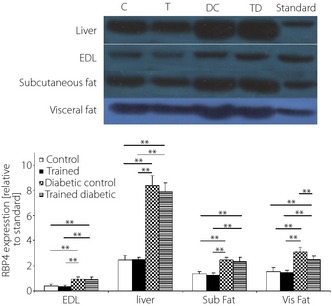
The effect of 7‐week endurance training on retinol‐binding protein 4 (RBP4) protein expression (relative to standard) determined by western immune blot in different tissues including muscle (extensor digitorum longus [EDL]), liver, subcutaneous fat (subfat) and visceral fat (visfat) at the end of the study. Data are presented for each group as means ± standard deviation (n = 6 rats in each group). *P < 0.05, **P < 0.01.
Exercise also significantly decreased RBP4 protein expression in visceral fat tissue in the TD group compared with the diabetic group. However, these reductions did not reach the levels of the C group. In contrast, exercise had no significant effect on RBP4 expression in the liver, muscle and subcutaneous fat tissue in the TD group (Table 4).
RBP4 Gene Expression
Diabetes induced RBP4 gene expression in adipose tissue, the liver and muscle. The highest increase was observed in visceral fat and the liver, respectively, whereas the muscle showed the lowest reduction (Figure 3). These findings were consistent with RBP4 expression in the liver, fat tissues and muscle.
Figure 3.
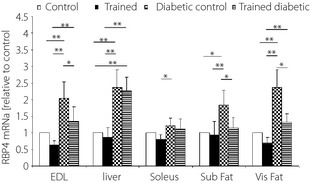
The effect of 7‐week endurance training on retinol‐binding protein 4 (RBP4) messenger ribonucleic acid expression (relative to control) determined by real‐time polymerase chain reaction in different tissues including muscles, liver, subcutaneous fat (subfat) and visceral fat (visfat) at the end of the study. Values are means ± standard deviation (n = 6 rats in each group). *P < 0.05, **P < 0.01.
Exercise significantly decreased RBP4 gene expression in fat tissues, whereas the highest change was observed in visceral fat. Exercise also induced a significant change in muscle RBP4 expression. However the effect of exercise on liver RBP4 mRNA expression was not significant (Figure 3).
Discussion
In the present study, we tried to answer the question: “reduction in which of the three tissues, liver, adipose tissue or muscle RBP4 expression” will be accompanied with a decrease in serum RBP4 concentration in response to endurance training in type 2 diabetic rats?”
In addition, simultaneous measurement of RBP4 expression in the three aforementioned tissues was carried out to investigate which tissue is a predominant source of serum RBP4 in the insulin resistant state.
It is notable that this is the first report showing the differential expression pattern of RBP4 between adipose tissue, liver and muscle in response to exercise training.
In the present study, the rat model for type 2 diabetes was developed by combining a HFD, which produced insulin resistance, and a low dose of STZ injection, which caused the initial β‐cell dysfunction. This kind of rat model could closely simulate the metabolic disturbances that occur in type 2 diabetes15. Thus, this model could be suitable for evaluating the effect of endurance exercise (which acts through modifying insulin resistance and serum RBP4) on mRNA RBP4 and its protein expression in different tissues. In addition, the fat we used to feed the rats in this experiment was tallow, which mainly consists of saturated (43%), monounsaturated fatty acids (50%) and polyunsaturated fatty acids (5%)16. The present findings showed that induction of diabetes caused a significant increase in bodyweight. In contrast, endurance training normalized bodyweight and improved lipid profile. We believe improvement of mitochondrial function and increased energy expenditure in different tissues is one of the mechanisms17 by which the 7‐week endurance exercise caused these changes in trained diabetic rats.
We found that induction of diabetes significantly enhanced insulin resistance and serum RBP4. These increases are accompanied with increased RBP4 expression at both a transcriptional and translational level in the liver, adipose tissues and muscle. The increased serum level of RBP4 in diabetic rats might be a consequence of its increased mRNA and protein expression in both the liver and adipose tissues, with the highest expression level in the liver followed by the second rate in the adipose tissues. Thus, in the present study, increased circulating RBP4 (10.21 in DC vs 3.52 in C, P < 0.000) could be mediated by increased RBP4 mRNA expression and its protein level mainly in the liver (Table 2).
This finding is supported by a study that reported RBP4 is predominantly expressed in the liver in rodents and rats19. However, our findings, which showed that adipose tissue is not the main organ to produce RBP4 in an insulin resistant state, are inconsistent with those showing that visceral fat is the major source of RBP4 production in an insulin resistant state and type 2 diabetes2.
One possible explanation could be related to the rat model we developed in the present study. Although this diabetic model was closely similar to the metabolic characteristics of type 2 diabetes, the question remains as to whether the RBP4 tissue expression pattern in an insulin resistant state as a result of induction of diabetes is still completely similar to real type 2 diabetes. Endurance exercise significantly improved insulin resistance, and this reduction was accompanied by dramatically reduced RBP4 in the circulation in diabetic trained rats compared with diabetic rats (10.22 vs 6.49, P < 0.000). These results support previous findings reporting interventions as the cause for reduction in serum RBP42, and suggesting that a decrease of serum RBP4 could be one of the mechanisms underlying the improvement of insulin resistance with exercise24. However, apart from RBP4, exercise also changed the serum level of other adipokines, such as adiponectin and leptin, which influence insulin resistance24. Perhaps measuring these adipokines in comparison with RBP4 would have given us more information in this regard. Therefore, the findings presented here should be considered with this limitation.
The most important and novel finding of the present study was that the 7‐week exercise resulted in a significant reduction in RBP4 expression in visceral adipose tissue of HFD–STZ induced diabetic rats. This reduction was accompanied by a parallel decrease in RBP4 expression in this tissue and serum RBP4 concentration. In contrast, endurance training was unable to change RBP4 mRNA level and its protein expression in the liver of diabetic rats. Thus, the effect of exercise on RBP4 protein expression was specific to visceral fat tissue and did not occur in the liver. These findings suggest that decreased adipose tissue RBP4 expression after endurance training might cause a reduction in serum RBP4, which in turn might cause a decrease in insulin resistance through different reported pathways24. It could be speculated that one mechanism by which endurance exercise reduced RBP4 expression in visceral fat tissue might be related to the reduced adipocyte size and fat mass, which was not measured in the present study. Consistent with our suggestion, previous studies showed that weight loss and exercise led to reduced adipocyte size, which in turn is an important determinant for the secretion of several adipokines25.
As the liver is the major tissue to produce and secrete RBP4, it was expected that endurance training should decrease liver RBP4 expression. However, this effect was not observed in the present study. One possible explanation for this finding is that the time and duration of the exercise session might not have been sufficient to induce significant changes in liver RBP4 protein expression. Based on the literature, the training effect is tissue specific, and any adaptive response to exercise training depends on the degree of involvement of tissue during exercise. As the liver is an inactive tissue during exercise, its involvement in exercise is limited to long duration workouts, and takes over 90 min to produce energy through the gluconeogenesis process. Because our exercise session took 40 min, the liver might not have been influenced. In contrast, visceral tissue is very active during this endurance exercise, in which the major substrate for energy supply is free fatty acid with two primary sources of visceral and subcutaneous fat tissues28. Furthermore, recent reports have shown that elevated serum RBP4 is closely associated with fatty liver29. Increased plasma RBP4 level might be a consequence of fat accumulation in the liver as a result of the induction of diabetes, and continuation of a HFD in diabetic rats and this period of exercise is not enough to reverse this metabolic disturbance. Our suggestion was supported by Vieira et al.31 who showed that just 12 weeks of exercise could completely eliminate hepatic esteatosis regardless of continuing the HFD. Thus, it might be possible that changes in liver RBP4 expression might present themselves during a longer exercise period. In contrast to diabetic rats, training did not change serum RBP4 concentration in trained rats. In other words, we showed that RBP4 levels were decreased after exercise only in the rats with a higher basic level of RBP4. This finding suggests that the influence of exercise on serum RBP4 concentration is dependent on its initial levels. It is conceivable that the basal RBP4 level in trained diabetic rats, but not in trained animals, might be high enough to be changed by 7 weeks of exercise training. This is also supported by other studies that showed that the effect of exercise seems to be more evident in those with high initial RBP42. Along with this evidence, in a previous study we also showed that the basic level of RBP4 was the only predictor of the after‐exercise plasma RBP4 concentration33.
Although our 7‐week endurance training significantly improved insulin resistance and reduced serum RBP4, it did not fully reverse them. In addition, mRNA RBP4 and its protein level in adipose tissue of trained diabetic rats did not return to the level of control rats. We suggest that the protective effect of exercise on adipose tissue RBP4 expression and serum RBP4 might be mediated through a reduction in visceral fat or reduction in adipose tissue inflammation. It has been reported that serum RBP4 was significantly correlated with visceral fat mass and visceral adipose RBP4 expression34. In addition, a decrease in serum RBP4 level achieved by exercise training predicts the improvement in insulin sensitivity35. Thus, the degree of reduction of body fat mass and adiposity, which was not measured in the present study, might be important in this regard. We assumed that the second mechanism by which exercise reduces adipose tissue RBP4 expression and serum RBP4 might involve improvement in the inflammation induced by diabetes and a HFD. This assumption was supported by evidence that showed a close connection between RBP4 expression and its protein level, and inflammatory markers36.
In addition, recent studies confirmed that the period of exercise intervention is a determinant factor in reducing inflammatory markers expression induced by a long‐term HFD10. We suggest that under the condition of a HFD, the impact of endurance training on RBP4 expression was moderate. Thus, a longer period of exercise program might be necessary to completely reverse the effect of continued consumption of HFD and induction of diabetes on mRNA RBP4 expression and its protein level in visceral fat tissue of diabetic mice in this experiment. Training intervention also resulted in a significant decrease in RBP4 mRNA level in subcutaneous fat tissue and muscle. However, the degree of changes in RBP4 mRNA expression was much higher in visceral fat than other tissues in the trained diabetic group. In this regard, the reduction in RBP4 mRNA in subcutaneous fat tissue and muscle was not reflected at the RBP4 protein level in these tissues. In other words, exercise induces muscle and subcutaneous tissues to decrease RBP4 mRNA expression without a decrease in its protein level. Several unknown regulatory mechanisms might be a reason for affecting the post‐transcriptional of RBP443. Furthermore, according to previous reports, gene expression levels do not necessarily correspond to protein expression44. Finally, the current study showed that 7‐week endurance training was able to reduce insulin resistance and serum RBP4, confirming its potential effect on RBP4 mRNA and protein expression in visceral fat tissue.
In conclusion, we found that the RBP4‐reducing effect of endurance training is predominantly mediated by reduction of RBP4 mRNA expression and its protein level in adipose tissue in diabetic rats induced by HFD and streptozotocin. In fact, the beneficial effect of 7‐week endurance training on insulin resistance and serum RBP4 might not be reflected by changes in RBP4 mRNA expression and its protein level in the liver.
Acknowledgements
The present study was supported by Endocrinology and Metabolism Research Center of Tehran University of Medical Sciences. We have no relevant financial or non‐financial relationship to disclose.
J Diabetes Invest 2014; 5: 484–491
References
- 1.Dall'Aglio E, Chang F, Chang H, et al Effect of exercise training and sucrose feeding on insulin‐stimulated glucose uptake in rats with streptozotocin‐induced insulin‐deficient diabetes. Diabetes 1983; 32: 165–168 [DOI] [PubMed] [Google Scholar]
- 2.Yang Q, Graham TE, Mody N, et al Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature 2005; 436: 356–362 [DOI] [PubMed] [Google Scholar]
- 3.Graham TE, Yang Q, Bluher M, et al Retinol‐binding protein 4 and insulin resistance in lean, obese, and diabetic subjects. N Engl J Med 2006; 354: 2552–2563 [DOI] [PubMed] [Google Scholar]
- 4.Takebayashi K, Suetsugu M, Wakabayashi S, et al Retinol binding protein‐4 levels and clinical features of type 2 diabetes patients. J Clin Endocrinol Metab 2007; 92: 2712–2719 [DOI] [PubMed] [Google Scholar]
- 5.Tan BK, Chen J, Lehnert H, et al Raised serum, adipocyte, and adipose tissue retinol‐binding protein 4 in overweight women with polycystic ovary syndrome: effects of gonadal and adrenal steroids. J Clin Endocrinol Metab 2007; 92: 2764–2772 [DOI] [PubMed] [Google Scholar]
- 6.Broch M, Vendrell J, Ricart W, et al Circulating retinol binding protein 4, insulin sensitivity, insulin secretion and insulin disposition index in obese and nonobese subjects. Diabetes Care 2007; 30: 1802–1806 [DOI] [PubMed] [Google Scholar]
- 7.Maghbooli Z, Hossein‐Nezhad A, Mirzaei K, et al Association between retinol‐binding protein 4 concentrations and gestational diabetes mellitus and risk of developing metabolic syndrome after pregnancy. Reprod Sci 2010; 17: 196–201 [DOI] [PubMed] [Google Scholar]
- 8.Craig RL, Chu WS, Elbein SC. Retinol binding protein 4 as a candidate gene for type 2 diabetes and prediabetic intermediate traits. Mol Genet Metab 2007; 90: 338–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Balagopal P, Graham TE, Kahn BB, et al Reduction of elevated serum retinol binding protein in obese children by lifestyle intervention: association with subclinical inflammation. J Clin Endocrinol Metab 2007; 92: 1971–1974 [DOI] [PubMed] [Google Scholar]
- 10.Bradley RL, Jeon JY, Liu FF, et al Voluntary exercise improves insulin sensitivity and adipose tissue inflammation in diet‐induced obese mice. Am J Physiol Endocrinol Metab 2008; 295: E586–E594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choi KM, Kim TN, Yoo HJ, et al Effect of exercise training on A_FABP, Lipocalin‐2 and RBP4 levels in obese women. Clin Endocrinol 2009; 70: 569–574 [DOI] [PubMed] [Google Scholar]
- 12.Wincey C, Marks V. A micro‐method for measuring glucose using the autoanalyzer and glucose‐oxidase. J Clin Pathol 1961; 14: 558–559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sharma AK, Srinivasan BP. Triple verses glimepiride plus metformin therapy on cardiovascular risk biomarkers and diabetic cardiomyopathy in insulin resistance type 2 diabetes mellitus rats. Eur J Pharm Sci 2009; 38: 433–444 [DOI] [PubMed] [Google Scholar]
- 14.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real‐time quantitative PCR and the 2‐ Delta C (T) method. Methods 2001; 25: 402–408 [DOI] [PubMed] [Google Scholar]
- 15.Srinivasan K, Viswanad KB, Lydia A, et al Combination of high‐fat diet‐fed and low‐dose streptozotocin‐treated rat: a model for type 2 diabetes and pharmacological screening. Pharmacol Res 2005; 52: 313–320 [DOI] [PubMed] [Google Scholar]
- 16.Nikooie R, Rajabi H, Gharakhanlu R, et al Exercise‐induced changes of MCT1 in cardiac and skeletal muscles of diabetic rats induced by high‐fat diet and STZ. J Physiol Biochem 2013; 69: 865–877 [DOI] [PubMed] [Google Scholar]
- 17.Henriksen EJ. Invited review: effects of acute exercise and exercise training on insulin resistance. J Appl Physiol 2002; 93: 788–796 [DOI] [PubMed] [Google Scholar]
- 18.Kadoglou NP, Iliadis F, Angelopoulou N, et al The anti‐inflammatory effects of exercise training in patients with type 2 diabetes mellitus. Eur J Cardiovasc Prev Rehabil 2007; 14: 837–843 [DOI] [PubMed] [Google Scholar]
- 19.Blander WS. Retinol‐binding protein: the serum transport protein for vitamin A. Endocr Rev 1989; 10: 308–316 [DOI] [PubMed] [Google Scholar]
- 20.Cho YM, Youn BS, Lee H, et al Plasma retinol‐binding protein‐4 concentrations are elevated in human subjects with impaired glucose tolerance and type 2 diabetes. Diabetes Care 2006; 29: 2457–2461 [DOI] [PubMed] [Google Scholar]
- 21.Lee DC, Lee JW, Im JA. Association of serum retinol biding protein 4 and insulin resistance in apparently healthy adolescnts. Metabolism 2007; 56: 327–331 [DOI] [PubMed] [Google Scholar]
- 22.Shepherd PR, Gnudi L, Tozzo E, et al Adipose cell hyperplasia and enhanced glucose disposal in transgenic mice overexpressing GLUT4 selectively in adipose tissue. J Biol Chem 1993; 268: 22243–22246 [PubMed] [Google Scholar]
- 23.Lim S, Choi SH, Jeong IK, et al Insulin‐Sensitizing Effects of Exercise on Adiponectin and Retinol‐Binding Protein‐4 Concentrations in Young and Middle‐Aged Women. J Clin Endocrinol Metab 2008; 93: 2263–2268 [DOI] [PubMed] [Google Scholar]
- 24.Takebayashi K, Aso Y, Inukai T. Role of retinol‐ binding protein 4 in the pathogenesis of type 2 diabetes. Endocrinol Metab 2008; 3: 1–13 [DOI] [PubMed] [Google Scholar]
- 25.Bluher M, Wilson‐Fritch L, Lesyzk J, et al Role of insulin action and cell size on protein expression patterns in adipocytes. J Biol Chem 2004; 279: 31902–31909 [DOI] [PubMed] [Google Scholar]
- 26.Skurk T, Alberti‐Huber C, Herder C, et al Relationship between adipocyte size and adipokines expression and secretion. J Clin Endocrinol Metab 2007; 92: 1023–1033 [DOI] [PubMed] [Google Scholar]
- 27.Katch FI, Clarkson PM, Kroll W, et al Effect of sit up exercise training on adipose cell size and adiposity. Res Q Exerc Sport 1984; 55: 242–247 [Google Scholar]
- 28.Michael N, Suzanne M, Douglas R, et al ACSMs advance exercise physiology, American collage of sport medicine, 2nd edn. Lippincott. Williams & Wilkins, Philadelphia,, 2011 [Google Scholar]
- 29.Seo JA, Kim NH, Park SY, et al Serum retinol‐binding 4 levels are elevated in non‐alcoholic fatty liver disease. Clin Endocrinol 2008; 68: 555–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu H, Jia W, Bao Y, et al Serum retinol binding protein 4 and nonalcoholic fatty liver disease in patients with type 2 diabetes mellitus. Diabetes Res Clin Pract 2008; 79: 2. [DOI] [PubMed] [Google Scholar]
- 31.Vieira VJ, Valentine RJ, Wilund KR, et al Effects of exercise and low‐fat diet on adipose tissue inflammation and metabolic complications in obese mice. Am J Physiol 2009; 296: E1164–E1171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ku YH, Han KA, Ahn H, et al Resistance exercise did not alter intramuscular adipose tissue but reduced retinol‐binding protein‐4 concentration in individuals with type 2 diabetes mellitus. J Int Med Res 2010; 38: 782–791 [DOI] [PubMed] [Google Scholar]
- 33.Mansouri M, Keshtkar A, Hasani‐Ranjbar S, et al The impact of one session resistance exercise on plasma adiponectin and RBP4 concentration in trained and untrained healthy young men. Endocr J 2011; 58: 861–868 e [DOI] [PubMed] [Google Scholar]
- 34.Kloting N, Graham TE, Berndt J, et al Serum retinol‐binding protein is more highly expressed in visceral than in subcutaneous adipose tissue and is a marker of intra‐abdominal fat mass. Cell Metab 2007; 6: 79–87 [DOI] [PubMed] [Google Scholar]
- 35.Kotnik P, Fischer‐Posovszky P, Wabitsch M. RBP4 a controversial adipokines. Eur J Endocrinol 2011; 165: 703–711 [DOI] [PubMed] [Google Scholar]
- 36.Park HS, Park JY, Yu R. Relationship of obesity and visceral adiposity with serum concentrations of CRP, TNF‐alpha and IL‐6. Diabetes Res Clin Pract 2005; 69: 29–35 [DOI] [PubMed] [Google Scholar]
- 37.Tilg H, Moschen AR. Inflammatory mechanisms in the regulation of insulin resistance. Mol Med 2008; 14: 222–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vieira VJ, Valentine RJ, Wilund KR, et al Effects of diet and exercise on metabolic disturbances in high‐fat diet‐fed mice. Cytokine 2009; 46: 339–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Friebe D, Neef M, Erbs S, et al Retinol binding protein 4 (RBP4) is primarily associated with adipose tissue mass in children. Int J Pediatr Obes 2010; 6: 346–352 [DOI] [PubMed] [Google Scholar]
- 40.Wellen KE, Hotamisligil GS. Obesity‐induced inflammatory changes in adipose tissue. J Clin Invest 2003; 112: 1785–1788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yao‐Borengasser A, Varma V, Bodles AM, et al Retinol binding protein 4 expression in humans: relationship to insulin resistance, inflammation, and response to pioglitazone. J Clin Endocrinol Metab 2007; 92: 2590–2597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kawanishi N, Yano H, Yokogawa Y, et al Exercise training inhibits inflammation in adipose tissue via both suppression of macrophage infiltration and acceleration of phenotypic switching from M1 to M2 macrophages in highfat‐diet‐induced obesemice. Exerc Immunol Rev 2010; 16: 105–118 [PubMed] [Google Scholar]
- 43.Ping F, Xiang HD, Li M, et al Effects of variation in retinol binding protein 4 gene and adipose specific expression of gestational diabetes in Beijing, China. Diabetes Res Clin Pract 2012; 97: 283–289 [DOI] [PubMed] [Google Scholar]
- 44.Baynard T, Vieira‐Potter VJ, Valentine RJ, et al Exercise training effects on inflammatory gene expression in white adipose tissue of young mice. Mediators Inflamm 2012; 2012: 767953. [DOI] [PMC free article] [PubMed] [Google Scholar]


