Abstract
Aims/Introduction
Six kinds of oral antidiabetic drugs (OADs), including the new dipeptidyl peptidase 4 (DPP‐4) inhibitors, are available. The present study aimed to define trends within the prescribing patterns of OADs, as well as changes in glycemic control in Japan over a 10‐year period from 2002 to 2011.
Materials and Methods
We carried out a cross‐sectional study using data of type 2 diabetes mellitus patients from 24 clinics for 2002, 2005, 2008 and 2011. OAD use was analyzed combined with clinical data.
Results
Sulfonylureas (SUs) were the most commonly used OAD, but their use for monotherapy markedly decreased over the study period. Biguanides (BGs) were the second most commonly used OAD, and their prescribing rate increased both for mono‐ and combination therapy. DPP‐4 inhibitors (DPP‐4I), released in 2009, were the third most commonly prescribed OAD in 2011 both for mono‐ and combination therapy. Among combination therapies, two OADs were mostly prescribed, but the use of three OADs and four OADs in 2011 was two‐ and 14.8‐fold those in 2002. These trends were accompanied by an improvement in average glycated hemoglobin from 7.5 ± 1.2% in 2002 to 7.1 ± 0.9% in 2011.
Conclusions
The OAD prescribing trend has moved away from monotherapy with SUs and toward combination therapies to achieve better glycemic control. Increased use of BGs and DPP‐4I was predominant in 2011. These trends were accompanied by an improvement of the glycated hemoglobin level.
Keywords: Glycemic control, Oral antidiabetic drugs, Type 2 diabetes mellitus
Introduction
Despite diabetes mellitus being the most common chronic disease with high mortality, morbidity and costs, the number of people with diabetes is still increasing in Japan and other countries. Although the benefits of tight control of blood glucose have been well recognized and supported with evidences of several studies1, the management of diabetes is complex, and considered to be not quite successful in a real‐world setting.
The availability of six kinds of oral antidiabetic drugs (OADs) including a new type of OAD, dipeptidyl peptidase 4 inhibitors (DPP‐4I), has broadened the choice for the treatment of diabetes. Before 1993, only insulin, sulfonylureas (SUs) and biguanides (BGs) were available in Japan, although the use of BGs was very low. In 1993, the first alpha‐glucosidase inhibitor (AGI) was released, a rapid‐acting secretagogue of insulin (Glinide) and pioglitazone (Pio) were released in 1999, and the newest SU, glimepiride, was released in 2000. DPP‐4I have been available since 2009, and a high dose of metformin (up to 2,250 mg) has been available since 2010. However, trends in the prescribing of OADs in Japan have not been well documented so far.
The Japan Diabetes Clinical Data Management (JDDM) study group, which is comprised of 73 clinics, has recorded clinical data in a database platform named CoDiC4 since 1999, so we can trace the clinical data and prescribing patterns. The present study aimed to use CoDiC data to gain insights into the trends of diabetic treatment, especially the use of OADs, and to examine any associated changes in glycemic control.
Materials and Methods
Ethical Considerations
The present study was approved by the ethics committee of the Japan Diabetes Clinical Data Management Study Group (JDDM), which also included outside members, such as lawyers and ethics experts. The JDDM operates as an aggregate organization under the supervision of the central analytical facility and an ethics committee. Informed consent was obtained from all patients at each participating institute, in accordance with the Guidelines for Epidemiological Study of the Ministry of Health, Labor and Welfare of Japan.
Patients and Methods
We carried out a cross‐sectional study by accessing data from the JDDM database for the years 2002, 2005, 2008 and 2011. The JDDM study group consists of nationwide diabetes specialists. The clinical data recorded in the CoDiC at each clinic were made anonymous and collected annually into the central analytical center. Kanatsuka studied the trends for the use of OADs during 2002–2004 using the JDDM database, and reported the increase of the use of BG and decrease of the use of SUs5. In the present study, we expanded the period to 2002–2011, and analyzed the data for type 2 diabetes patients from 24 clinics, which joined JDDM through this study period to lessen the selection bias of physicians. The inclusion criteria were outpatients with type 2 diabetes who were aged 15 years or older. The most recent data from May to July for each year were collected for analysis.
The types of therapy were divided into five categories: diet, OAD therapy, insulin, combination of insulin and OAD therapy, and therapy with glucagon‐like peptide‐1 receptor agonist (GLP‐1; mono‐ or combination therapy). We divided OADs into six categories: SUs, BGs, AGIs, Pio, Glinides and DPP4I.
For each OAD therapy, the prescribing rates and patterns in each study year were recorded. For the calculation of the prescribing rates, we calculated the number of patients prescribed each individual OAD divided by the total number of patients on OAD therapy for that year. For the calculation of the rate of a specific prescribing pattern, we used the number of patients with that pattern divided by the total number of patients on OAD therapy for that year. Prescribing patterns were separated into two major types, monotherapy and combination therapy (treatment with two or more OADs). Patients who were prescribed OADs with insulin were excluded for the study of the use of OADs.
Glycated hemoglobin (HbA1c) was measured by high‐performance liquid chromatography using either of the ADAMS A1c (Arkray, Kyoto, Japan) or HLC‐723 (Tosoh corp., Tokyo, Japan). The value of HbA1c, which is equivalent to the internationally used HbA1c (%) defined by the National Glycohemoglobin Standardization program, is expressed by adding 0.4% to the HbA1c (%) defined by the Japan Diabetes Society7.
Statistical Analysis
We used one‐way anova to test the statistical significance of the patients' characteristics, which were continuous variables. The data of continuous variables were presented as mean ± standard deviation. The Cochran–Armitage test was used to test the statistical significance of trends in the prescribing patterns and sex ratio in patients' characteristics from 2002 to 2011. The χ2‐test was used to compare the performance rate between two groups. The Bonferroni correction was applied for multiple comparisons. All statistical analyses were carried out with the statistical software package SPSS (SPSS Inc., Chicago, IL, USA). P‐values < 0.05 were considered to be statistically significant.
Results
Table 1 lists the patients' characteristics for the calendar years of 2002, 2005, 2008 and 2011; the upper half shows data for all patients and the lower half shows data for patients on OAD without insulin therapy. Sex, age, body mass index (BMI), HbA1c and duration of diabetes varied significantly with year, as evidenced by the one‐way anova test.
Table 1. Patients characteristics.
| 2002 | 2005 | 2008 | 2011 | P | |
|---|---|---|---|---|---|
| All of Type 2 DM | |||||
| No. patients | 12,529 | 17,565 | 19,776 | 22,961 | |
| Male (%) | 7,650 (61.1) | 10,967 (62.4) | 12,486 (63.1) | 14,683 (63.9) | <0.0001* |
| Age | 62.7 ± 11.1 | 63.3 ± 11.3 | 64.0 ± 11.5 | 65.2 ± 11.6 | 0.0006** |
| BMI | 24.1 ± 3.7 | 24.3 ± 3.9 | 24.5 ± 4.0 | 24.8 ± 4.2 | <0.0001** |
| HbA1c | 7.5 ± 1.3 | 7.5 ± 1.2 | 7.3 ± 1.1 | 7.2 ± 1.1 | <0.0001** |
| Duration of DM (years) | 11.3 ± 8.9 | 11.8 ± 8.9 | 12.1 ± 8.8 | 13.5 ± 9.1 | <0.0001** |
| Type 2 Diabetes with oral antidiabetic drugs without insulin therapy | |||||
| No. patients | 6,517 | 9,378 | 10,409 | 13,011 | |
| Male (%) | 4,076 (62.5) | 5,975 (63.7) | 6,659 (64.0) | 8,460 (65.0) | 0.007* |
| Age | 62.9 ± 10.9 | 63.2 ± 11.0 | 63.8 ± 11.2 | 65.1 ± 11.4 | 0.047** |
| BMI | 24.3 ± 3.7 | 24.5 ± 4.0 | 24.7 ± 4.1 | 24.8 ± 4.2 | <0.0001** |
| HbA1c | 7.5 ± 1.2 | 7.5 ± 1.1 | 7.2 ± 1.0 | 7.1 ± 0.9 | <0.0001** |
| Duration of DM (years) | 11.2 ± 8.2 | 11.4 ± 8.2 | 11.6 ± 8.2 | 12.7 ± 8.4 | <0.0001** |
Data are n, n (%) or mean ± standard deviation. *P‐value from Cochrane–Armitage test; **P‐value from one‐way analysis of variance across years. BMI, body mass index; DM, diabetes mellitus; HbA1c, glycated hemoglobin; OAD, oral antidiabetic drug.
The proportion of patients treated with each type of therapy in each year is listed in Table 2. The percentage of diet therapy significantly decreased, and that of OAD therapy significantly increased over the study period, especially in 2011 (both P < 0.001). The percentage of insulin therapy without OAD significantly decreased, and that of insulin + OAD significantly increased over the study period. The percentage of patients treated with GLP‐1 was 1.2% in 2011.
Table 2. Proportion of each type of therapy.
| 2002 | 2005 | 2008 | 2011 | P † | |
|---|---|---|---|---|---|
| Diet (%) | 3,162 (25.2) | 4,319 (24.6) | 4,887 (24.7) | 4,567 (19.9) | <0.0001 |
| OAD w/o insulin (%) | 6,517 (52.0) | 9,378 (53.4) | 10,409 (52.7) | 13,011 (56.7) | <0.0001 |
| Insulin w/o OAD (%) | 1,825 (14.6) | 2,322 (13.2) | 2,395 (12.1) | 2,359 (10.3) | <0.0001 |
| Insulin with OAD (%) | 1,025 (8.2) | 1,546 (8.8) | 2,085 (10.5) | 2,741 (11.9) | <0.0001 |
| GLP‐1 (%) | – | – | – | 283 (1.2) |
Data are n (%). †P‐value from Cochrane–Armitage test across years 2002–2011. OAD, oral antidiabetic drug; GLP‐1, glucagon‐like peptide‐1 receptor agonist; w/o, without.
Trends for the prescribing rates of individual OADs are shown in Table 3. SU was the most commonly used drug, but its use for monotherapy significantly decreased from 37.7% in 2002 to 12.5% in 2011. SUs prescribed for combination therapy increased significantly from 43.2% in 2002 to 52.0% in 2011. BGs were the second most frequently prescribed drug, both for monotherapy and combination therapy. BGs prescribed for combination therapy significantly increased from 28.7% in 2002 to 47.7% in 2011. The use of AGI for both monotherapy and combination therapy gradually decreased from 2002 to 2011. The use of Pio for both monotherapy and combination therapy increased from 2002 to 2008, but decreased in 2011 (P < 0.0001). The use of glinide increased for monotherapy from 2002 to 2005 and for combination therapy from 2002 to 2008, but decreased in 2011 for both therapy (P < 0.0001). In 2011, the proportion of patients prescribed DPP‐4Is was 6.8% for monotherapy and 30.7% for combination therapy, showing that DPP‐4Is were the third most frequently prescribed class of drug.
Table 3. Annual patterns of prescribed regimens in type 2 diabetes with oral antidiabetic drug without insulin.
| 2002 | 2005 | 2008 | 2011 | P † | |
|---|---|---|---|---|---|
| Total no. patients | 6,517 | 9,378 | 10,409 | 13,011 | |
| Monotherapy (%) | 3,444 (52.8) | 4,461 (47.6) | 4,381 (42.1) | 4,673 (35.9) | <0.0001 |
| SU (%) | 2,460 (37.7) | 2,913 (31.1 | 2,244 (21.6) | 1,631 (12.5) | <0.0001 |
| BG (%) | 220 (3.4) | 439 (4.7) | 831 (8.0) | 1,163 (8.9) | <0.0001 |
| AGI (%) | 362 (5.5) | 416 (4.4) | 424 (4.1) | 349 (2.7) | <0.0001 |
| Pio (%) | 89 (1.4) | 228 (2.4) | 380 (3.6) | 262 (2.0) | <0.0001 |
| Glinide (%) | 313 (4.8) | 465 (5.0) | 502 (4.8) | 389 (3.0) | <0.0001 |
| DPP‐4I (%) | – | – | – | 879 (6.8) | |
| Combination therapy (%) | 3,073 (47.2) | 4,917 (52.4) | 6,028 (57.9) | 8,338 (64.1) | <0.0001 |
| SU (%) | 2,818 (43.2) | 4,491 (47.9) | 5,225 (50.2) | 6,763 (52.0) | <0.0001 |
| BG (%) | 1,869 (28.7) | 3,296 (35.1) | 4,490 (43.1) | 6,200 (47.7) | <0.0001 |
| AGI (%) | 1,503 (23.1) | 2,003 (21.4) | 2,333 (22.4) | 1,822 (14.0) | <0.0001 |
| Pio (%) | 592 (9.1) | 1,572 (16.8) | 2,440 (23.4) | 2,300 (17.7) | <0.0001 |
| Glinide (%) | 160 (2.5) | 259 (2.8) | 394 (3.8) | 380 (2.9) | <0.0001 |
| DPP‐4I (%) | – | – | – | 3,995 (30.7) | |
| Any two OADs (n) (%) | 2,305 (35.4) | 3,283 (35.0) | 3,645 (35.0) | 4,338 (33.3) | 0.007 |
| Any three OADs (n) (%) | 740 (11.4) | 1,481 (15.8) | 1,940 (18.6) | 3,227 (24.8) | <0.0001 |
| Any four OADs (n) (%) | 28 (0.4) | 153 (1.6) | 443 (4.3) | 773 (5.9) | <0.0001 |
Data are n or n (%). †P‐value from Cochrane‐Armitage test across years 2002–2011. AGI, alfa‐glucosidase inhibitor; BG, biguanide; DPP‐4I, dipeptidyl peptidase 4 inhibitor; OAD, oral antidiabetic drug; Pio, pioglitazone; SU, sulfonylurea.
Table 3 shows the annual patterns of prescribed regimens. For each OAD, monotherapy use significantly decreased, and combination therapy significantly increased over the 10‐year period. Among the combination therapies, treatment with two OADs was the most common (35.4% in 2002, 35.0% in 2005 and 2008, and 33.3% in 2011; P = 0.007). The proportion of patients prescribed three OADs doubled over the 10‐year period from 11.4% in 2002 to 24.8% in 2011, and the proportion prescribed four OADs increased 14.8‐fold from 0.4% in 2002 to 5.9% in 2011.
Among all the regimens, SU plus BG combination therapy and SU monotherapy were the most common. The sum of the top five regimens comprised 77.9% of all the regimens in 2002, 70.3% in 2005 and 63.0% in 2008, and decreased to 52.4% in 2011, which showed that the wider variety of regimens were used as years progressed.
Table 4 shows the trends of the prescribing rates of individual OADs in patients subgrouped by age (<65 or ≥65 years). The trends were the same as those described earlier; however, BG and Pio were prescribed less, and AGIs and Glinides tended to be prescribed more for the patients aged 65 years or older than for those aged under 65 years. The proportions of patients prescribed SU in 2002 and 2005 were similar between the two age groups; however, the proportions were significantly larger in the older group in 2008 and 2011. DPP‐4Is were significantly less commonly used in the older group (P < 0.001).
Table 4. Age‐group analysis of annual patterns of prescribed oral antidiabetic drugs in patients with type 2 diabetes treated with oral antidiabetic drugs without insulin.
| 2002 | 2005 | 2008 | 2011 | P † | |
|---|---|---|---|---|---|
| Age < 65 years | |||||
| No. patients | 3,481 | 5,020 | 5,327 | 6,200 | |
| SU (%) | 2,799 (80.4) | 3,926 (78.2) | 3,776 (70.9) | 3,862 (62.3) | <0.0001 |
| BG (%) | 1,317 (37.8) | 2,273 (45.3) | 3,085 (57.9) | 4,052 (65.4) | <0.0001 |
| AGI (%) | 945 (27.1) | 1,223 (24.4) | 1,376 (25.8) | 893 (14.4) | <0.0001 |
| Pio (%) | 437 (12.6) | 1,099 (21.9) | 1,607 (30.2) | 1,301 (21.0) | <0.0001 |
| Glinide (%) | 225 (6.5) | 339 (6.8) | 390 (7.3) | 297 (4.8) | <0.0001 |
| DPP‐4I (%) | – | – | – | 2,519 (40.6) | – |
| Age ≧ 65 years | |||||
| No. patients | 3,036 | 4,358 | 5,082 | 6,811 | |
| SU (%) | 2,477 (81.6) | 3,478 (79.8) | 3,693 (72.7)* | 4,533 (66.6)*** | <0.0001 |
| BG (%) | 770 (25.4)*** | 1,462 (33.5)*** | 2,236 (44.0)*** | 3,312 (48.6)*** | <0.0001 |
| AGI (%) | 916 (30.2)** | 1,196 (27.4)*** | 1,381 (27.2) | 1,279 (18.8)*** | <0.0001 |
| Pio (%) | 244 (8.0)*** | 701 (16.1)*** | 1,213 (23.9)*** | 1,263 (18.5)*** | <0.0001 |
| Glinide (%) | 246 (8.1)* | 385 (8.8) | 506 (10.0) | 473 (6.9)*** | <0.0001 |
| DPP‐4I (%) | – | – | – | 2,359 (34.6)*** | – |
Data are n, n (%), or mean ± standard deviation. *P < 0.05, **P < 0.01, ***P < 0.001. Age < 65 years vs Age ≧ 65 years by χ2‐test. †P‐value from Cochrane–Armitage test across years 2002–2011. AGI, alpha‐glucosidase inhibitor; BG, biguanide; DPP‐4I, dipeptidyl peptidase 4 inhibitor; Pio, pioglitazone; SU, sulfonylurea.
The rate of achieving the HbA1c target of <7.0% was significantly improved from 32.2% in 2002 and 32.5% in 2005 to 43.2% in 2008 (P < 0.0001) and 48.9% in 2011 (P < 0.0001). Figure 1 shows the trends of mean HbA1c achieved for OAD therapies subgrouped by the number of OADs in the regimen. The mean HbA1c in each group had significantly decreased through the study period (P < 0.0001). The mean HbA1c in each year tended to be higher with the increase in the number of prescribed OADs (P < 0.0001), except four OADs, in 2005, 2008 and 2011, and yet the differences in HbA1c levels among groups observed in 2002 tended to diminish in 2005, 2008 and 2011.
Figure 1.
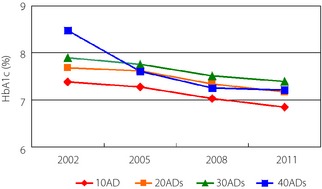
The change of mean glycated hemoglobin (HbA1c; %) of the oral antidiabetic drug (OAD) group subgrouped by the number of prescribed OADs. 1OAD, monotherapy of any OAD; 2OADs, combination therapy with any two OADs; 3OADs, combination therapy with any three OADs; 4OADs, combination therapy with any four OADs.
Discussion
This cross‐sectional study shows how the pattern of choice and number of OADs prescribed in Japan has changed in the 10 years from 2002 to 2011. The results indicate that SU monotherapy was markedly decreased, but SUs were still highly prescribed for combination therapy. There was a highly significant increase in the use of BGs both for monotherapy and combination therapy. These trends are consistent with the reports from the UK9, Italy11, France13, Taiwan14 and the USA15. The present study showed the trends of the increase of the use of BG and the decrease of that of SUs reported by Kanatsuka in 20065 had become more pronounced. Before the introduction of AGIs, Pio and Glinides in Japan, almost all prescribed OADs were SUs; although BGs were available, they were not widely used because of concerns about their side‐effects. As UK studies showed the effectiveness of metformin17, and the call for more intensive glycemic control1 as well as the control of postprandial hyperglycemia19 had been increased, the effectiveness of BGs were reassessed and BGs had also become more used in Japan. The obese population has been increasing in Japan during the study period21, and physicians have increasingly recognized the importance of insulin resistance as a pathophysiology of diabetes and have become more sensitive to risk of weight gain associated with the use of OADs. The dose of metformin available in Japan increased from 750 to 2,250 mg in 2009, which heightened the clinical effect of metformin and spurred the use of BG. Increased use of BGs was observed in both age groups (≥65 and <65 years) over the 10‐year period, although BGs and Pio were less prescribed for the older group compared with the younger group.
The present study also assessed the use of newly launched DPP‐4I. The release of DPP‐4I had a great impact on prescription patterns. Only 1 year after their release, DPP‐4I had become the third most used OAD both for monotherapy and combination therapy. In 2011 the proportion of diet therapy decreased and that of OAD therapy increased compared with earlier years. We consider this change to be the result of the release of DPP‐4I. The rapid increase of the use of DPP‐4I might also reflect the expectation of physicians that their use would avoid the side‐effects of previous OADs, such as weight gain and hypoglycemia22, while maintaining the insulin secretion ability of pancreatic β‐cells, as shown in rats and mice23. The OAD therapy might have been started earlier than before, encouraged by the low risk of hypoglycemia with DPP‐4I. The rapid early adoption of DPP‐4I into practice was also reported in the USA25, although several more years will be required to assess their place in therapy. In the older group, the use of DPP‐4I was 34.6%, which was less than the 40.6% usage in the younger group, despite the low risk of hypoglycemia associated with this drug. Physicians should be careful when prescribing a new drug for elderly patients.
The present study showed the increase of combination therapy, from 47.2% in 2002 to 64.1% in 2011. The use of three OADs in combination doubled, and that of four OADs increased 15‐fold in 10 years. In general, treatment of type 2 diabetes is carried out in a stepwise manner – initially with lifestyle modification, followed by the use of one OAD and then by a combination of two or more OADs or OAD with insulin is considered26. The United Kingdom Prospective Diabetes Study (UKPDS)27 reported that oral monotherapy is often effective at first, but combination therapy is ultimately necessary to achieve good glycemic control. Furthermore, with the availability of a wide variety of OADs, it is recommended that combination therapy be started early to achieve better glycemic control with a lower risk of side‐effects for each agent28. With the diversity of OADs and prescription patterns, the top five prescription patterns comprised just 52.4% of all treatment regimens in 2011; this percentage had gradually decreased from 77.9% in 2002.
As a consequence of these changes in OAD therapy, the proportion of patients achieving the target HbA1c level of <7.0% improved significantly from 32.2% in 2002 to 43.2% in 2008 and 48.9% in 2011. We speculate the factors relating to the improvement in 2008 were the increased use of BG and Pio, as the use of Pio as a combination therapy increased to 23.4% in 2008 compared with 9.1% in 2002 and 16.8% in 2005, and the use of BG both for monotherapy and combination therapy increased through the study period. The factors for the improvement in 2011 were speculated to be the introduction of DPP‐4I and a high dose of BG. The average HbA1c tended to be higher with the increase in the number of prescribed OADs, except for the four OADs group. However, the level was improved in every group over the study period (Figure 1).
The findings of the present study must be interpreted within the context of the limitations of the study design. This study was a retrospective analysis of OAD prescription patterns of diabetes specialists. Obviously, we can only describe a temporal association, and not cause and effect. We could not analyze the doses of prescribed OADs, or evaluate the appropriateness of therapy. Despite these limitations, the present study showed the prescription pattern in practice for a large number of patients with type 2 diabetes.
In conclusion, the OAD prescribing trend has moved away from monotherapy with SUs and toward combination therapies to achieve better glycemic control. Increased use of BGs and DPP‐4 inhibitors predominated in 2011 in Japan. These trends were accompanied by an improvement of HbA1c level.
Acknowledgements
The present study was supported by a grant from the Japan Diabetes Foundation. The CoDiC software system was constructed with the support of Novo Nordisk Pharm Ltd (Tokyo, Japan). The authors have no conflict of interest relevant to this article to declare. Professor Hirofumi Takagi (Dean and Professor, Faculty of Nursing, Toho University, Tokyo) and Mr Hajime Yamakage (Satista Co., Ltd.) are acknowledged for helpful advice on statistical methods. The authors thank the following members of JDDM who participated in this study (in alphabetical order): Dr Nobuyuki Abe, Dr Fumihiko Dake, Dr Hiroshi Fujiya, Dr Yoshihide Fukumoto, Dr Hiroshi Hayashi, Dr Koichi Hirao, Dr Koichi Iwasaki, Dr Koichi Kawai, Dr Mikihiko Kudo, Dr Yoshio Kurihara, Dr Hajime Maeda, Dr Takashi Miwa, Dr Yoko Notoya, Dr Gendai Lee, Dr Hideo Sasaki, Dr Sinichiro Shirabe, Dr Hiromichi Sugiyama, Dr Madoka Taguchi, Dr Masahiko Takai, Dr Hiroshi Takamura, Dr Hiroshi Takeda, Dr Noriharu Yagi, Dr Ritsuko Yamamoto and Dr Hiroki Yokoyama.
J Diabetes Invest 2014; 5: 581–587
References
- 1.The Diabetes Control and Complications Group . The effect of intensive treatment of diabetes on the development and progression of long term complications in insulin‐ dependent diabetes mellitus. N Engl J Med 1993; 329: 977–986 [DOI] [PubMed] [Google Scholar]
- 2.Ohkubo Y, Kishihata H, Araki E, et al Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with non‐insulin‐dependent diabetes mellitus: a randomized prospective 6‐year study. Diabetes Res Clin Pract 1995; 28: 103–117 [DOI] [PubMed] [Google Scholar]
- 3.UK Prospective Diabetes Study (UKPDS) Group . Intensive blood‐glucose control with sulphonylureas or insulin compared with conventional treatment and risk if complications in patients with type 2 diabetes (UKPDS33). Lancet 1998; 352: 837–853 [PubMed] [Google Scholar]
- 4.Kobayashi M, Yamazaki K, Hirao K, et al The status of diabetes control and anti‐diabetic drug therapy in Japan – a cross‐sectional survey of 17,000 patients with diabetes mellitus (JDDM1). Diabetes Res Clin Pract 2006; 73: 198–204 [DOI] [PubMed] [Google Scholar]
- 5.Kanatsuka A, Kawai K, Hirao K, et al Research on antihyperglycemic therapies in patients with type 2 diabetes mellitus in Japan (I): drug therapies and actual drug use. J Jpn Diabetes Soc 2006; 49: 409–415 (Japanese). [Google Scholar]
- 6.Kanatsuka A, Kawai K, Hirao K, et al Research on antihyperglycemic therapies in patients with type 2 diabetes mellitus in Japan (II): the effectiveness on glycemic control (JDDM7). J Jpn Diabetes Soc 2006; 49: 919–927 (Japanese). [Google Scholar]
- 7.Seino Y, Nanjo K, Tajima N, et al Report of the Committee on the classification and diagnostic criteria of diabetes mellitus. J Diabetes Invest 2010; 1: 212–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kashiwagi A, Kasuga M, Araki E, et al International clinical harmonization of glycated hemoglobin in Japan: from Japan diabetes society to national glycohemoglobin in standardization program values. J Diabetes Invest 2012; 3: 39–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walley T, Hughes D, Kendall H. Trends and influences on use of antidiabetic drugs in England, 1992–2003. Pharmacoepidemiol Drug Saf 2005; 14: 769–773 [DOI] [PubMed] [Google Scholar]
- 10.Filion KB, Joseph L, Boivin JF, et al Trends in the prescription of anti‐diabetic medications in the United Kingdom: a population‐based analysis. Pharmacoepidemiol Drug Saf 2009; 18: 973–976 [DOI] [PubMed] [Google Scholar]
- 11.Mazzaglia G, Yurgin N, Boye KS, et al Prevalence and antihyperglycemic prescribing trends for patients with type 2 diabetes in Italy: a 4‐year retrospective study from national primary care data. Pharmacol Res 2008; 57: 358–363 [DOI] [PubMed] [Google Scholar]
- 12.Baviera M, Monesi L, Marzona I, et al Trends in drug prescriptions to diabetic patients from 2000 to 2008 in Italy's Lombarsy Region: a large population‐based study. Diabetes Res Clin Pract 2011; 93: 123–130 [DOI] [PubMed] [Google Scholar]
- 13.Boyc KS, Yurgin N, Lage M. Trends in the prescription of antidiabetic medications in France: evidence from primary care physicians. Adv Ther 2007; 24: 803–813 [DOI] [PubMed] [Google Scholar]
- 14.Chiang CW, Chiu HF, Chen CY, et al Trends in the use of oral antidiabetic drugs by outpatients in Taiwan: 1997–2003. J Clin Pharm Ther 2006; 31: 73–82 [DOI] [PubMed] [Google Scholar]
- 15.Cohen FJ, Conklin J, Neslusan CA, et al Recent antihyperglycemic prescribing trends for U.S. privately insured patients with type 2 diabetes. Diabetes Care 2003; 26: 1847–1851 [DOI] [PubMed] [Google Scholar]
- 16.Boccuzzi SJ, Sung JCY, Wogen J, et al Utilization of oral hyperglycemic agents in a drug‐insured U.S. population. Diabetes Care 2001; 24: 1411–1415 [DOI] [PubMed] [Google Scholar]
- 17.UK Prospective Diabetes Study Group (UKPDS28) . A randomized trial of efficacy of early addition of metformin in sulphonylurea‐treated type 2 diabetes. Diabetes Care 1998; 21: 87–92 [DOI] [PubMed] [Google Scholar]
- 18.UK Prospective Diabetes Study (UKPDS) Group . Effect of intensive blood‐glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS34). Lancet 1998; 352: 854–865 [PubMed] [Google Scholar]
- 19.The DECODE‐study group on behalf of the European Diabetes Epidemiology Group . Is fasting glucose sufficient to define diabetes? Epidemiological data from 20 European studies. Diabetologia 1999; 42: 647–654 [DOI] [PubMed] [Google Scholar]
- 20.Qiao Q, Nakagami T, Tuomilehto J, et al; International Diabetes Epidemiology Group; DECODA Study Group . Comparison of the fasting and the 2‐h glucose criteria for diabetes in different Asian cohorts. Diabetologia 2000; 43: 1470–1475 [DOI] [PubMed] [Google Scholar]
- 21.Yoshiike N, Seino F, Tajima S, et al Twenty‐year changes in the prevalence of overweight in Japanese adults: the National Nutrition Survey 1976–1995. Obes Rev 2002; 3: 183–190 [DOI] [PubMed] [Google Scholar]
- 22.Karagiannis T, Paschos P, Paletas K, et al Dipeptidyl peptidase‐4 inhibitors for treatment of type 2 diabetes mellitus in the clinical setting; systematic review and meta‐analysis. BMJ 2012; 344: e1369. doi:10.1136/bmj.e1369 [DOI] [PubMed] [Google Scholar]
- 23.Inaba W, Mizukami H, Kamata K, et al Effect of long‐term treatment with the dipeptidyl peptidase‐4 inhibitor vildagliptin on islet endocrine cells in non‐obese type 2 diabetic Goto‐Kakizaki rats. Eur J Pharmacol 2012; 691: 297–306 [DOI] [PubMed] [Google Scholar]
- 24.Sato K, Nakamura A, Shirakawa J, et al Impact of the dipeptidyl peptidase‐4 inhibitor vildagliptin on glucose tolerance and β‐cell function and mass in insulin receptor substrate‐2‐knock‐out mice fed a high‐fat diet. Endocrinology 2012; 153: 1093–1102 [DOI] [PubMed] [Google Scholar]
- 25.Alexander CG, Sehgal NL, Moloney RM, et al National trends in treatment of type 2 diabetes mellitus, 1994–2007. Arch Intern Med 2008; 168: 2088–2094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Inzucchi SE, Bergenstal RM, Buse JB, et al Management of hyperglycemia in type 2 diabetes: a Patient‐Centered Approach, Position Statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2012; 35: 1364–1379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Turner RC, Cull CA, Frighi V, et al Glycemic control with diet, sulfonylurea, metformin or insulin in patients with type 2 diabetes mellitus: progressive requirement for multiple therapies (UKPDS49). UK Prospective Diabetes Study (UKPDS) group. JAMA 1999; 281: 2005–2012 [DOI] [PubMed] [Google Scholar]
- 28.Inzucchi SE. Oral antihyperglycemic therapy for type 2 diabetes. Scientific review. JAMA 2002; 287: 360–372 [DOI] [PubMed] [Google Scholar]
- 29.Kimmel B, Inzucchi SE. Oral agents for type 2 diabetes: an update. Clin Diabetes 2005; 23: 64–76 [Google Scholar]


