Abstract
Aims/Introduction
We assessed the tolerability, effectiveness and predictive parameters for the therapeutic usefulness of exenatide in obese Korean participants with type 2 diabetes. We also evaluated the characteristics of participants who respond adequately to glucagon‐like peptide‐1 (GLP‐1) analog therapy in terms of glycated hemoglobin (HbA1c) level reductions and weight loss.
Materials and Methods
This prospective, observational, single‐arm (exenatide b.i.d. in combination with both metformin and sulphonylurea), open‐label study of GLP‐1 analog treatment with close monitoring of metabolic parameters and weight changes was carried out for up to 22 weeks.
Results
Of the 110 enrolled obese participants, 37 participants dropped out during the 22‐week treatment period. A total of 73 participants completed the study (median age 55.0 years, interquartile range 44.0–65.0). The median duration of diabetes was 8.0 years (interquartile range 3.5–12.5) with a mean HbA1c value of 7.6% (interquartile range 7.00–8.55). The median body mass index was 30.78 kg/m2 (interquartile range 27.89–33.92). After 22 weeks, median changes from baseline for HbA1c levels and weight were −0.7% and −3.0 kg, respectively, which were significant. No severe hypoglycemic events were observed. Multivariate regression analysis showed that C‐peptide values were a significant independent predictor for a reduction in HbA1c levels (β = 0.865, P = 0.018) with exenatide BID in combination with both sulphonylurea and metformin in obese Korean participants with type 2 diabetes and insulin naïveté.
Conclusions
Clinical and laboratory parameters, such as insulin naïveté and preserved β‐cell function, should be taken into consideration as important factors in the choice of GLP‐1 analog when predicting GLP‐1 analog responsiveness. This trial was registered with the local committee at Yonsei University in Korea (4‐2011‐0032).
Keywords: Exenatide, C‐peptide, Type 2 Diabetes
Introduction
Under normal glucose or type 2 diabetes conditions, glucose status is mainly influenced by the pathophysiological nature of dysfunctional pancreatic β‐cells and increased peripheral insulin resistance1. Because insulin resistance is significantly associated with obesity, the risks of developing and aggravating diabetes are in parallel to the degree of excess bodyweight3. Until the advent of incretin‐based therapy, metformin had been the only glucose‐lowering drug that could bring about weight loss in diabetic patients. Glucagon‐like peptide‐1 (GLP‐1) analog, which has shown plenty of evidence of reducing HbA1c levels and bodyweight, has been gaining popularity for its potential pleiotropic effects on cardiovascular disorders, as well as for treatment of type 2 diabetes by modulating multiple gluco‐regulatory actions, such as increasing insulin secretion in response to glucose levels, suppressing hepatic glucagon action, increasing satiety and so on4. Despite this beneficial evidence, GLP‐1 analog has the inherent problems of needle injection and gastrointestinal adverse effects, such as nausea, vomiting and diarrhoea. In this regard, balancing the beneficial and adverse effects of GLP‐1 analog might be essential for the practical prescription of GLP‐1 analog in patients with type 2 diabetes.
However, clinical reports on the clinical efficacy and adverse effects of GLP‐1 analog in the Korean population are greatly lacking. Furthermore, there is no report regarding the characteristics of patients who respond adequately to GLP‐1 analog therapy in terms of glycated hemoglobin (HbA1c) level reductions and weight loss. Therefore, we analyzed the clinical and metabolic parameters in obese type 2 diabetic patients to characterize patients who are more likely to achieve not only glycaemic reduction, but also weight loss.
Methods
The present prospective, observational, single‐arm (exenatide b.i.d. in combination with both metformin and sulphonylurea [SU]), open‐label study of GLP‐1 analog treatment with close monitoring of metabolic parameters and weight changes was carried out for up to 22 weeks. The study protocol was reviewed by the local ethics committees (4‐2011‐0032). All of the participants provided written informed consent, and the study was carried out in accordance with the principles of the Declaration of Helsinki. Inclusion criteria were: patients with type 2 diabetes who were obese (>25 kg/m2) aged 25–75 years with inadequate glycemic control (defined as HbA1c ≥6.5% and ≤10%) on any oral hypoglycemic agents or insulin analogs treatment. We included patients with unchanged treatment regimen for at least 6 months before starting the study. Patients were excluded if they had a recent (≤6 months) significant history of cardiovascular events, including myocardial infarction, unstable angina, moderate to severe congestive heart failure and/or stroke. In addition, patients with a significant history or the presence of hepatic, renal, hematological or gastrointestinal disease, or treatment with a systemic corticosteroid in the past 12 weeks were excluded. Patients received exenatide 5 μg b.i.d. for the first 4 weeks, followed by 10 μg b.i.d. for the remaining 18 weeks while continuing their pre‐study metformin and SU dosages; however, previous pioglitazone, α‐glucosidase inhibitor, dipeptidyl peptidase 4 (DPP‐4) inhibitor and insulin regimens were discontinued. When exenatide was initiated, we changed the regimens to follow the Korean medical insurance reimbursement guideline as described in Methods S1 (Drug Switching Protocol). Clinic visits initially occurred at 8‐week intervals, and then at 14‐week intervals for the remainder of the study. If HbA1c levels were at or above 7.5% at the first visit after study enrolment, the SU dosage was escalated up to 8 mg of glimepiride and 120 mg gliclazide‐modified release. Laboratory measurement and homeostasis model assessment (HOMA) index calculation was followed as described in Methods S1 (Laboratory Measurements).
Effectiveness Assessments
The primary end‐point was the change in HbA1c levels and bodyweight from baseline to week 8 and week 22. Other end‐points included fasting plasma glucose (FPG) and fasting lipid concentrations (low‐density lipoprotein cholesterol, high‐density lipoprotein cholesterol, total cholesterol and triglycerides). HbA1c responders (marked as HbA1c[+]) were defined as those who showed negative delta HbA1c values, and weight responders (marked as Wt[+]) were defined as those who showed negative delta bodyweight over 22 weeks of exenatide treatment. Participants who completed the study were classified into three groups according to their reduction in HbA1c level and weight: reduction in both HbA1c level and weight (group I), only HbA1c level reduction (group II) and only weight loss (group III).
Tolerability and Hypoglycemia Assessments
Reasons for discontinuation of the study and treatment‐induced adverse events were recorded to assess tolerability and compliance. Monitoring of adverse events, including hypoglycemia, was surveyed by clinical coordination of the Lilly Call Center once a week for 8 weeks, and was voluntarily reported by the participants. In addition, the endocrinologists carried out surveys about the patients’ conditions in outpatient clinics during the scheduled visits. A hypoglycemic event was defined as a major episode that required treatment or admission (defined by blood glucose ≤60 mg/dL accompanied by neurological symptoms consistent with hypoglycemia or an episode requiring intervention with intravenous glucose).
Statistical Analysis
All statistical analyses were carried out with pasw statistics software (version 18.0; spss Inc., Chicago, IL, USA). Continuous variables with non‐normal distributions were expressed as medians (interquartile range [IQR]). Discrete variables were expressed as percentages using the χ2‐test. Statistical comparisons among groups based on HbA1c level and weight reduction were carried out using the Kruskal–Wallis test. We carried out Friedman's test to detect differences in repeatedly measured variables. Spearman's correlation coefficient was used to determine the relationship between HbA1c level and bodyweight changes, and the continuous variables. Multivariate regression analysis was used to estimate multiple correlations between HbA1c levels or weight changes, and clinical and laboratory risk factors. A variance inflation factor >10 suggests an erroneous model, and was not included in the models. Data with a P‐value <0.05 were considered significant.
Results
Baseline Clinical and Laboratory Participant Characteristics and Tolerability of Exenatide
Of the total of 110 intent‐to‐treat (ITT) participants who received more than a one‐time injection of exenatide b.i.d., 73 participants completed and 37 participants failed to complete the 22‐week study (Figure 1). Among the 37 participants who dropped out of the study, 10 and 25 participants discontinued the exenatide within 4 weeks and 5–8 weeks, respectively. After the first follow‐up visit, two participants additionally dropped out due to incompatibility with their time schedule or laboratory examinations. The most frequent reasons for discontinuation were: gastrointestinal adverse effects (AEs), such as nausea, vomiting and diarrhoea (12 participants [36.4%]); reluctance to have injections (7 participants [21.2%]); weight gain (2 participants [6.1%]); and uncontrolled hyperglycaemia (1 participant [3.0%]). Nine participants (27.3%) did not provide a reason for discontinuing the study. Table S1 shows the clinical and laboratory data of the 73 participants who completed the study (22 men and 51 women) and 37 participants who failed to complete the study (15 men and 22 women). The median age and duration of diabetes of those who completed the study were 55.0 years (IQR 44.0–65.0 years) and 8.0 years (3.5–12.5 years), respectively. Body mass index (BMI) was 30.78 kg/m2 (27.89–33.92). Initial levels of HbA1c, glycated albumin and fasting glucose were 7.6% (7.00–8.55%), 17.9% (14.9–23.0%) and 125.0 mg/dL (109–168 mg/dL), respectively. A total of 38 participants (52.05%) used insulin analogs. BMI (30.8 vs 29.0 kg/m2, P = 0.03) and bodyweight (80.0 vs 73 kg, P = 0.026) were statistically different between the groups who completed or withdrew from the study. In the multivariate logistic regression analysis investigating the predictive parameters for the discontinuation of exenatide therapy, no parameter predicted the discontinuation of exenatide therapy in the present study population.
Figure 1.
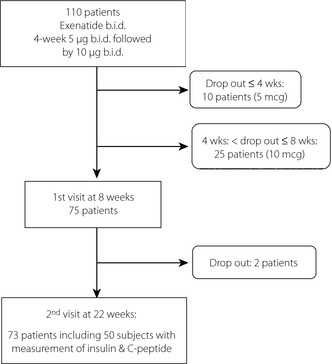
Flow diagrams showing the eligibility of participants under exenatide treatment.
Effects of Exenatide on Glycemia, Weight, Lipid Changes and Events of Major Hypoglycemia
A significant reduction in HbA1c level was observed over 22 weeks of exenatide b.i.d. treatment (Figure 2). The median changes in HbA1c from baseline were –0.4% at week 8 and −0.7% at week 22 (both P = 0.001, baseline vs all other time‐points). Overall, the HbA1c target of ≤7% was achieved in 53.4% (39/73) of participants. In addition, significant weight loss was also observed. The median changes in weight from baseline were −2.3 kg at week 8 and −3.0 kg at week 22 (P < 0.001, baseline vs all other time‐points). Total cholesterol levels decreased significantly (P < 0.001). No major hypoglycemic episodes as defined in the Methods section occurred during the study period.
Figure 2.
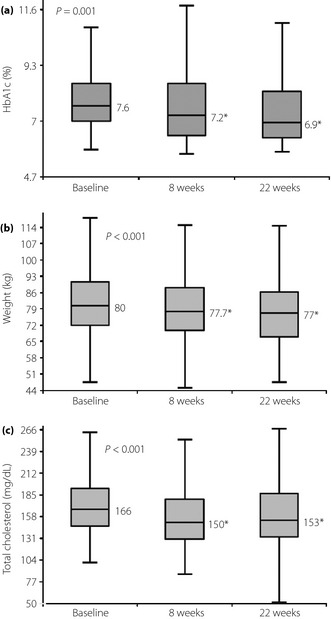
Changes in (a) glycated hemoglobin (HbA1c) level, (b) weight and (c) total cholesterol levels over 22 weeks of exenatide treatment. *P < 0.05 versus baseline.
Improvements of 52.1, 16.4, and 31.5% were observed in both HbA1c reduction and weight loss (group I), HbA1c only (group II), and weight loss only (group III), respectively, in these populations (Table 1, Figure 3). Except for use of insulin (36.8, 41.7, and 82.6% in groups I, II, and III, respectively, P = 0.002) and levels of fasting glucose (127.0, 138.0, and 109.0 mg/dL, respectively, P = 0.037) before study enrolment, there were no significant differences among the groups.
Table 1. Changes in anthropometric and biochemical parameters of the study population over 22 weeks of treatment with a exenatide, metformin, sulfonylurea combination.
| Characteristics | Response at 22 weeks later | P | |||||
|---|---|---|---|---|---|---|---|
| Group I | Group II | Group III | |||||
| HbA1c (+), Wt (+), n = 38 | HbA1c (+), Wt (−), n = 12 | HbA1c (−), Wt (+), n = 23 | |||||
| Median | IQR | Median | IQR | Median | IQR | ||
| Baseline | |||||||
| Age (years) | 55.5 | [46.8–64.8] | 58.5 | [54.3–67.0] | 47.0 | [36.0–65.0] | 0.187 |
| Sex (male %) | 13,34.2% | 4 (33.3%) | 6 (26.1%) | 0.794 | |||
| Durationo of diabetes (years) | 8.0 | [3.5–12.8] | 8.5 | [3.0–15.5] | 7.0 | [5.0–13.0] | 0.995 |
| Previous treatment (insulin, %) | 14 (36.8%) | 5 (41.7%) | 19 (82.6%) | 0.002 | |||
| Insulin dose | 69.0 | [43.0–93.0] | 78.0 | [52.0–95.0] | 46.0 | [36.0–87.0] | |
| BIA + OHA | 14 (36.8%) | 4 (33.3%) | 15 (65.2%) | ||||
| LLA + OHA | 1 (8.3%) | 4 (17.4%) | |||||
| Previous OHA treatment | |||||||
| metf + SU+/−a‐GI | 13 (34.2%) | 5 (41.7%) | 2 (8.7%) | ||||
| metf + piog | 2 (5.3%) | ||||||
| metf + DPP4‐l | 4 (10.5%) | ||||||
| metf + SU + DPP4‐I | 1 (2.6%) | 2 (16.7%) | |||||
| piog + SU +/− α‐GI | 3 (7.9%) | 2 (8.7%) | |||||
| piog + SU + metf | 1 (2.6%) | ||||||
| Weight (kg) | 79.5 | [71.9–91.5] | 77.8 | [71.5–96.1] | 80.0 | [73.0–88.9] | 0.972 |
| BMI (kg/m2) | 30.6 | [27.3–33.4] | 30.6 | [27.0–36.0] | 30.8 | [29.0–34.6] | 0.734 |
| SBP (mmHg) | 134.0 | [125.5–143.0] | 139.0 | [124.0–145.0] | 131.0 | [125.0–143.0] | 0.592 |
| DBP (mmHg) | 76.5 | [66.8–88.0] | 81.5 | [71.0–87.5] | 78.0 | [73.0–85.0] | 0.715 |
| HbA1c (%) | 7.6 | [–7.0–9.0] | 7.9 | [6.9–8.5] | 7.5 | [7.0–8.4] | 0.914 |
| GA (%) | 17.4 | [14.9–23.0] | 17.7 | [13.9–22.0] | 18.1 | [14.6–20.6] | 0.651 |
| Glucose (mg/dL) | 127.0 | [114.5–172.8] | 138.0 | [120.5–165.3] | 109.0 | [92.0–147.0] | 0.037 |
| BUN (mg/dL) | 14.6 | [12.1–21.1] | 14.0 | [12.2–16.4] | 13.1 | [10.8–18.2] | 0.488 |
| Creatinine (mg/dL) | 0.9 | [0.7–1.0] | 0.8 | [0.7–0.9] | 0.8 | [0.7–1.0] | 0.540 |
| T.chol (mg/dL) | 174.0 | [152.8–200.5] | 157.5 | [143.0–200.0] | 160.0 | [141.0–175.0] | 0.230 |
| TG (mg/dL) | 146.5 | [112.0–215.8] | 167.5 | [105.8–291.5] | 139.0 | [101.0–180.0] | 0.354 |
| HDL (mg/dL) | 45.0 | [38.8–51.3] | 41.0 | [38.3–53.3] | 44.0 | [39.0–54.0] | 0.876 |
| LDL (mg/dL) | 93.5 | [69.2–122.7] | 74.2 | [59.6–107.1] | 81.4 | [67.6–100.6] | 0.362 |
| 2nd visit (after 8 weeks) | |||||||
| Weight (kg) | 77.4 | [69.1–88.0] | 79.0 | [71.7–98.1] | 76.5 | [69.9–85.0] | 0.597 |
| BMI (kg/m2) | 30.3 | [26.7–33.2] | 30.4 | [27.6–36.4] | 30.5 | [26.5–33.3] | 0.635 |
| HBA1c (%) | 6.8 | [6.2–7.4] | 7.3 | [6.9–8.0] | 7.9 | [7.2–10.0] | 0.002 |
| GA (%) | 14.0 | [12.0–17.5] | 17.0 | [14.0–18.0] | 18.5 | [14.9–23.9] | 0.011 |
| Glucose (mg/dL) | 115.0 | [98.8–136.3] | 151.5 | [118.3–168.0] | 161.0 | [118.0–206.0] | 0.002 |
| T.chol (mg/dL) | 152.5 | [140.0–179.5] | 155.0 | [132.5–174.3] | 138.0 | [123.0–195.0] | 0.717 |
| TG (mg/dL) | 140.5 | [100.8–190.5] | 170.0 | [114.8–222.8] | 140.0 | [101.0–288.0] | 0.370 |
| HDL (mg/dL) | 41.0 | [36.0–46.0] | 40.0 | [31.5–49.0] | 39.0 | [31.0–50.0] | 0.907 |
| LDL (mg/dL) | 80.3 | [59.0–102.1] | 83.5 | [55.6–101.9] | 66.2 | [39.0–102.8] | 0.592 |
| 3rd visit (after 22 weeks) | |||||||
| Weight (kg) | 75.7 | [66.9–86.7 ] | 80.4 | [72.8–98.5] | 74.8 | [66.3–81.8] | 0.269 |
| BMI (kg/m2) | 28.8 | [25.7–32.7] | 31.0 | [27.4–36.1] | 29.3 | [26.4–32.0] | 0.282 |
| HBA1c (%) | 6.6 | [6.1–7.2] | 6.9 | [6.6–7.6] | 9.1 | [7.4–10.7] | <0.001 |
| GA (%) | 15.0 | [12.5–17.5] | 14.0 | [13.0–22.0] | 22.0 | [16.0–26.8] | 0.001 |
| Glucose (mg/dL) | 110.0 | [99.8–136.3] | 129.0 | [108.5–145.5] | 174.0 | [145.0–232.0] | <0.001 |
| T.chol (mg/dL) | 160.0 | [133.8–190.3] | 155.5 | [129.3–188.5] | 144.0 | [117.0–184.0] | 0.241 |
| TG (mg/dL) | 147.5 | [107.8–211.8] | 168.5 | [111.3–245.8] | 140.0 | [105.0–176.0] | 0.562 |
| HDL (mg/dL) | 39.5 | [35.8–42.0] | 39.0 | [34.5–53.8] | 42.0 | [33.0–48.0] | 0.727 |
| LDL (mg/dL) | 79.5 | [63.1–104.5] | 72.9 | [54.5–115.9] | 70.2 | [58.6–110.2] | 0.909 |
Non‐normal distribution expressed by median and interquartile range (IQR). a‐GI, alpha‐glucosidase inhibitor; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BIA, biphasic insulin analog; BMI, body mass index; BUN, blood urine nitrogen; DBP, diastolic blood pressure; DPP4‐I dipeptidyl peptidase 4 inhibitor; eGFR: estimated glomerular filtration rate; GA, glycated albumin; HbA1c, glycated hemoglobin; HbA1c (+), patients who had improved glycated hemoglobin levels; HDL, high‐density lipiprotein cholesterol; HLAA, long acting insulin analog; HOMA‐β, homeostasis model assessment for beta‐cell function; HOMA‐IR, homeostasis model assessment for insulin resistance; LDL, low‐density lipoprotein cholesterol; metf, metformin; piog, pioglitazone; SU, sulphonylurea; T.chol, total cholesterol; TG, triglyceride; WH ratio, waist‐to‐hip ratio;Wt (+), patients who had a weight reduction.
Figure 3.
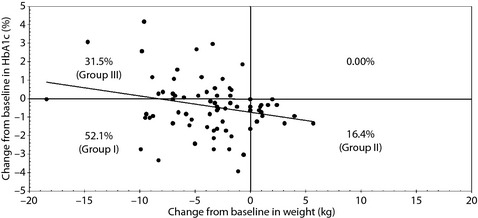
Efficacy of exenatide for glycated hemoglobin (HbA1c) level and weight changes over 22 weeks of combination treatment with exenatide, sulphonylurea and metformin. Group I, both HbA1c and weight responders; group II, HbA1c only responders; group III, weight only responders.
Predictive Independent Parameters to Achieve Optimal Glycemic Control or Weight Reduction with Exenatide in Combination with Metformin and SU
Of the 73 participants, the data of basal and stimulated insulin and C‐peptide levels within 3 months before initiation of exenatide were available in 50 participants. In 42 participants, serum insulin and C‐peptide levels were measured at the initiation of exenatide. Measurement of baseline insulin and C‐peptide was not included in the original protocol, and it caused these inconsistent results. Clinical and laboratory characteristics of the participants according to their HbA1c level reduction and weight loss responsiveness at 22 weeks with exenatide b.i.d. are summarized in Tables S2 and S3. Compared with the HbA1c(+) group, the HbA1c(−) group had significantly lower fasting glucose levels (responders vs non‐responders, 128.5 vs 109.0 mg/dL, P = 0.012), a larger proportion of previous insulin users (38.0% vs 82.6%, P < 0.001), higher baseline insulin levels (16.5% vs 32.4%, P < 0.001), higher HOMA of insulin resistance (IR) values (5.67 vs 11.3, P = 0.004), and higher HOMA of β‐cell function (β) levels (73.5 vs 331.9, P < 0.001). However, the HbA1c(−) group had significantly lower baseline C‐peptide levels (2.31 ng/mL vs 1.36 ng/mL, P < 0.001). In addition, there were no significant differences in clinical characteristics between the Wt(+) group and Wt(−) group.
We evaluated the correlation between serum delta HbA1c levels (changes in HbA1c between baseline and week 22), and various demographic and laboratory parameters (Table 2). There were significant correlations between delta HbA1c levels and age, initial HbA1c and serum fasting glucose levels, and several insulin secretory parameters, including HOMA‐β, basal and stimulated C‐peptide levels, and baseline insulin levels. Next, we also evaluated the correlation between serum delta bodyweight values (weight change across 22 weeks), and various demographic and laboratory parameters (Table 2). There were significant correlations between delta bodyweight values and serum fasting glucose, HOMA‐β, and baseline C‐peptide levels.
Table 2. Correlation between delta HbA1c over 22 weeks and clinical and laboratory parameters.
| Change of HbA1 c | Change of weight | |||
|---|---|---|---|---|
| Correlation coefficient | P‐value | Correlation coefficient | P‐value | |
| Age (years) | 0.263 | 0.024 | 0.001 | 0.996 |
| DM duration (years) | 0.067 | 0.576 | 0.068 | 0.568 |
| Height (cm) | −0.087 | 0.464 | −0.117 | 0.325 |
| Weight (kg) | −0.062 | 0.601 | −0.005 | 0.967 |
| BMI (kg/m2) | −0.023 | 0.850 | 0.053 | 0.658 |
| Waist (cm) | −0.014 | 0.905 | 0.075 | 0.530 |
| Hip (cm) | −0.049 | 0.678 | 0.096 | 0.419 |
| WH ratio | 0.050 | 0.672 | −0.069 | 0.563 |
| HbA1c (%) | 0.256 | 0.029 | −0.157 | 0.186 |
| GA (%) | 0.224 | 0.059 | −0.053 | 0.659 |
| Glucose (mg/dL) | 0.417 | <0.001 | −0.253 | 0.031 |
| Creatinine (mg/dL) | 0.015 | 0.900 | −0.071 | 0.550 |
| T.cholesterol (mg/dL) | 0.197 | 0.095 | −0.001 | 0.996 |
| TG (mg/dL) | 0.034 | 0.775 | −0.116 | 0.327 |
| HDL (mg/dL) | 0.031 | 0.792 | 0.092 | 0.441 |
| LDL (mg/dL) | 0.150 | 0.206 | 0.074 | 0.534 |
| HOMA‐β | −0.660 | <0.001 | 0.369 | 0.008 |
| HOMA‐IR | −0.271 | 0.057 | 0.095 | 0.511 |
| Baseline C‐peptide (ng/mL) | 0.497 | <0.001 | −0.280 | 0.047 |
| Simulated C‐peptide (ng/mL) | 0.284 | 0.044 | −0.204 | 0.151 |
| Baseline insulin (μU/mL) | −0.519 | <0.001 | 0.206 | 0.151 |
| Stimulated insulin (uU/mL) | −0.175 | 0.224 | 0.072 | 0.617 |
BMI, body mass index; DM, diabetes mellitus; GA, glycated albumin; HbA1c, glycated hemoglobin; HDL, high‐density lipiprotein cholesterol; HOMA‐β, homeostasis model assessment for beta‐cell function; HOMA‐IR, homeostasis model assessment for insulin resistance; LDL, low‐density lipoprotein cholesterol; T.cholesterol, total cholesterol; TG, triglyceride; WH ratio, waist‐to‐hip ratio.
To investigate the important factors in predicting the likelihood of achieving HbA1c level and weight reductions with exenatide BID in combination with metformin and SU in obese diabetic patients, a multiple regression analysis was carried out (Table 3). As described previously in Table 1, the HbA1c(−) group had a larger proportion of previous insulin users (82.6%). This exogenous insulin administration might have resulted in lower fasting glucose and increased insulin levels, which are associated with significantly higher HOMA‐IR and HOMA‐β values. In this regard, we included clinically important conventional variables and established parameters that are significantly correlated with delta HbA1c or bodyweight based on Table 2, and excluded significant factors that are influenced by exogenous insulin therapy, such as insulin and glucose levels. The analysis found C‐peptide level to be a significant independent predictor for reduction in HbA1c levels by treatment with exenatide in combination with SU and metformin (β = 0.865, P = 0.018). However, there was no significant association between GLP‐1 analog treatment and bodyweight reduction.
Table 3. Multivariable regression analysis between other glycaemic inidices and change of HbAlc Level a s dependent variables, over 22 weeks.
| Unstandardized coefficients | P‐value | 95.0% Confidence interval | |||
|---|---|---|---|---|---|
| B | Std. error | Lower | Upper | ||
| Age | 0.024 | 0.018 | 0.180 | −0.012 | 0.060 |
| HbA1c | 0.149 | 0.132 | 0.265 | −0.117 | 0.414 |
| Previeous insulin use | −0.246 | 0.585 | 0.676 | −1.427 | 0.934 |
| Baseline C‐peptide | 0.865 | 0.353 | 0.018 | 0.153 | 1.577 |
| Stimulated C‐peptide | −0.145 | 0.138 | 0.298 | −0.423 | 0.133 |
| HOMA‐β | 0.000 | 0.000 | 0.802 | −0.001 | 0.001 |
| HOMA‐IR | −0.033 | 0.018 | 0.071 | −0.070 | 0.003 |
HbA1c, glycated hemoglobin; HOMA‐ β, homeostasis model assessment for beta‐cell function; HOMA‐IR, homeostasis model assessment for insulin resistance.
We underwent subgroup analysis according to switching method, and included the switching method variable as an adjusting factor. We expected that serum insulin levels would be higher in patients who were previously treated with insulin than in those who were treated with oral hypoglycemic agents (OHA). The present results confirmed this hypothesis, because we found higher levels of insulin, HOMA‐β and HOMA‐IR, but lower levels of C‐peptide in the pre‐insulin use group than in the pre‐OHA use group (Table S4A). We classified patients according to previous drug use, as shown in Table 1. There were no differences in C‐peptide levels between the groups (Table S4B). We also divided the participants into subgroups based on the number of agents, and still did not find significant differences in C‐peptide levels between the groups (Table S4C). Table S5 shows the results of multiple regression analysis after adjusting for switching method. Table 3 and Table S5 showed consistent results.
Regarding the possible correlation between pre‐exenatide therapy with OHA, and HbA1c and bodyweight reduction, 35 participants (47.95%) used OHA. Except for two participants (2.7%) who were taking both metformin and pioglitazone, participants taking OHA were dependent on insulin secretagogue, such as SU and DPP‐4 inhibitor. To further investigate the predicting factors for bodyweight reduction without improvement of glycemic control, the 73 participants were classified into group III and the remaining groups (group I and II). The baseline C‐peptide (remaining group vs group III, 2.30 vs 1.36 ng/mL, P < 0.001), baseline insulin (16.5 vs 32.4 μU/mL, P < 0.001), glucose (139 vs 109 mg/dL, P = 0.012), HOMA‐β (73.5 vs 331.9, P < 0.001) and HOMA‐IR (5.67 vs 11.3, P = 0.004) were significantly different between the remaining groups (I and II) and group III. Group III tended to be younger than the remaining group (59.0 vs 54.0 years, P = 0.084). During the multivariate logistic regression analysis, weight reduction without improvement of glycemic control was used as a dependent variable, and conventional variables and established parameters that are significantly correlated with delta weight based on Table 2. such as age, HbA1c, HOMA‐β, HOMA‐IR, previous treatment of insulin and baseline C‐peptide, were entered as independent factors. To avoid the collinearity with HOMA values, baseline insulin level was not included. We found that the baseline C‐peptide level was also a significant independent predictor for reduction in bodyweight without improvement of glycemic control by treatment with exenatide in combination with SU and metformin (β = −1.348, P = 0.018).
Discussion
Secretory β‐cell dysfunction is a stronger contributing factor than insulin resistance in the development and aggravation of type 2 diabetes, and is known as a traditional characteristic of Korean type 2 diabetics1. Recent epidemiological data have also shown that Korean populations with BMI over 25 kg/m2 had been more prone to morbidity and mortality risk8. It is known that Asians generally have a slighter body build and less muscle mass than Caucasians. The World Health Organization recommends that cut‐off values in the definition of overweight and obesity should be lower for Asian than for Western populations. In this regard, the BMI categories in Korea are different from typical North American stratification. Recent epidemiological data have shown that the prevalence of obesity (BMI ≥25 kg/m2), severe obesity (BMI ≥30 kg/m2) and metabolic syndrome have rapidly increased in the past few decades in Korea8. Considering the pathophysiological characteristics and epidemiological trends of type 2 diabetes in the Korean population, we hypothesized that patients who are more obese and have reserved β‐cell function would be more likely to respond to GLP‐1 analog therapy. Based on this hypothesis, our attention was focused on investigating the expected tolerability/safety and effectiveness, as well as the predictive characteristics of patients who could achieve greater glycemic control and weight reduction with a GLP‐1 analog, by comparing indices related to β‐cell function and insulin resistance with clinically important conventional parameters, such as duration of diabetes, BMI, pre‐study medications and so on.
The present clinical study of obese Korean type 2 diabetics treated with exenatide b.i.d. with a combination of metformin and SU for up to 22 weeks showed two main findings. First, the study presents data on the tolerability, adverse effects and effectiveness of exenatide. Of 110 participants, 35 participants (31.9%) failed to continue on exenatide within 8 weeks (10 participants within 4 weeks, 25 between 5 and 8 weeks) of initiating exenatide b.i.d. treatment, which is a higher failure rate than previous reports carried out in Caucasian populations10. Of the 37 participants who withdrew from the study, the reasons for withdrawal included one participant (3.0%) with aggravated hyperglycemia and seven participants (21.2%) who were to reluctant to receive the exenatide injection. The remaining participants mainly withdrew because of gastrointestinal side‐effects, such as nausea, vomiting and diarrhoea, or injection repulsion. Based on this finding, caution should be exercised in treatment with exenatide in patients targeting intensive glycemic control; special attention needs to be paid to the patients’ tolerability. However, no additional patients discontinued exenatide after 8 weeks of treatment. Although participants who discontinued the drug were significantly less obese than participants who continued, no parameter predicted the discontinuation of exenatide therapy in the present study. In this regard, further studies targeting patients who tolerate exenatide well would be interesting and necessary to introduce a GLP‐1 analog into clinical practice. Regarding hypoglycemia, no major hypoglycemic episodes were observed in the present study, even though SUs were used in combination. The occurrence rate of hypoglycemia in Korean diabetes patients treated with exenatide was different from previous Diabetes Management for Improving Glucose Outcomes (AMIGO) clinical trials11. The hypoglycemia occurrence rate was lower in the present study than in the AMIGO studies. It might have been caused by a higher dropout rate than the AMIGO studies. Improvement in HbA1c levels and weight loss could be observed during the study period. Similar to a previous short‐term study of exenatide b.i.d. used with either metformin, SU or thiazolinedione, and a combination of metformin and SU in a Caucasian population10, 68.5% of the participants in the present study achieved a decrease in HbA1c. Despite the lack of a specific diet and exercise program, steady weight reduction (80.0 kg at baseline, 77.7 kg at the first visit and 77.0 kg at the second visit) during the 22‐week treatment period was observed in the present study. Of the participants who completed the study, 52.1% (n = 38) lost weight and improved their HbA1c levels. A HbA1c level ≤7% was achieved in 53.4% of participants who completed the study. However, the degree of bodyweight change was not different between those who achieved and did not achieve a HbA1c level ≤7%. In this regard, weight reduction and HbA1c reduction appear to be mutually exclusive in treatment with a GLP‐1 analog. With respect to lipid profiles, exenatide treatment was associated with a significant reduction in total cholesterol levels.
Second, the present study tried to provide the predictive characteristics of participants who would achieve a desirable glycemic index and bodyweight reduction. To appropriately evaluate β‐cell function, we used two specific indices that reflect insulin secretory capacity in the context of ambient insulin resistance. Among various factors assessed in the present study, age, high glucose parameters (HbA1c, fasting glucose) and β‐cell secretory parameters (HOMA‐β, fasting and stimulated C‐peptide levels, fasting insulin level) were significantly associated with a greater reduction in HbA1c levels with exenatide b.i.d. in combination with both SU and metformin in obese Korean type 2 diabetes patients, as seen in Table 2. In addition, insulin naïveté was significantly associated with HbA1c reduction responsiveness in obese Korean type 2 diabetics. This implies that clinical factors are still important in predicting responsiveness to GLP‐1 analogs in obese patients with type 2 diabetes. In the present study, delta HbA1c level was negatively associated with HOMA‐β and insulin levels, but was positively associated with C‐peptide levels. These conflicting data might have been influenced by higher usage of exogenous insulin analogs in the HbA1c(−)group. In this regard, we excluded insulin and glucose levels that might be influenced by exogenous insulin treatment in multiple regression analysis. Contrary to a previous efficacy report on the association of greater HbA1c reduction with insulin sensitivity (HOMA‐S) in a Caucasian population14, insulin secretory function of C‐peptide was associated with an adequate response of patients to GLP‐1 analog therapy. However, HOMA‐IR, as an independent variable, showed a tendency to influence the improvements of HbA1c in Korean type 2 diabetes patients treated with exenatide. Previous studies have shown that the reduced incretin effect itself and incretin receptor expression are not the primary events in the development of type 2 diabetes, but rather a consequence of the hyperglycemic state15. This phenomenon could be associated with suppressed insulin secretory function in type 2 diabetes. In summary, intact insulin secretory function accompanied with less use of exogenous insulin was the predictive parameter for achieving a desirable glycaemic index in Korean patients with type 2 diabetes.
Several recent studies including Korean type 2 diabetics have shown that treatment with GLP‐1 analogs and DPP‐4 inhibitors enhances pancreatic β‐cell function resulting in HbA1c level reductions17. Based on these results, we suggest that the addition of a GLP‐1 analog might be another option in poorly‐controlled Korean diabetic patients who had experienced pancreatic islet failure related to the main pathophysiological mechanism of development and aggravation in type 2 diabetes.
The present study had some limitations. First, this study was designed as an uncontrolled, open‐label, single center study, which limits its applicability and clinical relevance to the greater Korean population and to broader clinical practice. Second, not all participants were available for the evaluation of β‐cell function and insulin resistance, which could limit the interpretation of predictive characteristics that might apply well to those who were responders to the GLP‐1 analog. Third, insulin should have been discontinued before measuring insulin level to exclude exogenous insulin effect, which might confound the interpretation of insulin levels and HOMA values. However, this is the first well‐designed and documented study in the analysis and interpretation of GLP‐1 analog targeting Korean patients.
In conclusion, exenatide BID in combination with both metformin and SU was an effective option for obese Korean patients with suboptimally controlled type 2 diabetes. Notably, clinical and laboratory parameters, such as insulin naïveté, and preserved β‐cell function, such as C‐peptide levels, should be taken into consideration as important factors in the choice of GLP‐1 analog when predicting GLP‐1 analog response. Further studies are essential to investigate patients who tolerate the GLP‐1 analog well.
Supplementary Material
Table S1 | Anthropometric and clinical characteristics of participants who continued or discontinued the drug.
Table S2 | Anthropometric and clinical characteristics according to glycated hemoglobin improvement after 22 weeks.
Table S3 | Anthropometric and clinical characteristics according to weight improvement over 22 weeks.
Table S4A | HOMA index and C‐peptide levels according to previous treatment with insulin or oral hypoglycemic agents.
Table S4B | HOMA index and C‐peptide levels according to type of previous drug used.
Table S4C | HOMA index and C‐peptide levels according to switching method.
Table S5A | Multivariable regression analysis of other glycemic indices in which the dependent variable is the change in glycated hemoglobin during 22 weeks after adjusting for switching method.
Table S5B | Multivariable regression analysis of other glycemic indices in which the dependent variable is weight change during 22 weeks after adjusting for switching method.
Methods S1| Drug switching protocol.
Acknowledgements
All authors do not have any conflict of interest to declare. Statistical analyses in this study were supported by Jieun Kim (Basic Research Assistant Professor) and Min Woong Kang (Research Assistant) at Biostatistics Collaboration Unit, Yonsei University College of Medicine, Seoul, Korea. The authors thank Dong‐Su Jang BA (Medical Illustrator, Department of Research Affairs, Yonsei University College of Medicine, Seoul, Korea) for his help with the figures.
J Diabetes Invest 2014; 5: 554–562
References
- 1.Rhee SY, Woo JT. The prediabetic period: review of clinical aspects. Diabetes Metab J 2011; 35: 107–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nolan CJ, Damm P, Prentki M. Type 2 diabetes across generations: from pathophysiology to prevention and management. Lancet 2011; 378: 169–181 [DOI] [PubMed] [Google Scholar]
- 3.Field AE, Coakley EH, Must A, et al Impact of overweight on the risk of developing common chronic diseases during a 10‐year period. Arch Intern Med 2001; 161: 1581–1586 [DOI] [PubMed] [Google Scholar]
- 4.Ahren B. The future of incretin‐based therapy: novel avenues–novel targets. Diabetes Obes Metab 2011; 13(Suppl 1): 158–166 [DOI] [PubMed] [Google Scholar]
- 5.Yoon JS, Lee HW. Understanding the cardiovascular effects of incretin. Diabetes Metab J 2011; 35: 437–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim DJ, Lee MS, Kim KW, et al Insulin secretory dysfunction and insulin resistance in the pathogenesis of korean type 2 diabetes mellitus. Metabolism 2001; 50: 590–593 [DOI] [PubMed] [Google Scholar]
- 7.Byung‐Wan L, Jun H, Yim HJ, et al Dysfunctional pancreatic beta‐cells of critical stress play a more prominent role in the development of stress diabetes in critically burned Korean subjects. Metabolism 2010; 59: 1307–1315 [DOI] [PubMed] [Google Scholar]
- 8.Oh SW. Obesity and metabolic syndrome in Korea. Diabetes Metab J 2011; 35: 561–566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jee SH, Sull JW, Park J, et al Body‐mass index and mortality in Korean men and women. N Engl J Med 2006; 355: 779–787 [DOI] [PubMed] [Google Scholar]
- 10.Zinman B, Hoogwerf BJ, Duran Garcia S, et al The effect of adding exenatide to a thiazolidinedione in suboptimally controlled type 2 diabet es: a randomized trial. Ann Intern Med 2007; 146: 477–485 [DOI] [PubMed] [Google Scholar]
- 11.Buse JB, Henry RR, Han J, et al Effects of exenatide (exendin‐4) on glycemic control over 30 weeks in sulfonylurea‐treated patients with type 2 diabetes. Diabetes Care 2004; 27: 2628–2635 [DOI] [PubMed] [Google Scholar]
- 12.DeFronzo RA, Ratner RE, Han J, et al Effects of exenatide (exendin‐4) on glycemic control and weight over 30 weeks in metformin‐treated patients with type 2 diabetes. Diabetes Care 2005; 28: 1092–1100 [DOI] [PubMed] [Google Scholar]
- 13.Kendall DM, Riddle MC, Rosenstock J, et al Effects of exenatide (exendin‐4) on glycemic control over 30 weeks in patients with type 2 diabetes treated with metformin and a sulfonylurea. Diabetes Care 2005; 28: 1083–1091 [DOI] [PubMed] [Google Scholar]
- 14.Heald AH, Narayanan RP, Lowes D, et al HOMA‐S is Associated with Greater HbA1c Reduction with a GLP‐1 Analogue in Patients with Type 2 Diabetes. Exp Clin Endocrinol Diabetes 2012; 120: 420–423 [DOI] [PubMed] [Google Scholar]
- 15.Knop FK, Vilsboll T, Hojberg PV, et al Reduced incretin effect in type 2 diabetes: cause or consequence of the diabetic state? Diabetes 2007; 56: 1951–1959 [DOI] [PubMed] [Google Scholar]
- 16.Shu L, Matveyenko AV, Kerr‐Conte J, et al Decreased TCF7L2 protein levels in type 2 diabetes mellitus correlate with downregulation of GIP‐ and GLP‐1 receptors and impaired beta‐cell function. Hum Mol Genet 2009; 18: 2388–2399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Derosa G, Franzetti IG, Querci F, et al Exenatide plus metformin compared with metformin alone on beta‐cell function in patients with Type 2 diabetes. Diabet Med. 201229: 1515–1523 [DOI] [PubMed] [Google Scholar]
- 18.Chung HS, Lee MK. Efficacy of sitagliptin when added to ongoing therapy in korean subjects with type 2 diabetes mellitus. Diabetes Metab J 2011; 35: 411–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shin J, Chang JS, Kim HS, et al Effects of a 6‐month exenatide therapy on HbA1c and weight in Korean patients with Type 2 diabetes: a retrospective cohort study. Diabetes Metab J 2012; 36: 364–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 | Anthropometric and clinical characteristics of participants who continued or discontinued the drug.
Table S2 | Anthropometric and clinical characteristics according to glycated hemoglobin improvement after 22 weeks.
Table S3 | Anthropometric and clinical characteristics according to weight improvement over 22 weeks.
Table S4A | HOMA index and C‐peptide levels according to previous treatment with insulin or oral hypoglycemic agents.
Table S4B | HOMA index and C‐peptide levels according to type of previous drug used.
Table S4C | HOMA index and C‐peptide levels according to switching method.
Table S5A | Multivariable regression analysis of other glycemic indices in which the dependent variable is the change in glycated hemoglobin during 22 weeks after adjusting for switching method.
Table S5B | Multivariable regression analysis of other glycemic indices in which the dependent variable is weight change during 22 weeks after adjusting for switching method.
Methods S1| Drug switching protocol.


